This analysis of US Securities and Exchange Commission filings provides a contemporary estimate of research and development spending to develop 10 new cancer drugs.
Key Points
Question
What is the estimated research and development spending for developing a cancer drug?
Findings
In this analysis of US Securities and Exchange Commission filings for 10 cancer drugs, the median cost of developing a single cancer drug was $648.0 million. The median revenue after approval for such a drug was $1658.4 million.
Meaning
These results provide a transparent estimate of research and development spending on cancer drugs and show that the revenue since approval is substantially higher than the preapproval research and development spending.
Abstract
Importance
A common justification for high cancer drug prices is the sizable research and development (R&D) outlay necessary to bring a drug to the US market. A recent estimate of R&D spending is $2.7 billion (2017 US dollars). However, this analysis lacks transparency and independent replication.
Objective
To provide a contemporary estimate of R&D spending to develop cancer drugs.
Design, Setting, and Participants
Analysis of US Securities and Exchange Commission filings for drug companies with no drugs on the US market that received approval by the US Food and Drug Administration for a cancer drug from January 1, 2006, through December 31, 2015. Cumulative R&D spending was estimated from initiation of drug development activity to date of approval. Earnings were also identified from the time of approval to the present. The study was conducted from December 10, 2016, to March 2, 2017.
Main Outcomes and Measures
Median R&D spending on cancer drug development.
Results
Ten companies and drugs were included in this analysis. The 10 companies had a median time to develop a drug of 7.3 years (range, 5.8-15.2 years). Five drugs (50%) received accelerated approval from the US Food and Drug Administration, and 5 (50%) received regular approval. The median cost of drug development was $648.0 million (range, $157.3 million to $1950.8 million). The median cost was $757.4 million (range, $203.6 million to $2601.7 million) for a 7% per annum cost of capital (or opportunity costs) and $793.6 million (range, $219.1 million to $2827.1 million) for a 9% opportunity costs. With a median of 4.0 years (range, 0.8-8.8 years) since approval, the total revenue from sales of these 10 drugs since approval was $67.0 billion compared with total R&D spending of $7.2 billion ($9.1 billion, including 7% opportunity costs).
Conclusions and Relevance
The cost to develop a cancer drug is $648.0 million, a figure significantly lower than prior estimates. The revenue since approval is substantial (median, $1658.4 million; range, $204.1 million to $22 275.0 million). This analysis provides a transparent estimate of R&D spending on cancer drugs and has implications for the current debate on drug pricing.
Introduction
The cost of anticancer drugs continues to increase, with drugs routinely priced more than $100 000 per year of treatment1 and some nearing the $200 000 per year threshold.2 High drug prices have negative effects on patients and society, and groups of physicians, patients, and policymakers have voiced their opposition to these prices.3,4,5 However, one persistent argument in justification of high drug prices is the sizable outlay made by biopharmaceutical firms to develop new drugs. In a widely publicized analysis from the Tufts Center for the Study of Drug Development, the authors estimate $2.7 billion (inflation adjusted for 2017 US dollars) is needed to bring a single drug to the US market.6,7 This figure is more than 8 times higher than the $320.0 million (inflation adjusted for 2017 US dollars) estimate to develop one drug reached by the group Public Citizen.8 Given such divergent estimates, closer examination is warranted.
The Tufts group reached its estimate using private data from 10 pharmaceutical firms, which reported the number of compounds they had tested in phase 1 to 3 trials to yield their portfolio of approved drugs.6 By summing the cost of these studies, the Tufts group estimated that $1.4 billion was needed to develop a drug with an added $1.2 billion for cost of capital or opportunity cost (ie, the amount of money that would have been earned had the same sum been invested in the hands of money managers rather than used for drug development). In contrast, the analysis by Public Citizen drew on publicly available US Securities and Exchange Commission (SEC) research and development (R&D) filings for all major pharmaceutical firms during a 7-year period and divided the total expenditure across all companies by the number of new drugs approved for those companies in the subsequent 7 years.8
Both these methods have limitations. The Public Citizen analysis divides the total expenditures in a 7-year period with the total number of new drugs approved in the subsequent 7 years and does not reflect the actual spending to bring these particular drugs to market. The Tufts group’s analysis is opaque because the number of drugs pursued by firms and cost of clinical trials used in the analysis are not available for external scrutiny.7
We decided to update these estimates using a different approach. We focused on publicly traded pharmaceutical companies with only one US Food and Drug Administration (FDA)–approved drug. We used SEC filings from the approximate time of discovery or initial acquisition of the compound to drug approval to estimate the cost to bring one drug to market. Because, in all instances, these companies were simultaneously developing several compounds, our analysis considers the cost of failure (ie, we include R&D costs for each company’s entire portfolio of drugs, of which only one drug was ultimately approved).
Methods
We screened all new molecular entities approved for oncologic indications during a decade (January 1, 2006, through December 31, 2015) from the FDA website (https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm279174.htm) for drug products from a manufacturer that, at the time of FDA approval, had no other drugs on the market. This approach allows for an analysis of the cost of developing a single, new molecular entity from a portfolio of chemical agents. This study was not submitted for institutional review board approval because it did not involve health care records and all data are publicly available. Our study was conducted from December 10, 2016, to March 2, 2017.
For each approved drug, we calculated the total R&D spending from the first year of R&D activity to the year of approval. We defined the first year of R&D activity as follows. For companies that did not initially develop the compound but acquired it through purchase, we assessed R&D costs from the year of acquisition of the drug. For companies that self-originated the compound, we identified the first mention of the compound in the biomedical literature. The first mention of the compound was ascertained by searching Google Scholar and MEDLINE for the approved generic drug name and the preclinical chemical name. For example, the drug cabozantinib would have led to 2 searches: cabozantinib and XL184. We screened all results in Google Scholar and MEDLINE by year to ascertain the year of first publication. We then designated the start of R&D expenses to be 2 years before the initial mention to include additional time for preclinical development. This period was selected because it approximates the mean time from initiation of preclinical activity to the start of clinical trials.6 The year of drug approval was taken from the FDA website. The time from R&D initiation to drug approval provided us with the cumulative duration of R&D for each drug.
We then reviewed publicly available SEC 10-K filings, available at the SEC website (https://www.sec.gov/edgar/searchedgar/companysearch.html). The 10-K filings are annual reports that include comprehensive overview of the company’s financial situation and include audited financial statements.9 These were reviewed by one of us (S.M.), and all expenses listed as R&D were totaled for the cumulative duration of R&D for each drug. We calculated cost of capital (or opportunity costs) at 7% return. We chose 7% because bonds issued by pharmaceutical companies generally pay 1% to 5%,7 and experts have estimated 6% to 7% annual returns for equity investments.10 The total number of candidate drugs in clinical trials was also obtained from the 10-K filings. The median time since approval was estimated as the date of FDA approval to the last available SEC 10-K filings (December 31, 2015, or December 31, 2016) or until the company sold or licensed the compound to another company. From the SEC 10-K filings, we totaled the cumulative revenue since FDA approval for each drug. We also obtained the total of number of drugs in clinical development during the study period from the 10-K filings. Details about drugs in preclinical development are not consistently available in these filings. Six companies (developing the drugs ibrutinib, enzalutamide, brentuximab vedotin, irinotecan liposome, ruxolitinib, and pralatrexate) had collaborative agreements with another pharmaceutical company for the codevelopment and comarketing of these drugs. For these drugs, we included R&D expenditures from both companies but did not include revenues obtained as upfront or milestone payments. All dollars were inflation adjusted to 2017 US dollars using the Consumer Price Index.
Statistical analysis was performed using STATA software, version 13 (StataCorp). The Mann-Whitney test was used to compare groups. All tests are 2-tailed. A 2-sided P < .05 was considered to be significant. Analyses were not adjusted for multiple comparisons.
Results
We identified 13 companies that had no FDA-approved drug products on the US market at the time they received a cancer drug approval during the decade of 2007 and 2016. Three companies were excluded because they did not have available 10-K filings: Taiho Oncology (trifluridine and tipiracil), Nova Laboratories (oral mercaptopurine), and Gloucester Pharmaceuticals (romidepsin).
Ten drugs and companies are included in the current analysis. These 10 companies had no drug in any sector on the market at the time of approval. The Table details their cumulative R&D spending and revenue since approval. The 10 drugs were approved by the FDA between March 2007 and October 2015. Five (50%) drugs (ponatinib, brentuximab vedotin, cabozantinib, ruxolitinib, and eculizumab) were self-originated, whereas the other 5 drugs were acquired from another source. All but 1 (enzalutamide) of the 10 drugs have orphan drug designation from the FDA. The 10 companies had a median of 3.5 drugs in clinical development during the study period (range, 2-11).
Table. Oncologic Drugs and Pharmaceutical Manufacturers With a Single Oncologic Drug Approved by the FDA and Estimates of R&D Spending and the Revenues From Sales After FDA Approval.
| Drug (Manufacturer) | FDA Approval Date | No. of Drugs in Development |
R&D Start Date | Basis of FDA Approval | Orphan Drug Exclusivity | Time to Approval, y | Total R&D Costs in Millions, $a | R&D Costs, Including 7% per Annum Cost of Capital, in Millions, $a | Time Since Approval, y | Revenue Since Approval in Millions, $a | Revenue as Part of R&D Spending, % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eculizumab (Alexion Pharmaceuticalsb) | March 2007 | 3 | January 1992 | Regular (other) | Yes | 15.2 | 817.6 | 1088.0 | 8.8 | 12 987.8 | 1588.5 |
| Pralatrexate (Allos Therapeutics) | September 2009 | 3 | December 2002c | Accelerated (RR) | Yes | 6.8 | 178.2 | 217.4 | 3.0 | 304.8d | 171.0 |
| Brentuximab vedotin (Seattle Genetics) | August 2011 | 3 | January 2001 | Accelerated (RR) | Yes | 10.6 | 899.2 | 1119.2 | 5.3 | 1034.3 | 115.0 |
| Ruxolitinib (Incyte Corporation) | November 2011 | 5 | January 2004 | Regular (other) | Yes | 7.8 | 1097.8 | 1374.3 | 5.1 | 2251.5 | 205.1 |
| Enzalutamide (Medivation) | August 2012 | 2 | August 2005c | Regular (OS) | No | 7.0 | 473.3 | 554.9 | 4.0 | 21 068.3d | 4451.4 |
| Vincristine liposome (Talon Therapeutics) | September 2012 | 4 | May 2006c | Accelerated (RR) | Yes | 6.3 | 157.3 | 203.6 | 0.8 | 204.1d | 129.8 |
| Cabozantinib (Exelixis) | November 2012 | 11 | January 2004 | Regular (PFS) | Yes | 8.8 | 1950.8 | 2601.7 | 4.1 | 341.9 | 17.5 |
| Ponatinib (Ariad Pharmaceuticals) | December 2012 | 3 | January 2007 | Accelerated (RR) | Yes | 5.9 | 480.1 | 548.4 | 4.1 | 5457.9d | 1136.8 |
| Ibrutinib (Pharmacyclics) | November 2013 | 4 | April 2006c | Accelerated (RR) | Yes | 7.6 | 328.1 | 388.7 | 1.3 | 22 275.0d | 6789.1 |
| Irinotecan liposome (Merrimack Pharmaceuticals) | October 2015 | 5 | December 2009c | Regular (OS) | Yes | 5.8 | 815.8 | 959.8 | 1.3 | 1065.2 | 130.6 |
Abbreviations: FDA, US Food and Drug Administration; OS, overall survival; PFS, progression-free survival; R&D, research and development; RR, response rate.
Inflation adjusted for 2017 US dollars.
Alexion Pharmaceuticals later had 2 drug approvals (asfotase alfa for hypophosphatemia and kanuma sebelipase alfa for lysosomal acid lipase in October and December 2015) in the last quarter of 2015. We did not include revenues from the sales of these 2 drugs for this analysis.
Compound acquired by the company on this date.
Manufacturer or compound sold or licensed to a different company, and the revenue since approval includes the acquisition price. Ponatinib was acquired by Takeda Pharmaceuticals for $5.2 billion, ibrutinib by AbbVie for $21.5 billion, enzalutamide by Pfizer for $14 billion, vincristine liposome for $203.3 million, and pralatrexate by Spectrum Pharmaceuticals for $205.3 million.
The median time to develop a drug was 7.3 years (range, 5.8-15.2 years). Five companies (50%) developed drugs that received accelerated approval from the FDA on the basis of response rate, a measure of tumor shrinkage. Five drugs (50%) received regular approval: 2 on the basis of improvements in overall survival (enzalutamide and irinotecan liposome), 1 on the basis of improvement in progression-free survival (cabozantinib), and 2 based on other measures (ruxolitinib based on reduction of spleen volume and eculizumab based on stabilization of hemoglobin concentration). Five of the 10 drugs (50%) act on a novel target (ibrutinib, brentuximab vedotin, ruxolintinib, cabozantinib, and eculizumab), whereas the other 5 (50%) are next-in-class drugs with a mechanism of action similar to a previously approved drug.
The median cost of development of a single drug in 2017 US dollars was $648.0 million (range, $157.3 million to $1950.8 million), and the mean cost of development was $719.8 million (95% CI, $336.0 million to $1104.0 million). Drugs that received accelerated approval cost less to develop than those that received regular approval, although this finding was not statistically significant (median, $328.1 million [range, $157.3 million to $899.2 million] vs $817.6 million [range, $473.3 million to $1950.8 million]; P = .08). The R&D spending on novel drugs was higher than next-in-class drugs (median, $899.2 million [range, $328.1 million to $1950.8 million] vs $473.3 million [range, $157.3 million to $815.8 million]; P = .047). Self-originated drugs had higher R&D spending compared with acquired drugs (median, $899.2 million [range, $480.1 million to $1950.8 million] vs $328.1 million [range, $157.3 million to $815.8 million]; P = .02). We also estimated the cost of drug development to include a 7% per annum cost of capital (or opportunity costs); the median cost in 2017 US dollars was $757.4 million (range, $203.6 million to $2601.7 million), and the mean cost in 2017 US dollars was $905.6 million (95% CI, $391.0 million to $1420.0 million). As sensitivity analysis, we estimated the drug development costs to include a 9% per annum cost of capital; the median cost was $793.6 million (range, $219.1 million to $2827.1 million), and the mean cost was $969.4 million (95% CI, $408.6 to $1530.3 million). Similarly, with a 5% per annum cost of capital, the median cost was $723.0 million (range, $189.1 million to $2395.1 million), and the mean cost was $846.9 million (95% CI, $373.9 million to $1319.9 million).
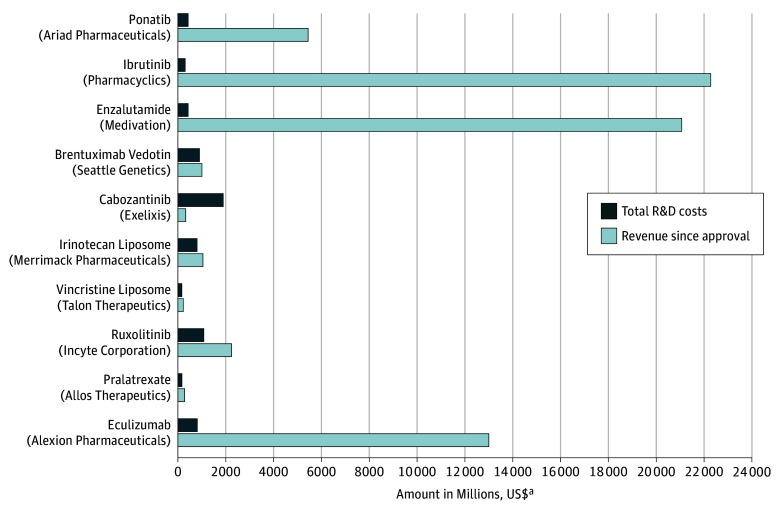
From the time of approval to December 2016 (or until the company sold or licensed the compound to another company), the total revenue of these 10 drugs was $67.0 billion. The median revenue for these companies in 2017 US dollars was $1658.4 million (range, $204.1 million to $22 275.0 million), and the mean was $6699.1 million (95% CI, $403.0 million to $12 996.0 million). Five of the 10 drugs (ponatinib, ibrutinib, enzalutamide, vincristine liposome, and pralatrexate) were acquired by other pharmaceutical companies, with acquisition prices in 2017 US dollars ranging from $204.1 million to $21 500.0 million. The median time since regulatory approval was 4.0 years (range, 0.8-8.8 years). Nine of the 10 drugs (90%) had higher revenues compared with R&D spending (range, 17.5%-6789.1%), and for 4 drugs (ponatinib, ibrutinib, enzalutamide, and eculizumab), the revenue from sales was more than 10-fold higher than R&D spending. The Figure compares drug development costs with revenue after approval by company. Given that total spending (including a 7% cost of capital) to develop these drugs was $9 billion and total revenue to date was $67 billion, the postapproval revenue was more than 7-fold higher than the R&D spending.
Figure. Comparison of Drug Development Costs With Revenue Earned After Approval.
R&D indicates research and development.
aAdjusted to 2017 US dollars.
Discussion
Our results seek to provide clarity and refine the estimate of the cost to develop a single oncologic drug. Specifically, we found that the cost to develop one cancer drug is approximately $648.0 million ($757.4 million when opportunity costs are included), a figure that falls between prior estimates but is significantly smaller than a widely publicized figure of $2.7 billion.
Other analysts have used similar methods with notable differences. For instance, Public Citizen summed R&D spending across major pharmaceutical companies over a 7-year period8 and divided by the number of approval in the subsequent 7-year period. However, this approach compares the cost of developing future drugs against the number of approvals in a 7-year period—a denominator that varies.11 Other attempts at R&D estimation have summed total R&D for 15 years among the largest pharmaceutical companies and divided by the total number of new molecular entities approved during that time.12 This approach, however, places heavy weight on large companies, which, by virtue of having had consistent and large profit margins, may have become less efficient, undergone expansions in staff, or introduced redundant costs.13 In addition, these companies may be providing estimates of R&D that include the cost of phase 4 trials for approved drugs, which, at least in some studies, may be used to bolster the sales of products off label14 rather than R&D costs used solely to pursue novel FDA approvals.15 Our analysis may even approximate the true cost of R&D at major pharmaceutical companies if these additional charges are removed because previous work16 has suggested that the cost to develop a drug between large and small firms is comparable.
Our analysis also has advantages over the Tufts analysis. The Tufts analysis lacks transparency and is difficult to judge on its merits.7 It cannot be properly analyzed without knowing the specific drug products investigated, yet this has been deemed proprietary information and is governed by confidentiality agreements.
There are several external observations that support the generalizability of our analysis. First, we find that drugs take median of 7.3 years from compound discovery to approval, with a wide range (6-15 years) that overlaps other estimates of the time required for drug development.17 Second, we analyzed drugs approved in the past decade, and 60% of drugs were approved based on progression-free survival or response rate (ie, surrogate clinical end points), whereas 40% were approved based on overall survival, patient-reported outcomes, or other end points. These percentages are similar to the fraction of all recent cancer drugs approved based on a surrogate.1,18 Third, our analysis examined drugs that were mixed between novel (50%) and next in class (50%), and again this percentage was similar to the percentage of all new approvals that are next in class.1 In other words, although small, our data set is similar to the general characteristics of approved cancer drugs.
One of the additional observations made in our analysis is that postapproval revenue from sales for these drugs is sizable. Revenue since approval ranged from $204.1 million to $22 275.0 million. All 10 drugs are currently protected by patents and/or market exclusivity, with the median time since approval of 4 years. Prior work19 has shown that the mean length of market exclusivity for oncologic drugs is 14.3 years; thus, these revenues will continue to increase over time. Our analysis showing revenue after approval far surpasses R&D spending is consistent with prior analysis by Yu and colleagues20 revealing that excess revenues earned from premium drug pricing in the United States are in excess of the total global R&D spending for many large pharmaceutical companies.
Some of the examples in the Table are notable. Ariad Pharmaceuticals, the maker of the tyrosine kinase inhibitor ponatinib, has earned more than $5 billion in revenue from this drug. Ponatinib has a complex regulatory history.21 The drug was initially approved for patients with chronic myeloid leukemia who were refractory to 1 prior tyrosine kinase inhibitor therapy. Almost immediately, a strong safety concern emerged, with 24% to 48% of patients who took the drug in a pivotal study21 developing arterial and venous thromboembolism. The drug was then withdrawn from the market before being reintroduced several months later for a narrow indication: chronic myeloid leukemia with the T315I gatekeeper mutation or for those in whom all other tyrosine kinase inhibitors (imatinib, dasatinib, nilotinib, and bosutinib) failed. Despite this turmoil and limited market share, Ariad Pharmaceuticals has reaped sizable revenue in part because of aggressive price increases that have drawn the attention of the US Congress.22,23
Alexion Pharmaceuticals’ drug eculizumab is also notable. The drug was initially approved for a rare disease: paroxysmal nocturnal hemoglobinuria. It later received approval for a supplementary indication: atypical hemolytic uremic syndrome. Both these uses concern small market sizes, yet, largely through pricing, eculizumab has been able to generate more than $12 billion in revenue, 15-fold higher than the R&D spending to develop the drug (Table). At one point, eculizumab was the most expensive drug.24 The cases of ponatinib and eculizumab show that blockbuster drugs (ie, those with returns greater than a billion dollars) can be created despite incredibly small market share.
A more measured history is that of Exelixis. Exelixis initially pursued cabozantinib for medullary thyroid cancer, an indication that received FDA approval in 2012. Market share for this rare malignant tumor is limited, and the company simultaneously pursued the drug for other indications, including prostate cancer.25 However, highly promising phase 2 results in that cancer25 failed to be corroborated in phase 3,26 after which the company laid off 70% of its staff.27 However, despite these setbacks, Exelixis still retains 9 years of exclusivity for cabozantinib and has expanded approved indications to include renal cancer. In time, the company is ensured a profit. Together, these 3 cases are illustrative of the diverse paths and setbacks companies face in the road to drug approval, but, despite the challenges, all demonstrate high profitability.
Strengths and Limitations
Our analysis has several strengths. By focusing solely on companies that have brought a single drug to market, we were able to sum R&D filings that led to a single approval. As indicated in the Table, all these companies were pursuing several drugs. Thus, our analysis includes the cost of pursuing a portfolio of candidate compounds to yield one drug (ie, considers the cost of development failure).
There are critical limitations to our work. First, our data set is small, containing approximately 15% of all new molecular entities approved for cancer during this time. To take advantage of the unique sample of the drug market, we limited our study to companies that were pursuing their first approval. This method removed R&D costs used to bolster the sales of prior approved products but necessarily limited our sample size. Second, although we used SEC filings, which are subject to strict guidelines and regulation, it is possible that some companies overemphasized or underemphasized R&D costs through unintentional errors. Without access to primary company data, we cannot be sure. Third, our analysis pertains only to cancer drugs, which have had a steady pace of drug approvals in recent years, and cannot be extrapolated to other sectors in which drug development may be harder for biological reasons (eg, Alzheimer disease). Furthermore, cancer medications are costly, and revenue cannot be extrapolated to other sectors. Fourth, R&D spending permits firms to obtain various tax breaks, which can subsidize expenses by up to 15%, and these are not accounted for in our analysis. Nevertheless, despite these limits, our analysis provides important information for this ongoing discussion. Ultimately, the greatest limitation to our analysis is one shared by all the prior analyses6,8 we have discussed: we still lack total R&D transparency. The cost to develop a drug is a measurable concept, and with open access to pharmaceutical company records, it can be done with some precision. We favor an analysis of the question that brings all stakeholders together to produce a precise, transparent estimate to inform policy.
Conclusions
Prior estimates for the cost to develop one new drug span from $320.0 million to $2.7 billion. We analyzed R&D spending for pharmaceutical companies that successfully pursued their first drug approval and estimate that it costs $648.0 million to bring a drug to market. In a short period, development cost is more than recouped, and some companies boast more than a 10-fold higher revenue than R&D spending—a sum not seen in other sectors of the economy. Future work regarding the cost of cancer drugs may be facilitated by more, not less, transparency in the biopharmaceutical industry.
References
- 1.Mailankody S, Prasad V. Five years of cancer drug approvals: innovation, efficacy, and costs. JAMA Oncol. 2015;1(4):539-540. [DOI] [PubMed] [Google Scholar]
- 2.Wertheimer A, Bush P. Ask the pharmacists: sticker shock at the pharmacy counter. phillycom. 2017. http://www.philly.com/philly/health/Ask-the-pharmacists-Sticker-shock-at-the-pharmacy-counter.html. Accessed July 31, 2017.
- 3.Prasad V, De Jesús K, Mailankody S. The high price of anticancer drugs: origins, implications, barriers, solutions. Nat Rev Clin Oncol. 2017;14(6):381-390. [DOI] [PubMed] [Google Scholar]
- 4.Experts in Chronic Myeloid Leukemia . The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts. Blood. 2013;121(22):4439-4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tefferi A, Kantarjian H, Rajkumar SV, et al. In support of a patient-driven initiative and petition to lower the high price of cancer drugs. Mayo Clin Proc. 2015;90(8):996-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: new estimates of R&D costs. J Health Econ. 2016;47:20-33. [DOI] [PubMed] [Google Scholar]
- 7.Avorn J. The $2.6 billion pill–methodologic and policy considerations. N Engl J Med. 2015;372(20):1877-1879. [DOI] [PubMed] [Google Scholar]
- 8.Young B, Surrusco M. Rx R&D Myths: The Case Against the Drug Industry’s R&D “Scare Card.” Washington, DC: Public Citizen’s Congress Watch; 2001. https://www.citizen.org/sites/default/files/rdmyths.pdf. Accessed July 31, 2017.
- 9.US Securities and Exchange Commission . Form 10-K. Washington, DC: US Securities & Exchange Commission; June 26, 2009. [Google Scholar]
- 10.Buffett EC. Expect 6%-7% returns from the market. The Street. https://www.thestreet.com/story/10084972/1/buffett-expect-6-7-returns-from-the-market.html. Accessed July 31, 2017.
- 11.Gassman AL, Nguyen CP, Joffe HV. FDA regulation of prescription drugs. N Engl J Med. 2017;376(7):674-682. [DOI] [PubMed] [Google Scholar]
- 12.Herper M. The truly staggering cost of inventing new drugs. Forbes. https://www.forbes.com/sites/matthewherper/2012/02/10/the-truly-staggering-cost-of-inventing-new-drugs/#5f0fb73d4a94. Published February 10, 2012. Accessed July 31, 2017.
- 13.Paul SM, Mytelka DS, Dunwiddie CT, et al. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat Rev Drug Discov. 2010;9(3):203-214. [DOI] [PubMed] [Google Scholar]
- 14.Gagnon MA, Lexchin J. The cost of pushing pills: a new estimate of pharmaceutical promotion expenditures in the United States. PLoS Med. 2008;5(1):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sox HC, Rennie D. Seeding trials: just say “no”. Ann Intern Med. 2008;149(4):279-280. [DOI] [PubMed] [Google Scholar]
- 16.DiMasi J, Grabowski H. The cost of biopharmaceutical R&D: is biotech different? Manage Decis Econ. 2007;28(4-5):469-479. doi: 10.1002/mde.1360 [DOI] [Google Scholar]
- 17.Dranove D, Meltzer D. Do important drugs reach the market sooner? Rand J Econ. 1994;25(3):402-423. [Google Scholar]
- 18.Kim C, Prasad V. Cancer drugs approved on the basis of a surrogate end point and subsequent overall survival: an analysis of 5 years of US Food and Drug Administration approvals. JAMA Intern Med. 2015;175(12):1992-1994. [DOI] [PubMed] [Google Scholar]
- 19.Wang B, Liu J, Kesselheim AS. Variations in time of market exclusivity among top-selling prescription drugs in the United States. JAMA Intern Med. 2015;175(4):635-637. [DOI] [PubMed] [Google Scholar]
- 20.Yu N, Helms Z,Bach P. R&D costs for pharmaceutical companies do not explain elevated US drug prices. Health Affairs Blog. http://healthaffairs.org/blog/2017/03/07/rd-costs-for-pharmaceutical-companies-do-not-explain-elevated-us-drug-prices/. Published March 7, 2017. Accessed July 31, 2017.
- 21.Prasad V, Mailankody S. The accelerated approval of oncologic drugs: lessons from ponatinib. JAMA. 2014;311(4):353-354. [DOI] [PubMed] [Google Scholar]
- 22.Hagen T. Ponatinib (CML) pricing gets a blast from Congress. OncLive. http://www.onclive.com/web-exclusives/ponatinib-cml-pricing-gets-a-blast-from-congress. Published October 21, 2016. Accessed July 31, 2017.
- 23.Derrick J. Bernie Sanders and Rep. Cummings to Ariad: explain why Iclusig now costs $199,000 a year. Benzinga. https://www.benzinga.com/general/biotech/16/10/8584112/bernie-sanders-and-rep-cummings-to-ariad-explain-why-iclusig-now-costs. Published October 20, 2016. Accessed July 31, 2017.
- 24.Nordrum A. Drug prices: world’s most expensive medicine costs $440,000 a year: but is it worth the expense? http://www.ibtimes.com/drug-prices-worlds-most-expensive-medicine-costs-440000-year-it-worth-expense-2302609. Published February 13, 2016. Accessed July 31, 2017.
- 25.Smith DC, Smith MR, Sweeney C, et al. Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J Clin Oncol. 2013;31(4):412-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith M, De Bono J, Sternberg C, et al. Phase III study of cabozantinib in previously treated metastatic castration-resistant prostate cancer: COMET-1. J Clin Oncol. 2016;34(25):3005-3013. [DOI] [PubMed] [Google Scholar]
- 27.Lee S. Exelixis to lay off 70 percent of staff after prostate cancer drug bombs. SFGate. http://blog.sfgate.com/techchron/2014/09/02/exelixis-to-lay-off-70-percent-of-staff-after-prostate-cancer-drug-bombs/. Published September 2, 2014. Accessed July 31, 2017.