Key Points
Question
Does implementation of a “surgeon on service” model, that is, maintaining a dedicated inpatient pediatric otolaryngologist, improve health care quality at an urban, academic pediatric medical center?
Findings
In this analysis of medical records of pediatric tracheostomy patients and survey of their treating physician trainees before and after implementation of a surgeon on service model, we found that time to tracheostomy was reduced by 43% after implementation of the model, and fiscal analysis demonstrated that this program was productive.
Meaning
The surgeon on service model represents an alternative approach to improving quality at urban, academic pediatric medical centers.
Abstract
Importance
The traditional practice model for pediatric otolaryngologists at high-volume academic centers is to simultaneously balance outpatient care responsibilities with those of the inpatient service, emergency department, and ambulatory care clinics. This model leads to challenges with care coordination, timeliness of nonemergency operative care, and consistent participation in care and consultation at the attending surgeon level. The “surgeon on service” (SOS) model—where faculty members rotate to manage the inpatient service in lieu of outpatient responsibilities—has been described as one method to address this conundrum. The operational and economic feasibility of the SOS model has been demonstrated; however, its impact on care coordination, time from consultation to surgical care, and length of stay (LOS) have not been evaluated.
Objective
To determine the impact of the SOS model on the quality principles of timeliness and efficiency of tracheostomy tube placement and to determine if the SOS model is fiscally feasible in an academic pediatric otolaryngology practice.
Design, Setting, and Participants
Medical record review of patients undergoing tracheostomy in a pediatric academic medical center and survey of their treating physician trainees, comparing the 6-month SOS pilot phase (postimplementation, January-June 2016) with the 6-month preimplementation period (January-June 2015).
Intervention
Implementation of the SOS model.
Main Outcomes and Measures
Time to tracheostomy, frequency of successful coordination of tracheostomy with gastrostomy tube placement, total LOS, productivity measured in work relative value units, and responses to trainee surveys.
Results
Of the 41 patients included in the study (24 boys and 17 girls; mean age, 3 years; range, 3 months to 17 years), 15 were treated before SOS implementation, and 26 after. Also included were 21 trainees. Before SOS implementation, median time to tracheostomy was 7 days (range, 2-20 days); after SOS implementation, it was 4 days (range, 1-10 days) (difference between the medians, before to after, −3 days; 95% CI, −5 to 0 days). There was no significant difference in overall LOS or ability to coordinate tracheostomy with gastrostomy tube placement. Preimplementation trainee surveys cited dissatisfaction with the communication channels to the primary team when the consulting surgeon was not immediately available to perform tracheostomy. No challenges were reported after implementation. Productivity was comparable to that in the outpatient setting.
Conclusions and Relevance
In this study, the presence of a rotating inpatient pediatric otolaryngologist was a productive approach to patient care associated with more timely performance of tracheostomy. Other benefits were an improved balance of service with education to trainees and a better perception of communication with consulting services.
This review of pediatric otolaryngology medical records and physician trainee surveys compares time to tracheostomy and other factors before vs after implementation of a “surgeon on service” program at a large pediatric academic medical center.
Introduction
Otolaryngologists practicing at urban, academic medical centers are required to balance supervision and provision of hospital-based inpatient and emergency department (ED) care with care-access needs at ambulatory care practice locations, which are often in satellite centers. In large metropolitan areas, practice growth and expansion to satellite locations improves access to subspecialists, but it also challenges the ability of a finite group of attending surgeons to provide supervision and oversight to the hospital-based practice at the primary academic hospital. This has affected timeliness to completion of semielective surgical procedures for inpatients, especially tracheostomy, at our hospital.
The hospitalist model is a widely accepted mode of care delivery for medical patients. It has a proven track record of decreased length of stay (LOS), reduced hospital cost, improved patient satisfaction scores, and improved perception of intraprofessional communication and teamwork. The first introduction of a hospitalist care model to surgical practice demonstrated improved outcomes through comanagement of patients between medically trained hospitalists and orthopedic surgeons. Implementation of a hospitalist model in general surgery has similarly improved timeliness of care as measured by ED LOS and time to appendectomy. A full-time hospitalist model in an adult otolaryngology–head and neck surgery department has demonstrated similar success. While the experience of a pediatric otolaryngology hospitalist model has not been extensively reported, a group at the Boston Children’s Hospital was the first to report a care delivery model using a rotating dedicated attending surgeon to provide supervision and oversight to the hospital-based practice. That study demonstrated improved oversight and high volume of billable patient care encounters that supported the financial viability of such a model. However, with the complexity of hospital-based practice reimbursement structures and revenue cycles, and disparity in the number of faculty members, trainees, and advanced practice clinicians in academic centers, the financial feasibility of adopting such a model may not be fully generalizable. Aside from financial feasibility, it has been demonstrated that the clinical, educational, and organizational practice needs of the hospital-based practice are tacitly improved by having an attending surgeon provide oversight. To our knowledge, the impact of such a care delivery model on the principles of quality outlined by the Institute of Medicine (IOM), specifically pertaining to the principles of timeliness and efficiency, has not been studied in pediatric otolaryngology.
The purpose of this study was to investigate whether the presence of a dedicated, rotating inpatient attending surgeon, the “surgeon on service” (SOS), improved the quality of care in a way that was sufficiently productive and fiscally feasible in our urban practice. Using timeliness of tracheostomy and facilitation of coordinated surgical care as quality measures, we hypothesized that the SOS model would result in a decreased time to tracheostomy and that more tracheostomies could be performed in conjunction with gastrostomy tube placement by pediatric surgery. Similar to the Boston Children’s Hospital study, we predicted that this model would be fiscally sound owing to the capture of previously unbilled procedures and patient encounters.
Methods
Intervention
The SOS model was implemented at our medical center in September 2015 in a pilot study. Similar to the model at Boston Children’s Hospital described by Adil and colleagues, an attending pediatric otolaryngologist was designated to provide clinical provision and oversight of the hospital-based practice, which included otolaryngology inpatient service, existing and new consults, ED consults, and all surgical procedures generated from these consultations between 7:00 am and 5:00 pm for a 1-week rotation. The responsibilities of the SOS surgeon were 3-fold: (1) perform daily rounds of inpatients, staff all inpatient and ED consults, and perform and/or supervise all resultant bedside and surgical procedures; (2) provide consultations and bedside procedures at the affiliated Women’s Hospital, which is connected to the primary hospital by a pedestrian walkway; and (3) provide educational and clinical oversight of trainees, which included 2 fellows (postgraduate year 6 [PGY6]), 3 residents (2 PGY3, 1 PGY1 or PGY2), and advanced practice clinicians. The SOS model was modified slightly from that described by Adil et al in that the surgeon was also permitted to maintain 1 half day of ambulatory clinic and 1 half day of prescheduled operative cases per week at the primary hospital site. We chose to modify the model to determine the feasibility of maintaining care access in our urban setting, where access to care for publicly funded patients is managed primarily by academic and publicly funded health clinics. Because this was a pilot study to test the feasibility of the SOS model in our practice without disruption of our care access model for those with well-established practices and referral patterns, we chose to have 1 surgeon serve in the SOS role for the pilot study duration.
Data Collection and Analysis
After implementation of the SOS model, a retrospective analysis was conducted to compare the preimplementation period of January 1 through June 30, 2015, with the postimplementation period of January 1 through June 30, 2016. The model was assessed for its effect on timeliness and efficiency of care as well as productivity. Ann & Robert H. Lurie Children’s Hospital of Chicago institutional review board approval was obtained prior to initiation of the pilot study analysis, waiving written informed consent from both patients and trainees because all records and surveys were reviewed retrospectively.
Patient data included in the analysis were from those who underwent tracheostomy tube placement as the outcome of an inpatient consultation in the 6-month preimplementation period and 6-month postimplementation period. To assess the IOM quality principle of timeliness, the number of days between the decision for tracheostomy and the actual surgical procedure were compared between the preimplementation and postimplementation periods. The date of decision for tracheostomy was defined as the date on which the patient was deemed medically stable for surgery by the primary service, and the legal guardian agreed to and consented for surgery.
To assess the IOM quality principle of efficiency, the preimplementation and postimplementation groups were further evaluated for gastrostomy tube placement during the concurrent hospital care episode and whether the surgical procedures were coordinated during the same surgical anesthetic episode. Patients were included for analysis if they underwent both tracheostomy and gastrostomy tube placement within a single admission. Patients were excluded if gastrostomy tube placement was not performed during the same hospital care episode. Efficiency was also assessed by measuring the impact of SOS model implementation on LOS by comparing total LOS in the preimplementation and postimplementation periods.
To determine if the SOS model of care was fiscally viable, productivity was measured in work relative value units (wRVUs). The monthly wRVUs for the SOS surgeon was normalized to the monthly median wRVUs for the remainder of the division. Total wRVUs for the 6-month postimplementation period were also compared for the SOS surgeon and the remainder of the division.
Impact of the SOS model on trainees was retrospectively assessed through review of comments on internal annual resident and fellow end-of-year program assessment surveys.
Statistical Analysis
All collected data were maintained in an Excel spreadsheet. Statistical analysis was performed using effect size and 95% confidence intervals (CIs).
Results
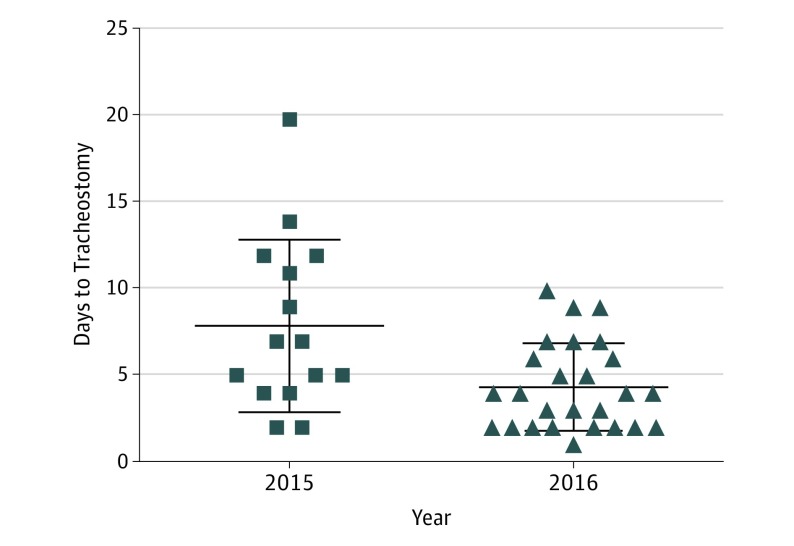
During the preimplementation period, 15 tracheostomies were performed, while 26 tracheostomies were performed postimplementation. Median time to tracheostomy in the preimplementation period was 7 days (range, 2-20 days). This was significantly longer than the median time to tracheostomy of 4 days (range, 1-10 days) postimplementation (difference between medians, before to after, −3 days; 95% CI, −5 to 0 days) (Figure 1). While tracheostomies were performed in a more timely fashion in the postimplementation period, there was no difference in LOS between the 2 time periods: 66.8 days in the preimplementation period and 70.6 days in the postimplementation period (difference between medians, before to after, −4.1 days; 95% CI, −43.6 to 31.8 days).
Figure 1. Time to Tracheostomy.
Distribution of time to tracheostomy prior to implementation of the “surgeon on service” (SOS) model (2015) and after SOS implementation (2016). The difference between median times to tracheostomy, before and after SOS implementation, was −3 days (95% CI, −5 to 0 days).
Of the 15 tracheostomies performed in the preimplementation period, 7 patients underwent gastrostomy tube placement during the same admission. Of these, 3 gastrostomy tubes were placed during the same anesthetic episode (43%). In the postimplementation period, 16 patients had gastrostomy tube placement during the same admission as tracheostomy. Of these, 12 were coordinated under the same anesthetic episode (75%). The frequency of coordination before and after implementation was not significantly different (attributable risk 0.32; 95% CI, −0.03 to 0.78).
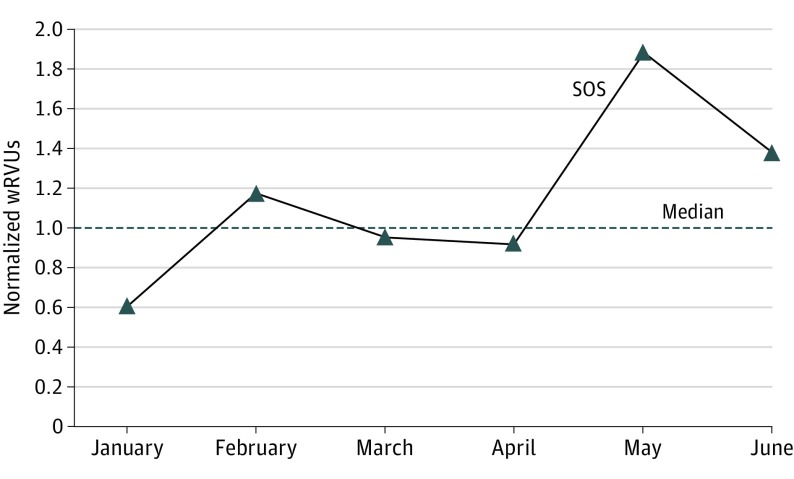
Productivity analysis revealed that the normalized monthly wRVUs for the SOS surgeon were near or above the median wRVUs for the rest of the division (range, 0.6-1.9) (Figure 2). Total normalized SOS wRVUs for the 6-month postimplementation period were greater than that of the rest of the division (1.09).
Figure 2. Monthly wRVUs of the SOS Normalized to the Median Value for the Rest of the Division.
SOS indicates surgeon on service; wRVUs, work relative value units.
A review of fellowship and residency trainee survey responses from the preimplementation period noted frequent challenges and dissatisfaction with the communication channels to the primary team when the consulting surgeon was not immediately available to perform tracheostomy. Trainees noted further dissatisfaction when the lag time to schedule tracheostomy was dependent on either the surgeon’s open block time or the availability of another surgeon to assume care. This problem was no longer cited in the annual program review held during the postimplementation period.
Discussion
While the growth of satellite ambulatory clinical practices for academic pediatric medical centers improves patient access to subspecialty care in large, urban communities, it also challenges the physician’s ability to simultaneously supervise patient care responsibilities at the primary hospital location. Such a limitation is in conflict with a growing body of literature demonstrating a patient safety benefit to oversight of trainees by an attending physician. Similar to the study from Boston Children’s Hospital, our motivation to implement the SOS model was to provide continuity of care to a high-acuity patient population and to balance satellite practices. The demonstration of the SOS model’s tacit benefit to clinical care and education at Boston Children’s Hospital, along with acceptable measures of wRVU productivity, provided a platform for buy-in for members of our division to pilot the model.
Building on the success of the Boston Children’s Hospital study, we sought to determine if implementation of the SOS model improved the quality of patient care in a way that was productive. Selection of days to tracheostomy for children with prolonged ventilation as a measure of timely care was chosen for 2 reasons: (1) tracheostomy is the most frequent nonurgent surgery resulting from inpatient consultation at our hospital, and (2) there was an institutional perception of delay in scheduling this surgery. Prior to SOS implementation, scheduling of nonurgent and nonemergency procedures such as tracheostomy was often delayed by the surgeon’s conflicting clinical ambulatory care responsibilities or lack of available block time. Because such a delay has significant downstream medical and financial implications, the team frequently was left to search for an available surgeon to assume care. Residents and fellows are the consistent hospital-based team and were often put in the dissatisfying position of managing this conundrum. Since the concerns about scheduling that had been reported by the trainees were no longer reported after implementation of the SOS model, we concluded that this model anecdotally addressed a significant service-over-education concern of our trainees, which is common in busy urban pediatric practices.
Implementation of the SOS model was associated with improved quality of care as demonstrated by a decrease in the median time from decision to placement of a tracheostomy tube by 43%. This timely performance of surgery was realized despite an increased overall volume of tracheostomies performed during the SOS pilot study period. Such a finding is advantageous, given that recent literature has suggested that early tracheostomy reduces health care costs, morbidity, overall duration of mechanical ventilation, and LOS in the intensive care unit (ICU) among pediatric patients. In a study by Halloway et al, each additional day awaiting tracheostomy in the pediatric ICU (PICU) was associated with a 0.5-day increase in ICU LOS and a 1.9-day increase in overall LOS. This suggests that even a modest reduction in time to tracheostomy has the potential for a large impact on health care costs.
Despite improvement in time to tracheostomy, there was no significant improvement in overall LOS among the patients analyzed. This finding is likely multifactorial in that a large portion of patients analyzed were in the neonatal ICU, and their degree of prematurity likely had a larger bearing on LOS than time awaiting procedure. Owing to the small sample size, analysis of the PICU population alone was not possible in this study. It should further be mentioned that most of the studies investigating early pediatric tracheostomy define “early” as within 14 days of intubation. The present study did not specifically investigate early vs late tracheostomy, which pertains more to the decision making of the critical care team. Instead, we investigated whether provision of timelier tracheostomy was possible with the SOS model of care. Such a finding is significant because the availability of the surgical team is essential in the event that the critical care team decides on early tracheostomy.
In addition to measures of timeliness, we also assessed whether the SOS model improved the ability to perform combination tracheostomy and gastrostomy tube placement in eligible patients. Such ability to combine procedures in a single anesthetic episode is advantageous both from a cost-containment perspective and to address the growing concern about the effect of anesthesia on the developing brain. Owing to small sample size, we were unable to conclude whether the SOS model definitively improved the ability to coordinate surgical cases, but the trend toward an increased frequency of combination cases suggests a potential area for further investigation.
The Boston Children’s Hospital study clearly demonstrated that a dedicated, rotating inpatient surgeon can provide a high enough volume of productive work to make it worthwhile to have an assigned attending surgeon serve in this role. The group at the Boston Children’s Hospital found a similar wRVU measure of productivity between the physician on service and those in the traditional practice model. In comparison, we found that the wRVUs in our model were greater than the median value for the rest of the physicians in the division, demonstrating that the model is sound from a productivity standpoint. Such a finding highlights the magnitude of missed opportunities in the form of uncaptured evaluation and management services and bedside procedures at our hospital. Our model was modified from that of the Boston Children’s Hospital by permitting the SOS surgeon to maintain 1 half day of outpatient clinic at the main hospital location and 1 half day of elective surgical cases per week. This factor may explain the above-median wRVUs seen in our model compared with the similar measure of productivity found in the Boston Children’s Hospital model. Such a modification demonstrates the feasibility of successful implementation in institutions with varying or lower inpatient volumes or urban practices such as ours that have a mission to provide access to care for publicly funded patients.
Despite demonstrated wRVU productivity, a perceived barrier to adoption of an SOS model at other pediatric otolaryngology centers may be that wRVUs do not fully represent revenue required to support salary-sustaining income in some compensation models. Having a single surgeon serve in the role of SOS during the study period allowed us to gain unique insights. For example, we found that the revenue generated by the SOS surgeon was at the median salary of the entire division for the 2016 fiscal year. Even in instances where inpatient volume and compensation models cannot independently support an SOS surgeon, justification of the role for the downstream revenue to the hospital may be considered. Subsidization of hospital-based physicians has been warranted in medicine owing to its implications for downstream revenue secondary to reduced LOS and increased throughput. Further analysis of the impact of an SOS model on these efficiency measures may provide rationale for similar support.
Zalzal and Shah, in their commentary on the Boston Children’s Hospital model, attributed part of the success to the large number of advanced-practice clinicians. We demonstrated that the SOS model can be successfully implemented without this resource; however, it should be noted that the attending surgeon in our model shared much of the burden of the day-to-day work, such as bedside procedures and completion of consults. From a quality-of-life perspective for the attending surgeon assigned to the SOS role, an advanced-practice clinician may be of great benefit. As a result of our pilot study, our institution has added a nurse practitioner to our inpatient service complement.
Although we did not formally survey beyond our department for purposes of this study, the overall impression of the SOS model was positive across the institution. For example, informal feedback from consulting services was positive, citing improved ability to have face-to-face contact with attending physicians, closed-loop conversations about patient care, and improved availability for care conferences for complex-care patients. In addition, the frequency, caliber, and quality of our interprofessional collaboration and communication was recognized at the highest administrative institutional level, to the extent that other surgical subspecialties are now being asked to consider this model.
The attending pediatric otolaryngologists cited improved perceived quality of life owing to elimination of the need to balance ambulatory practice with daytime coverage of hospital-based urgent and emergency care and consultations. Formal quality-of-life evaluations of the faculty could not be conducted owing to the retrospective nature of this study; this represents a potential area of further research. Similarly, the effect of the SOS model on patient and parent satisfaction represents a potential patient-centered quality measure that can be investigated in the future.
It should be noted that while the 6-month pilot study used a single surgeon in the SOS role, our division has since adopted coverage of the SOS model among all surgeons on a weekly, rotating basis. Transition from a single physician to a rotating team within the SOS model has not affected the productivity or the quality of care provided.
Conclusions
In conclusion, in our academic pediatric otolaryngology practice, the implementation of a dedicated, rotating inpatient physician improved the quality of patient care as measured by time to tracheostomy. Continuation of the SOS model is justified by its productivity. Further investigation into measures of efficiency, including facilitation of combination surgical procedures performed with other services and reduction in LOS in the PICU population are areas of further potential research.
References
- 1.White HL, Glazier RH. Do hospitalist physicians improve the quality of inpatient care delivery? a systematic review of process, efficiency and outcome measures. BMC Med. 2011;9:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514-517. [DOI] [PubMed] [Google Scholar]
- 3.Wachter RM, Goldman L. Zero to 50,000: the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. [DOI] [PubMed] [Google Scholar]
- 4.Huddleston JM, Long KH, Naessens JM, et al. ; Hospitalist-Orthopedic Team Trial Investigators . Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1):28-38. [DOI] [PubMed] [Google Scholar]
- 5.Maa J, Carter JT, Gosnell JE, Wachter R, Harris HW. The surgical hospitalist: a new model for emergency surgical care. J Am Coll Surg. 2007;205(5):704-711. [DOI] [PubMed] [Google Scholar]
- 6.Austin MT, Diaz JJ Jr, Feurer ID, et al. Creating an emergency general surgery service enhances the productivity of trauma surgeons, general surgeons and the hospital. J Trauma. 2005;58(5):906-910. [DOI] [PubMed] [Google Scholar]
- 7.Russell MS, Eisele D, Murr A. The otolaryngology hospitalist: a novel practice paradigm. Laryngoscope. 2013;123(6):1394-1398. [DOI] [PubMed] [Google Scholar]
- 8.Adil E, Xiao R, McGill T, Rahbar R, Cunningham M. A chief of service rotation as an alternative approach to pediatric otolaryngology inpatient care. JAMA Otolaryngol Head Neck Surg. 2014;140(9):809-814. [DOI] [PubMed] [Google Scholar]
- 9.Zalzal GH, Shah RK. The chief of service rotation: for each, their own. JAMA Otolaryngol Head Neck Surg. 2014;140(9):815-816. [DOI] [PubMed] [Google Scholar]
- 10.Institute of Medicine Crossing the Quality Chasm: A new Health System for the 21st Century. Washington, DC: National Academies Press; 2001. [PubMed] [Google Scholar]
- 11.Trowbridge RL, Almeder L, Jacquet M, Fairfield KM. The effect of overnight in-house attending coverage on perceptions of care and education on a general medical service. J Grad Med Educ. 2010;2(1):53-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shojania KG, Fletcher KE, Saint S. Graduate medical education and patient safety: a busy—and occasionally hazardous—intersection. Ann Intern Med. 2006;145(8):592-598. [DOI] [PubMed] [Google Scholar]
- 13.Cram P, Hillis SL, Barnett M, Rosenthal GE. Effects of weekend admission and hospital teaching status on in-hospital mortality. Am J Med. 2004;117(3):151-157. [DOI] [PubMed] [Google Scholar]
- 14.Holloway AJ, Spaeder MC, Basu S. Association of timing of tracheostomy on clinical outcomes in PICU patients. Pediatr Crit Care Med. 2015;16(3):e52-e58. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Koo CH, Lee SY, et al. Effect of early vs. late tracheostomy on clinical outcomes in critically ill pediatric patients. Acta Anaesthesiol Scand. 2016;60(9):1281-1288. [DOI] [PubMed] [Google Scholar]
- 16.Liu CC, Rudmik L. A cost-effectiveness analysis of early vs late tracheostomy. JAMA Otolaryngol Head Neck Surg. 2016;142(10):981-987. [DOI] [PubMed] [Google Scholar]
- 17.Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23(3):876-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel P, Sun L. Update on neonatal anesthetic neurotoxicity: insight into molecular mechanisms and relevance to humans. Anesthesiology. 2009;110(4):703-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Istaphanous GK, Howard J, Nan X, et al. Comparison of the neuroapoptotic properties of equipotent anesthetic concentrations of desflurane, isoflurane, or sevoflurane in neonatal mice. Anesthesiology. 2011;114(3):578-587. [DOI] [PubMed] [Google Scholar]
- 20.Gregory D, Baigelman W, Wilson IB. Hospital economics of the hospitalist. Health Serv Res. 2003;38(3):905-918. [DOI] [PMC free article] [PubMed] [Google Scholar]