Key Points
Question
Does gamification, the application of game design elements such as points and levels in nongame contexts, that uses insights from behavioral economics to enhance social incentives increase physical activity among families in the community?
Findings
In this randomized clinical trial of 200 adults comprising 94 families, participants in the gamification arm had significantly greater physical activity during the 12-week intervention than participants in the control arm, including the proportion of days that step goals were achieved and the change in the mean daily steps.
Meaning
Gamification designed to leverage insights from behavioral economics to enhance social incentives could offer a promising approach to improve daily health behaviors.
Abstract
Importance
Gamification, the application of game design elements such as points and levels in nongame contexts, is often used in digital health interventions, but evidence on its effectiveness is limited.
Objective
To test the effectiveness of a gamification intervention designed using insights from behavioral economics to enhance social incentives within families to increase physical activity.
Design, Setting, and Participants
The Behavioral Economics Framingham Incentive Trial (BE FIT) was a randomized clinical trial with a 12-week intervention period and a 12-week follow-up period. The investigation was a community-based study between December 7, 2015, and August 14, 2016. Participants in the modified intent-to-treat analysis were adults enrolled in the Framingham Heart Study, a long-standing cohort of families.
Interventions
All participants tracked daily step counts using a wearable device or a smartphone, established a baseline, selected a step goal increase, and received daily individual feedback on goal performance by text message or email for 24 weeks. Families in the gamification arm could earn points and progress through levels based on physical activity goal achievement during the 12-week intervention. The game design was meant to enhance collaboration, accountability, and peer support.
Main Outcomes and Measures
The primary outcome was the proportion of participant-days that step goals were achieved during the intervention period. Secondary outcomes included the proportion of participant-days that step goals were achieved during the follow-up period and the change in the mean daily steps during the intervention and follow-up periods.
Results
Among 200 adults comprising 94 families, the mean age was 55.4 years, and 56.0% (n = 112) were female. During the intervention period, participants in the gamification arm achieved step goals on a significantly greater proportion of participant-days (0.53 vs 0.32; adjusted difference, 0.27; 95% CI, 0.20-0.33; P < .001) and had a significantly greater increase in the mean daily steps compared with baseline (1661 vs 636; adjusted difference, 953; 95% CI, 505-1401; P < .001) than the control arm. During the follow-up period, physical activity in the gamification arm declined but remained significantly greater than that in the control arm for the proportion of participant-days achieving step goals (0.44 vs 0.33; adjusted difference, 0.12; 95% CI, 0.05-0.19; P < .001) and the mean daily steps compared with baseline (1385 vs 798; adjusted difference, 494; 95% CI, 170-818; P < .01).
Conclusions and Relevance
Gamification designed to leverage insights from behavioral economics to enhance social incentives significantly increased physical activity among families in the community.
Trial Registration
clinicaltrials.gov Identifier: NCT02531763
This randomized clinical trial tests the effectiveness of a gamification intervention designed using insights from behavioral economics to enhance social incentives within families to increase physical activity.
Introduction
More than half of the adults in the United States do not obtain enough physical activity and are at higher risk for cardiovascular disease. Gamification, the application of game design elements such as points and levels in nongame contexts, is increasingly being used in digital health interventions to promote changes in health behaviors, such as physical activity. Whereas interest in these approaches is growing, evidence on their effectiveness is limited, and most applications have not appropriately leveraged principles from theories of health behavior.
The rapidly expanding availability of mobile technologies such as wearable devices and smartphones provides a platform to monitor daily health behaviors and to deploy interventions on a broader scale. By incorporating insights from behavioral economics, approaches could be designed to anticipate predictable barriers to behavior change. Social incentives, or the influences that motivate individuals to adjust their behaviors based on social ties or connections, are ubiquitous and could be leveraged within gamification interventions to provide a scalable, low-cost approach to increase engagement. Evidence indicates that individual health behaviors are influenced by social networks, but more rigorous and prospective evaluations in community settings are lacking.
Our objective was to conduct a randomized clinical trial to test the effectiveness of a gamification intervention that used insights from behavioral economics to enhance social incentives, such as collaboration, accountability, and peer support, to increase physical activity. To test this intervention among family networks in the community, we conducted the first clinical trial among adults enrolled in the Framingham Heart Study, a long-standing cohort of families.
Methods
Study Design
The Behavioral Economics Framingham Incentive Trial (BE FIT) was a randomized clinical trial conducted between December 7, 2015, and August 14, 2016, consisting of a 2-week run-in period, a 12-week intervention period, and a 12-week follow-up period. The investigation was a community-based study among families in the Framingham Heart Cohort. The trial protocol is available in Supplement 1 and was approved by the institutional review boards at the University of Pennsylvania and Boston University Medical Center.
The study was conducted using Way to Health, a research platform at the University of Pennsylvania used previously for physical activity interventions. Participants accessed the study website to create an account, provide online informed consent, and complete baseline eligibility surveys. Eligible participants either downloaded a smartphone application (Moves; ProtoGeo Oy or Fitbit; Fitbit, Inc) or were mailed a wrist-worn wearable device (Fitbit Flex; Fitbit, Inc) to track step counts, and they authorized access for their data to be captured for the study. Prior work has demonstrated that these devices accurately track step counts.
Participants
Recruitment occurred from September 23, 2015, to February 26, 2016, using email and telephone calls. Adults already enrolled in the Framingham Heart Study were eligible to participate if they were 18 years or older, had an active email address, had access to either a smartphone or computer compatible with one of the activity tracking devices, and had at least one other family member enrolled in the Framingham Heart Study who was also eligible to participate with them. Participants were excluded if they were already participating in a physical activity study, had been told not to exercise by a physician, were currently pregnant, or had at least one fall with significant injury in the past year or if there was any other reason why participation was unsafe or infeasible. Forty-six individuals who wanted to participate did not have a family member also interested in participating. We obtained institutional review board approval to allow these individuals to participate in a singleton arm that was similar to the control arm. This was conducted outside of the main trial; therefore, their outcomes data were not analyzed. Characteristics of these participants are listed in eTable 1 in Supplement 2. Participants who received a wearable device were allowed to keep it; no other financial compensation was offered.
Baseline Step Count
Before randomization, participants were told to spend a few weeks getting accustomed to their device. During this run-in period, we estimated a baseline step count using the second week of data. The first week of data was ignored to diminish the potential upward bias of the estimate from higher activity during initial device use. To prevent potential downward bias, we ignored any daily values less than 1000 steps because evidence indicates that these values are unlikely to represent capture of actual activity. If less than 4 days of data were available during the second week (n = 9), the participant was contacted to inquire about any issues, and the run-in period was extended until 4 days of data were captured. Nine participants who began with the Moves smartphone application switched to Fitbit (4 to the smartphone application and 5 to the wearable device). These participants had their baseline step count estimated using only data from the new device.
Randomization and Goal Selection
Families composed of 2 or 3 members were randomly assigned to a study arm. A computerized random number generator was used with block sizes of 2 families stratified on family size.
After randomization but before participants learned of study arm assignment, each participant was informed of his or her baseline step count and was asked to select a step goal increase of 33%, 40%, or 50% or any goal at least 1000 steps greater than baseline. After all family members selected a step goal increase, the family was informed of study arm instructions. All investigators, statisticians, and data analysts were masked to study arm assignments until the study and analysis were completed.
Interventions
All participants selected whether to receive study communications by text message, email, or both. During the entire 24-week study, all participants (including those in the control arm) received daily feedback on whether or not they had achieved their step goal on the prior day. Participants in the control arm received no other intervention.
Participants in the gamification arm were entered into a game with their family for 12 weeks that was designed using insights from behavioral economics to address predictable barriers to behavior change and to enhance social incentives. First, participants electronically signed a commitment pledge to try their best to achieve their step goal. Precommitment has been demonstrated to motivate behavior change. Second, every Monday, the family was endowed with 70 points (10 for each day of the upcoming week). Each day, the family was informed of the one member who was selected at random to represent their team. If that member achieved his or her step goal on the prior day, the family kept its points; otherwise, 10 points were lost. This design leveraged the following 3 important psychological principles: individuals tend to be more motivated by losses than gains, behavior is often better sustained by variable than constant reinforcement, and individuals tend to be more motivated for aspirational behavior around temporal landmarks, such as the beginning of the week (the fresh start effect). Third, each individual had 5 lifelines to use on days when they were sick or activity was infeasible. This element allowed for some forgiveness and enabled individuals to seek help from a family member. Fourth, if the family had 50 points or more at the end of the week, they advanced up a level (bronze, silver, gold, and platinum). If not, the family dropped a level. This design creates achievable goal gradients (the notion that the next highest level was attainable), a sense of social status, progression through the game, and longer-term loss aversion for families that reached higher levels. Families began at bronze and were informed of their new level on Monday. Families were not told how other families were doing. Fifth, families were informed that if they finished the intervention period at the gold or platinum level they each would receive a coffee mug with the study logo as a reward (eFigure in Supplement 2).
Outcome Measures
The primary outcome was the proportion of participant-days that step goals were achieved during the 12-week intervention period. Secondary outcomes included the proportion of participant-days that step goals were achieved during the 12-week follow-up period and the change in the mean daily steps from baseline during the intervention and follow-up periods.
Statistical Analysis
A priori, we estimated that a sample of at least 170 participants (85 per study arm) would ensure 80% power to detect a 0.15 difference in the proportion of participant-days that step goals were achieved between study arms, with a 2-sided α level of .05 and accounting for clustering at the level of the family. This calculation was based on previous studies and assumed a standard deviation of 0.30, an intracluster correlation coefficient between family members of 0.24, and an 8% dropout rate.
After randomization, 6 participants were deemed ineligible and were not started in any intervention. One participant assigned to the control arm lost interest; therefore, the family member no longer had a group in which to participate. Two participants assigned to the gamification arm did not select a goal or receive study arm assignment; thus, their 2 family members also no longer had a group with which to participate. All other randomly assigned participants who received an intervention (n = 200) were included in the modified intent-to-treat analysis.
For each participant on each day of the study (participant-day level), the number of steps achieved was obtained as a continuous variable, and this number was used to estimate the mean daily steps. These data were dichotomized at the participant-day level to create a binary variable indicating whether or not each participant achieved his or her step goal, and this variable was used to estimate the proportion of participant-days that step goals were achieved.
Data could be missing for any day if a participant did not use the activity tracking device or did not upload data. For the main analysis, we used multiple imputation (using the mice package in the R Project for Statistical Computing) for data that were missing and step values less than 1000. Evidence indicates that step values less than 1000 may not represent accurate data capture, and our group has accounted for these values as missing data in prior work. Five imputations were conducted using the following predictors of missing data: baseline step count, study arm, calendar month fixed effects, week in the study, and a binary variable indicating the weekday or weekend. Results were combined using standard rules by Rubin. Secondary analyses were conducted using collected data without multiple imputation, both with and without step values less than 1000.
Unadjusted analyses estimated the proportion of participant-days that step goals were achieved and the change in the mean daily steps from baseline by study arm for the intervention and follow-up periods and for each week. In adjusted analyses, models were fit with generalized estimating equations (using the geeglm function in the R Project for Statistical Computing) with an exchangeable correlation structure to account for the correlation within a family cluster and among a participant’s repeated observations. The bootstrap method, resampling participants within each arm 150 times, was used to estimate 95% CIs and P values. The prespecified main model also adjusted for baseline steps and calendar month fixed effects. As a sensitivity analysis, the main model was estimated by also adjusting for the tracking device (wearable device or smartphone) of participant activity.
Hypothesis tests were 2 sided using a significance level of .05. Analyses were conducted using R, version 3.2.3; R Project for Statistical Computing. A data and safety monitoring board met before the start of the study and after the intervention and follow-up periods were completed.
Results
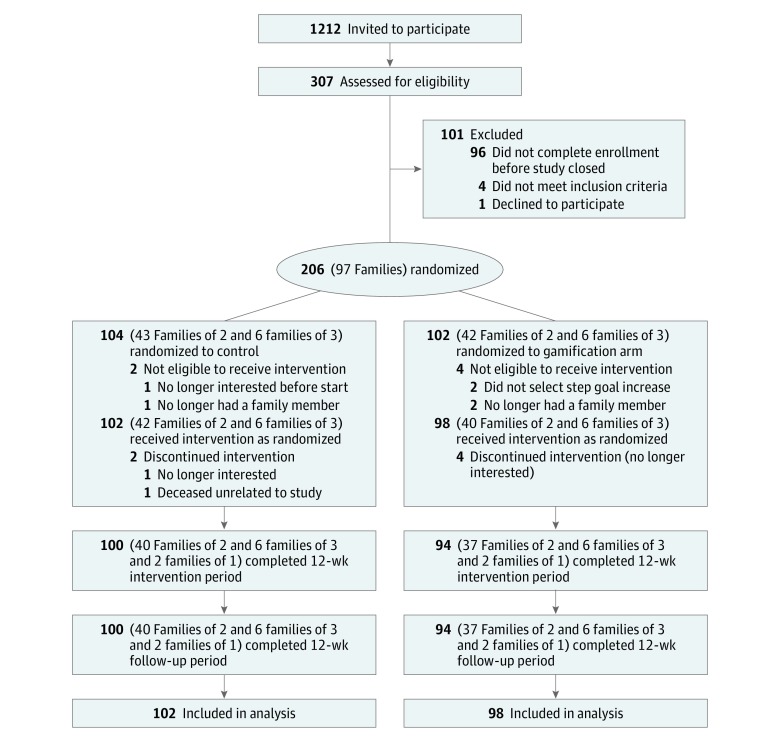
Among 1212 adults invited to participate, 307 were assessed for eligibility. Two hundred adults comprising 94 families were randomized and received an intervention (Figure 1). The mean age of participants was 55.4 years, and 56.0% (n = 112) were female (Table 1). Other participant characteristics were similar between study arms. The mean (SD) numbers of participant baseline daily steps were 7662 (3378) in the control arm and 7244 (3368) in the gamification arm, which were not significantly different (P = .41). The percentage increase in participant step goals from baseline was not significantly different between study arms (P = .74), with a mean (SD) of 2722 (1740) steps in the control arm and 2340 (1153) steps in the gamification arm. Almost 40% (79 of 200) of participants selected a custom step goal, and on average these goals were a 49.5% increase from baseline. Family structure and device use among study participants are available, as well as characteristics of individuals who did not enroll (eTables 2, 3, and 4 in Supplement 2).
Figure 1. Study Flow CONSORT Diagram.
The control arm received daily feedback for 24 weeks. The gamification arm received daily feedback for 24 weeks and a gamification intervention for the first 12 weeks. CONSORT indicates Consolidated Standards of Reporting Trials.
Table 1. Characteristics of Study Participantsa.
| Variable | Control (n = 102) |
Gamification (n = 98) |
|---|---|---|
| Sociodemographics | ||
| Age, mean (SD), y | 56.2 (10.1) | 55.7 (9.7) |
| Female, No. (%) | 60 (58.8) | 52 (53.1) |
| White non-Hispanic race/ethnicity, No. (%) | 102 (100) | 98 (100) |
| Education, No./total No. (%) | ||
| High school graduate | 5/98 (5.1) | 6/92 (6.5) |
| Some college | 16/98 (16.3) | 23/92 (25.0) |
| College graduate | 77/98 (78.6) | 63/92 (68.5) |
| Marital status, No./total No. (%) | ||
| Single | 13/99 (13.1) | 7/92 (7.6) |
| Married | 79/99 (79.8) | 78/92 (84.8) |
| Other | 7/99 (7.1) | 7/92 (7.6) |
| Annual household income, No./total No. (%) | ||
| <$55 000 | 9/75 (12.0) | 11/77 (14.3) |
| $55 000 to $100 000 | 26/75 (34.7) | 27/77 (35.1) |
| >$100 000 | 40/75 (53.3) | 39/77 (50.6) |
| Baseline Measures | ||
| Atrial fibrillation, No. (%) | 0 | 2 (2.0) |
| BMI, mean (SD) | 26.9 (4.9) | 27.2 (5.1) |
| Cardiovascular disease, No. (%) | 2 (2.0) | 7 (7.1) |
| Current smoker, No./total No. (%) | 3/99 (3.0) | 6/92 (6.5) |
| Diabetes, No./total No. (%) | 4/98 (4.1) | 4/92 (4.3) |
| Framingham Risk Score, mean (SD) | 8.2 (8.1) | 10.8 (10.6) |
| Hypertension, No./total No. (%) | 15/99 (15.2) | 24/92 (26.1) |
| Hyperlipidemia, No./total No. (%) | 18/99 (18.2) | 22/92 (23.9) |
| Physical Activity Measures | ||
| Baseline step count, mean (SD) | 7662 (3776) | 7244 (3368) |
| Step goal selection, No. (%) | ||
| 33% Increase from baseline | 34 (33.3) | 27 (27.6) |
| 40% Increase from baseline | 13 (12.7) | 17 (17.3) |
| 50% Increase from baseline | 15 (14.7) | 15 (15.3) |
| Set custom goal | 40 (39.2) | 39 (39.8) |
| Increase from baseline to goal, mean (SD) steps | 2722 (1740) | 2340 (1153) |
| Step tracking device, No. (%) | ||
| Wearable device | 82 (80.4) | 74 (75.5) |
| Smartphone | 20 (19.6) | 24 (24.5) |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
Characteristics were not available or not provided by all participants; therefore, the denominators reflect available data.
Ninety-seven percent (194 of 200) of participants completed the entire 24-week study. During the intervention period, step data that were missing or had values less than 1000 steps per day represented 12.7% (1090 of 8568 participant-days) of observations in the control arm and 10.1% (835 of 8232 participant-days) of observations in the gamification arm. During the follow-up period, these percentages increased to 37.0% (3166 of 8568) and 42.1% (3462 of 8232), respectively (eTable 5 in Supplement 2).
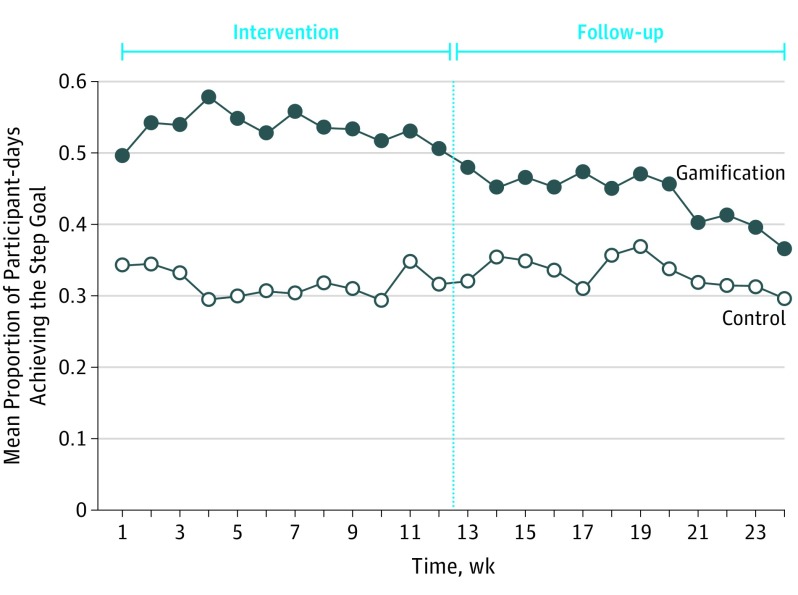
The mean (SD) unadjusted proportions of participant-days that step goals were achieved during the intervention period were 0.32 (0.24) in the control arm and 0.53 (0.29) in the gamification arm (Table 2). The proportion achieving step goals remained constant throughout the intervention period for both study arms but declined for the gamification arm during the follow-up period (Figure 2). The mean (SD) unadjusted proportions of participant-days that step goals were achieved during the follow-up period were 0.33 (0.20) in the control arm and 0.44 (0.22) in the gamification arm.
Table 2. Physical Activity Outcomesa.
| Variable | Mean (SD) | Main Modelb | Main Model Adjusted by Devicec | |||
|---|---|---|---|---|---|---|
| Control | Gamification | Gamification Effect (95% CI)d | P Value | Gamification Effect (95% CI)d | P Value | |
| Baseline | ||||||
| Steps per day | 7662 (3776) | 7244 (3368) | NA | NA | NA | NA |
| Intervention, wk 1-12 | ||||||
| Proportion of participant-days achieving the step goal | 0.32 (0.24) | 0.53 (0.29) | 0.27 (0.20-0.33) | <.001 | 0.26 (0.20-0.33) | <.001 |
| Steps per day | 8298 (3836) | 8905 (3382) | 953 (505-1401) | <.001 | 1004 (545-1463) | <.001 |
| Follow-up, wk 13-24 | ||||||
| Proportion of participant-days achieving the step goal | 0.33 (0.20) | 0.44 (0.22) | 0.12 (0.05-0.19) | <.001 | 0.12 (0.05-0.19) | <.001 |
| Steps per day | 8460 (3186) | 8629 (2783) | 494 (170-818) | .003 | 523 (184-862) | .003 |
Abbreviation: NA, not applicable.
All data presented represent the main analysis using multiple imputation for missing data and step values less than 1000.
Adjusted for baseline step count, repeated measures, calendar month fixed effects, and team random effect.
Adjusted for baseline step count, repeated measures, calendar month fixed effects, team random effect, and step tracking device.
Adjusted for baseline. The gamification arm is compared with the control arm during the specified periods. The 95% CIs were obtained using the bootstrap method.
Figure 2. Unadjusted Proportion of Participant-days That Step Goals Were Achieved by Study Arm and Week.
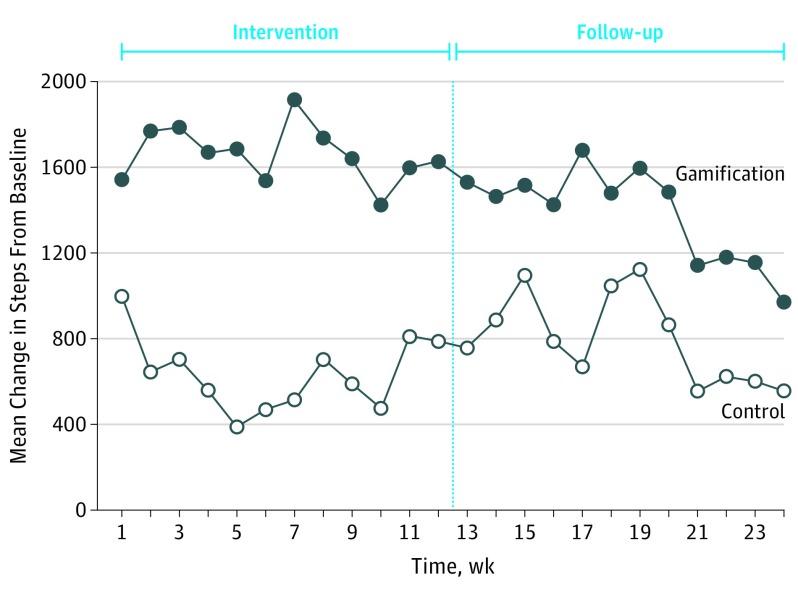
The unadjusted mean (SD) steps per day during the intervention period were 8298 (3836) in the control arm and 8905 (3382) in the gamification arm (Table 2). In the control arm, the change in daily steps from baseline was near 1000 in week 1, declined to less than 400 by week 5, and then increased to near 800 by week 12 (Figure 3). In the gamification arm, the change in daily steps from baseline began near 1550 in week 1 and ranged between 1400 and 1900 during the rest of the intervention period. The unadjusted mean (SD) steps per day during the follow-up period were 8460 (3186) in the control arm and 8629 (2783) in the gamification arm.
Figure 3. Unadjusted Mean Change in Daily Steps From Baseline by Study Arm and Week.
In the main adjusted model (Table 2), the gamification arm achieved step goals on a significantly greater proportion of participant-days than the control arm during the intervention period (adjusted difference, 0.27; 95% CI, 0.20-0.33; P < .001) and during the follow-up period (adjusted difference, 0.12; 95% CI, 0.05-0.19; P < .001). The gamification arm also had a significantly greater change in the mean daily steps than the control arm during the intervention period (adjusted difference, 953; 95% CI, 505-1401; P < .001) and during the follow-up period (adjusted difference, 494; 95% CI, 170-818; P = .003). Sensitivity analyses that adjusted for the tracking device of participant activity demonstrated similar results (Table 2), as did secondary analyses using only collected data (eTable 6 and eTable 7 in Supplement 2).
One participant in the control arm died for reasons deemed by the institutional review boards and data and safety monitoring board as unrelated to the study. No other major adverse events were reported (eTable 8 in Supplement 2). Most participants in both study arms had positive perceptions about their experiences in the study, and many stated that they would continue to use their activity tracking devices after the study concluded (eTable 9 in Supplement 2).
Discussion
In this trial, we found that gamification designed using insights from behavioral economics to enhance social incentives within families significantly increased physical activity during the 12-week intervention. The mean increase from baseline among participants in the gamification arm was approximately 1700 steps, which is almost an additional 1 mile per day. During the 12-week follow-up period, physical activity levels among participants in the gamification arm declined over time but overall remained significantly greater than those in the control arm. These findings demonstrate the potential for leveraging incentives within social networks to change health behaviors. The use of wearable devices and smartphones may offer a scalable approach to deliver these types of interventions on a broader scale. Because this intervention offered only trivial material reward (a coffee mug), it could be deployed more widely at low cost.
The results of this trial expand our understanding of using gamification and social incentives to change health behaviors and reveal important implications for the design of future interventions. First, an important element of our study was the use of principles from behavioral economics within the gamification design to address predictable barriers to behavior change. While gamification is used widely across various industries, evidence on its effectiveness is inconsistent, and most study designs have not appropriately incorporated theories from health behavior. Our findings indicate that these approaches could be more effective if designed to leverage insights from behavioral economics.
Second, although several retrospective studies have identified associations between social networks and health behaviors, there have been few studies evaluating effects prospectively. Zhang and colleagues have conducted 2 randomized trials testing various forms of social feedback and found associated increases in gym attendance and self-reported exercise. However, these studies were conducted among graduate students, and financial incentives were also offered, making the effect of social incentives more challenging to isolate and apply to the general community. In addition, Zhang and colleagues tested interventions among groups of anonymous individuals. The use of family networks is a strength of our study design because these relationships are widespread and long-standing.
Third, approaches using financial incentives have raised concern regarding their potential to create effects that do not persist over time. A study by Finkelstein and colleagues randomly assigned 800 adults to control, activity monitoring, cash incentives, or charity incentives. Over 6 months, the 2 incentive groups had greater physical activity than the controls. However, these effects and the use of the activity tracking device declined during the 6 months after incentives ceased. In our study using social incentives, 97.0% (194 of 200) of participants completed the 12-week intervention, and none dropped out during the 12-week follow-up period. Overall during the follow-up period, physical activity levels remained significantly greater in the gamification arm. However, activity levels in the gamification arm declined in the follow-up period, more quickly after week 20. Future studies could evaluate ways to increase longer-term sustainability, such as testing different forms of social incentives (eg, competition or support) and the interaction of social incentive–based approaches with other intervention designs such as financial incentives or social comparisons.
Limitations
Our study is subject to some limitations. First, participants were members of the Framingham Heart Study, had European ancestry, and needed either a smartphone or a computer, which may limit generalizability. While more physical activity is associated with greater health benefits for all individuals, future studies should evaluate the approaches tested in the present study among samples that are more diverse, sedentary, and high risk. Second, while enrollment rates were significantly higher than those of previous similar studies, future interventions will need to identify how to engage an even broader population. Third, we evaluated physical activity using step counts and did not have data on other measures of physical activity, device wear time, or other health outcomes. Step counts are most commonly displayed by wearable devices and have been successfully used in interventions to improve clinical outcomes across different populations. However, a systematic review of studies among individuals with type 2 diabetes found that improvements in physical activity, including steps and minutes of activity, did not influence clinical outcomes. Future studies could evaluate both changes in step counts and other clinical outcomes over longer periods. Fourth, while effects were sustained during follow-up overall, activity in the gamification arm declined after week 20, and further evaluations are needed to determine longer-term sustainability. Fifth, we did not test the effect of the intervention in nonfamily networks.
Conclusions
Compared with a control group of families in the community, a social incentive–based gamification intervention among families was effective at increasing physical activity. Our findings suggest that gamification may offer a promising approach to change health behaviors if designed using insights from behavioral economics to enhance social incentives.
Trial Protocol
eTable 1. Characteristics of Participants in the Singleton Arm
eTable 2. Family Structure for Participants in the Control and Gamification Arms
eTable 3. Characteristics of Participants That Received an Intervention Based on the Activity Tracking Device Used
eTable 4. Characteristics of All Individuals That Were Invited to Participate and Comparisons to Those That Participated as a Team With Their Family and Those That Participated Individually
eTable 5. Missing Data Rates by Arm and Study Period
eTable 6. Adjusted Models Using Only Collected Data Without Multiple Imputation
eTable 7. Adjusted Models Using Only Collected Data Without Multiple Imputation but Excluding Values Less Than 1000
eTable 8. Safety Data
eTable 9. Participant End of Study Survey Responses by Arm
eFigure. Coffee Mug Reward for Participants in the Gamification Arm Ending the Intervention in the Gold or Platinum Status
References
- 1.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Impact of physical activity on the world’s major non-communicable disease. Lancet. 2012;380:219-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Q, Cogswell ME, Flanders WD, et al. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA. 2012;307(12):1273-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding D, Lawson KD, Kolbe-Alexander TL, et al. ; Lancet Physical Activity Series 2 Executive Committee . The economic burden of physical inactivity: a global analysis of major non-communicable diseases. Lancet. 2016;388(10051):1311-1324. [DOI] [PubMed] [Google Scholar]
- 4.Mohan D, Schell J, Angus DC. Not thinking clearly? play a game, seriously. JAMA. 2016;316(18):1867-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cugelman B. Gamification: what it is and why it matters to digital health behavior change developers. JMIR Serious Games. 2013;1(1):e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller AS, Cafazzo JA, Seto E. A game plan: gamification design principles in mHealth applications for chronic disease management. Health Informatics J. 2016;22(2):184-193. [DOI] [PubMed] [Google Scholar]
- 7.Brown M, O’Neill N, van Woerden H, Eslambolchilar P, Jones M, John A. Gamification and adherence to web-based mental health interventions: a systematic review. JMIR Ment Health. 2016;3(3):e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamari J, Koivisto J, Sarsa H Does gamification work? a literature review of empirical studies on gamification. In: Proceedings of the 47th Hawaii International Conference on System Sciences; January 6-9, 2014; Waikoloa, HI. [Google Scholar]
- 9.Lister C, West JH, Cannon B, Sax T, Brodegard D. Just a fad? gamification in health and fitness apps. JMIR Serious Games. 2014;2(2):e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards EA, Lumsden J, Rivas C, et al. Gamification for health promotion: systematic review of behaviour change techniques in smartphone apps. BMJ Open. 2016;6(10):e012447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel MS, Asch DA, Volpp KG. Wearable devices as facilitators, not drivers, of health behavior change. JAMA. 2015;313(5):459-460. [DOI] [PubMed] [Google Scholar]
- 12.Loewenstein G, Brennan T, Volpp KG. Asymmetric paternalism to improve health behaviors. JAMA. 2007;298(20):2415-2417. [DOI] [PubMed] [Google Scholar]
- 13.Patel MS, Asch DA, Rosin R, et al. Framing financial incentives to increase physical activity among overweight and obese adults: a randomized, controlled trial. Ann Intern Med. 2016;164(6):385-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel MS, Asch DA, Rosin R, et al. Individual vs team-based financial incentives to increase physical activity: a randomized, controlled trial. J Gen Intern Med. 2016;31(7):746-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel MS, Volpp KG, Rosin R, et al. A randomized trial of social comparison feedback and financial incentives to increase physical activity. Am J Health Promot. 2016;30(6):416-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asch DA, Rosin R. Engineering social incentives for health. N Engl J Med. 2016;375(26):2511-2513. [DOI] [PubMed] [Google Scholar]
- 17.Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007;357(4):370-379. [DOI] [PubMed] [Google Scholar]
- 18.Christakis NA, Fowler JH. The collective dynamics of smoking in a large social network. N Engl J Med. 2008;358(21):2249-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson SE, Steptoe A, Wardle J. The influence of partner’s behavior on health behavior change: the English Longitudinal Study of Ageing. JAMA Intern Med. 2015;175(3):385-392. [DOI] [PubMed] [Google Scholar]
- 20.Asch DA, Volpp KG. On the Way to Health. LDI Issue Brief. 2012;17(9):1-4. [PubMed] [Google Scholar]
- 21.Case MA, Burwick HA, Volpp KG, Patel MS. Accuracy of smartphone applications and wearable devices for tracking physical activity data. JAMA. 2015;313(6):625-626. [DOI] [PubMed] [Google Scholar]
- 22.Bassett DR Jr, Wyatt HR, Thompson H, Peters JC, Hill JO. Pedometer-measured physical activity and health behaviors in U.S. adults. Med Sci Sports Exerc. 2010;42(10):1819-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang M, Zhu W, Tudor-Locke C, Ainsworth B. Experimental determination of effectiveness of an individual information–centered approach in recovering step-count missing data. Meas Phys Educ Exerc Sci. 2004;9(4):233-250. [Google Scholar]
- 24.Rowe DA, Mahar MT, Raedeke TD, Lore J. Measuring physical activity in children with pedometers: reliability, reactivity, and replacement of missing data. Pediatr Exerc Sci. 2004;16(4):343-354. [Google Scholar]
- 25.Ariely D, Wertenbroch K. Procrastination, deadlines, and performance: self-control by precommitment. Psychol Sci. 2002;13(3):219-224. [DOI] [PubMed] [Google Scholar]
- 26.Rogers T, Milkman KL, Volpp KG. Commitment devices: using initiatives to change behavior. JAMA. 2014;311(20):2065-2066. [DOI] [PubMed] [Google Scholar]
- 27.Skinner BF. The Behavior of Organisms : An Experimental Analysis New York, NY: Appleton-Century-Crofts; 1938. [Google Scholar]
- 28.Dai H, Milkman KL, Riis J. The fresh start effect: temporal landmarks motivate aspirational behavior. Manage Sci. 2014;60(10):2563-2582. [Google Scholar]
- 29.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: Wiley; 1987. [Google Scholar]
- 30.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44(4):1049-1060. [PubMed] [Google Scholar]
- 31.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York, NY: Chapman & Hall; 1993. [Google Scholar]
- 32.Davison AC, Hinkley DV. Bootstrap Methods and Their Application. Cambridge, England: Cambridge University Press; 1997. [Google Scholar]
- 33.Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296-2304. [DOI] [PubMed] [Google Scholar]
- 34.Howe KB, Suharlim C, Ueda P, Howe D, Kawachi I, Rimm EB. Gotta catch’em all! Pokémon GO and physical activity among young adults: difference in differences study. BMJ. 2016;355:i6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Brackbill D, Yang S, Centola D. Efficacy and causal mechanism of an online social media intervention to increase physical activity: results of a randomized controlled trial. Prev Med Rep. 2015;2:651-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, Brackbill D, Yang S, Becker J, Herbert N, Centola D. Support or competition? how online social networks increase physical activity: a randomized controlled trial. Prev Med Rep. 2016;4:453-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finkelstein EA, Haaland BA, Bilger M, et al. Effectiveness of activity trackers with and without incentives to increase physical activity (TRIPPA): a randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4(12):983-995. [DOI] [PubMed] [Google Scholar]
- 38.Fox CS, Hwang SJ, Nieto K, et al. Digital connectedness in the Framingham Heart Study. J Am Heart Assoc. 2016;5(4):e003193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sattelmair J, Pertman J, Ding EL, Kohl HW III, Haskell W, Lee IM. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation. 2011;124(7):789-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baskerville R, Ricci-Cabello I, Roberts N, Farmer A. Impact of accelerometer and pedometer use on physical activity and glycaemic control in people with type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2017;34(5):612-620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Characteristics of Participants in the Singleton Arm
eTable 2. Family Structure for Participants in the Control and Gamification Arms
eTable 3. Characteristics of Participants That Received an Intervention Based on the Activity Tracking Device Used
eTable 4. Characteristics of All Individuals That Were Invited to Participate and Comparisons to Those That Participated as a Team With Their Family and Those That Participated Individually
eTable 5. Missing Data Rates by Arm and Study Period
eTable 6. Adjusted Models Using Only Collected Data Without Multiple Imputation
eTable 7. Adjusted Models Using Only Collected Data Without Multiple Imputation but Excluding Values Less Than 1000
eTable 8. Safety Data
eTable 9. Participant End of Study Survey Responses by Arm
eFigure. Coffee Mug Reward for Participants in the Gamification Arm Ending the Intervention in the Gold or Platinum Status