This randomized clinical trial investigates the effect of liraglutide added to clozapine or olanzapine treatment of schizophrenia spectrum disorders in patients with prediabetes who are overweight or obese.
Key Points
Question
Can the glucagon-like peptide-1 receptor agonist liraglutide reduce body weight and improve cardiometabolic disturbances in antipsychotic-treated patients?
Findings
In this randomized clinical trial of 103 patients with schizophrenia spectrum disorders treated with clozapine or olanzapine, glucose tolerance improved significantly and 30 liraglutide-treated patients with prediabetes (63.8%) developed normal glucose tolerance compared with 8 placebo-treated patients (16.0%). Liraglutide induced a placebo-subtracted body weight loss of 5.3 kg.
Meaning
Glucagon-like peptide-1 receptor agonists offer a novel and effective approach to mitigation of some of the cardiometabolic adverse effects associated with the commonly used and efficacious antipsychotic drugs clozapine and olanzapine.
Abstract
Importance
Compared with the general population, patients with schizophrenia have a 2- to 3-fold higher mortality rate primarily caused by cardiovascular disease. Previous interventions designed to counteract antipsychotic-induced weight gain and cardiometabolic disturbances reported limited effects.
Objectives
To determine the effects of the glucagon-like peptide-1 receptor agonist liraglutide added to clozapine or olanzapine treatment of schizophrenia spectrum disorders.
Design, Setting, and Participants
This randomized clinical double-blind trial enrolled participants at 2 clinical sites in Denmark. Of 214 eligible participants with a schizophrenia spectrum disorder, 103 were randomized to liraglutide or placebo. Participants received stable treatment with clozapine or olanzapine, were overweight or obese, and had prediabetes. Data were collected from May 1, 2013, through February 25, 2016.
Interventions
Treatment for 16 weeks with once-daily subcutaneous injection of liraglutide or placebo. Trial drug therapy was titrated during the first 2 weeks of the study.
Main Outcomes and Measures
The primary end point was change in glucose tolerance estimated by a 75-g oral glucose tolerance test result. Secondary end points included change in body weight and cardiometabolic parameters.
Results
Of the 103 patients undergoing randomization (60 men [58.3%] and 43 women [41.7%]), 97 were included in the efficacy analysis, with a mean (SD) age of 42.5 (10.5) years and mean (SD) body mass index (calculated as weight in kilograms divided by height in meters squared) of 33.8 (5.9). The liraglutide and placebo groups had comparable characteristics (mean [SD] age, 42.1 [10.7] vs 43.0 [10.5] years; 30 men in each group; mean [SD] body mass index, 33.7 [5.1] vs 33.9 [6.6]). A total of 96 randomized participants (93.2%) completed the trial. Glucose tolerance improved in the liraglutide group compared with the placebo group (P < .001). Altogether, 30 liraglutide-treated participants (63.8%) developed normal glucose tolerance compared with 8 placebo-treated participants (16.0%) (P < .001; number needed to treat, 2). Body weight decreased with liraglutide compared with placebo (−5.3 kg; 95% CI, −7.0 to −3.7 kg). Reductions in waist circumference (−4.1 cm; 95% CI, −6.0 to −2.3 cm), systolic blood pressure (−4.9 mm Hg; 95% CI, −9.5 to −0.3 mm Hg), visceral fat (−250.19 g; 95% CI, −459.9 to −40.5 g), and low-density lipoprotein levels (−15.4 mg/dL; 95% CI, −23.2 to −7.7 mg/dL) occurred with liraglutide compared with placebo. Adverse events with liraglutide affected mainly the gastrointestinal tract.
Conclusions and Relevance
Liraglutide significantly improved glucose tolerance, body weight, and cardiometabolic disturbances in patients with schizophrenia spectrum disorders treated with clozapine or olanzapine.
Trial Registration
clinicaltrials.gov Identifier: NCT01845259
Introduction
Patients with schizophrenia have a 2- to 3-fold higher mortality compared with the general population. Somatic diseases, primarily cardiovascular disease, account for an estimated 60% of the excess mortality. A high prevalence of obesity, metabolic disturbances, and type 2 diabetes among patients with schizophrenia largely explains the increased risk for cardiovascular morbidity and increased mortality. Antipsychotic medications are effective for treating schizophrenia but also induce weight gain, metabolic disturbances, and type 2 diabetes. Clozapine and olanzapine are 2 of the most effective antipsychotics, and clozapine is used for treatment-resistant schizophrenia. Unfortunately, clozapine and olanzapine induce the greatest weight gain and confer a higher risk for metabolic disturbances compared with all other psychotropic medications. Clozapine and olanzapine are associated with increased appetite and delayed satiety signaling and sedation, which can lead to body weight gain and an increased risk for metabolic disturbances. Previous studies have demonstrated limited effects for counteracting antipsychotic-induced weight gain with adjunct pharmacologic treatments, behavioral interventions, or switch of antipsychotics.
Glucagon-like peptide-1 (GLP-1) is an incretin hormone secreted from the L cells in the intestinal mucosa in response to nutrients. Glucagon-like peptide-1 stimulates insulin secretion and inhibits glucagon secretion, thereby lowering plasma glucose levels. In 2009, the GLP-1 receptor agonist liraglutide (1.8 mg once daily) was approved for treatment of type 2 diabetes. In 2015, a high dose of liraglutide (3 mg once daily) was approved for treatment of obesity owing to its inhibitory effects on appetite and food intake.
The potential mitigating effects of GLP-1 receptor agonists in antipsychotic-treated patients require further elucidation. The present trial investigated the effect of 1.8 mg of liraglutide once daily added to stable treatment with clozapine or olanzapine in patients with a schizophrenia spectrum disorder (schizoaffective disorder excluded) who were overweight or obese and had prediabetes.
Methods
Study Overview
This investigator-initiated randomized clinical trial was conducted from May 1, 2013 (first patient, first visit), through February 25, 2016 (last patient, last visit), at 2 clinical sites in Denmark. A copy of the study protocol is found in Supplement 1, and details have been published previously. The trial was approved by the Scientific-Ethical Committee of the Capital Region of Denmark and the Danish Health Authority and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. Before participants underwent examination, they were provided oral and written information and gave written informed consent.
Participants
Eligible participants were adults aged 18 to 65 years who were receiving stable treatment with clozapine or olanzapine and diagnosed with a schizophrenia spectrum disorder (schizoaffective disorder excluded) according to The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines or DSM-IV-TR. Furthermore, the participants had prediabetes and a body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) of 27 or greater. Prediabetes was defined as an elevated fasting plasma glucose level of 110 to 125 mg/dL (to convert to mmol/L, multiply by 0.0555), elevated glycated hemoglobin level of 6.1% to 6.4% (to convert to mmol/mol, subtract 2.15% and multiply by 10.929), and/or impaired glucose tolerance with a 2-hour plasma glucose level of at least 140 mg/dL during a 75-g oral glucose tolerance test. Main exclusion criteria consisted of type 2 diabetes (glycated hemoglobin level of at least 6.5%), treatment with antidiabetic medications, substance abuse, or serious somatic illnesses (eg, pancreatitis or heart disease). A detailed list of all inclusion and exclusion criteria is presented in eTable 1 in Supplement 2.
Study Design
At baseline and after 16 weeks of treatment, all participants completed a 4-hour, 75-g oral glucose tolerance test. Blood samples were obtained using a cannula inserted in an antecubital vein before oral intake of the glucose load and at specific times thereafter (15 and 10 minutes before intake; at intake [0]; and 5, 10, 15, 20, 30, 40, 50, 60, 90, 120, 150, 180, and 240 minutes after intake). Blood samples were analyzed for plasma levels of glucose, C-peptide, and glucagon. A glucose oxidase method using a glucose analyzer (2300 Stat Plus analyzer; YSI Inc) measured plasma glucose levels. Whole blood samples were distributed in serum tubes containing a clot activator and chilled tubes containing EDTA. To prevent degradation, a dipeptidyl peptidase 4 inhibitor (valine pyrrolidide) was added to the EDTA-containing tubes, with a final concentration of 0.01 mmol/L. The serum samples were left to coagulate at room temperature. The plasma and serum samples were stored at −20°C and −80°C, respectively. Analyses of C-peptide and glucagon were performed using a 2-sided electrochemiluminescence immunoassay (ADIVA Centaur XP; Siemens) and a C-terminal glucagon-specific antibody (code No. 4305).
After completing the oral glucose tolerance test, body composition was evaluated using dual-energy x-ray absorptiometry (DXA), and 4 rating scales were administered. The DXA measured body composition by subdividing the soft tissue into fat and lean subcompartments. A scanner (Lunar Prodigy; GE Healthcare) with Encore software (version 16) was used. The CoreScan software option (GE Healthcare) was applied to calculate the amount of visceral fat in the android region from the level of the iliac crest up to 20% of the distance to the chin. The rating scales evaluated (1) quality of life (the Schizophrenia Quality of Life Scale), (2) illness severity (Clinical Global Impressions Scale severity score), (3) daily functioning (Global Assessment of Functioning scale), and (4) alcohol consumption (Alcohol Use Disorders Identification Test).
After the baseline examinations, eligible participants were randomly assigned in a double-blind manner to 16 weeks of treatment with subcutaneously injected liraglutide or placebo provided in prefilled pen injectors. The participants followed a fixed uptitration schedule of 0.6 mg per week to a daily dose of 1.8 mg. Participants who did not tolerate uptitration to 1.8 mg of liraglutide continued to receive 1.2 mg/d (n = 3). Every 4 weeks from the day of randomization, participants had blood samples obtained; body weight, waist circumference, and blood pressure measured; and adverse events and alcohol consumption recorded using the Alcohol Use Disorders Identification Test.
End Points
The primary end point was change in glucose tolerance estimated from the plasma glucose excursions (all time values) after the oral glucose tolerance test from baseline to week 16. Secondary end points included changes in glycemia (fasting plasma glucose levels, impaired glucose tolerance, and glycated hemoglobin levels), body weight, waist circumference, and blood pressure; secretion of C-peptide and glucagon evaluated during the oral glucose tolerance test; beta cell function, insulin sensitivity, and beta cell function evaluated using the homeostatic model assessment 2; body composition evaluated using DXA; lipid profile; liver function; measures of quality of life and daily functioning; severity of the psychiatric disease; and alcohol consumption from baseline to the end of trial.
Statistical Analysis
The study was initially designed as an exploratory study. However, before the study commenced, a sample size of 96 patients (48 in each group) was estimated based on the primary end point and values from participants with and without impaired glucose tolerance measured in a prior study (eMethods in Supplement 2). All statistical analyses except the post hoc sensitivity analyses were performed with treatment groups still blinded. Before dividing participants into 2 groups, the statistical analysis plan was uploaded at clinicaltrials.gov and the data set was locked. Statistical analyses were performed using SAS software (version 9.4; SAS Institute). The hypothesis tests were 2 sided, and the level of significance was 5%. All efficacy analyses were performed using a modified intention-to-treat principle in which all randomized participants who received at least 1 dose of the trial compound and had at least 1 assessment after baseline were included. All safety analyses were performed in an intention-to-treat sample in which all randomized participants who received at least 1 dose of the trial compound were included. For the primary end point, a repeated mixed-model analysis of covariance was used to analyze change in glucose tolerance from week 0 to week 16 for the liraglutide and the placebo groups. All changes in secondary end points from baseline to the end of the trial were analyzed using repeated mixed-model analyses for continuous outcomes and mixed-model logistic regression for categorical outcomes. To adjust for multiple testing, the Bonferroni correction was applied. For comparison between the 2 groups, the covariates age, sex, illness duration, treatment group (olanzapine, clozapine, or both), and baseline Clinical Global Impressions Scale severity score and BMI were included in the analyses together with the baseline value of the relevant variable. Exploratory analyses and post hoc sensitivity analyses (eMethods and eTables 2-9 in Supplement 2) were performed to assess the robustness of the primary analyses. The number needed to treat for normalization of prediabetes status was calculated dividing 1 by the risk difference. Effect sizes (Cohen d) for lowering glycated hemoglobin levels and reducing body weight were calculated by dividing the difference of the means in change from baseline to end point (treatment − placebo) by the pooled SD.
Results
Trial Population and Baseline Characteristics
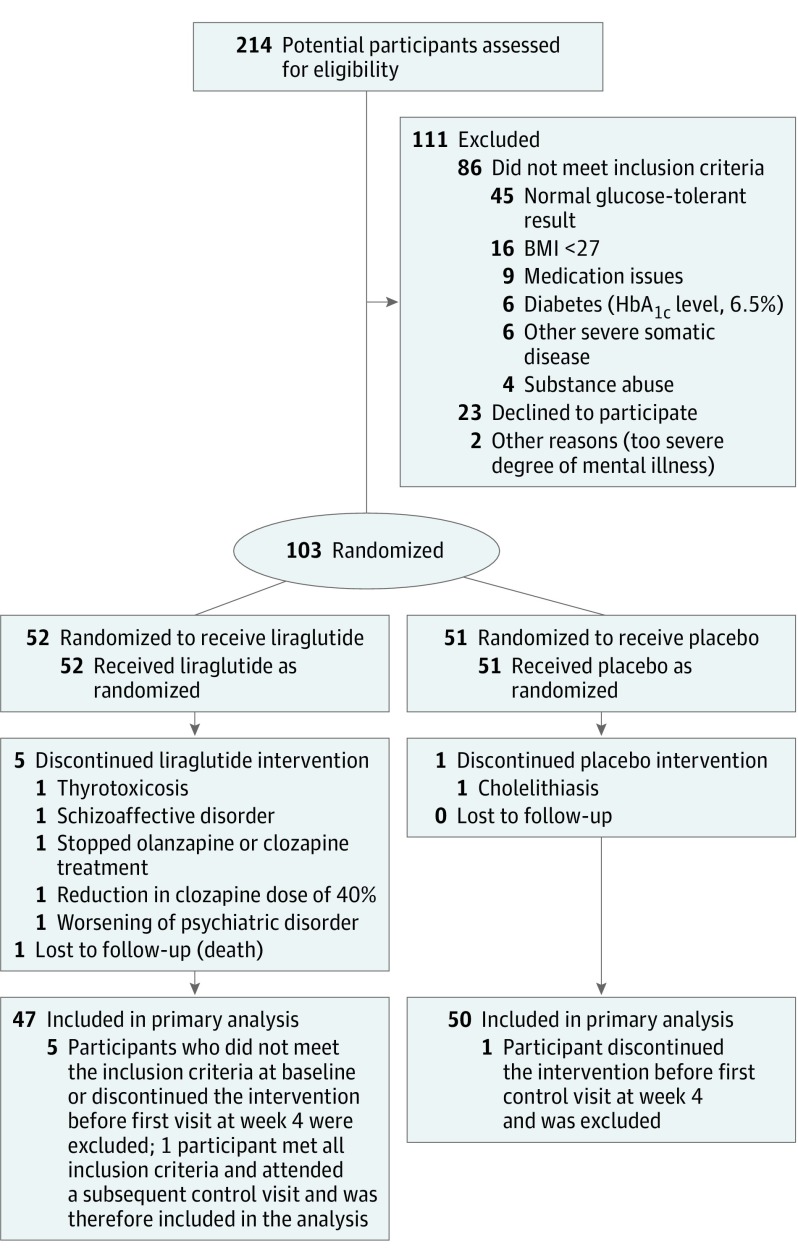
Of 214 eligible participants with a schizophrenia spectrum disorder, 103 (60 men [58.3%] and 43 women [41.7%]; mean [SD] age, 42.5 [10.5] years) were randomized to liraglutide or placebo treatment (Figure 1). One participant in each group withdrew from the trial. In the liraglutide group, an additional 3 participants were randomized in error and 1 participant died before the end of the study (Figure 1). These participants withdrew from the study before any follow-up visits were conducted and therefore were not included in the efficacy analyses. One participant with follow-up data until week 12 (excluded owing to a large clozapine dose reduction) was included in the efficacy analyses (Figure 1). Altogether, 96 participants (93.2%) completed the trial (Figure 1), including 46 of the 52 participants (88.5%) randomized to liraglutide and 50 of the 51 participants (98.0%) randomized to placebo (P = .11). The randomization resulted in comparable groups at baseline (Table 1 and eTable 2 and eFigure 1 in Supplement 2).
Figure 1. Flowchart of Study Participants.
All randomized participants who received at least 1 dose of the trial compound (liraglutide or placebo) and who had at least 1 assessment after baseline were included in the efficacy analyses. Five participants from the liraglutide group and 1 from the placebo group were excluded from the analyses. Another participant in the liraglutide group was excluded after the first assessment and was therefore included in the efficacy analyses without completing the 16 weeks of treatment. All participants who were randomized and had received at least 1 dose of liraglutide or placebo were included in the safety analyses. BMI indicates body mass index (calculated as weight in kilograms divided by height in meters squared); HbA1c,hemoglobin A1c. To convert hemoglobin A1c to mmol/mol, subtract 2.15% and multiply by 10.929.
Table 1. Baseline Characteristics.
| Characteristic | Treatment Groupa | |
|---|---|---|
| Liraglutide (n = 47) |
Placebo (n = 50) |
|
| Sociodemographic | ||
| Age, mean (SD), y | 42.1 (10.7) | 43.0 (10.5) |
| Male, No. (%) | 30 (63.8) | 30 (60.0) |
| Diagnosis, No. (%) | ||
| Schizophrenia | 46 (97.9) | 50 (100) |
| Schizotypal disorder | 0 | 0 |
| Psychosis | 1 (2.1) | 0 |
| Duration of diagnosis, mean (SD), y | 15.5 (9.4) | 15.1 (8.8) |
| Treatment, No. (%) | ||
| Olanzapine | 15 (31.9) | 6 (12.0) |
| Clozapine | 32 (68.1) | 41 (82.0) |
| Clozapine and olanzapine | 0 | 3 (6.0) |
| Dose, median (IQR), mg | ||
| Olanzapine | 17.5 (17.5) | 13.8 (10.0) |
| Clozapine | 300.0 (200.0) | 312.5 (212.5) |
| Clinical characteristics, mean (SD) | ||
| Body weight, kg | 103.3 (16.1) | 102.4 (23.9) |
| Waist circumference, cm | 117.3 (12.4) | 115.9 (15.1) |
| BMI | 33.7 (5.1) | 33.9 (6.6) |
| Systolic blood pressure, mm Hg | 125.9 (10.5) | 125.2 (14.1) |
| Diastolic blood pressure, mm Hg | 84.3 (9.8) | 84.6 (7.6) |
| Prediabetes criteria, No. (%)b | ||
| Elevated fasting plasma glucose level | 14 (29.8) | 15 (30.0) |
| Elevated glycated hemoglobin level | 6 (12.8) | 6 (12.0) |
| Impaired glucose tolerance | 45 (95.7) | 48 (96.0) |
| >1 Criterion of prediabetes | 14 (29.8) | 17 (34.0) |
| Glucose metabolism | ||
| Glycated hemoglobin level, % | 5.6 (0.4) | 5.5 (0.4) |
| Fasting plasma glucose level, median (IQR), mg/dL | 100.9 (18.0) | 102.7 (16.2) |
| Fasting C-peptide secretion, median (IQR), ng/mL | 3.4 (1.0) | 3.7 (1.3) |
| Fasting glucagon secretion, mean (SD), pg/mL | 21.3 (13.2 | 23.0 (14.6) |
| Insulin resistance, median (IQR)c | 2.6 (0.86) | 2.9 (1.0) |
| Beta cell function, median (IQR), %c | 143.7 (50.5) | 148.8 (41.9) |
| Insulin sensitivity, median (IQR), %c | 38.7 (12.0) | 34.2 (12.6) |
| 2-h, 75-g OGTT finding, median (IQR), mg/dL | 165.8 (46.8) | 171.2 (41.4) |
| Body composition | ||
| Visceral fat, mean (SD), g | 2129 (882) | 2003 (906) |
| Android to gynoid fat ratio, median (IQR) | 1.2 (0.25) | 1.2 (0.3) |
| Total body fat, median (IQR), % | 41.5 (11.4) | 40.9 (10.4) |
| Cholesterol level, median (IQR), mg/dL | ||
| Total | 204.6 (69.5) | 196.9 (42.5) |
| LDL | 123.5 (57.9) | 127.4 (42.5) |
| HDL | 42.5 (19.3) | 42.5 (23.2) |
| VLDL | 30.9 (11.6) | 30.9 (15.4) |
| Fasting triglyceride level, median (IQR), mg/dL | 177.0 (150.4) | 177.0 (123.9) |
| Rating scales, mean (SD) score | ||
| SQLSd | ||
| Psychosocial | 39.0 (25.0) | 38.4 (26.2) |
| Motivation and energy | 31.0 (24.6) | 37.7 (26.8) |
| Adverse effects | 27.9 (20.7) | 25.3 (19.4) |
| CGI-Se | 3.6 (0.7) | 3.8 (0.7) |
| GAFf | 49.4 (9.3) | 47.2 (8.5) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CGI-S, Clinical Global Impressions Scale severity score; GAF, Global Assessment of Functioning scale; HDL, high-density lipoprotein; IQR, interquartile range (calculated by subtracting the 25th percentile value from the 75th percentile value); LDL, low-density lipoprotein; OGTT, oral glucose tolerance test; SQLS, Schizophrenia Quality of Life Scale; VLDL, very low-density lipoprotein.
SI conversion factors: To convert cholesterol levels to mmol/L, multiply by 0.0259; C-peptide to nmol/L, multiply by 0.331; glucagon to ng/L, multiply by 1.0; glucose levels to mmol/L, multiply by 0.0555; glycated hemoglobin levels to mmol/mol, subtract 2.15 and multiply by 10.929; and triglyceride levels to mmol/L, multiply by 0.0113.
Baseline values were calculated for participants who were randomized, received at least 1 dose of the trial compound (liraglutide or placebo), and had at least 1 assessment after baseline. For dose of olanzapine, fasting plasma glucose level, 2-hour OGTT value, android to gynoid fat ratio, total body fat composition, HDL and VLDL cholesterol levels, triglyceride level, and homeostatic model assessment 2 (HOMA2) measures, differences were based on logarithmic transformed data because of skewed distributions. No significant differences between groups were noted for any of the characteristics except treatment with clozapine, olanzapine, or both (P = .02).
Defined as an elevated fasting plasma glucose level of 110 to 125 mg/dL, elevated glycated hemoglobin of 6.1% to 6.4%, and/or impaired glucose tolerance with a 2-hour value of at least 140 mg/dL during a 75-g OGTT. Calculations are based on the number of participants included in the efficacy analyses.
Estimated using HOMA2 measures.
Scores range from 0 to 100, with higher scores indicating poorer quality of life.
Scores range from 0 to 7, with higher scores indicating higher illness severity.
Scores range from 0 to 100, with higher scores indicating a higher function of daily living.
Glycemic Control
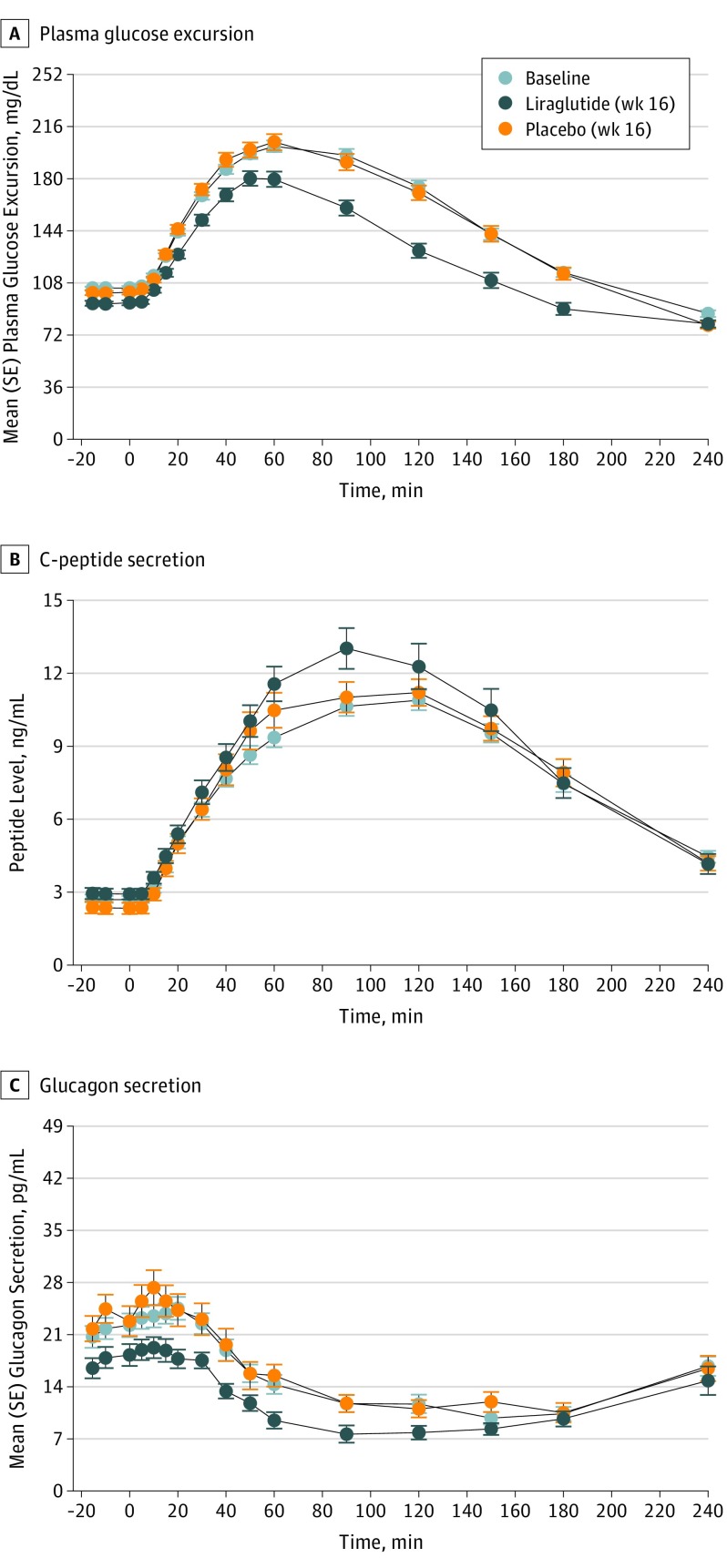
After 16 weeks of treatment, the primary end point of glucose tolerance evaluated by the oral glucose tolerance test improved significantly for liraglutide compared with placebo (P < .001) (Figure 2). A 23% larger reduction of the 2-hour plasma glucose level during the oral glucose tolerance test occurred in the liraglutide group compared with the placebo group (0.77; 95% CI, 0.70-0.85; P < .001). The improvements in glycemic control were robust in sensitivity analyses, examining (1) no covariate adjustments (P < .001) and (2) all randomized patients (n = 103) included and assumed to have no change in values from baseline to week 16 in the 6 patients with no postbaseline assessments (P < .001) (eTable 5 in Supplement 2). In the liraglutide group, 30 participants (63.8%) changed status from prediabetes to normal glucose tolerance compared with 8 (16.0%) in the placebo group, corresponding to a number needed to treat of 2 (Table 2). The liraglutide group had significantly reduced glycated HbA1c level (−0.2%; 95% CI, −0.3 to −0.1%; P < .001) and relative change in fasting plasma glucose level (0.90; 95% CI, 0.88 to 0.95; P < .001) compared with the placebo group (Table 2). An increased C-peptide secretion and a decreased glucagon secretion during the oral glucose tolerance test was found with liraglutide compared with placebo (C-peptide, P = .01; glucagon, P = .01). After adjusting for covariates, the differences in the oral glucose tolerance test results were no longer significant for C-peptide (P = .06) or glucagon secretion (P = .13) (Figure 2 and eTable 5 in Supplement 2). With use of the homeostatic model assessment 2 model, an improved beta cell function for the liraglutide group was found, but no change in insulin sensitivity occurred (Table 2).
Figure 2. Oral Glucose Tolerance Test Results.
Plasma glucose excursion, C-peptide level, and glucagon secretion were evaluated at fixed time points before and after ingestion of oral glucose (0 indicates intake). Values represent the mean calculated by a repeated mixed-model analysis. When comparing the liraglutide group with the placebo group, the model includes the covariates age; sex; illness duration; treatment with olanzapine, clozapine, or both; baseline body mass index; and baseline Clinical Global Impressions Scale severity score. The analyses include all participants who were randomized, received at least 1 dose of the trial compound (liraglutide or placebo), and had at least 1 assessment after baseline. To convert C-peptide to nanomoles per liter, multiply by 0.331; glucagon to nanograms per liter, multiply by 1.0; and glucose levels to millimoles per liter, multiply by 0.0555.
Table 2. Change in End Points From Baseline to Week 16a.
| Characteristic | Liraglutide Treatment Group (n = 47) |
Placebo Treatment Group (n = 50) |
Estimated Treatment Difference, Liraglutide vs Placebo (95% CI)b | P Valuec |
|---|---|---|---|---|
| Clinical, mean (SE) | ||||
| Body weight, kg | −4.7 (0.5) | 0.5 (0.7) | −5.3 (−7.0 to −3.7) | <.001d |
| Waist circumference, cm | −4.0 (0.6) | 0.5 (0.7) | −4.1 (−6.0 to −2.3) | <.001d |
| BMI | −1.6 (1.2) | 0.08 (0.2) | −1.8 (−2.4 to −1.3) | <.001d |
| Systolic blood pressure, mm Hg | −1.4 (2.0) | 1.1 (1.8) | −4.9 (−9.5 to −0.3) | .04 |
| Diastolic blood pressure, mm Hg | 0.5 (1.5) | 2.4 (1.1) | −3.0 (−6.8 to 0.9) | .13 |
| Prediabetes status, No. (%)e | −30 (63.8) | −8 (16.0) | 9.2 (2.6 to 32.7) | <.001d |
| Elevated fasting plasma glucose level | −13 (85.7) | −6 (40.0) | 2.1 (0.9 to 3.3) | <.001d |
| Elevated glycated hemoglobin level | −5 (83.3) | 0 (0.0) | NA (too few events) | NA (too few events) |
| Impaired glucose tolerance | −28 (37.8) | −6 (12.5) | 2.1 (0.8 to 3.5) | .002d |
| Glucose metabolism | ||||
| Glycated hemoglobin level, % | −0.2 (0.04) | 0.06 (0.04) | −0.2 (−0.3 to −0.1) | <.001d |
| Fasting plasma glucose level, relative change | 0.90 | 0.99 | 0.90 (0.88 to 0.95) | <.001d |
| Fasting C-peptide level, mean (SE), ng/mL | 0.26 (0.15) | −0.20 (0.16) | 0.46 (−0.02 to 0.94) | .06 |
| Fasting glucagon level, mean (SE), pg/mL | −4.6 (2.4) | 2.0 (2.8) | −4.7 (−8.6 to −0.05) | .02 |
| Insulin resistancef | 1.02 | 0.96 | 1.08 (0.96 to 1.22) | .21 |
| Beta cell functionf | 1.28 | 0.99 | 1.29 (1.18 to 1.42) | <.001d |
| Insulin sensitivityf | 0.99 | 1.04 | 0.93 (0.82 to 1.06) | .26 |
| 2-h, 75-g OGTT value | 0.47 | 0.95 | 0.77 (0.70 to 0.85) | <.001d |
| Body composition | ||||
| Visceral fat, mean (SE), g | −315.8 (75.3) | −24.0 (41.7) | −250.19 (−459.9 to −40.5) | .02 |
| Android to gynoid fat ratio | 0.99 | 1.01 | 0.98 (0.94 to 1.01) | .23 |
| Total body fat | 0.91 | 0.99 | 0.96 (0.94 to 0.99) | .01 |
| Cholesterol level | ||||
| Total, mean (SE), mg/dL | −19.3 (3.5) | 3.5 (3.1) | −19.3 (−30.9 to −7.7) | <.001d |
| LDL, mean (SE), mg/dL | −15.4 (3.1) | −2.3 (1.9) | −15.4 (−23.2 to −7.7) | <.001d |
| HDL | 0.95 | 0.99 | 0.96 (0.90 to 1.03) | .27 |
| VLDL | 0.94 | 0.94 | 0.93 (0.79 to 1.10) | .39 |
| Fasting triglyceride level | 0.88 | 0.97 | 0.90 (0.76 to 1.07) | .22 |
| Rating scales | ||||
| SQLSg | ||||
| Psychosocial | −1.8 (2.2) | −2.6 (2.3) | 2.2 (−5.0 to 9.3) | .55 |
| Motivation and energy | −1.5 (3.2) | −0.8 (2.5) | −3.5 (−12.3 to 5.2) | .42 |
| Adverse effects | −5.5 (3.2) | −2.5 (2.5) | 4.0 (−3.6 to 11.6) | .30 |
| CGI-Sh | −0.002 (0.08) | 0.09 (0.07) | −0.1 (−0.3 to 0.2) | .49 |
| GAFi | −0.3 (0.4) | 0.8 (0.6) | −1.7 (−3.5 to 0.05) | .06 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CGI-S, Clinical Global Impressions Scale severity score; GAF, Global Assessment of Functioning scale; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NA, not applicable; OGTT, oral glucose tolerance test; SQLS, Schizophrenia Quality of Life Scale; VLDL, very low-density lipoprotein.
SI conversion factors: To convert cholesterol levels to mmol/L, multiply by 0.0259; C-peptide to nmol/L, multiply by 0.331; glucagon to ng/L, multiply by 1.0; and glucose levels to mmol/L, multiply by 0.0555.
Differences in means (SE from baseline to week 16) were calculated using a mixed-model analysis of covariance for participants who were randomized, received at least 1 dose of the trial compound (liraglutide or placebo), and had at least 1 assessment after baseline. For fasting plasma glucose level, 2-h value during the OGTT, android to gynoid fat ratio, total body fat, HDL and VLDL cholesterol levels, triglyceride level, and homeostatic model assessment 2 (HOMA2) measures, the relative change from baseline is presented.
Estimated treatment differences between groups were calculated using a mixed-model analysis of covariance. The model includes the baseline value of the relevant variable together with the covariates age; sex; illness duration; treatment with olanzapine, clozapine, or both; baseline BMI; and baseline CGI-S.
Refer to the estimated treatment difference. To adjust for multiple testing, Bonferroni correction was applied.
Indicates that a given P value remained significant after Bonferroni adjustments with P < .002 (.05/number of tests = 26).
Indicates number of patients who experienced change from prediabetes status to normal glucose tolerance at week 16. Differences between the groups were analyzed using mixed-model logistic regression and are presented as the proportion of patients who developed normal glucose tolerance at week 16 in numbers (percentage) and odds ratios.
Estimated using HOMA2 measures.
Scores range from 0 to 100, with higher scores indicating poorer quality of life.
Scores range from 0 to 7, with higher scores indicating higher illness severity.
Scores range from 0 to 100, with higher scores indicating a higher function of daily living.
Body Weight and Metabolic Variables
At week 16, the mean weight loss difference between liraglutide and placebo treatment was −5.3 kg (95% CI, −7.0 to −3.7 kg). Compared with placebo, mean differences for liraglutide compared with placebo were significant for reduced waist circumference (−4.1 cm; 95% CI, −6.0 to −2.3 cm; P < .001), BMI (−1.8; 95% CI, −2.4 to −1.3; P < .001), systolic blood pressure (−4.9 mm Hg; 95% CI, −9.5 to −0.3 mm Hg; P = .04), and levels of total cholesterol (−19.3 mg/dL; 95% CI, −30.9 to −7.7 mg/dL; P < .001) and low-density lipoprotein cholesterol (−15.4 mg/dL; 95% CI, −23.2 to −7.7 mg/dL; P < .001). Furthermore, liraglutide reduced visceral fat (−250.19 g; 95% CI, −459.9 to −40.5 g; P = .02) and caused a reduction of total body fat (relative change, 0.96; 95% CI, 0.94 to 0.99; P = .01) evaluated by the DXA scan (Table 2). With the exception of systolic blood pressure and body composition measures, these changes remained significant after Bonferroni correction and in sensitivity analyses (eTable 6 in Supplement 2). Effect size for lowering glycated hemoglobin levels was 1.0 (95% CI, 0.61 to 1.46); for reducing body weight, 1.1 (95% CI, 0.77 to 1.64).
Adverse Events
No differences were found regarding liver function, but a small, significant increase of amylase level (1.27 vs 0.98 U/L [to convert to microkatals per liter, multiply by 0.0167; P < .001) was found in the liraglutide group (eTable 3 in Supplement 2). The most common adverse events with liraglutide were related to the gastrointestinal tract, and rates were high in both groups (Table 3) and most prevalent at the beginning of treatment. The nausea rate was significantly higher in the liraglutide group (31 of 50 [62%] vs 16 of 50 [32%]; P = .008) but diminished over time (eTable 4 in Supplement 2). No effect of nausea with respect to body weight reduction, BMI, waist circumference, and reduction of visceral fat was found in subgroup analyses comparing participants who reported nausea with those who did not. Fewer serious adverse events occurred in the liraglutide group compared with the placebo group (6 of 51 [11.5%] vs 13 of 51 [25.5%]). No difference in the occurrence of somatic serious adverse events was found (Table 3). The most common serious adverse event was exacerbation of the psychiatric disease and voluntary admission to a psychiatric hospital. No differences were found between liraglutide and placebo with respect to quality of life, daily function, psychiatric disease severity, or alcohol intake (Table 2 and eTable 3 in Supplement 2).
Table 3. Adverse Events and Serious Adverse Eventsa.
| Adverse Event or Reaction | No. (%) of Participants | P Valueb | |
|---|---|---|---|
| Liraglutide Treatment Group (n = 52) |
Placebo Treatment Group (n = 51) |
||
| Gastrointestinal tract | |||
| Nausea | 31/50 (62.0) | 16/50 (32.0) | .008 |
| Diarrhea | 10/50 (20.0) | 7/50 (14.0) | .42 |
| Constipation | 18/50 (36.0) | 11/50 (22.0) | .16 |
| Vomiting | 15/49 (30.6) | 9/50 (18.0) | .09 |
| Dyspepsia | 17/50 (34.0) | 11/50 (22.0) | .54 |
| Abdominal pain | 23/50 (46.0) | 13/50 (26.0) | .06 |
| Cardiovascular | |||
| Palpitation | 1/50 (2.0) | 0 | .25 |
| Orthostatic hypotension | 4/49 (8.2) | 0 | .04 |
| Infection | |||
| Upper respiratory tract | 9/50 (18.0) | 9/50 (18.0) | .82 |
| Influenza virus | 1/50 (2.0) | 2/51 (3.9) | .47 |
| Others | 2/50 (4.0) | 0 | .21 |
| Nervous system | |||
| Fatigue | 1/50 (2.0) | 1/50 (2.0) | .81 |
| Headache | 10/50 (20.0) | 8/50 (16.0) | .51 |
| Dizziness | 4/50 (8.0) | 3/51 (5.9) | .95 |
| Injection site | |||
| Hematoma or pain | 0 | 3/51 (5.9) | .13 |
| Musculoskeletal disorder | 3/49 (6.1) | 2/51 (3.9) | .47 |
| Other | 2/50 (4.0) | 6/51 (11.8) | .20 |
| Hypoglycemiac | 13/50 (26.0) | 7/50 (14.0) | .22 |
| Serious adverse events | |||
| Total No. | 6/51 (11.8) | 13/51 (25.5) | .04 |
| Somatic disease | |||
| Tonsillitis | 0 | 1/50 (2.0) | .39 |
| Pneumonia | 2 (3.9) | 0 | .40 |
| Bacterial gastroenteritis | 0 | 1/50 (2.0) | .40 |
| Cholelithiasis | 0 | 1/50 (2.0) | .40 |
| Hashimoto disease | 0 | 1/50 (2.0) | .40 |
| Death | 1/52 (1.9) | 0 | .30 |
| Psychiatric disease | |||
| Admission to hospital for worsening of schizophrenia | 3/50 (6.0) | 951 (17.7) | .08 |
All participants who received at least 1 dose of liraglutide or placebo were included in the safety analyses.
Calculated using exact logistic regression adjusting for antipsychotic treatment (olanzapine, clozapine, or both).
Based on self-reported cases of hypoglycemia or plasma glucose levels less than 54 mg/dL (to convert to mmol/L, multiply by 0.0555) measured during the trial. All cases of measured hypoglycemia happened during the final oral glucose tolerance test in participants who had received placebo.
Discussion
In overweight or obese patients with schizophrenia spectrum disorders and prediabetes, 16 weeks of liraglutide as an adjunctive treatment to stable treatment with clozapine or olanzapine significantly improved glucose tolerance and glycemic control. At the end of the trial, the placebo-subtracted body weight loss was 5.3 kg. The weight loss was further supported by concurrent reductions in waist circumference and visceral fat evaluated using DXA. These reductions are particularly relevant because increased waist circumference and visceral fat accumulation are strongly associated with insulin resistance, diabetes development, and consequent cardiovascular disease. Compared with placebo, liraglutide reduced body weight and lowered glycated hemoglobin levels with large effect sizes of 1.0 and 1.1, respectively, and improved several cardiometabolic variables, including systolic blood pressure and total and low-density lipoprotein cholesterol levels. Consistent with the proposed mechanisms of liraglutide for improving glycemic control, increased levels of C-peptide and suppressed levels of glucagon in the liraglutide group were found. Homeostatic model assessment 2 calculations indicated an increased beta cell function; however, no improvement in insulin resistance or insulin sensitivity occurred.
Although the participants included in this study received ongoing treatment with antipsychotic compounds, the improvements induced by liraglutide were consistent with the findings of previous studies in individuals with no known psychiatric illness. Adverse events in the liraglutide group were similar to those previously described in individuals without schizophrenia spectrum disorders. Most adverse events were transient gastrointestinal tract effects, particularly nausea. The higher nausea rate in the liraglutide group was not associated with a larger body weight reduction. One participant, a man in his 60s with a long duration of schizophrenia, died unexpectedly after 3 days of acute illness. No causal relationship was established to treatment with liraglutide. Altogether, the liraglutide group experienced significantly fewer serious adverse events, with exacerbation of patients’ psychiatric disease being the most common cause, and no differences in quality of life, daily functioning, or psychiatric disease severity were found.
Other interventions for antipsychotic-induced weight gain and metabolic disturbances have been examined previously. Several meta-analyses of adjunct pharmacologic intervention to antipsychotic medications found metformin hydrochloride to be superior to placebo and more effective than comparators, but metformin only induced a body weight loss of approximately 3 kg for 2 to 4 months. In meta-analyses of other interventions, such as adjunctive treatment with topiramate, switch of antipsychotic medication, and behavioral interventions, similar or somewhat smaller effects on antipsychotic-induced body weight gain and less consistent improvements across metabolic parameters were found.
Limitations and Strengths
Most antipsychotic-treated patients experience weight gain and metabolic disturbances, although large interindividual differences exist. In this trial, a selected group of patients treated with the antipsychotic compounds clozapine or olanzapine were included, which could be viewed as a limitation. Clozapine and olanzapine were selected because they induce the greatest weight gain and metabolic abnormalities. Furthermore, because clozapine and olanzapine are among the most efficacious antipsychotic compounds, switch to other antipsychotics is often not possible. The participants also fulfilled criteria for prediabetes, which further limits generalizability. However, prediabetes is considered to be a strong predictor for later development of diabetes and is associated with an increased risk for cardiovascular disease. In the present study, only participants with a glycated hemoglobin level of at least 6.5% were characterized as having type 2 diabetes, allowing participants with a 2-hour glucose level greater than 199 mg/dL during the oral glucose tolerance test to be included in the study. However, in sensitivity analyses in which these participants (n = 24) were excluded, the significant effects of liraglutide persisted (eFigure 2 and eTable 9 in Supplement 2).
Strengths of the study include the randomized, placebo-controlled design and double-blinding of treatment. Although the participants had a severe mental illness, had to accept daily subcutaneous injection, and had relatively high rates of nausea, the dropout rate remained very low (7 participants [6.8%]) compared with previous reports of nonpsychiatric populations treated with liraglutide. In our study, participants were treated with stable doses of clozapine or olanzapine for more than 6 months before inclusion. Because antipsychotic-induced weight gain and metabolic disturbances are most profound at the beginning of treatment, further research is needed to determine whether liraglutide can be used as a preventive adjunctive treatment during the emergence of weight gain and metabolic abnormalities. Furthermore, a head-to-head study with the best-studied comparator for this purpose (metformin or topiramate) would be relevant. Last, several GLP-1 receptor agonists are currently available for the treatment of type 2 diabetes, and 1 randomized clinical trial recently reported that the once-weekly GLP-1 receptor agonist exenatide did not promote weight loss or improve metabolic disturbances in antipsychotic-treated patients. However, the number of participants was small and the intervention period was short. Further research is also needed to determine the safety and effectiveness of different GLP-1 receptor agonists in antipsychotic-treated patients. Whether the recently established benefits of liraglutide treatment for cardiovascular disease can be extended to patients with schizophrenia spectrum disorder is beyond this study to establish; however, the results of the present study are encouraging.
Conclusions
In patients with schizophrenia spectrum disorder, once-daily subcutaneous injection with the GLP-1 receptor agonist liraglutide as adjunctive treatment to clozapine or olanzapine was a safe and effective pharmacologic intervention. Liraglutide improved glucose tolerance and metabolic variables and induced a body weight loss superior to that of placebo without adversely affecting the mental status of the participants.
Trial Protocol
eTable 1. Inclusion and Exclusion Criteria
eTable 2. Additional Baseline Characteristics
eTable 3. Additional Change in End Points Between Baseline and Week 16
eTable 4. Prevalence of the Most Common Adverse Events During the 16 Weeks
eTable 5. Sensitivity Analyses of the Plasma Glucose Excursion, C-Peptide, and Glucagon Secretion During the Oral Glucose Tolerance Test Between Baseline and Week 16
eTable 6. Sensitivity Analyses for the Secondary End Points Between Baseline and Week 16
eTable 7. Mean Changes for the Major Outcomes Between Baseline and Week 16 by Olanzapine and Clozapine Treatment
eTable 8. Mean Changes for the Major Outcomes Between Baseline and Week 16 by Change in Dose of Olanzapine or Clozapine
eTable 9. Mean Changes for the Major Outcomes Between Baseline and Week 16 by the 2-Hour Value During the OGTT
eMethods. Data Analysis
eFigure 1. Baseline Oral Glucose Tolerance Test for the Liraglutide and the Placebo Groups
eFigure 2. Exploratory Analyses for the Primary Outcome
References
- 1.Lawrence D, Hancock KJ, Kisely S. The gap in life expectancy from preventable physical illness in psychiatric patients in Western Australia: retrospective analysis of population based registers. BMJ. 2013;346:f2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123-1131. [DOI] [PubMed] [Google Scholar]
- 3.Laursen TM, Munk-Olsen T, Vestergaard M. Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr Opin Psychiatry. 2012;25(2):83-88. [DOI] [PubMed] [Google Scholar]
- 4.Hennekens CH, Hennekens AR, Hollar D, Casey DE. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115-1121. [DOI] [PubMed] [Google Scholar]
- 5.Henderson DC, Vincenzi B, Andrea NV, Ulloa M, Copeland PM. Pathophysiological mechanisms of increased cardiometabolic risk in people with schizophrenia and other severe mental illnesses. Lancet Psychiatry. 2015;2(5):452-464. [DOI] [PubMed] [Google Scholar]
- 6.Leucht S, Cipriani A, Spineli L, et al. . Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962. [DOI] [PubMed] [Google Scholar]
- 7.Allison DB, Mentore JL, Heo M, et al. . Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156(11):1686-1696. [DOI] [PubMed] [Google Scholar]
- 8.Correll CU, Lencz T, Malhotra AK. Antipsychotic drugs and obesity. Trends Mol Med. 2011;17(2):97-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796. [DOI] [PubMed] [Google Scholar]
- 10.Maayan L, Vakhrusheva J, Correll CU. Effectiveness of medications used to attenuate antipsychotic-related weight gain and metabolic abnormalities: a systematic review and meta-analysis. Neuropsychopharmacology. 2010;35(7):1520-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caemmerer J, Correll CU, Maayan L. Acute and maintenance effects of non-pharmacologic interventions for antipsychotic associated weight gain and metabolic abnormalities: a meta-analytic comparison of randomized controlled trials. Schizophr Res. 2012;140(1-3):159-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mukundan A, Faulkner G, Cohn T, Remington G. Antipsychotic switching for people with schizophrenia who have neuroleptic-induced weight or metabolic problems. Cochrane Database Syst Rev. 2010;(12):CD006629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizuno Y, Suzuki T, Nakagawa A, et al. . Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiedorowicz JG, Miller DD, Bishop JR, Calarge CA, Ellingrod VL, Haynes WG. Systematic review and meta-analysis of pharmacological interventions for weight gain from antipsychotics and mood stabilizers. Curr Psychiatry Rev. 2012;8(1):25-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409-1439. [DOI] [PubMed] [Google Scholar]
- 16.Madsbad S. A review of head-to-head comparisons of GLP-1 receptor agonists. Diabetes Obes Metab. 2016;18(4):317-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pi-Sunyer X, Astrup A, Fujioka K, et al. ; SCALE Obesity and Prediabetes NN8022-1839 Study Group . A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11-22. [DOI] [PubMed] [Google Scholar]
- 18.Astrup A, Rössner S, Van Gaal L, et al. ; NN8022-1807 Study Group . Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374(9701):1606-1616. [DOI] [PubMed] [Google Scholar]
- 19.Nuffer WA, Trujillo JM. Liraglutide: a new option for the treatment of obesity. Pharmacotherapy. 2015;35(10):926-934. [DOI] [PubMed] [Google Scholar]
- 20.Blonde L, Russell-Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1-5 studies. Diabetes Obes Metab. 2009;11(suppl 3):26-34. [DOI] [PubMed] [Google Scholar]
- 21.Ishøy PL, Knop FK, Vilsbøll T, Glenthøj BY, Ebdrup BH. Sustained weight loss after treatment with a glucagon-like peptide-1 receptor agonist in an obese patient with schizophrenia and type 2 diabetes. Am J Psychiatry. 2013;170(6):681-682. [DOI] [PubMed] [Google Scholar]
- 22.Ebdrup BH, Knop FK, Ishøy PL, et al. . Glucagon-like peptide-1 analogs against antipsychotic-induced weight gain: potential physiological benefits. BMC Med. 2012;10:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayfield K, Siskind D, Winckel K, et al. . Glucagon-like peptide-1 agonists combating clozapine-associated obesity and diabetes. J Psychopharmacol. 2016;30(3):227-236. [DOI] [PubMed] [Google Scholar]
- 24.Ishøy PL, Knop FK, Broberg BV, et al. . Effect of GLP-1 receptor agonist treatment on body weight in obese antipsychotic-treated patients with schizophrenia: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2017;19(2):162-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen JR, Vedtofte L, Holst JJ, et al. . Does a GLP-1 receptor agonist change glucose tolerance in patients treated with antipsychotic medications? design of a randomised, double-blinded, placebo-controlled clinical trial [published correction appears in BMJ Open. 2015;5(5):e004227corr1]. BMJ Open. 2014;4(3):e004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 28.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 29.Wewer Albrechtsen NJ, Hartmann B, Veedfald S, et al. . Hyperglucagonaemia analysed by glucagon sandwich ELISA: nonspecific interference or truly elevated levels? Diabetologia. 2014;57(9):1919-1926. [DOI] [PubMed] [Google Scholar]
- 30.Heymsfield SB, Wang J, Heshka S, Kehayias JJ, Pierson RN. Dual-photon absorptiometry: comparison of bone mineral and soft tissue mass measurements in vivo with established methods. Am J Clin Nutr. 1989;49(6):1283-1289. [DOI] [PubMed] [Google Scholar]
- 31.Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990;51(6):1106-1112. [DOI] [PubMed] [Google Scholar]
- 32.Kaul S, Rothney MP, Peters DM, et al. . Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity (Silver Spring). 2012;20(6):1313-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilkinson G, Hesdon B, Wild D, et al. . Self-report quality of life measure for people with schizophrenia: the SQLS. Br J Psychiatry. 2000;177:42-46. [DOI] [PubMed] [Google Scholar]
- 34.Busner J, Targum SD. The Clinical Global Impressions Scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4(7):28-37. [PMC free article] [PubMed] [Google Scholar]
- 35.Aas IH. Global Assessment of Functioning (GAF): properties and frontier of current knowledge. Ann Gen Psychiatry. 2010;9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption–II. Addiction. 1993;88(6):791-804. [DOI] [PubMed] [Google Scholar]
- 37.Foghsgaard S, Vedtofte L, Mathiesen ER, et al. . The effect of a glucagon-like peptide-1 receptor agonist on glucose tolerance in women with previous gestational diabetes mellitus: protocol for an investigator-initiated, randomised, placebo-controlled, double-blinded, parallel intervention trial. BMJ Open. 2013;3(10):e003834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neeland IJ, Turer AT, Ayers CR, et al. . Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308(11):1150-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Hert M, Detraux J, van Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114-126. [DOI] [PubMed] [Google Scholar]
- 40.Astrup A, Carraro R, Finer N, et al. ; NN8022-1807 Investigators . Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond). 2012;36(6):843-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng W, Li XB, Tang YL, Xiang YQ, Wang CY, de Leon J. Metformin for weight gain and metabolic abnormalities associated with antipsychotic treatment: meta-analysis of randomized placebo-controlled trials. J Clin Psychopharmacol. 2015;35(5):499-509. [DOI] [PubMed] [Google Scholar]
- 42.Correll CU, Maayan L, Kane J, Hert MD, Cohen D. Efficacy for psychopathology and body weight and safety of topiramate-antipsychotic cotreatment in patients with schizophrenia spectrum disorders: results from a meta-analysis of randomized controlled trials. J Clin Psychiatry. 2016;77(6):e746-e756. [DOI] [PubMed] [Google Scholar]
- 43.Zheng W, Xiang YT, Xiang YQ, et al. . Efficacy and safety of adjunctive topiramate for schizophrenia: a meta-analysis of randomized controlled trials. Acta Psychiatr Scand. 2016;134(5):385-398. [DOI] [PubMed] [Google Scholar]
- 44.Deng C. Effects of antipsychotic medications on appetite, weight, and insulin resistance. Endocrinol Metab Clin North Am. 2013;42(3):545-563. [DOI] [PubMed] [Google Scholar]
- 45.Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383(9922):1068-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.American Diabetes Association 2. Classification and diagnosis of diabetes. Diabetes Care. 2016;39(suppl 1):S13-S22. [DOI] [PubMed] [Google Scholar]
- 47.Marso SP, Daniels GH, Brown-Frandsen K, et al. ; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Inclusion and Exclusion Criteria
eTable 2. Additional Baseline Characteristics
eTable 3. Additional Change in End Points Between Baseline and Week 16
eTable 4. Prevalence of the Most Common Adverse Events During the 16 Weeks
eTable 5. Sensitivity Analyses of the Plasma Glucose Excursion, C-Peptide, and Glucagon Secretion During the Oral Glucose Tolerance Test Between Baseline and Week 16
eTable 6. Sensitivity Analyses for the Secondary End Points Between Baseline and Week 16
eTable 7. Mean Changes for the Major Outcomes Between Baseline and Week 16 by Olanzapine and Clozapine Treatment
eTable 8. Mean Changes for the Major Outcomes Between Baseline and Week 16 by Change in Dose of Olanzapine or Clozapine
eTable 9. Mean Changes for the Major Outcomes Between Baseline and Week 16 by the 2-Hour Value During the OGTT
eMethods. Data Analysis
eFigure 1. Baseline Oral Glucose Tolerance Test for the Liraglutide and the Placebo Groups
eFigure 2. Exploratory Analyses for the Primary Outcome