Key Points
Question
What are the natural history and growth kinetics of low-risk papillary thyroid cancers in patients undergoing active surveillance in the United States?
Findings
In this cohort study of 291 patients undergoing active surveillance for low-risk papillary thyroid cancer, most cancers remained stable over several years of observation. Serial measurements of tumor volume defined the rate of growth for each tumor and can facilitate early identification of tumor growth.
Meaning
Tumor volume kinetics can inform the timing of surveillance imaging and therapeutic interventions for patients with papillary thyroid cancer undergoing active surveillance.
Abstract
Importance
Active surveillance of low-risk papillary thyroid cancer (PTC) is now an accepted alternative to immediate surgery, but experience with this approach outside of Japan is limited. The kinetics (probability, rate, and magnitude) of PTC tumor growth under active surveillance have not been well defined.
Objective
To describe the kinetics of PTC tumor growth during active surveillance.
Design, Setting, and Participants
Cohort study of 291 patients undergoing active surveillance for low-risk PTC (intrathyroidal tumors ≤1.5 cm) with serial tumor measurements via ultrasonography at a tertiary referral center in the United States.
Intervention
Active surveillance.
Main Outcomes and Measures
The cumulative incidence, rate, and magnitude of the change in tumor diameter or volume, as well as associations with patient and tumor characteristics.
Results
Of the 291 patients, 219 (75.3%) were women; mean (SD) age was 52 (15) years. During a median (range) active surveillance of 25 (6-166) months, growth in tumor diameter of 3 mm or more was observed in 11 of 291 (3.8%) patients, with a cumulative incidence of 2.5% (2 years) and 12.1% (5 years). No regional or distant metastases developed during active surveillance. In all cases, 3-dimensional measurements of tumor volume allowed for earlier identification of growth (median, 8.2 months; range, 3-46 months before increase in tumor diameter). In multivariable analysis, both younger age at diagnosis (hazard ratio per year, 0.92; 95% CI, 0.87-0.98; P = .006) and risk category at presentation (hazard ratio for inappropriate, 55.17; 95% CI, 9.4-323.19; P < .001) were independently associated with the likelihood of tumor growth. Of the tumors experiencing volume growth, kinetics demonstrated a classic exponential growth pattern, with a median doubling time of 2.2 years (range, 0.5-4.8 years; median r2 = 0.75; range, 0.42-0.99).
Conclusions and Relevance
The rates of tumor growth during active surveillance in a US cohort with PTCs measuring 1.5 cm or less were low. Serial measurement of tumor volumes may facilitate early identification of tumors that will continue to grow and thereby inform the timing of surveillance imaging and therapeutic interventions.
This cohort study examines the probability, rate, and magnitude of tumor growth in patients with papillary thyroid cancer during active surveillance.
Introduction
The recent dramatic increase in thyroid cancer incidence in the United States and other countries has been characterized as an “epidemic of diagnosis” rather than an epidemic of disease. With wider use of diagnostic and imaging technologies, many small, subclinical papillary thyroid cancers (PTCs) are now being detected. If never diagnosed and treated, most (estimated as 50%-90%) of these PTCs would not go on to cause symptoms or death. Recognizing this indolent behavior, American Thyroid Association guidelines now endorse active surveillance as an alternative to immediate thyroidectomy in properly selected patients with very low-risk tumors.
In landmark Japanese studies establishing the safety of active surveillance for papillary thyroid microcarcinomas (<1.0-cm diameter), 10% to 15% of patients experienced tumor growth, usually within 5 years. Growth of 3 mm—the smallest difference reliably measured with ultrasonography—was also the threshold for surgical intervention. Beyond this single binary end point, the kinetics (probability, rate, and magnitude) of PTC growth during active surveillance have not been well described. A more dynamic characterization of tumor growth based on 3-D volume measurements may allow for earlier determination of whether a PTC is stable or growing. Such information would be of value in more precise tailoring of surveillance imaging and, if needed, intervention, in patients undergoing active surveillance. In this study, we describe tumor volume kinetics in 291 patients undergoing active surveillance for low-risk PTCs (≤1.5 cm) at a tertiary referral center in the United States.
Methods
We identified a cohort of 291 patients with PTC prospectively followed with active surveillance at Memorial Sloan Kettering Cancer Center, New York, New York (eFigure 1 in the Supplement and Table 1), with
Table 1. Cohort Characteristics of 291 Patients With Low-Risk Papillary Thyroid Cancer With Active Surveillance for More Than 6 Months.
| Variable | Value | Tumor Diameter Increase ≥3 mm (n = 11) |
Tumor Volume Increase ≥50% (n = 36) |
|---|---|---|---|
| Age at diagnosis, y | |||
| Median (range) | 51 (20-86) | 42 (31-57) | 46 (20-86) |
| Mean (SD) | 52 (15) | 41.8 (9.6) | 46.4 (14.1) |
| Sex, No. (%) | |||
| Female | 219 (75.3) | 10 (90.9) | 29 (80.6) |
| Male | 72 (24.7) | 1 (9.1) | 7 (19.4) |
| Cytology, No. (%) | |||
| Bethesda category VI | 243 (83.5) | 9 (81.8) | 26 (72.2) |
| Bethesda category V | 48 (16.5) | 2 (18.2) | 10 (27.8) |
| Tumor size, No. (%) | |||
| ≤1.0 cm | 232 (79.7) | 9 (81.8) | 31 (86.1) |
| 1.1-1.5 cm | 59 (20.3) | 2 (18.2) | 5 (13.9) |
| History of autoimmune thyroid disease, No. (%) | |||
| None | 250 (85.9) | 7 (63.6) | 28 (77.8) |
| Autoimmune thyroiditis | 38 (13.1) | 4 (36.4) | 8 (22.2) |
| Graves disease | 3 (1.0) | 0 | 0 |
| Family history of thyroid cancer, No. (%) | |||
| Yes | 40 (13.7) | 0 | 1 (2.8) |
| No | 251 (86.3) | 11 (100) | 35 (97.2) |
| Personal history of another cancer, No. (%) | |||
| Yes | 52 (17.9) | 1 (9.1) | 4 (11.1) |
| No | 239 (82.1) | 10 (90.9) | 32 (88.9) |
| Radiation exposure, No. (%) | |||
| Yes | 2 (0.7) | 0 | 0 |
| No | 289 (99.3) | 11 (100) | 36 (100) |
| Appropriateness for active surveillance, risk category, No. (%) | |||
| Ideal | 13 (4.5) | 0 | 1 (2.8) |
| Appropriate | 273 (93.8) | 9 (81.8) | 33 (91.7) |
| Inappropriate | 5 (1.7) | 2 (18.2) | 2 (5.6) |
| Duration of active surveillance, mo | |||
| Median (range) | 25 (6-166) | 38 (12-54) | 27 (20-68) |
| Mean (SD) | 29 (19) | 34.1 (15.5) | 29.7 (16.1) |
| Increase in tumor diameter ≥3 mm during observation, No. (%) | |||
| Yes | 11 (3.8) | 11 (100) | 11 (30.6) |
| No | 280 (96.2) | 0 | 25 (69.4) |
| Increase in tumor volume >50% during observation, No. (%) | |||
| Yes | 36 (12.4) | 11 (100) | 36 (100) |
| No | 255 (87.6) | 0 | 0 |
| Metastasis detected during observation, No. (%) | |||
| Yes | 0 | 0 | 0 |
| No | 291 (100) | 11 (100) | 36 (100) |
| Status at final follow-up, No. (%) | |||
| Continue on active surveillance | 279 (95.9) | 6 (54.5) | 31 (86.1) |
| Surgery for increase in tumor size | 5 (1.7) | 5 (45.5) | 5 (13.9) |
| Surgery despite stable tumor size | 5 (1.7) | 0 | 0 |
| Lost to follow-up | 2 (0.7) | 0 | 0 |
Bethesda system for reporting thyroid cytopathology: category VI (malignant), category V (suspicious for malignancy).
thyroid nodules classified as PTC (Bethesda category VI), or suspicious for PTC (Bethesda category V) with suspicious ultrasonographic characteristics (hypoechoic with irregular margins, microcalcifications, or taller-than-wide shape),
tumor size 1.5 cm or less in maximal dimension at diagnosis,
no clinical or radiographic evidence of extrathyroidal extension, invasion of local structures, or regional or distant metastases,
thyroid-stimulating hormone level within the reference range,
cytopathology interpretation by Memorial Sloan Kettering Cancer Center thyroid cytopathologists,
ultrasonography examinations by Memorial Sloan Kettering Cancer Center radiologists every 6 months for 2 years, then yearly, and
minimum of 6 months’ follow-up at Memorial Sloan Kettering Cancer Center.
At diagnosis, patients were classified under a previously described clinical framework as ideal, appropriate, or inappropriate for active surveillance. Surgery was recommended if the primary tumor increased 3 mm or more in greatest dimension over baseline or if there was evidence for extrathyroidal extension or nodal or distant metastases. Tumor volumes were measured and calculated relative to prebiopsy baseline. A meaningful change in volume was defined as a greater than 50% increase from baseline. The study was approved by the Memorial Sloan Kettering Cancer Center Institutional Review Board. Written informed consent for prospective data collection and tumor acquisition was obtained from 249 participants. Data from the remaining 42 participants were collected and analyzed after a waiver was obtained from the Memorial Sloan Kettering Cancer Center Institutional Review Board for retrospective data collection. None of the participants received financial compensation.
Statistical Analysis
Continuous data are presented as means (SDs) or medians (ranges), as appropriate for each variable. Tumor growth rates were calculated by fitting a least-squares regression model to log-transformed tumor volume measurements and plotted on semi-log curves. Doubling time was calculated by dividing log (2) by log (1 + growth rate). The volume (milliliters) of the tumor was calculated using the ellipsoid equation: π/6 × length × width × height. Percentage change in volume was calculated relative to the baseline before the initial fine-needle aspiration. A meaningful change in tumor volume was defined as a greater than 50% increase from baseline. Results are presented as hazard ratios (HRs) with 95% CIs. The cumulative incidence of tumor diameter or volume growth was calculated using the Kaplan-Meier method, and statistical testing was performed using the log rank test or univariate Cox regression analysis. Multivariable Cox regression was used to analyze clinical and pathologic factors associated with tumor growth, with the model including all covariates with P values <.05 on univariate analysis. Analyses were performed using SPSS, version 24.0 (IBM Corp) and R, version 3.3.1 (R Foundation for Statistical Computing).
Results
Cohort Characteristics
A total of 291 patients with low-risk PTC were followed with active surveillance for a median of 25 months (range, 6-166 months) (median age at diagnosis, 51 years [range, 20-86 years], 75.3% women, 79.7% with tumors ≤1.0 cm, and 20.3% with tumors 1.1-1.5 cm maximal diameter) (Table 1). Few patients (13 [4.5%]) were classified as ideal candidates for observation (age >60 years, solitary papillary microcarcinoma within an otherwise normal thyroid gland). Most patients (273 [93.8%]) were classified as appropriate for observation on the basis of either age (<60 years old), the presence of other benign appearing nodules or ultrasonographic characteristics of thyroiditis, or close proximity to the thyroid capsule (≤2 mm of normal thyroid tissue between the nodule and the thyroid capsule).
At the time of writing, 279 (95.9%) patients remained under active surveillance, 5 (1.7%) underwent thyroidectomy after an increase in tumor size of 3 mm or more, 5 (1.7%) elected to undergo surgery despite no increase in tumor size, and 2 (0.7%) were lost to follow-up. No regional or distant metastases developed during active surveillance.
Five patients (1.7%) classified as inappropriate for observation refused surgery at the time of diagnosis and elected to be monitored with observational management despite ultrasonographic features suspicious for minor extrathyroidal extension into overlying strap muscles (n = 3) or subcentimeter suspicious lymph nodes adjacent to the thyroid in the central or lateral neck (n = 2). Two of these patients underwent surgery for an increase in tumor diameter and tumor volume after 12 and 18 months of active surveillance, respectively.
Six patients demonstrating a tumor diameter increase of 3 mm or more had not had surgery at the time of writing (2 were scheduled for thyroid surgery, 2 were planning to schedule surgery at a more convenient time, and 2 whose tumors increased in diameter by 3 mm after more than 4 years refused surgery). Among the 10 patients who underwent surgery, none of the cancers had histologically adverse features, such as extrathyroidal extension, lymphovascular invasion, or metastatic lymph node involvement. No patients received radioactive iodine ablation. None of the patients had biochemical or structural evidence of residual or recurrent disease during a mean of 7.3 (range, 3-32) months of postoperative follow-up.
Changes in Tumor Size During Active Surveillance
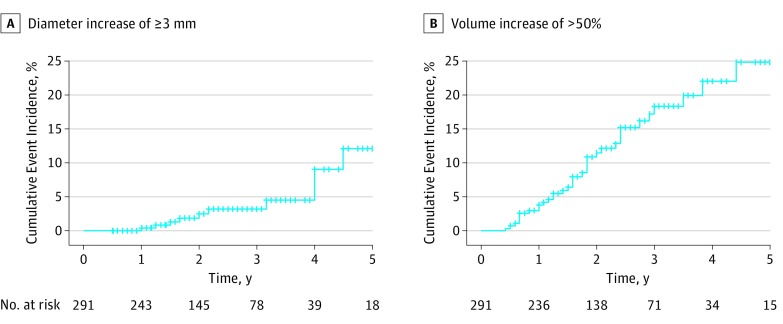
Tumor diameter growth of 3 mm or more was observed in 11 of 291 (3.8%) patients. The cumulative incidence of growth of 3 mm or more was 2.5% at 2 years and 12.1% at 5 years. The cumulative incidence of volume increase greater than 50% was 11.5% at 2 years and 24.8% at 5 years (Figure 1). Volume increased by more than 50% in 36 patients, was stable in 229 patients, decreased by more than 50% in 19 patients, and could not be determined in 7 patients (Figure 2). All tumors experiencing 3-mm growth also demonstrated a volume increase greater than 50% (mean increase, 178%; range, 96%-364%).
Figure 1. Cumulative Incidence of Increase in Tumor Diameter and Volume Among Papillary Thyroid Cancers During Active Surveillance.
A, Time to increase in tumor diameter of 3 mm or more. B, Increase in tumor volume of more than 50%.
Figure 2. Percentage Change in Tumor Volume During Active Surveillance for Each Patient.
Of 284 patients undergoing active surveillance with complete 3-D measurements, tumor size decreased >50% in 19 patients (6.7%), was stable in 229 (80.2%), and increased >50% in 36 (12.7%). In 7 patients (2.4%), tumor volume could not be calculated because of the lack of reproducible 3-D measurements. Tumor diameter increased ≥3 mm in 11 (3.8%).
Clinical Correlates of Tumor Growth
On multivariable Cox regression, younger age at diagnosis (HR per year, 0.92; 95% CI, 0.87-0.98; P = .006) (Table 2) and risk category (HR for inappropriate, 55.17; 95% CI, 9.40-323.19; P < .001) were independently associated with the likelihood of 3-mm diameter growth. Patients younger than 50 years at diagnosis had a nearly 5-fold likelihood of experiencing tumor growth compared with patients 50 years or older (27.3% vs 4.6% at 5 years; HR, 4.5; 95% CI, 1.2-17.0; P = .03) (eFigures 2 and 3 in the Supplement). Similar results were observed for tumor volume, with both younger age (HR, 0.97; 95% CI, 0.94-0.99; P = .01) and risk category (HR for inappropriate, 10.54; 95% CI, 2.39-46.53; P = .002) independently associated with the likelihood of 50% volume growth. Tumor size (<1.0 cm vs 1.0-1.5 cm) at presentation was not associated with an increase in either volume or diameter (HR, 0.93; 95% CI, 0.36-2.41 and HR, 1.94; 95% CI, 0.41-9.23, respectively).
Table 2. Cox Regression for Factors Associated With Change in Tumor Diameter or Volume During Active Surveillance.
| Covariate | Cox Regression, OR (95% CI)a | |
|---|---|---|
| Univariate | Multivariableb | |
| Tumor Diameter Growth of ≥3 mm | ||
| Age, y | 0.93 (0.89-0.98) | 0.92 (0.87-0.98) |
| Appropriateness for AS | ||
| Appropriate | 1 [Reference] | 1 [Reference] |
| Ideal | 0 | 0 |
| Inappropriate | 37.68 (7.13-199.03)c | 55.17 (9.40-323.19)c |
| Sex | ||
| Male | 1 [Reference] | |
| Female | 1.62 (0.58-4.55) | |
| Cytology | ||
| Bethesda category V | 1 [Reference] | |
| Bethesda category VI | 0.81 (0.42- 0.91) | |
| Tumor maximal diameter (continuous) | 1.09 (0.88-1.35) | |
| Tumor maximal diameter (category) | ||
| <1.0 cm | 1 [Reference] | |
| 1.0-1.5 cm | 1.94 (0.41-9.23) | |
| Tumor Volume Growth of >50% During Observation | ||
| Age, y | 0.97 (0.94-0.99) | 0.97 (0.94-0.99) |
| Appropriateness for AS | ||
| Appropriate | 1 [Reference] | 1 [Reference] |
| Ideal | 0.41 (0.06-2.99) | 0.67 (0.08-5.39) |
| Inappropriate | 6.12 (1.45-25.75)c | 10.54 (2.39-46.53)c |
| Sex | ||
| Male | 1 [Reference] | |
| Female | 1.09 (0.74-1.62) | |
| Cytology | ||
| Bethesda category V | 1 [Reference] | |
| Bethesda category VI | 0.49 (0.24-0.99) | 0.43 (0.21-0.87) |
| Tumor maximal diameter (continuous) | 0.94 (0.82-1.07) | |
| Tumor maximal diameter (category) | ||
| <1.0 cm | 1 [Reference] | |
| 1.0-1.5 cm | 0.93 (0.36-2.41) | |
Abbreviation: AS, active surveillance.
Empty cells indicate measure was not applicable.
On multivariable Cox regression, both patient age at diagnosis and risk category at presentation were independently associated with the likelihood of tumor growth in either diameter or volume.
Patients initially classified as inappropriate for active surveillance, either owing to radiographic suspicion for invasion of tumor beyond the thyroid capsule or location of the tumor in close proximity to the trachea or recurrent laryngeal nerve, were more likely to experience an increase in tumor diameter of 3 mm or more and an increase in tumor volume of 50% or more than were patients classified as receiving appropriate surveillance.
Volume Growth Kinetics
Each tumor experiencing a greater than 50% increase in volume demonstrated growth kinetics consistent with classic exponential growth patterns (Figure 3), with a median doubling time of 2.2 years (range, 0.5-4.8 years) (median r2 = 0.75; range, 0.42-0.99). Among these tumors, the mean (SD) time to 50% volume increase was 22.9 (15.0) months. Among the 11 tumors experiencing 3 mm or more diameter growth, the mean time to reach this threshold was 34.1 (15.5) months. In all cases, a 50% increase in volume preceded the 3-mm or more increase in tumor diameter by a median of 8.2 months (range, 3-46 months).
Figure 3. Tumor Measurements in Representative Patients.
A, Measurements during active surveillance for all 36 patients demonstrating a greater than 50% increase. Markers on the lines (eg, triangles, bars) indicate individual patient measures. B, Pattern of exponential growth in 1 patient (r2 = 0.72; tumor volume doubling time [DT], 4 years; diameter, from 10 to 13 mm; volume, from 0.3 to 0.5 mL). C, Pattern of exponential growth in another patient (r2 = 0.90; DT, 2.7 years; diameter, from 6 to 9 mm; volume, from 0.05 to 0.1 mL). D, Measurements in a patient with stable disease (r2 = 0.98; diameter, from 15 to 13 mm; volume, from 1.0 to 0.9 mL).
Discussion
We report the outcomes of 291 patients undergoing active surveillance for PTC in the United States. Consistent with previous reports from Japan, our findings confirm that only 10% to 15% of small PTCs will increase in tumor diameter by 3 mm or more during the first 5 years of active surveillance and that an increase in tumor size is more likely in younger patients. In this study, we extend these observations by demonstrating that (1) the kinetics of PTC volume growth follow classic exponential growth patterns, indicating that growth can be accurately modeled, (2) tumor volume measurements appear to identify growth before the tumor diameter reaches the 3-mm threshold, (3) small PTCs above the traditional 1.0-cm cutoff (1.0-1.5 cm) show a similarly low likelihood of growth, (4) a small percentage of patients experience tumor regression, (5) active surveillance can be successfully implemented outside of a Japanese population, and (6) our institution’s previously described clinicopathologic classification of appropriateness for active surveillance was associated with likelihood of growth.
Limitations
There is little doubt that the success of our active surveillance management program depends on the availability of specialized and highly skilled radiologists who are an integral part of our thyroid cancer disease management team. A senior radiologist reviews all images prepared by the ultrasonography technician and compares them with previous ultrasonographic images before the patient is released from the suite. This approach minimizes the expected variation between examiners and examinations and also allows us to be confident that the serial 3-D measurements used to calculate the tumor value fall within the ±3-mm range of variation that we expected. In the few patients who did not have reliable 3-D measurements (2.4%) (Figure 2), we used the maximum diameter of the nodule as the indicator of tumor growth. With experience, expertise, and careful attention to detail, we are confident that these types of ultrasonographic examinations can be done outside of major medical centers. The other important limitation was that the number of patients in the inappropriate for active surveillance category was small, and therefore the association between this variable and outcomes will require further validation as we accrue more patients.
Conclusions
As the number of small, incidentally detected PTCs continues to increase, new approaches are needed to avoid overtreatment of tumors that would otherwise remain indolent and asymptomatic while identifying the small percentage of such tumors that will continue to grow. Because PTCs appear to follow predictable growth kinetics under active surveillance, serial measurements of tumor volume hold significant promise in triaging patients to observation vs surgery. Additional studies will helpful to determine the clinical significance of mild growth in PTC diameter and volume and further refine the thresholds for intervention.
eFigure 1. STROBE Diagram
eFigure 2. Increase in Tumor Volume and Tumor Diameter by Decade of Age
eFigure 3. Cumulative Incidence of Increased Tumor Size
References
- 1.Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140(4):317-322. [DOI] [PubMed] [Google Scholar]
- 2.Brito JP, Al Nofal A, Montori VM, Hay ID, Morris JC. The impact of subclinical disease and mechanism of detection on the rise in thyroid cancer incidence: a population-based study in Olmsted County, Minnesota during 1935 through 2012. Thyroid. 2015;25(9):999-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies L, Ouellette M, Hunter M, Welch HG. The increasing incidence of small thyroid cancers: where are the cases coming from? Laryngoscope. 2010;120(12):2446-2451. [DOI] [PubMed] [Google Scholar]
- 4.Udelsman R, Zhang Y. The epidemic of thyroid cancer in the United States: the role of endocrinologists and ultrasounds. Thyroid. 2014;24(3):472-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. Worldwide thyroid-cancer epidemic? the increasing impact of overdiagnosis. N Engl J Med. 2016;375(7):614-617. [DOI] [PubMed] [Google Scholar]
- 6.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito Y, Miyauchi A, Oda H. Low-risk papillary microcarcinoma of the thyroid: a review of active surveillance trials. Eur J Surg Oncol. 2017;16:30370-0. [DOI] [PubMed] [Google Scholar]
- 8.Brito JP, Ito Y, Miyauchi A, et al. A clinical framework to facilitate risk stratification when considering an active surveillance alternative to immediate biopsy and surgery in papillary microcarcinoma. Thyroid. 2016;26(1):144-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyauchi A. Clinical trials of active surveillance of papillary microcarcinoma of the thyroid. World J Surg. 2016;40(3):516-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brauer VF, Eder P, Miehle K, Wiesner TD, Hasenclever H, Paschke R. Interobserver variation for ultrasound determination of thyroid nodule volumes. Thyroid. 2005;15(10):1169-1175. [DOI] [PubMed] [Google Scholar]
- 11.Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. 2014;24(1):27-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. STROBE Diagram
eFigure 2. Increase in Tumor Volume and Tumor Diameter by Decade of Age
eFigure 3. Cumulative Incidence of Increased Tumor Size