Key Points
Question
Can the symptoms of laryngopharyngeal reflux (LPR) improve without the use of medication?
Findings
In this cohort study that included 184 patients, there was no significant difference in reflux symptom index reduction between patients treated with alkaline water, a plant-based, Mediterranean-style diet, and standard reflux precautions vs those treated with proton pump inhibitors (PPI) and standard reflux precautions.
Meaning
The symptoms of LPR can improve with alkaline water and a plant-based diet with results not significantly different from the use of a standard PPI regimen.
This cohort study examines the efficacy of a dietary approach to treating laryngopharyngeal reflux using alkaline water, a plant-based, Mediterranean-style diet, and standard reflux precautions compared with the traditional treatment approach using proton pump inhibition.
Abstract
Importance
Laryngopharyngeal reflux (LPR) is a common disorder with protean manifestations in the head and neck. In this retrospective study, we report the efficacy of a wholly dietary approach using alkaline water, a plant-based, Mediterranean-style diet, and standard reflux precautions compared with that of the traditional treatment approach of proton pump inhibition (PPI) and standard reflux precautions.
Objective
To determine whether treatment with a diet-based approach with standard reflux precautions alone can improve symptoms of LPR compared with treatment with PPI and standard reflux precautions.
Design, Setting, and Participants
This was a retrospective medical chart review of 2 treatment cohorts. From 2010 to 2012, 85 patients with LPR that were treated with PPI and standard reflux precautions (PS) were identified. From 2013 to 2015, 99 patients treated with alkaline water (pH >8.0), 90% plant-based, Mediterranean-style diet, and standard reflux precautions (AMS) were identified. The outcome was based on change in Reflux Symptom Index (RSI).
Main Outcomes and Measures
Recorded change in the RSI after 6 weeks of treatment.
Results
Of the 184 patients identified in the PS and AMS cohorts, the median age of participants in each cohort was 60 years (95% CI, 18-82) and 57 years (95% CI, 18-93), respectively (47 [56.3%] and 61 [61.7%] were women, respectively). The percentage of patients achieving a clinically meaningful (≥6 points) reduction in RSI was 54.1% in PS-treated patients and 62.6% in AMS-treated patients (difference between the groups, 8.05; 95% CI, −5.74 to 22.76). The mean reduction in RSI was 27.2% for the PS group and 39.8% in the AMS group (difference, 12.10; 95% CI, 1.53 to 22.68).
Conclusions and Relevance
Our data suggest that the effect of PPI on the RSI based on proportion reaching a 6-point reduction in RSI is not significantly better than that of alkaline water, a plant-based, Mediterranean-style diet, and standard reflux precautions, although the difference in the 2 treatments could be clinically meaningful in favor of the dietary approach. The percent reduction in RSI was significantly greater with the dietary approach. Because the relationship between percent change and response to treatment has not been studied, the clinical significance of this difference requires further study. Nevertheless, this study suggests that a plant-based diet and alkaline water should be considered in the treatment of LPR. This approach may effectively improve symptoms and could avoid the costs and adverse effects of pharmacological intervention as well as afford the additional health benefits associated with a healthy, plant-based diet.
Introduction
Laryngopharyngeal reflux (LPR) has been implicated as a cause of multiple symptoms and diseases of the head and neck. Chronic dysphonia, excessive throat clearing, persistent cough, globus pharyngeus, dysphagia, as well as chronic sinusitis, subglottic stenosis, laryngospasm, and even carcinoma have been linked to LPR. Treatment of this disease, however, has remained controversial, with few studies demonstrating that the current predominant regimen of proton pump inhibition (PPI) has a statistical advantage over other therapeutic modalities. Moreover, the diagnosis and treatment of LPR using this approach has significant economic ramifications on society, with PPI therapy alone costing more than 13 billion dollars in the United States in 2009.
Laryngopharyngeal reflux is thought to be primarily mediated through a process of exposure of the laryngopharynx to an acidic environment in the presence of pepsin. Pepsin, which can be active and reactivated up to a pH of 8.0, is presumed to play a key role in causing ongoing damage to the macroenvironment and microenvironment of the cellular structure of the laryngopharynx. Exposure of pepsin to alkaline water with a pH level greater than 8 has been shown to inactivate pepsin, suggesting that alkaline water might be useful as an adjunct treatment for patients with LPR. A pilot study using a low-acid diet demonstrated a possible improvement in symptoms and findings. Pepsin, which is secreted as the zymogen pepsinogen from the chief cells in the stomach, is thus a potential target for LPR treatment.
Pepsinogen secretion is regulated by the concentration of amino acids in the stomach, gastrin secretion, and vagal stimulation. The typical American diet is high in animal protein content with up to 35% of calories from animal-based protein. A primarily plant-based diet low in animal protein lowers the gastric load of amino acids, which may indirectly lead to decreased activity of pepsin by decreasing gastrin secretion. Previous treatment strategies have aimed at counteracting acid, either through diet or pharmacological inhibition. To our knowledge, no study to date has suggested a dietary approach aimed at decreasing pepsin secretion to potentially control the symptoms of laryngopharynx acid reflux disease (LPRD). Given the current gold standard of PPI use, we hypothesized that the treatment of LPR with PPI would be significantly better than treatment with the plant-based diet. In this preliminary study, we describe and compare the effects of alkaline water, a Mediterranean-style diet, and standard reflux precautions with those of PPI therapy and standard reflux precautions.
Methods
Study Design and Participants
The New York Medical College institutional review board reviewed and approved the study prior to its initiation. A waiver of informed consent of study participants was also granted because participant data were protected and deidentified. A retrospective medical chart review of all patients from the senior author (C.H.Z.) who were diagnosed with LPR from 2010 to 2015 was performed. Patients who had a single diagnosis of LPR or a dual diagnosis of LPR with dysphagia, dysphonia, or cough were first identified from the electronic medical record system using the International Classification of Diseases, Ninth Revision (ICD-9) codes 530.11 and 478.79 for LPR, 787.2 for dysphagia, 748.42 for dysphonia, and 786.2 for cough. A total of 822 consecutive patients were extracted from 2010 to 2012 and 848 patients from 2013 to 2015. The patient cohort from 2010 to 2012 was treated with either esomeprazole twice daily or dexlansoprazole daily and standard reflux diet and precautions (PS), which prohibited coffee, tea, chocolate, soda, greasy, fried, fatty and spicy foods, and alcohol. The second cohort from 2013 to 2015 was treated with alkaline water (pH >8.0), a plant-based, Mediterranean-style diet, and standard reflux precautions. Patients were given both verbal and written instructions to replace all beverages with alkaline water and to eat a 90% to 95% plant-based diet consisting of vegetables, fruits, whole grains, and nuts with less than 5% to 10% from animal-based products until the follow-up at 6 weeks. Typically, this entailed 2 to 3 meals per week containing 3 to 4 ounces of meat and minimal intake of dairy.
Of the 1670 patients identified, 698 patients were included in the study. Primary inclusion criteria were as follows: (1) presented to the practice with LPR symptoms and had not begun treatment and a pretreatment Reflux Symptom Index (RSI) of 10 or greater; (2) treatment with 1 of the 2 aforementioned regimens; (3) documentation showing compliance with either dietary or pharmacological treatment; and (4) 1 posttreatment follow-up RSI at 6 weeks. Patients were asked to pay specific attention to the meals containing animal-based products and were considered compliant only if they were limiting such products to 2 to 3 times per week. Compliance was documented through a questionnaire and verbal history taking by the senior author.
Rigorous exclusion criteria were subsequently used to select well-matched samples and to minimize bias and eliminate confounding factors. Of the patients identified, 193 were excluded owing to a concomitant diagnosis of a benign disorder causing dysphonia, which included muscle tension dysphonia, unilateral vocal fold paralysis or paresis, vocal fold scar, subglottic stenosis, Reinke edema, anterior web, nodules, cyst, polyp, granuloma, and ectasia. One hundred sixty seven patients were excluded for concurrent presumed neuropathic cough or prior treatment with a neuropathic pain medication (amitriptyline, gabapentin, and/or tramadol). Forty patients were excluded for a history of laryngeal malignant abnormality and/or radiation therapy; 38 patients for a concurrent diagnosis of allergic rhinitis, sinusitis, or recent upper respiratory illness; 35 patients for current smoking; 33 patients owing to an unrelated diagnosis causing dysphagia, such as cricopharyngeal dysfunction or cervical osteophytes; and 8 patients owing to a nasopharyngeal or oropharyngeal mass.
Data Collection and Assessment of LPR
The tool used to assess the presence of LPR was the RSI. The severity of LPR and any change in symptoms following treatment were determined by the RSI. To confirm adherence to diet and medication, historical data was reviewed from outpatient follow-up visits. The mean pretreatment RSI and mean posttreatment RSI for each group was calculated.
Patient Cohorts and Statistical and Power Analyses
Patients were placed in 1 of 2 primary treatment cohorts based on when they presented to the practice: (1) a PPI inhibitor and standard reflux precautions (PS) and (2) alkaline water, a plant-based, Mediterranean-style diet, and standard reflux precautions (AMS). Patient responses were measured at baseline and at 6 weeks after initiation of treatment. The objective was to determine if there was a significant difference between the standard treatment and the new dietary approach.
Statistical analysis was performed using SAS (version 9.4, SAS institute), NCSS (version 10, SAS institute), and GraphPad Prism (version 6.0, SAS institute). The baseline distribution of RSI category and symptom presentation was compared for the 2 groups using the Mantel-Haenszel test for RSI and the χ2 test for symptom presentation, with a significance level set at 5%.
As suggested by Lien et al, a patient with 6-point or greater reduction in the RSI score was considered a clinical responder. Using this criterion, patients in each cohort were grouped as responders or nonresponders and differences between the means of the 2 cohorts (PS and AMS) and 95% CIs were determined.
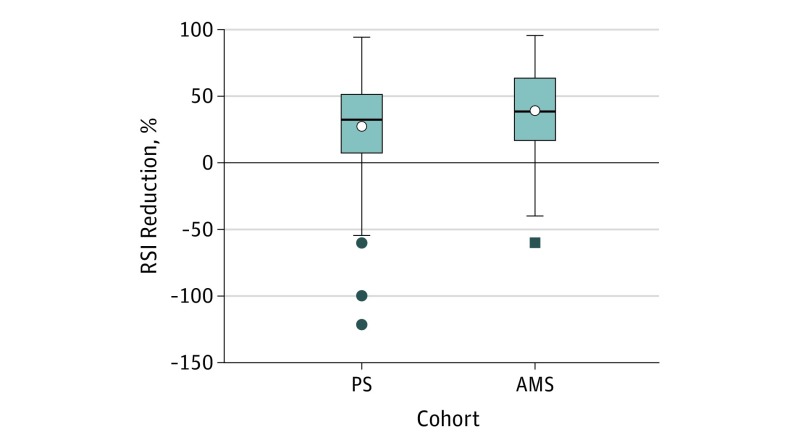
As an additional, relative quantification of the effect of the various treatments, patient responses at 6 weeks were also represented as “percent decrease” in RSI from initial scores. Using this definition, a patient whose RSI score decreased from 20 to 10 would have a 50% reduction. If the RSI decreased to 5, there would be a 75% reduction. Thus, a 100% reduction would reflect a complete relief of symptoms. If a patient’s symptoms worsened, the change in RSI would be reflected as negative results. Thus, a doubling of RSI score would be a 100% decrease. Cohorts were analyzed in their entirety. The percent reduction for each group was determined and the percent difference between the groups and 95% CI were calculated (Figure).
Figure. Percent Reduction in RSI.
PS Indicates proton pump inhibitor and standard reflux precautions; AMS, Mediterranean/plant-based diet, alkaline water, and standard reflux precautions. The box contains the twenty-fifth to the seventy-fifth percentile. The horizontal, bisecting line is the median, the open circles indicate the mean. The whiskers were calculated using the Tukey method and the solid circles and/or square indicate outliers.
Power analyses were performed for both the 6-point reduction in RSI scores and the percent reduction in RSI scores using the Decision Support Systems research tool. There was wide variability in patient response to LPR treatment measured by change in RSI score, both in the literature and in our own clinical experience. Furthermore, there can be a significant placebo effect in response to treatment. The values that were used for the power analysis reflect this variability. For the 6-point reduction in RSI scores, a percentage, 2-sample power analysis was performed. Using a 50% response-to-treatment rate in the PS cohort and a 25% placebo effect in the AMS cohort, it was calculated that 60 patients per cohort would have 81.6% power at an α of 5%. Increasing the cohort size to 75 participants would provide 89.3% power. Allowing for patient dropout and noncompliance, we sought to enroll approximately 75 patients.
For the percent decrease in RSI, an average, 2-sample power analysis was performed. Using a 30% decrease in RSI in the PS cohort (standard deviation [SD], 30%) and a 15% decrease in RSI (placebo effect) (SD, 30%) in the AMS cohort, it was calculated that 60 patients per cohort would have 78.2% power at an α of 5%. Increasing the cohort size to 75 participants would provide 86.5% power. Allowing for patient dropout and noncompliance, we sought to enroll approximately 75 patients.
Results
A total of 184 patients were identified who underwent 1 of the 2 previously delineated treatment regimens for LPR. There were 85 patients in the PS cohort and 99 patients in the AMS cohort. The characteristics of the PS and AMS cohorts were similar (Table 1). The median age of participants in each cohort was 60 years (95% CI, 18-82) and 57 years (95% CI, 18-93), respectively. Follow-up time after initiation of treatment was approximately 6 weeks.
Table 1. Characteristics of Patients in Each Cohort.
| Characteristic | PS Cohort (n = 85) | AMS Cohort (n = 99) |
|---|---|---|
| Reason for exclusion, patients, No.(%) | ||
| Dysphonia caused by coexisting diagnosis | 93 (48.2) | 100 (51.8) |
| NC or prior treatment with neuropathic medication | 86 (51.5) | 81 (48.5) |
| Prior laryngeal malignant abnormality or RT | 22 (55) | 18 (45) |
| Coexisting AR, sinusitis, or recent URI | 18 (47.4) | 20 (52.6) |
| Current smoker | 16 (45.7) | 19 (54.3) |
| Dysphagia caused by coexisting diagnosis | 17 (51.5) | 16 (48.5) |
| Nasopharyngeal or oropharyngeal mass | 5 (62.5) | 3 (37.5) |
| Sex, No. (%) | ||
| Male | 38 (44.7) | 38 (38.3) |
| Female | 47 (56.3) | 61 (61.7) |
| Age, median (range), y | 60 (18-82) | 57 (18-93) |
| Type of PPI, No. (%) | ||
| Esomeprazole | 39 (45.9) | |
| Dexlansoprazole | 46 (54.1) | |
| PPI-naive, No. (%) | 36 (42.4) | 44 (44.4) |
| Baseline RSI, No. (%)a | ||
| <11 | 6 (7.1) | 10 (10.1) |
| 11-20 | 44 (51.2) | 51 (51.5) |
| 21-30 | 23 (27.1) | 28 (28.3) |
| >30 | 12 (14.1) | 10 (10.1) |
| Cohort, mean (SD) | 20 (8.2) | 19 (7.4) |
| Primary symptom, No. (%)b | ||
| Cough | 32 (37.6) | 27 (27.3) |
| Dysphagia | 24 (28.2) | 38 (38.4) |
| Dysphonia | 29 (34.1) | 34 (34.3) |
Abbreviations: AMS, alkaline water, Mediterranean-style diet, standard reflux precautions; AR, allergic rhinitis; NC, neuropathic cough; PS, proton pump inhibitor and standard reflux precautions; RSI, reflux symptom index; RT, radiation therapy; URI, upper respiratory infection.
P = .41, calculated using the Mantel-Haenszel method.
P = .23, calculated using χ2 test.
Using the 6-point reduction (improvement) in RSI score as a response to treatment, patients in the PS cohort did not experience a response that was statistically better or worse than those patients in the AMS cohort at 6 weeks (Table 2). Of 85 patients in the PS cohort, 46 (54%) achieved a 6-point reduction in RSI score, compared with 62 (62.6%) in the AMS cohort (differences in proportion achieving a 6-point reduction in RSI score, 8.51; 95% CI, −5.74 to 22.76). Using reduction in RSI score as a continuous variable to assess response, there was no statistical difference between the mean reductions in the PS cohort (mean, 5.92; 95% CI, 4.10 to 7.76) and the AMS cohort (mean, 7.05; 95% CI, 5.95 to 8.14) (difference in means, 1.12; 95% CI, −1.00 to 3.24).
Table 2. Patient Responders Using a 6-Point Reduction in RSI After 6 Weeks of Treatment.
| Variable | Treatment Group | |
|---|---|---|
| PS | AMS | |
| No. of patients | 85 | 99 |
| No. of responders | 46 | 62 |
| No. of nonresponders | 39 | 37 |
| Percentage of patients with decrease in RSI ≥6 | 54 | 63 |
| Difference in percentage of patients with decrease in RSI ≥6 (95% CI) | 8.51 (−5.74 to 22.76) | |
Abbreviations: AMS, alkaline water, Mediterranean-style diet, standard reflux precautions; PS, proton pump inhibitor and standard reflux precautions; RSI, reflux symptom index.
As an additional measure of response to treatment, the percent reduction in RSI for the 2 cohorts was compared. The mean percent reduction in RSI score in the PS cohort was 27.2, compared with 39.8 in the AMS cohort. The difference in percent reduction between groups was statistically significant in favor of the AMS cohort (difference, 12.10; 95% CI, 1.53 to 22.68) (Table 3) (Figure).
Table 3. Mean Percent Reduction in RSI After 6 Weeks of Treatment.
| Variable | Treatment Group, Mean % (95% CI) | |
|---|---|---|
| PS (n = 85) | AMS (n = 99) | |
| Pretreatment RSI | 20.2 (18.4-22) | 19.1 (17.6-20.6) |
| Posttreatment RSI | 14.3 (12.4-16.2) | 12.1 (10.4-13.7) |
| RSI percent reduction | 27.2 (18.5-35.9) | 39.3 (33.1-45.5) |
| Difference in RSI percent reduction between groups | 12.1 (1.5-22.7) | 12.1 (1.5-22.7) |
Abbreviations: AMS, alkaline water, Mediterranean-style diet, standard reflux precautions; PS, proton pump inhibito and standard reflux precautions; RSI, reflux symptom index.
Discussion
Our data demonstrate that treatment with PPI therapy is not significantly more effective than a wholly dietary approach; however, the data do suggest that the change in RSI scores with the dietary approach could be of clinical importance. No difference was noted between the 2 cohorts in the proportion achieving a 6-point or greater reduction in RSI scores, a clinically meaningful change. However, the 95% CI suggests that the true difference between the treatment groups in the proportion reaching a 6-point reduction could be as high as 22.76, which would be a clinically significant difference.
When percent reduction in RSI was used to compare the 2 treatment groups, a statistically greater mean percent reduction was seen with AMS treatment. Since there is no standard to assess the clinical importance of a given percent reduction in RSI scores, the clinical meaning of this difference requires further study.
Using historical controls of standard reflux precautions for gastroesophageal reflux disease (GERD) symptoms alone, prior studies have demonstrated little clinical change in reflux incidence with these lifestyle approaches. This study indicates that, by supplementing with alkaline water and a Mediterranean-style diet, effective control of symptoms as defined by the RSI may be obtained without PPI use. Other benefits of this diet-based approach include decreased risk for and improved control of cardiovascular disease, diabetes, stroke, and cancer, and avoiding the risks of drug interaction or complication.
Our patient population included individuals with different presenting symptoms, including cough, dysphagia, and dysphonia. The current study was not powered to measure subgroup responses in a statistically significant manner. The response to different treatments (PS vs AMS) by subgroups of patients with particular presenting symptom may be clinically relevant, and will be the focus of subsequent studies.
In the past 3 decades, the pharmacological treatment of LPRD has focused on PPI, which is the most effective drug therapeutic approach to date. Use of PPI allows for the suppression of hydrogen ion secretion and thus elevation of gastric pH. While response rates to PPI are highly variable across patients, improvement and normalization of symptoms have been achieved in numerous studies. Patients with GERD who are treated with PPIs have a high rate of erosive lesion healing, and PPIs are much more effective than other medications in alleviating symptoms. Proton pump inhibitors also play an important role in cases of refractory LPRD, complicated disease, and poor diet control.
Despite the pervasive use of PPI therapy and reflux precautions, the gold standard treatment for LPRD remains elusive. Many publications report a range of responses to PPI therapy and often use unvalidated or inconsistent outcome measures. Meta-analyses show very little difference in pretreatment and posttreatment symptoms compared with placebo, and standard practice shifts to twice daily, then 3 times daily, in cases of refractory LPRD. A substantial number of patients demonstrate relative resistance to PPIs. Studies on surgical treatment with fundoplication have also been inconclusive. Nonpharmacological approaches have been studied as well. Koufman reported that a strict, low-acid diet may have benefits on the symptoms and findings of PPI-resistant LPRD. Alkaline water has been suggested as an effective adjunct, presumably by inactivating pepsin and neutralizing the acidic environment.
A major shortcoming of LPRD studies is the lack of standardization. Specifically, there is no gold standard diagnostic test for LPRD, with many practitioners using a variety of diagnostic tools. Twenty-four-hour double-probe ambulatory pH monitoring has been suggested as a standard for diagnosis. A newer oropharyngeal pH probe specifically designed to measure oropharyngeal pH variation has also been developed. Other modalities, such as the RSI, are based on subjective measures. The RSI, a self-administered survey that consists of scaling 9 items that represent various symptoms of LPRD, has been proposed as a reliable tool for the first-line assessment of suspected LPRD and for treatment guidance. Proponents advocate that it is highly reproducible and exhibits both construct-based and criterion-based clinical validity. However, the RSI is not without its limitations. Park et al reported that the RSI had low specificity and there was no significant difference in the RSI between their test and control groups, while Friedman et al showed that it was of poor diagnostic utility for prediction of RYAN score, a weighted measure of reflux parameters akin to the DeMeester score for GERD.
An important consideration prior to the start of PPI therapy in any patient is the potential of detrimental effects. According to several studies, prescription of PPIs is inappropriate in 40% to 80% of individuals. Proton pump inhibitors can cause adverse drug effects, such as abdominal pain, nausea, diarrhea and constipation. In addition, they have been associated with a variety of other adverse events, including fundic gland polyps secondary to hypergastrinemia, hypomagnesemia, hypocalcemia, bone fractures, decreased absorption of vitamin B12, diarrhea, and pneumonia, though evidence for the clinical significance of these relationships is limited. Importantly, a landmark data-mining study published in 2015 concluded that GERD patients treated with PPI have an increased association with myocardial infarction and a 2-fold increase in association with cardiovascular mortality on survival analyses. Dementia and stroke have also been linked to PPI use.
A recent study suggested that medical error is currently the third leading cause of death in the United States. Drug reactions and interactions account for a considerable percentage of these deaths. Proton pump inhibitors have several purported interactions with other medications, the most widely studied of which are clopidogrel and methotrexate. Thus, for an individual patient, it is pertinent to address the indication of use and balance the risks and benefits of therapy.
Over the past 30 years, LPRD has been implicated as a cause and cofactor in numerous head and neck conditions, such as chronic cough, asthma, and chronic sinusitis. In addition, LPRD has been shown to have an influence in respiratory disorders, such as subglottic stenosis, laryngospasm, vocal cord dysfunction, asthma, and upper aerodigestive tract malignant diseases. The prevalence of esophagitis and Barrett esophagus in patients with LPRD is 12% and 7%, respectively. Some experts believe that these numbers warrant esophagoscopy for all patients with LPRD, especially in the setting of a more than 6-fold increase in esophageal adenocarcinoma over the past 3 decades. Reavis et al reported that symptoms of LPRD, specifically chronic cough, correlate better with the presence of esophageal adenocarcinoma than typical GERD symptoms. Laryngopharynx acid reflux disease may also be a potential cofactor in the development of laryngeal carcinoma, particularly in nonsmokers.
The gravity of the potential sequelae of LPRD has led to considerable advances in the biological understanding of LPRD. Johnston and Koufman implicated changes in E-cadherin, EGF, and carbonic anhydrase subtype ratios and the presence of pepsin in laryngeal epithelial cells in the pathogenesis of LPRD. There is a consensus that the disease process is mediated and propagated by pepsin, detection of which in throat sputum has been posited as a reliable biological marker of LPRD. Initially thought to be activated by acid, it is now known that pepsin can function up to a pH of 8.0. Uptake of pepsin by laryngeal epithelial cells results in a shift in the normal acid-mediated stress protein response. In a nonacidic environment, exposure of hypopharyngeal cells to pepsin induces the expression of proinflammatory cytokines involved in the inflammation of the esophageal epithelium owing to reflux. In rat models, amino acids, particularly alanine and glutamine, have been shown to aggravate esophagitis by shifting the intraluminal pH to the optimal pH for the proteolytic activity of pepsin. Though discussed extensively as a possible candidate for antagonistic therapy, to date, no therapy has targeted pepsin in the treatment of LPRD or GERD.
Limitations
There are a number of limitations for this study, including the inherent biases of retrospective chart reviews, such as selection, information, and exclusion group biases. As rigorous as exclusion criteria were, patients with dual diagnoses may have been enrolled in the study, thus confounding results. Confirmation of the diagnosis of LPRD with objective measures, such as pH testing, was inconsistent among patients, and data on pH testing was insufficient to include in this pilot study. The placebo effect of both PPI and diet should also be recognized, although most patients were not naive to LPRD and had received prior treatment. In addition, the study design did not differentiate between the effects of alkaline water and diet. Determining adherence to dietary guidelines was not strictly standardized, with variations in the extent of adherence across patients, and potential recall bias must also be considered. Another potential confounding factor is weight loss, which was a frequent finding not adequately controlled for. The validity of self-reported tools such as the RSI scores has also been questioned in the literature.
Randomized clinical trials are necessary to corroborate the results of this study. Future prospective studies will focus on correlating subjective findings with objective measures, such as levels of pepsin in the oropharynx prior to and following treatment with either diet or PPI. Other objectives will include differentiating the effects of alkaline water from a low-animal protein diet and identifying certain subgroups of patients with reflux-associated laryngeal symptoms who are more likely to benefit from dietary vs PPI treatment. Clinicians should also be educated on the significance of the dietary modifications used in this study in the treatment of LPRD as well as the potential overall benefit to population health.
Conclusions
Laryngopharyngeal reflux has become a common diagnosis in the otolaryngologist’s office. Treatment frequently involves PPI therapy, which comes at a high price, both with cost and potential adverse effects. Our data suggest that the effect of PPI on RSI scores among patients with LPR is not significantly better than that of alkaline water and a plant-based, Mediterranean-style diet. In fact, our data suggest that the plant based approach is at least as good, if not better, than PPI therapy. Thus, we recommend that a patient with suspected LPR at least attempt a dietary approach prior to any pharmacological intervention.
References
- 1.Karkos PD, Wilson JA. Empiric treatment of laryngopharyngeal reflux with proton pump inhibitors: a systematic review. Laryngoscope. 2006;116(1):144-148. [DOI] [PubMed] [Google Scholar]
- 2.Francis DO, Rymer JA, Slaughter JC, et al. High economic burden of caring for patients with suspected extraesophageal reflux. Am J Gastroenterol. 2013;108(6):905-911. [DOI] [PubMed] [Google Scholar]
- 3.Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci. 2011;56(4):931-950. [DOI] [PubMed] [Google Scholar]
- 4.Johnston N, Dettmar PW, Bishwokarma B, Lively MO, Koufman JA. Activity/stability of human pepsin: implications for reflux attributed laryngeal disease. Laryngoscope. 2007;117(6):1036-1039. [DOI] [PubMed] [Google Scholar]
- 5.Koufman JA, Johnston N. Potential benefits of pH 8.8 alkaline drinking water as an adjunct in the treatment of reflux disease. Ann Otol Rhinol Laryngol. 2012;121(7):431-434. [DOI] [PubMed] [Google Scholar]
- 6.Koufman JA. Low-acid diet for recalcitrant laryngopharyngeal reflux: therapeutic benefits and their implications. Ann Otol Rhinol Laryngol. 2011;120(5):281-287. [DOI] [PubMed] [Google Scholar]
- 7.Hirschowitz BI. The control of pepsinogen secretion. Ann N Y Acad Sci. 1967;140(2):709-723. [DOI] [PubMed] [Google Scholar]
- 8.Campbell TC. Dietary protein, growth factors, and cancer. Am J Clin Nutr. 2007;85(6):1667. [DOI] [PubMed] [Google Scholar]
- 9.Guilloteau P, Le Meuth-Metzinger V, Morisset J, Zabielski R. Gastrin, cholecystokinin and gastrointestinal tract functions in mammals. Nutr Res Rev. 2006;19(2):254-283. [DOI] [PubMed] [Google Scholar]
- 10.Qian JM, Rowley WH, Jensen RT. Gastrin and CCK activate phospholipase C and stimulate pepsinogen release by interacting with two distinct receptors. Am J Physiol. 1993;264(4 Pt 1):G718-G727. [DOI] [PubMed] [Google Scholar]
- 11.Lien HC, Wang CC, Lee SW, et al. Responder definition of a patient-reported outcome instrument for laryngopharyngeal reflux based on the US FDA guidance. Value Health. 2015;18(4):396-403. [DOI] [PubMed] [Google Scholar]
- 12.Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI). J Voice. 2002;16(2):274-277. [DOI] [PubMed] [Google Scholar]
- 13.Habermann W, Schmid C, Neumann K, Devaney T, Hammer HF. Reflux symptom index and reflux finding score in otolaryngologic practice. J Voice. 2012;26(3):e123-e127. [DOI] [PubMed] [Google Scholar]
- 14.Reichel O, Dressel H, Wiederänders K, Issing WJ. Double-blind, placebo-controlled trial with esomeprazole for symptoms and signs associated with laryngopharyngeal reflux. Otolaryngol Head Neck Surg. 2008;139(3):414-420. [DOI] [PubMed] [Google Scholar]
- 15.Steward DL, Wilson KM, Kelly DH, et al. Proton pump inhibitor therapy for chronic laryngo-pharyngitis: a randomized placebo-control trial. Otolaryngol Head Neck Surg. 2004;131(4):342-350. [DOI] [PubMed] [Google Scholar]
- 16.Festi D, Scaioli E, Baldi F, et al. Body weight, lifestyle, dietary habits and gastroesophageal reflux disease. World J Gastroenterol. 2009;15(14):1690-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Estruch R, Ros E, Martínez-González MA. Mediterranean diet for primary prevention of cardiovascular disease. N Engl J Med. 2013;369(7):676-677. [DOI] [PubMed] [Google Scholar]
- 18.Orlich MJ, Singh PN, Sabaté J, et al. Vegetarian dietary patterns and mortality in Adventist Health Study 2. JAMA Intern Med. 2013;173(13):1230-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiba T, Kudara N, Abiko Y, et al. Effects of proton pump inhibitors in patients with laryngopharyngeal reflux disease. Hepatogastroenterology. 2011;58(110-111):1580-1582. [DOI] [PubMed] [Google Scholar]
- 20.Guo H, Ma H, Wang J. Proton pump inhibitor therapy for the treatment of laryngopharyngeal reflux: a meta-analysis of randomized controlled trials. J Clin Gastroenterol. 2016;50(4):295-300. [DOI] [PubMed] [Google Scholar]
- 21.Semmanaselvan K, Mukaddam QI, Naik M. An open label, prospective, single centre study to evaluate the efficacy and safety of fixed dose combination of rabeprazole (enteric-coated, EC) 20 mg + domperidone (sustained release, SR) 30 mg capsule in treatment of patients with laryngopharyngeal reflux disease. J Assoc Physicians India. 2015;63(7):27-32. [PubMed] [Google Scholar]
- 22.Khan M, Santana J, Donnellan C, Preston C, Moayyedi P. Medical treatments in the short term management of reflux oesophagitis. Cochrane Database Sys Rev. 2007;18(2):Cd003244. [DOI] [PubMed] [Google Scholar]
- 23.Kahrilas PJ. Maximizing outcome of extraesophageal reflux disease. Am J Manag Care. 2000;6(16)(suppl):S876-S882. [PubMed] [Google Scholar]
- 24.Liu C, Wang H, Liu K. Meta-analysis of the efficacy of proton pump inhibitors for the symptoms of laryngopharyngeal reflux. Braz J Med Biol Res. 2016;49(7):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Megwalu UC. A systematic review of proton-pump inhibitor therapy for laryngopharyngeal reflux. Ear Nose Throat J. 2013;92(8):364-371. [DOI] [PubMed] [Google Scholar]
- 26.Reimer C, Bytzer P. Management of laryngopharyngeal reflux with proton pump inhibitors. Ther Clin Risk Manag. 2008;4(1):225-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amin MR, Postma GN, Johnson P, Digges N, Koufman JA. Proton pump inhibitor resistance in the treatment of laryngopharyngeal reflux. Otolaryngol Head Neck Surg. 2001;125(4):374-378. [DOI] [PubMed] [Google Scholar]
- 28.Carroll TL, Nahikian K, Asban A, Wiener D. Nissen fundoplication for laryngopharyngeal reflux after patient selection using dual pH, full column impedance testing: a pilot study. Ann Otol Rhinol Laryngol. 2016;125(9):722-728. [DOI] [PubMed] [Google Scholar]
- 29.Mazzini Gda S, Gurski RR. Impact of laparoscopic fundoplication for the treatment of laryngopharyngeal reflux: review of the literature. Int J Otolaryngol. 2012;2012:291472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope. 1991;101(4 Pt 2)(suppl 53):1-78. [DOI] [PubMed] [Google Scholar]
- 31.Park KH, Choi SM, Kwon SU, Yoon SW, Kim SU. Diagnosis of laryngopharyngeal reflux among globus patients. Otolaryngol Head Neck Surg. 2006;134(1):81-85. [DOI] [PubMed] [Google Scholar]
- 32.Friedman M, Hamilton C, Samuelson CG, et al. The value of routine pH monitoring in the diagnosis and treatment of laryngopharyngeal reflux. Otolaryngol Head Neck Surg. 2012;146(6):952-958. [DOI] [PubMed] [Google Scholar]
- 33.Batuwitage BT, Kingham JG, Morgan NE, Bartlett RL. Inappropriate prescribing of proton pump inhibitors in primary care. Postgrad Med J. 2007;83(975):66-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu WH, Wu IC, Kuo CH, et al. Influence of proton pump inhibitor use in gastrointestinal polyps. Kaohsiung J Med Sci. 2010;26(2):76-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ngamruengphong S, Leontiadis GI, Radhi S, Dentino A, Nugent K. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol. 2011;106(7):1209-1218. [DOI] [PubMed] [Google Scholar]
- 36.Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ. 2008;179(4):319-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang YX, Metz DC. Safety of proton pump inhibitor exposure. Gastroenterology. 2010;139(4):1115-1127. [DOI] [PubMed] [Google Scholar]
- 38.Fashner J, Gitu AC. Common gastrointestinal symptoms: risks of long-term proton pump inhibitor therapy. FP Essent. 2013;413:29-39. [PubMed] [Google Scholar]
- 39.Lambert AA, Lam JO, Paik JJ, Ugarte-Gil C, Drummond MB, Crowell TA. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS One. 2015;10(6):e0128004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah NH, LePendu P, Bauer-Mehren A, et al. Proton pump inhibitor usage and the risk of myocardial infarction in the general population. PLoS One. 2015;10(6):e0124653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gomm W, von Holt K, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73(4):410-416. [DOI] [PubMed] [Google Scholar]
- 42.Sehested TS, Fosbøl EL, Hansen PW, Charlot MG, Torp-Pedersen C, Gislason GH. Abstract 18462: Proton pump inhibitor use increases the associated risk of first-time ischemic stroke. Circulation. 2016;134:A18462-A. [Google Scholar]
- 43.Makary MA, Daniel M. Medical error-the third leading cause of death in the US. BMJ. 2016;353:i2139. [DOI] [PubMed] [Google Scholar]
- 44.Ho PM, Maddox TM, Wang L, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA. 2009;301(9):937-944. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki K, Doki K, Homma M, et al. Co-administration of proton pump inhibitors delays elimination of plasma methotrexate in high-dose methotrexate therapy. Br J Clin Pharmacol. 2009;67(1):44-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maronian NC, Azadeh H, Waugh P, Hillel A. Association of laryngopharyngeal reflux disease and subglottic stenosis. Ann Otol Rhinol Laryngol. 2001;110(7 Pt 1):606-612. [DOI] [PubMed] [Google Scholar]
- 47.Loughlin CJ, Koufman JA. Paroxysmal laryngospasm secondary to gastroesophageal reflux. Laryngoscope. 1996;106(12 Pt 1):1502-1505. [DOI] [PubMed] [Google Scholar]
- 48.Murry T, Tabaee A, Owczarzak V, Aviv JE. Respiratory retraining therapy and management of laryngopharyngeal reflux in the treatment of patients with cough and paradoxical vocal fold movement disorder. Ann Otol Rhinol Laryngol. 2006;115(10):754-758. [DOI] [PubMed] [Google Scholar]
- 49.Eryuksel E, Dogan M, Golabi P, Sehitoglu MA, Celikel T. Treatment of laryngopharyngeal reflux improves asthma symptoms in asthmatics. J Asthma. 2006;43(7):539-542. [DOI] [PubMed] [Google Scholar]
- 50.Kilic M, Ozturk F, Kirmemis O, et al. Impact of laryngopharyngeal and gastroesophageal reflux on asthma control in children. Int J Pediatr Otorhinolaryngol. 2013;77(3):341-345. [DOI] [PubMed] [Google Scholar]
- 51.Hur C, Miller M, Kong CY, et al. Trends in esophageal adenocarcinoma incidence and mortality. Cancer. 2013;119(6):1149-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reavis KM, Morris CD, Gopal DV, Hunter JG, Jobe BA. Laryngopharyngeal reflux symptoms better predict the presence of esophageal adenocarcinoma than typical gastroesophageal reflux symptoms. Ann Surg. 2004;239(6):849-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gill GA, Johnston N, Buda A, et al. Laryngeal epithelial defenses against laryngopharyngeal reflux: investigations of E-cadherin, carbonic anhydrase isoenzyme III, and pepsin. Ann Otol Rhinol Laryngol. 2005;114(12):913-921. [DOI] [PubMed] [Google Scholar]
- 54.Johnston N, Bulmer D, Gill GA, et al. Cell biology of laryngeal epithelial defenses in health and disease: further studies. Ann Otol Rhinol Laryngol. 2003;112(6):481-491. [DOI] [PubMed] [Google Scholar]
- 55.Johnston N, Yan JC, Hoekzema CR, et al. Pepsin promotes proliferation of laryngeal and pharyngeal epithelial cells. Laryngoscope. 2012;122(6):1317-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Samuels TL, Johnston N. Pepsin as a causal agent of inflammation during nonacidic reflux. Otolaryngol Head Neck Surg. 2009;141(5):559-563. [DOI] [PubMed] [Google Scholar]
- 57.Nagahama K, Nishio H, Yamato M, Takeuchi K. Orally administered L-arginine and glycine are highly effective against acid reflux esophagitis in rats. Med Sci Monit. 2012;18(1):BR9-BR15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagahama K, Yamato M, Nishio H, Takeuchi K. Essential role of pepsin in pathogenesis of acid reflux esophagitis in rats. Dig Dis Sci. 2006;51(2):303-309. [DOI] [PubMed] [Google Scholar]
- 59.Naiboglu B, Durmus R, Tek A, Toros SZ, Egeli E. Do the laryngopharyngeal symptoms and signs ameliorate by empiric treatment in patients with suspected laryngopharyngeal reflux? Auris Nasus Larynx. 2011;38(5):622-627. [DOI] [PubMed] [Google Scholar]