Key Points
Question
What is the prevalence of hearing loss among 9- to 11-year-old children in the Netherlands?
Findings
Using audiometry data from the prospective Generation R cohort study of 5368 children, the estimated prevalence of sensorineural hearing loss was 7.8%. Associations were found with maternal educational level and a history of recurrent otitis media.
Meaning
These results show that progressive sensorineural hearing loss might have an unexpectedly early onset, prior to known risk factors, such as noise exposure, and that early childhood risk factors, such as recurrent otitis media, already influence hearing acuity.
Abstract
Importance
Hearing loss (HL), a major cause of disability globally, negatively affects both personal and professional life.
Objective
To describe the prevalence of sensorineural hearing loss (SNHL) among a population-based cohort of 9- to 11-year-old children, and to examine potential associations between purported risk factors and SNHL in early childhood.
Design, Setting, and Participants
The study was among the general, nonclinical, pediatric community within the city of Rotterdam, the Netherlands, and was conducted between 2012 and 2015 as a cross-sectional assessment within the Generation R Study, a population-based longitudinal cohort study from fetal life until adulthood. Participants are children of included pregnant women in the Generation R Study with an expected delivery date between April 2002 and January 2006. They form a prenatally recruited birth cohort.
Main Outcomes and Measures
Pure-tone air-conduction hearing thresholds were obtained at 0.5, 1, 2, 3, 4, 6, and 8 kHz, and tympanometry was performed in both ears. Demographic factors and parent-reported questionnaire data, including history of otitis media, were also measured.
Results
A total of 5368 participants with a mean age of 9 years 9 months (interquartile range, 9 years 7 months–9 years 11 months) completed audiometry and were included in the analyses. A total of 2720 were girls (50.7%), and 3627 (67.6%) were white. Most of the participants (4426 children [82.5%]) showed normal hearing thresholds 15 dB HL or less in both ears. Within the cohort, 418 children (7.8%) were estimated to have SNHL (≥16 dB HL at low-frequency pure-tone average; average at 0.5, 1, and 2 kHz or high-frequency pure-tone average; average at 3, 4, and 6 kHz in combination with a type A tympanogram) in at least 1 ear, most often at higher frequencies. In multivariable analyses, a history of recurrent acute otitis media and lower maternal education were associated with the estimated SNHL at ages 9 to 11 years (odds ratio, 2.0 [95% CI. 1.5-2.8] and 1.4 [95% CI, 1.1-1.7], respectively).
Conclusions and Relevance
Within this cohort study in the Netherlands, 7.8% of the children ages 9 to 11 years had low-frequency or high-frequency HL of at least 16 dB HL in 1 or both ears. A history of recurrent acute otitis media and lower maternal education seem to be independent risk factors for presumed SNHL in early childhood.
This Dutch population-based cohort study describes the prevalence of sensorineural hearing loss among children 9 to 11 years old and examines potential associations between purported risk factors and sensorineural hearing loss in early childhood.
Introduction
Hearing loss (HL) can have an impact on communication and relationships on a personal level and can negatively affect education and occupation. Globally, HL is the second leading cause of years lived with disability, making HL an important issue for society as well. Although most apparent in later stages of life, it is probable that hearing acuity gradually declines with age and should therefore be studied at young ages as well. The prevalence of low-frequency or high-frequency HL in at least 1 ear (≥16 dB HL) was 14.9% among children aged 12 to 19 years in the cross-sectional National Health and Nutrition Examination Survey III (NHANES III) in 1988 to 1994. The prevalence rose to 19.5% in NHANES 2005-2006 and the prevalence of bilateral HL (≥16 dB HL) was 5.5%. A cross-sectional cluster sample survey of 6240 7- to 11-year-old Australian children published in 2010 showed 0.9% of the children having bilateral slight to mild sensorineural HL (SNHL) of 16 to 40 dB HL in low and high frequencies. There is a large spread in prevalence among the published literature, which consists mostly of cross-sectional studies. The prospective cohort study Avon Longitudinal Study of Parents and Children (ALSPAC) found a prevalence of mild and high-frequency bilateral HL of 0.5% at the age of 11 years.
To determine whether an increase of the prevalence of acquired HL in children is present, and if so, which factors are associated, there is a need for more longitudinal population-based studies with accurate outcome measures and covering numerous potential risk factors. Previous cross-sectional studies have observed associations between a history of otitis media and permanent HL among young adults. Other associations have been found for sex, socioeconomic status, and the use of personal music players (PMPs). A recent study among 9-year-olds showed that most seldom or never listen to music with headphones. Exposure to loud music, such as through PMPs and concert and party attendance, rises significantly around the age of 11 to 13 years.
Our purpose is to perform a reliable longitudinal hearing evaluation, with repeated measurements, that has the ability to study associations with the development of permanent HL. To do this accurately, it is necessary to determine the baseline situation. With the current study, we aim to assess the prevalence of SNHL among 9- to 11-year-old children in a large cohort study, preceding the exposure to risk factors for acquired HL, such as smoking, alcohol use, and exposure to loud music. Furthermore, we aimed to examine potential associations with purported risk factors of early childhood, predominantly from socioeconomic background and medical history.
Methods
Design and Sample
The current study was performed as part of the Generation R Study, a longitudinal cohort study that enrolled nearly 10 000 children born in Rotterdam, the Netherlands. Pregnant women with an expected delivery date between April 2002 and January 2006 were eligible for participation. The children born from these pregnancies form a prenatally included birth cohort that will be followed at least until young adulthood. The study is designed to identify early environmental and (epi)genetic causes and causal pathways leading to normal and abnormal growth, development, and health during fetal life, childhood, and adulthood. The research aims, follow-up rates, and measurements are described in more detail by Jaddoe et al. All children who were not withdrawn or lost to follow-up from the cohort at the start of the examination phase at the age of 9 to 11 years were eligible for participation, resulting in the invitation of 8548 children (eFigure 1 in the Supplement). Participants were invited to the research center to undergo various physical examinations and measurements and received extensive questionnaires on medical history, family history, environmental factors, and lifestyle. A total of 5862 children underwent testing at the research center (68.6% of those invited). These children did not differ in year of birth from the children who did not participate or who participated solely by questionnaires (eTable 1 in the Supplement). The participating children more often had higher educated mothers (59.3% [2953 children] vs 46.0% [586 children]; absolute difference of 13.3% [95% CI, 10.3%-16.4%]), were more often white (68.2% [3898 children] vs 52.6% [1777 children]; absolute difference of 15.6% [95% CI 13.6%-17.7%]), and had more equal boy-girl distribution (49.7% [2913] boys among participating children vs 52.1 [2024] boys among nonparticipating children; absolute difference of 2.4% [95% CI, 0.37%-4.42%]) than children who did not visit the research center. Audiometric measurements included pure-tone audiometry and tympanometry. Of the 5862 children visiting the research center, 5434 (92.7%) participated in pure-tone audiometry. Data were collected between April 2012 and October 2015.
Oral and written informed consent of the parents was collected for all measurements. Participants received a small incentive for participation (a simple backpack), but there was no financial compensation. The general design, all research aims, and the specific measurements within the Generation R Study have been approved by the Medical Ethics Committee of the Erasmus Medical Center, Rotterdam.
Pure-Tone Audiometry
Hearing levels were obtained in a sound-proofed booth that met the maximum permissible ambient sound pressure levels of ISO standard 8253-1. In all participants, hearing was measured with a clinical audiometer (Decos audiology workstation; version 210.2.6 with AudioNigma interface) and TDH-39P earphones with MX-41/AR ear cushions. Calibration met the reference equivalent sound pressure levels specified by ISO standard 389-1. The calibration of all equipment was checked every 6 months. Pure-tone air-conduction thresholds were obtained at frequencies 0.5, 1, 2, 3, 4, 6, and 8 kHz by dedicated research assistants who were trained by a member of the Speech and Hearing Center. All thresholds were measured according to the shortened ascending method based on ISO standard 8253-1, which means that thresholds were defined by the intensity level at which the tone was heard in 2 out of 3 ascents, resulting in a true clinical audiogram. The right or left ear was alternately tested first, and the measurement variance was 5 dB HL. When no response could be obtained, even at maximum stimulation level, the threshold was set to 5 dB above the maximum stimulation level. Owing to time constraints within the tight schedule of the examination day, masking was not applied, and bone conduction thresholds were not measured.
The air-conduction pure-tone thresholds were averaged at low frequencies (0.5, 1, and 2 kHz) for a low-frequency pure-tone average (LPTA), and at high frequencies (3, 4, and 6 kHz) for a high-frequency pure-tone average (HPTA), as described by Niskar et al. Normal hearing was defined as both LPTA and HPTA 15 dB or less in both ears, regardless of tympanometry results. Increased hearing levels included slight (16-25 dB HL), mild (26-40 dB HL), moderate (41-55 dB HL), moderately severe (56-70 dB HL), severe (71-90 dB HL), or profound (≥91 dB HL), in accordance with the American Speech-Language-Hearing Association guidelines and in line with other prevalence studies.
Tympanometry
After air-conduction testing was completed, tympanometry (Interacoustics AT235h; stimulus frequency, 226 Hz) was performed for both ears, unless a contraindication, such as otorrhea or recent ear surgery, was present. Ear-canal volume, static compliance, middle ear pressure, and gradient were automatically calculated during the pressure sweep. Ears with an ear canal volume smaller than 0.3 mL were excluded to avoid ear canal collapse or occlusive cerumen influencing the results. Tympanograms were categorized as described by Jerger, assessing a value of at least 0.25 mL as normal compliance and a value between −100 and 100 daPa as normal middle ear pressure. Middle ear function was judged by the tympanograms to distinguish between conductive hearing loss (CHL) and SNHL in the absence of bone conduction threshold measurements. Any HL in combination with a type A tympanogram (suggesting normal middle ear function) was considered SNHL, since it is unlikely, although possible, that conductive hearing impairment would be present in ears with normal tympanograms. Loss of middle ear function, presented via type B and type C tympanograms, in combination with HL, was categorized as CHL, although mixed or underlying SNHL could not be excluded.
Demographic Covariates and Otitis Media
Demographic information of the participants and information on maternal education were collected via questionnaires at different time points, as part of the general study. Sex, age, race/ethnicity, gestational age at time of birth, and maternal education were selected to analyze their possible association with HL based on literature. Because of the large variety in races/ethnicity in the Generation R Study, race/ethnicity was grouped as white and nonwhite. Maternal education was used as a marker of socioeconomic status, categorized as lower (did not follow or finish higher education) or higher (finished higher education, namely, higher vocational education or university). A history of acute otitis media was determined based on parent-reported otitis media at the ages of 2 and 6 months and 1, 2, 3, 4, and 5 years. Otitis media was classified as having no history of otitis media, acute otitis media, or recurrent acute otitis media (eFigure 2 in the Supplement).
Statistical Analysis
Pure-tone averages (means [SDs]) were evaluated in the context of the accompanying tympanogram, and between-group differences were analyzed using independent samples t-tests. We calculated prevalence estimates and 95% CIs for unilateral and bilateral HL at low and high frequencies (LFHL and HFHL, respectively). Sensorineural hearing loss was the main interest in this study, and therefore only children with presumed SNHL and children with normal hearing were included in further analyses. Statistical analyses using independent samples t-tests compared the proportions of demographic and exposure variables between children with presumed SNHL and those with normal hearing. Univariable and multivariable logistic regression analyses were carried out to assess associations between the demographic and exposure variables (sex, age, gestational age at birth, maternal education, and otitis media) and the presence of presumed SNHL by calculating odds ratios (ORs) with 95% CIs. IBM SPSS Statistics software (version 21.0 for Windows) was used for data management and analyses, and an α = .05 was used for statistical significance.
Results
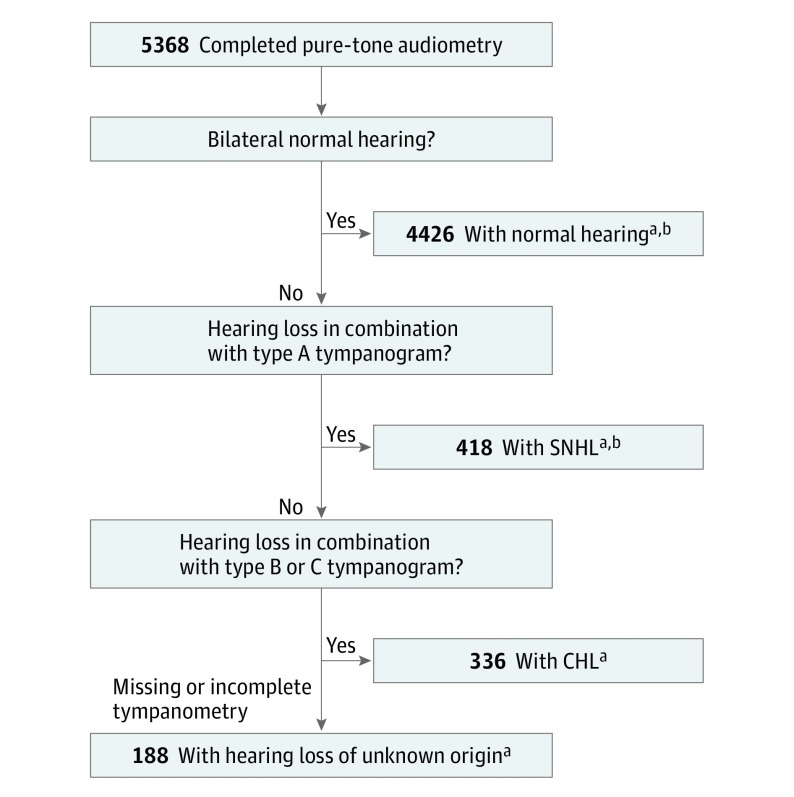
A total of 5368 participants with a mean age of 9 years 9 months (interquartile range, 9 years 7 months–9 years 11 months) completed audiometry and were included in the analyses. A total of 2720 (50.7%) were girls, and 67.6% (3627) were white. Children who completed audiometry at all frequencies in both ears were included in our analyses (n = 5368). Nearly 4800 of these children completed tympanometry (88.2%), of which 4344 right ears (80.9%) and 4298 left ears (80.1%) could be classified using the Jerger classification. The included 5368 children were grouped based on their audiological and tympanometry results, shown in Figure 1. The demographics of the children with normal hearing, SNHL, and CHL are presented in Table 1.
Figure 1. Flowchart of Classification in Groups Based on Audiological and Tympanometry Results.
CHL indicates conductive hearing loss; SNHL, sensorineural hearing loss.
aIncluded in prevalence estimate calculations.
bIncluded in regression analyses to assess associations between demographic and exposure variables and SNHL.
Table 1. Characteristics of the Participantsa.
| Characteristic | No. (%) | NH vs SNHL, Difference (95% CI)d | CHL, No. (%)e | NH vs CHL, Difference (95% CI)d | |
|---|---|---|---|---|---|
| NHb | SNHLc | ||||
| No. | 4426 | 418 | 336 | ||
| Age, median (IQR) | 9 y 9 mo (9 y 7 mo to 9 y 11 mo) | 9 y 9 mo (9 y 7mo to 9 y 11 mo) | 0.01 (−0.03 to 0.04) | 9 y 9 mo (9 y 7 mo to 9 y 11 mo) | 0.04 (−0.01 to 0.07) |
| Sex | |||||
| Male | 2199 (49.7) | 203 (48.6) | 1.1% (−3.9% to 6.1%) | 154 (45.8) | 3.9% (−1.7% to 9.4%) |
| Female | 2227 (50.3) | 215 (51.4) | NA | 182 (54.2) | NA |
| Gestational age at birth, mean (SD), wk | 39.8 (1.9) | 39.6 (2.0) | 0.15 (−0.04 to 0.34) | 39.8 (2.1) | 0.02 (−0.23 to 0.19) |
| Race/ethnicity | |||||
| White | 2996 (67.7) | 272 (65.1) | 2.6% (−2.2% to 7.4%) | 226 (67.3) | 0.1% (−5.1% to 5.3%) |
| Nonwhite | 1332 (30.1) | 136 (32.5) | NA | 100 (29.8) | NA |
| Unknown | 98 (2.2) | 10 (2.4) | NA | 10 (3.0) | NA |
| Maternal education | |||||
| Higher | 2351 (51.8) | 183 (43.8) | 7.0% (1.5% to 12.5%) | 174 (51.8) | 1.0% (−4.8% to 6.8%) |
| Lower | 1501 (34.3) | 161 (38.5) | NA | 120 (35.7) | NA |
| Unknown | 574 (13.9) | 74 (17.7) | NA | 42 (12.5) | NA |
| History of otitis mediaf | |||||
| No | 1827 (41.3) | 144 (34.4) | 6.4% (1.3% to 11.5%) | 96 (28.6) | 12.4% (7.0% to 17.8%) |
| AOM | 1902 (43.0) | 174 (41.6) | NA | 162 (48.2) | NA |
| rAOM | 444 (10.0) | 67 (16.0) | NA | 48 (14.3) | NA |
| Unknown | 253 (5.7) | 33 (7.9) | NA | 30 (8.9) | NA |
Abbreviations: AOM, acute otitis media; CHL, conductive hearing loss; HPTA, high-frequency pure-tone average; HL, hearing level; IQR, interquartile range; LPTA, low-frequency pure-tone average; NA, not applicable; NH, normal hearing; rAOM, recurrent acute otitis media; SNHL, sensorineural hearing loss.
The participants with hearing loss of unknown origin, because of incomplete or missing tympanometry (n = 188) are not shown.
Hearing thresholds of 15 dB HL or less at both LPTA and HPTA in both ears.
Children with hearing thresholds of 16 dB HL or higher at LPTA or HPTA in either ear with a normal tympanogram, indicating SNHL.
Differences were calculated based on participants with complete data regarding the variable.
Children with hearing thresholds ≥16 dB HL at LPTA or HPTA in either ear with a type B or type C tympanogram, indicating CHL.
Based on parent reports up to the age of 5 years.
Audiological Results
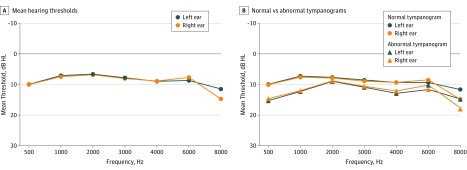
The mean (SD) LPTA of the total cohort was 8.10 (5.60) dB HL for right ears and 7.91 (5.78) dB HL for left ears (Figure 2A, and eTable 2 in the Supplement). The mean HPTAs were 8.22 (6.16) dB HL and 8.51 (6.18) dB HL for right and left ears, respectively. Tympanograms were most often of type A (3289 [75.7%] for right ears and 3301 [76.8%] for left ears), and 66.6% of the participants had bilateral type A tympanograms. Of the total studied cohort, 4426 children (82.5%) had normal hearing. Presumed SNHL (≥16 dB HL with a type A tympanogram) in at least 1 ear was present in 418 children (7.8%) at LPTA and/or HPTA, namely 7.7% (203) in boys and 7.9% (215) in girls. In 47 of these children (0.9% of the cohort) HL was of mild degree or worse (≥26 dB HL). More specifically, 33 children (0.6% of the cohort) had HL in the mild category (26-40 dB HL), 7 children (0.1%) had moderate SNHL, 5 children (0.1%) had moderately severe SNHL, and 2 children (0.0%) had profound SNHL. Sensorineural hearing loss was unilateral in 350 children (6.5%), and 68 children (1.3% of the cohort) had bilateral HL of at least 16 dB HL. In 103 of those children (1.9% of the cohort), HL was present at low and high frequencies in the same ear (all unilateral).
Figure 2. Mean Hearing Thresholds of the Right and Left Ears.
A, Total study cohort of 5368 participants. B, Normal vs abnormal tympanograms.
Within ears with normal middle ear function (type A tympanograms), the average LPTA was 7.09 (4.79) dB HL in right ears and 6.84 (4.92) dB HL in left ears (eTable 2 in the Supplement). Low-frequency hearing loss was present in 3.9% of the right ears and 3.7% of the left ears. Table 2 shows hearing levels of children with bilateral normal tympanograms. High-frequency hearing loss was 7.49 (5.80) dB HL for right ears and 7.67 (5.47) dB HL for left ears, with a prevalence of 5.4% HFHL in right ears and 5.3% HFHL in left ears (Table 2).
Table 2. Hearing Levels Based on Low-Frequency Pure-Tone Average in 2631 Children With Bilateral Normal Tympanogramsa.
| Left Ear | Right Ear, % (95% CI) | ||
|---|---|---|---|
| Normal (≤15 dB HL) | Slight (16-25 dB HL) | ≥Mild (≥26 dB HL) | |
| Low Frequencyb | |||
| Normal (≤15 dB HL) | 94.4 (93.5-95.3) | 2.2 (1.6-2.8) | 0.1 (0.0-0.3) |
| Slight (16-25 dB HL) | 2.0 (1.5-2.6) | 1.0 (0.6-1.4) | 0.0 (0.0-0.1) |
| ≥Mild (≥26 dB HL) | 0.0 (0.0-0.1) | 0.0 (0.0-0.1) | 0.2 (0.0-0.3) |
| High Frequencyc | |||
| Normal (≤15 dB HL) | 92.5 (91.5-93.5) | 2.7 (2.0-3.3) | 0.2 (0.0-0.4) |
| Slight (16–25 dB HL) | 2.8 (2.2-3.5) | 1.1 (0.7-1.5) | 0.2 (0.1-0.4) |
| ≥Mild (≥26 dB HL) | 0.2 (0.0-0.3) | 0.2 (0.1-0.4) | 0.2 (0.0-0.3) |
Abbreviation: HL, hearing level.
Hearing levels presented as prevalence (95% CI) per category.
Low-frequency pure-tone average = (500 + 1000 + 2000) Hz/3.
High-frequency pure-tone average = (3000 + 4000 + 6000) Hz/3.
Abnormal tympanograms were found in 1055 (24.3%) of the right ears and 997 (23.2%) of the left ears. Type B and type C tympanograms were bilaterally present in, respectively, 139 (3.6%) and 287 (7.3%) children. Children with tympanograms of types B and C had worse average hearing thresholds than children with type A tympanograms (mean difference in right ear, 2.9 dB HL [95% CI, 2.5-3.3]; mean difference in left ear, 3.5 dB HL [95% CI, 3.0-3.9]; eTable 2 in the Supplement). Increased hearing thresholds were more prevalent among these ears (153 [29.8%] at low frequencies and 149 [29.0%] at high frequencies in either ear), resulting in 336 children (6.3% of the cohort) fulfilling the criteria of CHL.
Purported Risk Factors in Young Children (Ages 9-11 Years) With SNHL
There were no significant differences in sex (1.1% [95% CI, −3.9% to 6.1%]), age (0.01 [95% CI, −0.03 to 0.04]), gestational age at birth (0.15 weeks [95% CI, −0.04 to 0.34 weeks]), and race/ethnicity (2.6% [95% CI, −2.2% to 7.4%]) between children with SNHL and those with normal hearing (Table 1). Higher maternal education was less prevalent in the group with SNHL than in the group with normal hearing (mean difference, 7.0%; 95% CI, 1.5%-12.5%). A history of otitis media was present more often in children with SNHL than in children with normal hearing (mean difference, 6.4%; 95% CI, 1.3%-11.5%). Associations between several available risk indicators and presumed SNHL were statistically evaluated. Sex, age, gestational age at birth, and race/ethnicity were not associated. Recurrent acute otitis media was significantly associated with presumed SNHL (OR, 1.9; 95% CI, 1.4-2.6), whereas merely a history of otitis media (without being of recurrent origin) was not (OR, 1.2; 95% CI, 0.9-1.5). Another significant association was found between the educational status of the mother and the presence of presumed SNHL, with OR, 1.3 (95% CI, 1.1-1.7) for maternal education of a lower level. These positive associations remained present in a multivariable analysis, while adjusting for sex, age, gestational age, and race/ethnicity, indicating independence of the associations (OR, 1.4 [95% CI, 1.1-1.7], for lower maternal education, and OR, 2.0 [95% CI, 1.5-2.8], for recurrent otitis media).
Discussion
To our knowledge, this study is the first large population-based study to examine hearing acuity among school-aged children born in the Netherlands. It demonstrates that 7.8% of the children were estimated to have SNHL in low or high frequencies in at least 1 ear, which was similar in boys and girls. Most of the HLs were unilateral and of slight degree (16-25 dB HL). The prevalence of mild or more severe HL of our study was 0.9%, and bilateral SNHL was present in 1.3%. The latter is slightly higher than the prevalence of bilateral SNHL that was reported in previous large studies among children of similar age, namely 0.5% and 0.9%. Altogether, 17.5% of the cohort had hearing thresholds exceeding 15 dB HL (7.8% SNHL, 6.3% CHL, and 3.5% HL of unknown origin). This is roughly comparable with the prevalence of 16.0% HL among 12- to 13-year-old children in the NHANES studies, which did not distinguish between SNHL and CHL.
To achieve optimal comparability between our study and most of the previously published literature, we chose to use the most frequently used HL categorization of low and high frequencies as described by Niskar et al. This criterion includes even small HLs in either low- or high-frequency regions. Most of the HLs in the current study were of slight degree and are probably subclinical. Although the clinical relevance of these slight losses might be uncertain, they are indicative of mild cochlear damage, which in turn might be relevant regarding the young age of the participants and their future. Moreover, cochlear synaptopathy (hidden HL) does not, or does slightly, elevate hearing thresholds in pure-tone audiometry, while significantly contributing to hearing-in-noise difficulties and tinnitus. Therefore, even though HL is only of slight degree in pure-tone audiometry, possible effects should not be underestimated.
We found that recurrent acute otitis media was associated with the presumed SNHL, which is in line with some, but not all, previous studies among children and young adults. Limited evidence exists that inflammatory mediators present in otitis media can cause changes in the auditory structures, including causing cochlear damage and SNHL over and above CHL. Maternal education was associated with worse hearing acuity as well, showing that increased odds were present for lower maternal educational status. This could be related to the more general socioeconomic status, which is partly determined by educational level among other factors. Also, maternal education has been found to be a prognostic indicator for a child’s success with a cochlear implant. Together with associations between socioeconomic variables and HL that have been found before, maternal education and socioeconomic status seem important factors in relation to hearing. This could be a topic of further studies, while including other household variables and preferably modifiable factors in search of possible targets for prevention.
The current study was conducted as part of the Generation R Study, which, besides having a large sample size, has the benefits of being closely related to clinical practice and comprises a large number of determinants. Testing was performed by dedicated personnel with a small variance, and accordingly we do not expect this variance to explain the presented results. Although this is a longitudinal study, the audiometric assessments described herein were cross-sectional. As a result, we cannot be certain whether the found HLs were acquired or congenital. Most of the included children probably participated in the universal newborn hearing screening that is available in the Netherlands, but these measurements are less sensitive for slight HL compared with pure-tone audiometry and are unfortunately not available within the Generation R Study. Hearing evaluation within the Generation R Study will be repeatedly measured in future assessments, and longitudinal data will become available in the future. This will provide information on the permanence and the course of the detected hearing losses. In addition, it gives the possibility to assess the test-retest variability and reliability, for instance, due to attentive factors within the children.
Limitations
Our audiometry was limited by the lack of bone-conduction hearing thresholds owing to strict time constraints in conducting audiological measurements among several other assessments within the Generation R Study. Therefore, tympanometry served as a measure to estimate middle ear function to differentiate between CHL and SNHL. Our main interest was to focus on permanent HL, without possible interference of middle ear pathology. To achieve this, we chose to include only those children with type A tympanograms in the main analyses on presumed SNHL and to accept the decrease in sample size. Also, there was a small but significant difference between the participants of the study and those who chose not to participate, and maternal educational level was missing in a noteworthy proportion of the participants. This might influence the generalizability of the results. However, because an association was found between lower maternal education and presumed SNHL, and those who participated in the study seemed higher educated than those who did not, the current presented prevalence of SNHL is probably rather an underestimation than an overestimation.
Sensorineural hearing loss, and likely acquired SNHL, is not only a problem of the older adolescents, but it also occurs at younger ages as well. It is possible that the acquired HL that becomes apparent at older age actually starts to develop at a younger age. More important, identifying HL at an early stage could prevent communicational and educational difficulties. The association of recurrent acute otitis media with presumed SNHL suggests a possible effect thereof. However, it would be too strong of a claim within this study to state that acquired HL was caused by ear infections earlier in life. Further studies must evaluate the association between otitis media and HL, while taking other confounding factors into account. More detailed information on possible risk factors is required, which would ideally be collected in an objective and not a self- or parent-reported manner.
Conclusions
In this cross-sectional assessment of a population-based prospective birth cohort study, 7.8% of children were estimated to have low- or high-frequency SNHL in either ear, with a prevalence of 0.9% of mild or more severe SNHL (≥26 dB HL). A history of recurrent acute otitis media and lower maternal educational status were associated with the presence of presumed SNHL.
eFigure 1. Flow chart of the Generation R Study participation
eFigure 2. Flow chart of questionnaire data used to define otitis media phenotypes
eTable 1. Characteristics and comparison of study participants vs. non-participants
eTable 2. Hearing thresholds for the total study cohort, and for ears with normal and abnormal tympanogram
References
- 1.Arlinger S. Negative consequences of uncorrected hearing loss: a review. Int J Audiol. 2003;42(suppl 2):2S17-20. [PubMed] [Google Scholar]
- 2.Bess FH, Dodd-Murphy J, Parker RA. Children with minimal sensorineural hearing loss: prevalence, educational performance, and functional status. Ear Hear. 1998;19(5):339-354. [DOI] [PubMed] [Google Scholar]
- 3.Lieu JE. Speech-language and educational consequences of unilateral hearing loss in children. Arch Otolaryngol Head Neck Surg. 2004;130(5):524-530. [DOI] [PubMed] [Google Scholar]
- 4.Mathers C, Smith A, Concha M Global burden of hearing loss in the year 2000. 2003:1-30. http://www.who.int/healthinfo/statistics/bod_hearingloss.pdf. Accessed August 18, 2017.
- 5.Rigters SC, Metselaar M, Wieringa MH, Baatenburg de Jong RJ, Hofman A, Goedegebure A. Contributing determinants to hearing loss in elderly men and women: results from the population-based Rotterdam Study. Audiol Neurootol. 2016;21(suppl 1):10-15. [DOI] [PubMed] [Google Scholar]
- 6.Agrawal Y, Platz EA, Niparko JK. Prevalence of hearing loss and differences by demographic characteristics among US adults: data from the National Health and Nutrition Examination Survey, 1999-2004. Arch Intern Med. 2008;168(14):1522-1530. [DOI] [PubMed] [Google Scholar]
- 7.Shargorodsky J, Curhan SG, Curhan GC, Eavey R. Change in prevalence of hearing loss in US adolescents. JAMA. 2010;304(7):772-778. [DOI] [PubMed] [Google Scholar]
- 8.Cone BK, Wake M, Tobin S, Poulakis Z, Rickards FW. Slight-mild sensorineural hearing loss in children: audiometric, clinical, and risk factor profiles. Ear Hear. 2010;31(2):202-212. [DOI] [PubMed] [Google Scholar]
- 9.Jiang W, Zhao F, Guderley N, Manchaiah V. Daily music exposure dose and hearing problems using personal listening devices in adolescents and young adults: a systematic review. Int J Audiol. 2016;55(4):197-205. [DOI] [PubMed] [Google Scholar]
- 10.le Clercq CM, van Ingen G, Ruytjens L, van der Schroeff MP. Music-induced hearing loss in children, adolescents, and young adults: a systematic review and meta-analysis. Otol Neurotol. 2016;37(9):1208-1216. [DOI] [PubMed] [Google Scholar]
- 11.Hall AJ, Midgley E, Steer C, Humphriss R. Prevalence and risk factors for mild and high-frequency bilateral sensorineural hearing loss at age 11 years old: a UK prospective cohort study. Int J Audiol. 2011;50(11):809-814. [DOI] [PubMed] [Google Scholar]
- 12.de Beer BA, Graamans K, Snik AF, Ingels K, Zielhuis GA. Hearing deficits in young adults who had a history of otitis media in childhood: use of personal stereos had no effect on hearing. Pediatrics. 2003;111(4, pt 1):e304-e308. [DOI] [PubMed] [Google Scholar]
- 13.Job A, Raynal M, Tricoire A, Signoret J, Rondet P. Hearing status of French youth aged from 18 to 24 years in 1997: a cross-sectional epidemiological study in the selection centres of the army in Vincennes and Lyon. Rev Epidemiol Sante Publique. 2000;48(3):227-237. [PubMed] [Google Scholar]
- 14.Hunter LL, Margolis RH, Rykken JR, Le CT, Daly KA, Giebink GS. High frequency hearing loss associated with otitis media. Ear Hear. 1996;17(1):1-11. [DOI] [PubMed] [Google Scholar]
- 15.Båsjö S, Möller C, Widén S, Jutengren G, Kähäri K. Hearing thresholds, tinnitus, and headphone listening habits in nine-year-old children. Int J Audiol. 2016;55(10):587-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knobel KA, Lima MC. Influences of age, gender, and parents’ educational level in knowledge, behavior and preferences regarding noise, from childhood to adolescence. Noise Health. 2014;16(73):350-360. [DOI] [PubMed] [Google Scholar]
- 17.Warner-Czyz AD, Cain S. Age and gender differences in children and adolescents’ attitudes toward noise. Int J Audiol. 2016;55(2):83-92. [DOI] [PubMed] [Google Scholar]
- 18.Hofman A, Jaddoe VWV, Mackenbach JP, et al. . Growth, development and health from early fetal life until young adulthood: the Generation R Study. Paediatr Perinat Epidemiol. 2004;18(1):61-72. [DOI] [PubMed] [Google Scholar]
- 19.Kooijman MN, Kruithof CJ, van Duijn CM, et al. . The Generation R Study: design and cohort update 2017. Eur J Epidemiol. 2016;31(12):1243-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaddoe VWV, van Duijn CM, Franco OH, et al. . The Generation R Study: design and cohort update 2012. Eur J Epidemiol. 2012;27(9):739-756. [DOI] [PubMed] [Google Scholar]
- 21.Niskar AS, Kieszak SM, Holmes A, Esteban E, Rubin C, Brody DJ. Prevalence of hearing loss among children 6 to 19 years of age: the Third National Health and Nutrition Examination Survey. JAMA. 1998;279(14):1071-1075. [DOI] [PubMed] [Google Scholar]
- 22.Clark JG. Uses and abuses of hearing loss classification. ASHA. 1981;23(7):493-500. [PubMed] [Google Scholar]
- 23.Jerger J. Clinical experience with impedance audiometry. Arch Otolaryngol. 1970;92(4):311-324. [DOI] [PubMed] [Google Scholar]
- 24.Kostić M, Ribarić Jankes K, Trotić R, Ries M, Ledić B, Bedeković V. Clinical and audiological findings in children with acute otitis media. Acta Otolaryngol. 2015;135(7):645-650. [DOI] [PubMed] [Google Scholar]
- 25.Wake M, Tobin S, Cone-Wesson B, et al. . Slight/mild sensorineural hearing loss in children. Pediatrics. 2006;118(5):1842-1851. [DOI] [PubMed] [Google Scholar]
- 26.Khairi Md Daud M, Noor RM, Rahman NA, Sidek DS, Mohamad A. The effect of mild hearing loss on academic performance in primary school children. Int J Pediatr Otorhinolaryngol. 2010;74(1):67-70. [DOI] [PubMed] [Google Scholar]
- 27.Liberman MC, Kujawa SG. Cochlear synaptopathy in acquired sensorineural hearing loss: manifestations and mechanisms. Hear Res. 2017;S0378-5955(16)30250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mittal R, Lisi CV, Gerring R, et al. . Current concepts in the pathogenesis and treatment of chronic suppurative otitis media. J Med Microbiol. 2015;64(10):1103-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braveman PA, Cubbin C, Egerter S, et al. . Socioeconomic status in health research: one size does not fit all. JAMA. 2005;294(22):2879-2888. [DOI] [PubMed] [Google Scholar]
- 30.Polat B, Başaran B, Kara HC, Ataş A, Süoğlu Y. The impact of social and demographic features on comprehensive receptive and expressive performance in cochlear implant patients. Kulak Burun Bogaz Ihtis Derg. 2013;23(2):90-95. [DOI] [PubMed] [Google Scholar]
- 31.Cupples L, Ching TY, Button L, et al. . Language and speech outcomes of children with hearing loss and additional disabilities: identifying the variables that influence performance at five years of age [published online September 14, 2017]. Int J Audiol. doi: 10.1080/14992027.2016.1228127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Ploeg CP, Uilenburg NN, Kauffman-de Boer MA, Oudesluys-Murphy AM, Verkerk PH. Newborn hearing screening in youth health care in the Netherlands: national results of implementation and follow-up. Int J Audiol. 2012;51(8):584-590. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flow chart of the Generation R Study participation
eFigure 2. Flow chart of questionnaire data used to define otitis media phenotypes
eTable 1. Characteristics and comparison of study participants vs. non-participants
eTable 2. Hearing thresholds for the total study cohort, and for ears with normal and abnormal tympanogram