Abstract
Importance
The function of rods and cones in children born extremely preterm has not yet been fully investigated.
Objective
To compare retinal function via full-field electroretinographic (ffERG) recordings in 6.5-year-old children born extremely preterm with children born at term.
Design, Setting, and Participants
A subcohort study was conducted from July 1, 2010, to January 15, 2014, of the national Extremely Preterm Infants in Sweden Study, including preterm children (<27 weeks’ gestational age) and children born at term, at 6.5 years of age and living in the Uppsala health care region in Sweden. Full-field electroretinography was performed binocularly, using DTL electrodes and electroretinographic (ERG) protocols with flash strengths of 0.009, 0.17, 3.0, and 12.0 candelas (cd)/s/m2, together with 30-Hz flicker and 3.0 cd/s/m2 single-cone flash.
Main Outcomes and Measures
The ffERG recordings were analyzed, and their associations with gestational age and retinopathy of prematurity were examined.
Results
Adequate ffERG recordings were obtained from 52 preterm children (19 girls and 33 boys; mean [SD] age at examination, 6.6 [0.1] years) and 45 children born at term (22 girls and 23 boys; mean [SD] age at examination, 6.6 [0.1] years). Lower amplitudes of the combined rod and cone responses (the a-wave of the dark-adapted ERG protocol of 3.0 cd/s/m2: mean difference, –48.9 μV [95% CI, –80.0 to –17.9 μV]; P=.003; the a-wave of the dark-adapted ERG protocol of 12.0 cd/s/m2: mean difference, –55.7 μV [95% CI, –92.5 to –18.8 μV]; P = .004), as well as of the isolated cone response (30-Hz flicker ERG: mean difference, –12.1 μV [95% CI, –22.5 to –1.6 μV]; P = .03), were found in the preterm group in comparison with the group born at term. The implicit time of the combined rod and cone responses (the a-wave of the dark-adapted ERG protocol of 12.0 cd/s/m2) was longer (mean difference, 1.2 milliseconds [95% CI, 0.3-2.0 milliseconds]; P = .01) in the preterm group, as were the isolated cone responses (30-Hz flicker ERG: mean difference, 1.2 milliseconds [95% CI, 0.5-1.8 milliseconds]; P < .001), than in the group born at term. No association was found between the ffERG recordings and gestational age or retinopathy of prematurity in the preterm group.
Conclusions and Relevance
Both rod function and cone function were reduced in children born extremely preterm when compared with children born at term. There was no association with retinopathy of prematurity in the preterm group, which suggests that being born extremely preterm may be one of the main reasons for a general retinal dysfunction.
This subcohort study of the Extremely Preterm Infants in Sweden Study compares retinal function via full-field electroretinography recordings in 6.5-year-old children born extremely preterm with that of children born at term.
Key Points
Question
How does extreme preterm birth affect retinal function in children who are 6.5 years of age?
Findings
In this subcohort study of the Extremely Preterm Infants in Sweden Study, children 6.5 years of age who were born extremely preterm (<27 weeks’ gestational age) had reduced function of both rods and cones when compared with children born at term, according to full-field electroretinography recordings.
Meaning
A reduced function of both rods and cones is reported in children born extremely preterm, compared with children born at term, which may contribute to the various visual problems of this new population as they age.
Introduction
Advanced neonatal care enables the survival of very preterm children with low gestational age (GA) and has resulted in a new group of children growing up today. The national population-based Extremely Preterm Infants in Sweden Study (EXPRESS) investigates the mortality and long-term morbidity of children born extremely preterm (born before GA of 27 weeks during 2004-2007). In the neonatal period, severe retinopathy of prematurity (ROP) was found in 35% of the infants, 20% of whom were treated for ROP. Among the infants surviving to 1 year of age, 55% had major neonatal morbidities such as ROP, periventricular leukomalacia, severe intraventricular hemorrhage, and bronchopulmonary dysplasia. At a recent 6.5-year follow-up, ophthalmologic problems such as low visual acuity (VA), high refractive errors, and strabismus were found in 38% of the children who were born extremely preterm.
The retina is still histologically immature at birth, and the development process continues for several years. At around 4 years of age, the fovea shows histologic signs of maturation but has not reached adult cone density. The function of the developing retinal maturation is reflected by the full-field electroretinographic (ffERG) recordings showing larger amplitudes and shorter implicit times with increasing age. In very preterm infants (born <32 weeks’ GA), during the neonatal period, the macular photoreceptors show signs of underdevelopment compared with those of children born at term, when examined with spectral-domain optical coherence tomography. Central macular thickness has recently been reported as thicker in a subcohort of 6.5-year-old children born extremely preterm, participating in the EXPRESS, in comparison with children born at term. Furthermore, retinal function is reduced in preterm infants, with smaller electroretinographic (ERG) amplitudes compared with infants born at term.
Several ERG studies have investigated the retinal function in preterm infants and children, but, to our knowledge, no study has examined a group consisting only of children born extremely preterm. The aim of our study was to evaluate the combined rod and cone function and isolated cone function assessed with ffERG in children born extremely preterm and to compare the recordings with children born at term. The second aim was to investigate the association between ffERG recordings in the preterm group and GA and ROP.
Methods
Study Participants
The original cohort consisted of 87 preterm children and 66 children born at term, all of whom were 6.5 years of age, living in the Uppsala health care region in Sweden, and participating in the EXPRESS. The children born at term were age matched with and born in the same region as the preterm children, and met the inclusion criteria of GA of 37 weeks or more and birth weight of 2500 g or more. The study was performed according to the Declaration of Helsinki, with approval from the Regional Ethics Boards of Lund University. The parents of the participating children provided prior written consent, and the children provided verbal consent.
Screening and Grading of ROP
The preterm children were screened for ROP in the neonatal period; ROP was graded according to the International Committee for the Classification of Retinopathy of Prematurity. Mild ROP was defined as stages 1 and 2, and severe ROP as stages 3 to 5. Indication for treatment followed the recommendations of the Early Treatment for Retinopathy of Prematurity study.
Intraventricular Hemorrhage and Periventricular Leukomalacia
All preterm children underwent cranial ultrasonography during the neonatal period. Intraventricular hemorrhage was graded according to Papile et al, and periventricular leukomalacia was diagnosed according to de Vries et al.
Eye Examination
At the 6.5-year follow-up, the children underwent monocular VA testing with habitual correction using logMAR Lea Hyvärinen symbol charts. An automated refractor measurement in cycloplegia (using eye drops of cyclopentolate hydrochloride, 0.85%, and phenylephrine hydrochloride, 1.5%) was performed, and the spherical equivalent (SE) was calculated. All examinations took place between July 1, 2010, and January 15, 2014, in Uppsala University Hospital.
ffERG Recordings
Full-field ERG was performed with the Espion Ganzfeld system (Diagnosys LCC). The recordings were performed in accordance with the recommendations of the International Society for Clinical Electrophysiology of Vision, except the light-adaption time, which was excluded to adapt the examination to children.
Full-field ERG was performed binocularly using DTL electrodes with previous installation of anesthetic eye drops (tetracaine hydrochloride, 1%). Reference electrodes were placed on the skin of the right and left zygomatic bones. A ground electrode was positioned on the back of 1 hand. Electrical impedance of less than 10 kΩ of all electrodes was required. Pupils were dilated with eye drops (cyclopentolate hydrochloride, 0.85%, and phenylephrine hydrochloride, 1.5%), and at least 8 mm of dilation was required before the recordings were made.
During the ffERG recordings, 6 protocols with different flash strengths from light-emitting diode light sources were used (Table 1). The light intensity of the flashes was measured in candela-seconds per square meter (cd/s/m2).
Table 1. Six Full-Field Electroretinographic Protocols.
| Flash Strength, cd/s/m2 | Nomenclature | Type of Photoreceptor Response |
|---|---|---|
| 0.009 | Dark-adapted 0.009 ERG | Rods |
| 0.17 | Dark-adapted 0.17 ERG | Rods and cones |
| 3.0 | Dark-adapted 3 ERG | Rods and cones |
| 12.0 | Dark-adapted 12 ERG | Rods and cones |
| 3.0 | 30-Hz flicker ERG | Cones |
| 3.0 | 3 Single-cone flash ERG | Cones |
Abbreviation: ERG, electroretinography.
Dark adaptation during 20 minutes was performed. The dark-adapted protocols were assessed with at least 6 recordings and the 30-Hz flicker and 3.0 single-cone flash ERG with at least 2 recordings, ensuring the responses were reproducible. Mean calculations of the 6 ffERG recordings of the dark-adapted protocols and the 2 recordings of the 30-Hz flicker and 3.0 single-cone flash ERG were performed, from which the final ffERG curve was established and from which amplitudes and the implicit times of the a-wave and the b-wave were measured. Cone stimulation with 30-Hz flicker and 3.0 single-cone flash ERG were performed with a background dome light of 34 cd/m2 and room light without previous light adaptation. The a-wave was measured and analyzed in the combined rod and cone protocols: 0.17, 3.0, and 12.0 ERG. An amplitude greater than 1 μV was required for inclusion. The b-wave was recorded and analyzed in all ERG protocols. The b-wave in 30-Hz flicker is referred to as the peak of the wave.
The ffERG signals were filtered through a band-pass filter of 0.3 to 300 Hz, and artifact rejection was used, excluding responses greater than 1000 µV. The child had to be seated in a relaxed position before the recordings were started. The examiner also encouraged the child and monitored the ERG responses throughout the assessment. Fixation and cooperation (detection of larger eye movements and/or blinks) were both monitored with an infrared camera in the Ganzfeld dome and were evaluated by the examiner during the recordings. The inclusion criteria for the ffERG recordings were adequate cooperation without a lot of large eye and body movements. Furthermore, ffERG recordings with present alternating-current disturbances were excluded.
Statistical Analysis
Statistical calculations were performed with SPSS, version 22 (IBM Corp), and R, version 3.2.3 (The R Foundation). Mean (SD) values and ranges were calculated for the continuous data. Linear mixed models were performed to analyze the ffERG recordings (all 6 protocols included) between the preterm and control groups, as well as within the preterm and control groups. The data were validated and considered acceptable before entering in the parametric regression model. In the linear mixed models, the eye (right and left eye) and group (preterm and control group) were the fixed factors, and the participants were the random factors. Initially, the preterm group was compared with the control group regarding the ffERG recordings, with adjustment for sex and refraction (SE). The results from the regression analysis were initially presented as the mean difference between the groups with 95% CIs. Thereafter, linear mixed models were performed within the preterm and control groups separately for the explanatory variables VA, SE, eye (right and left), and sex for each of the ffERG values. In the preterm group, the explanatory variables GA, birth weight, and ROP (present or not present) vs ffERG recordings were compared according to the linear mixed models. Further analyses within the preterm group were performed, dividing ROP into the following groups: no ROP, mild ROP, severe ROP, severe untreated ROP, and severe treated ROP. The results from the linear mixed models were presented with 95% CIs. P < .05 was considered statistically significant. There was no adjustment for multiplicity, so the interpretation of P values should be descriptive. The number of children included was not based on any formal sample size calculation, and thus the study was not powered to show statistical differences.
Results
Of the original EXPRESS cohort of children in the Uppsala health care region, 73 of 87 preterm children (84%) and 64 of 66 children born at term (97%) participated in the present study at the 6.5-year follow-up. Full-field ERG recordings (according to the inclusion criteria) were obtained in at least 1 eye in 52 preterm children (50 right eyes and 48 left eyes) and 45 children born at term (45 right eyes and 45 left eyes), who comprised the study population. Background data on this population and the mean VA and the mean SE are described in Table 2. Owing to missing data on the SE of 1 child in the control group, the results of 44 right and left eyes are presented in Table 2.
Table 2. Demographic Data During the Neonatal Period and at the 6.5-Year Follow-up.
| Characteristic | Preterm Group (n = 52) |
Control Group (n = 45) |
|---|---|---|
| Age at examination, mean (SD) [range], y | 6.6 (0.1) [6.3 to 6.7] | 6.6 (0.1) [6.3 to 6.7] |
| Sex, No. | ||
| Boys | 33 | 23 |
| Girls | 19 | 22 |
| GA, mean (SD) [range], wk | 25 (1.1) [22 to 26] | 40 (1.1) [37 to 41] |
| Week, No. | ||
| 22 | 1 | NA |
| 23 | 5 | NA |
| 24 | 10 | NA |
| 25 | 17 | NA |
| 26 | 19 | NA |
| BW, mean (SD) [range], g | 766 (175) [492 to 1218] | 3636 (427) [2740 to 4360] |
| ROP stages, No. | ||
| None | RE: 21; LE: 20 | NA |
| Mild | RE: 20; LE: 23 | NA |
| Severe | RE: 11; LE: 9 | NA |
| Treatment for ROP | RE: 6; LE: 5 | NA |
| logMAR VA, mean (SD) [range]a | RE: 0.13 (0.20) [0.00 to 0.70]; LE: 0.12 (0.31) [−0.10 to 2.00] | RE: 0.03 (0.06) [−0.10 to 0.20]; LE: 0.03 (0.07) [−0.10 to 0.20] |
| Snellen fraction VA, mean (range) | RE: 20/25 (20/100 to 20/20); LE: 20/26 (20/2000 to 20/16) | RE: 20/21 (20/32 to 20/16); LE: 20/21 (20/32 to 20/16) |
| Refraction, spherical equivalent, mean (SD) [range]b | RE: 1.59 (2.34) [−7.63 to 7.50]; LE: 1.18 (3.44) [−13.25 to 7.38] | RE: 1.39 (0.70) [−0.25 to 4.00]; LE: 1.35 (0.64) [−0.25 to 3.63] |
Abbreviations: BW, birth weight; GA, gestational age; LE, left eye; NA, not applicable; RE, right eye; ROP, retinopathy of prematurity; VA, visual acuity.
Preterm group: 50 right eyes and 48 left eyes; control group: 45 right eyes and 45 left eyes.
Preterm group: 50 right eyes and 48 left eyes; control group: 44 right eyes and 44 left eyes.
Only 4 children had severe intraventricular hemorrhage (grades 3-4), and none had a diagnosis of neonatal periventricular leukomalacia, so further statistical analyses of the association between these conditions and the ffERG responses could not be performed. The preterm children had lower VA (logMAR VA mean difference, 0.082 [95% CI, 0.017-0.147]; P = .02) and higher SE values (mean difference, 0.944 [95% CI, 0.297-1.592]; P = .005) than the children in the control group, according to the mixed model analyses. Three children in the preterm group had myopia less than –3 diopters (D), and 8 children had hypermetropia greater than 3.0 D. In the control group, no children had myopia and 3 children had hypermetropia greater than 3.0 D.
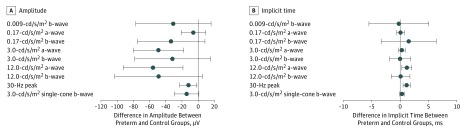
The mean (SD) values and ranges of the ffERG recordings (right and left eyes) in the preterm and control groups are presented in Table 3. The preterm children had lower amplitudes of the a-wave of the dark-adapted 3.0-cd/s/m2 protocol (mean difference, –48.9 μV [95% CI, –80.0 to –17.9 μV]; P = .003) and of the a-wave of the dark-adapted 12.0-cd/s/m2 protocol (mean difference, –55.7 μV [95% CI, –92.5 to –18.8 μV]; P = .004), as well as of the peak of 30-Hz flicker (mean difference, –12.1 μV [95% CI, –22.5 to –1.6 μV]; P = .03), when compared with the control group (Figure 1).
Table 3. Full-Field Electroretinographic Recordings in the Right and Left Eyes of the Preterm and Control Groups.
| ERG Protocol | Mean (SD) [range] | |||
|---|---|---|---|---|
| Preterm Group (n = 52) |
Control Group (n = 45) |
|||
| Right Eye | Left Eye | Right Eye | Left Eye | |
| Dark-adapted 0.009 ERG | ||||
| b-Wave, μV | 291.0 (108.2) [38.5 to 561.9] |
271.6 (121.5) [65.8 to 666.2] |
333.0 (124.7) [129.3 to 693.6] |
317.3 (111.5) [134.8 to 579.7] |
| b-Wave, ms | 93.1 (12.8) [71.0 to 130.0] |
94.5 (14.1) [74.5 to 160.0] |
93.0 (12.6) [63.5 to 127.5] |
94.3 (16.1) [71.0 to 139.0] |
| Dark-adapted 0.17 ERG | ||||
| a-Wave, μV | −94.4 (50.6) [−226.7 to −15.8] |
−78.5 (43.9) [−197.1 to −1.6] |
−96.2 (47.8) [−207.4 to −3.4] |
−98.3 (44.6) [−187.4 to −25.7] |
| a-Wave, ms | 25.3 (2.6) [13.0 to 30.5] |
25.2 (2.9) [17.0 to 30.5] |
25.3 (2.2) [17.5 to 30.5] |
25.3 (1.3) [19.0 to 27.5] |
| b-Wave, μV | 365.0 (116.7) [133.4 to 656.6] |
351.1 (108) [131.9 to 577.3] |
410.5 (111.5) [162.1 to 663.3] |
382.5 (111.7) [163.3 to 625.1] |
| b-Wave, ms | 71.5 (12.6) [43.5 to 85.0] |
73.1 (11.9) [44.0 to 85.0] |
69.9 (13.2) [43.5 to 85.0] |
69.9 (12.7) [43.0 to 85.0] |
| Dark-adapted 3 ERG | ||||
| a-Wave, μV | −220.2 (78.0) [−395.9 to −81.9] |
−201.8 (81.4) [−443.0 to 32.9] |
−256.9 (86.6) [−453.5 to −114.5] |
−263.7 (81.5) [−423.3 to −113.7] |
| a-Wave, ms | 15.3 (2.1) [12.5 to 28.0] |
15.2 (0.8) [11.0 to 16.5] |
15.0 (0.4) [14.0 to 16.0] |
15.0 (0.5) [13.5 to 16.0] |
| b-Wave, μV | 450.5 (130.1) [180.8 to 796.4] |
431.4 (118.1) [234.6 to 669.9] |
484.2 (128.2) [174.8 to 772.6] |
457.5 (120.5) [217.6 to 736.5] |
| b-Wave, ms | 52.6 (4.7) [44.5 to 60.0] |
53.1 (4.6) [44.0 to 60.0] |
53.1 (5.3) [43.5 to 60.0] |
53.3 (5.3) [43.5 to 60.0] |
| Dark-adapted 12 ERG | ||||
| a-Wave, μV | −252.5 (93.0) [−482.9 to −51.6] |
−232.2 (70.9) [−380.3 to −90.3] |
−300.8 (106.2) [−527.3 to −103.9] |
−295.4 (106.3) [−522.5 to −90.9] |
| a-Wave, ms | 13.6 (2.4) [10.5 to 27.0] |
13.8 (2.9) [10.0 to 25.5] |
12.6 (1.8) [10.0 to 19.0] |
12.5 (1.4) [10.0 to 19.5] |
| b-Wave, μV | 441.1 (135.2) [208.8 to 691.0] |
421.2 (110.5) [234.2 to 657.5] |
495.7 (156.0) [236.1 to 905.6] |
458.6 (143.0) [185.4 to 784.3] |
| b-Wave, ms | 49.8 (3.9) [40.0 to 55.0] |
50.3 (4.2) [40.5 to 55.0] |
49.7 (5.0) [39.5 to 55.0] |
50.2 (4.9) [38.5 to 55.0] |
| 30-Hz flicker ERG | ||||
| b-Wave, μV | 78.6 (30.4) [26.9 to 164.4] |
67.3 (22.5) [17.4 to 122.5] |
85.8 (23.2) [32.1 to 164.3] |
80.6 (22.1) [33.9 to 119.1] |
| b-Wave, ms | 28.3 (1.6) [25.5 to 32.0] |
28.6 (2.0) [24.0 to 32.0] |
27.5 (1.4) [25.0 to 31.0] |
27.3 (1.2) [25.0 to 30.5] |
| 3 Single-cone flash ERG | ||||
| b-Wave, μV | 111.2 (42.4) [34.9 to 228.1] |
106.2 (38.4) [36.9 to 193.2] |
126.7 (36.8) [43.3 to 221.0] |
122.1 (37.8) [48.8 to 212.0] |
| b-Wave, ms | 30.5 (1.3) [28.0 to 35.0] |
29.9 (1.5) [25.0 to 33.0] |
30.0 (1.1) [27.0 to 32.5] |
29.9 (1.0) [27.0 to 32.0] |
Abbreviation: ERG, electroretinography.
Figure 1. Mean Difference of the Full-Field Electroretinographic (ffERG) Recordings Between the Preterm and Control Groups.
Mean differences of each ffERG protocol. Dots indicate the mean difference and bars indicate the 95% CIs.
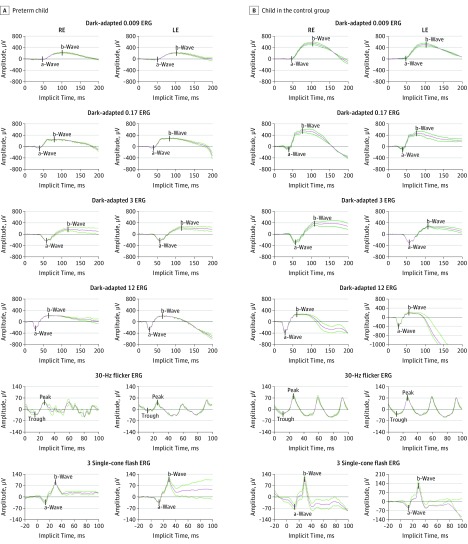
Implicit times of the a-wave of the dark-adapted 12.0-cd/s/m2 ERG protocol (mean difference, 1.2 milliseconds [95% CI, 0.3-2.0 milliseconds]; P = .01) and of the peak of 30-Hz flicker ERG (mean difference, 1.2 milliseconds [95% CI, 0.5-1.8]; P < .001) were longer in the preterm children than the control group (Figure 1). The ffERG recordings for the 6 protocols from a child born extremely preterm and a child born at term are shown in Figure 2. The mean value of all ffERG recordings is shown as the middle trace, whereas the 2 other traces show the reproducibility of the recordings.
Figure 2. The 6 Full-Field Electroretinographic (ffERG) Protocols of 2 Study Participants.
Amplitudes and implicit times of the a-waves and b-waves of the 6 ffERG protocols of a child born extremely preterm (A) and a child born at term (B). The mean value of the ffERG recordings is the middle trace. ERG indicates electroretinography; LE, left eye; and RE, right eye.
Within the preterm group, there was no association between GA, birth weight, previous ROP, stage of ROP, or treated ROP for any of the ffERG values. When investigating the association between the 6 ffERG protocols and sex, VA, and SE in the preterm and the control groups, there was only a small number of P values below .05 (preterm group: SE and amplitude of the a-wave of dark-adapted 0.17 ERG [P = .02], SE and implicit time of the a-wave of the dark-adapted 0.17 ERG [P = .011], VA and amplitude of the b-wave of 3 single-cone flash ERG [P = .02]; control group: sex and implicit time of the peak 30-Hz ficker (P = .004), SE and implicit time of the a-wave of the dark-adapted 12 ERG [P = .02], VA and implicit time of the a-wave of the dark-adapted 3 ERG [P = .003]), indicating no systemic associations. Furthermore, there was no difference in the ERG recordings between the right and left eyes in either the preterm group or the control group.
Discussion
This population-based study revealed both reduced rod and cone functions in 6.5-year-old children born extremely preterm when compared with age- and region-matched children born at term. The preterm children had lower amplitudes of the a-wave of combined rod-cone response (the dark-adapted 3.0 and 12.0-cd/s/m2 ERG protocols) as well as of the peak of the isolated cone response (30-Hz flicker ERG) compared with the control group. Furthermore, implicit times were longer in the a-wave of combined rod and cone response (the dark-adapted 12.0-cd/s/m2 ERG protocol) and in the peak of the cone response (30-Hz flicker ERG). The a-wave measures the response from the photoreceptors, and the b-wave reflects primarily the bipolar cells’ response. Consequently, our findings revealed a general dysfunction of the rod and cone photoreceptors, as well as that of the cone bipolar cells in children 6.5 years of age who were born extremely preterm.
The results of our study are in line with those of several ffERG studies, revealing a retinal dysfunction in infants and children who were born preterm. However, most of these studies reported a predominantly negative association with rod function, and Fulton et al have described that cone function was less affected by preterm birth than was rod response. In contrast, our study found that cone function was also reduced, which might be explained by the extreme prematurity of the study group, resulting in an early arrest of the development of cone photoreceptors and bipolar cells. The creation of cone photoreceptors starts before the formation of the rod photoreceptors. Cones have been identified as early as fetal week 8, the same time that the inner plexiform layer is created. When an extremely preterm infant is born, as early as fetal week 22, all retinal layers and cell types can be identified histologically in the parafovea. However, retinal vascularization is not complete at this stage, and important steps of further retinal development take place between fetal weeks 25 and 38. During this period, photoreceptors develop more distinct inner and outer segments, the avascular zone in the fovea is created, and the outer plexiform layer, as well as the retinal vasculature, extend to the retinal periphery. It can be assumed that the remaining abnormal retinal development in this cohort of children born extremely preterm explains both their reduced rod and cone functions at 6.5 years of age. Furthermore, oxygen exposure has been identified to affect the rod photoreceptor morphology and to reduce rod function in rat retinas. Hence, exposure to oxygen would also be a potential reason for an arrest of normal retinal development.
Recently published studies, in which multifocal ERG was performed and visual evoked potentials recorded, also describe a reduced electrophysiological response in children born preterm in comparison with children born at term. Those findings suggest that there is reduced macular function and affected visual pathways in preterm children.
Within the preterm group of our study, there was no association between the ffERG recordings and GA, stage of ROP, or laser-treated ROP. Several ERG studies of prematurely born infants and children reveal different conclusions regarding the association with GA, ROP, and treated ROP. However, the studies are not comparable because the GA range, the number of preterm children, the age at examination, and the ERG methods were all different. Åkerblom et al reported a positive correlation between the rod and cone response and increasing GA in preterm children with a GA of between 22 and 32 weeks at birth. Several articles have reported that previous ROP reduces the rod function and that there is an association between the severity of ROP and the dysfunction of rod photoreceptors. Furthermore, both rod dysfunction and cone dysfunction are seen in infants with treated ROP. Our study encompassed a homogenous group of children, born within a very narrow range of GA. Furthermore, a large number (35 of 52 [67%]) of the preterm children had ROP diagnosed during the neonatal period, and only 6 children had received laser therapy for ROP (1 eye also received cryotherapy). These facts might explain the lack of association between retinal dysfunction and GA or ROP in this cohort.
The preterm children in our study had lower VA and higher values of refraction (SE) than did those in the control group, which is in line with results of previous studies. High refractive errors and axial length affect the results of ERG in children and adults. When the preterm and control groups were compared regarding the ffERG recordings, an adjustment for refractive errors was performed, thus ensuring that the results were not affected by high values of SEs. An adjustment for sex was also performed because there was an unequal distribution of girls and boys in the cohort, and a sex-associated difference in ffERG measurements has been reported. In our study, however, there was no significant association between the ffERG values and sex in the preterm and control groups.
Limitations
A limitation of the study was the small number of children who had been treated for ROP. The conclusion regarding the effect of treated ROP is therefore limited.
Conclusions
This cohort of children 6.5 years of age who were born extremely preterm in a very narrow range of GA had a reduced function of both rods and cones. Although amplitudes and implicit times of the ffERG in premature infants increase and develop over time, there seems to be an arrest in the normal retinal development that appears to persist later into childhood, with reduced rod and cone functions. This finding accords with previously reported retinal structural and morphologic disturbances present after birth and during adolescence in the prematurely born child. Rod and cone disorders are known to result in night vision problems, loss of the visual field, reduced color vision, and photophobia. It can be speculated how reduced retinal function may be expressed in this new, vulnerable population of children as they age. To be able to provide correct habilitation, long-term ophthalmologic follow-up of these children is therefore necessary.
References
- 1.Fellman V, Hellström-Westas L, Norman M, et al. ; EXPRESS Group . One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA. 2009;301(21):2225-2233. [DOI] [PubMed] [Google Scholar]
- 2.EXPRESS Group Incidence of and risk factors for neonatal morbidity after active perinatal care: extremely preterm infants study in Sweden (EXPRESS). Acta Paediatr. 2010;99(7):978-992. [DOI] [PubMed] [Google Scholar]
- 3.Holmström GE, Källen K, Hellström A, et al. Ophthalmologic outcome at 30 months’ corrected age of a prospective Swedish cohort of children born before 27 weeks of gestation: the Extremely Preterm Infants in Sweden Study. JAMA Ophthalmol. 2014;132(2):182-189. [DOI] [PubMed] [Google Scholar]
- 4.Austeng D, Källen KB, Ewald UW, Jakobsson PG, Holmström GE. Incidence of retinopathy of prematurity in infants born before 27 weeks’ gestation in Sweden. Arch Ophthalmol. 2009;127(10):1315-1319. [DOI] [PubMed] [Google Scholar]
- 5.Hellgren KM, Tornqvist K, Jakobsson PG, et al. Ophthalmologic outcome of extremely preterm infants at 6.5 years of age: Extremely Preterm Infants in Sweden Study (EXPRESS) [published online March 24, 2016]. JAMA Ophthalmol. doi: 10.1001/jamaophthalmol.2016.0391 [DOI] [PubMed] [Google Scholar]
- 6.Hendrickson A, Drucker D. The development of parafoveal and mid-peripheral human retina. Behav Brain Res. 1992;49(1):21-31. [DOI] [PubMed] [Google Scholar]
- 7.Hendrickson A, Possin D, Vajzovic L, Toth CA. Histologic development of the human fovea from midgestation to maturity. Am J Ophthalmol. 2012;154(5):767-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birch DG, Anderson JL. Standardized full-field electroretinography: normal values and their variation with age. Arch Ophthalmol. 1992;110(11):1571-1576. [DOI] [PubMed] [Google Scholar]
- 9.Fulton AB, Hansen RM, Westall CA. Development of ERG responses: the ISCEV rod, maximal and cone responses in normal subjects. Doc Ophthalmol. 2003;107(3):235-241. [DOI] [PubMed] [Google Scholar]
- 10.Vajzovic L, Rothman AL, Tran-Viet D, Cabrera MT, Freedman SF, Toth CA. Delay in retinal photoreceptor development in very preterm compared to term infants. Invest Ophthalmol Vis Sci. 2015;56(2):908-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molnar A, Holmström G, Larsson E. Macular thickness assessed with spectral domain OCT in a population-based study of children: normative data, repeatability and reproducibility and comparison with time domain OCT. Acta Ophthalmol. 2015;93(5):470-475. [DOI] [PubMed] [Google Scholar]
- 12.Mactier H, Dexter JD, Hewett JE, Latham CB, Woodruff CW. The electroretinogram in preterm infants. J Pediatr. 1988;113(3):607-612. [DOI] [PubMed] [Google Scholar]
- 13.Zhou X, Huang X, Chen H, Zhao P. Comparison of electroretinogram between healthy preterm and term infants. Doc Ophthalmol. 2010;121(3):205-213. [DOI] [PubMed] [Google Scholar]
- 14.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 15.International Committee for the Classification of Retinopathy of Prematurity The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123(7):991-999. [DOI] [PubMed] [Google Scholar]
- 16.Early Treatment For Retinopathy Of Prematurity Cooperative Group Revised indications for the treatment of retinopathy of prematurity: results of the Early Treatment For Retinopathy Of Prematurity randomized trial. Arch Ophthalmol. 2003;121(12):1684-1694. [DOI] [PubMed] [Google Scholar]
- 17.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529-534. [DOI] [PubMed] [Google Scholar]
- 18.de Vries LS, Eken P, Dubowitz LM. The spectrum of leukomalacia using cranial ultrasound. Behav Brain Res. 1992;49(1):1-6. [DOI] [PubMed] [Google Scholar]
- 19.Hyvärinen L, Näsänen R, Laurinen P. New visual acuity test for pre-school children. Acta Ophthalmol (Copenh). 1980;58(4):507-511. [DOI] [PubMed] [Google Scholar]
- 20.McCulloch DL, Marmor MF, Brigell MG, et al. ISCEV standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol. 2015;130(1):1-12. [DOI] [PubMed] [Google Scholar]
- 21.Dawson WW, Trick GL, Litzkow CA. Improved electrode for electroretinography. Invest Ophthalmol Vis Sci. 1979;18(9):988-991. [PubMed] [Google Scholar]
- 22.Brown KT, Wiesel TN. Localization of origins of electroretinogram components by intraretinal recording in the intact cat eye. J Physiol. 1961;158:257-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown KT, Watanabe K. Isolation and identification of a receptor potential from the pure cone fovea of the monkey retina. Nature. 1962;193:958. [DOI] [PubMed] [Google Scholar]
- 24.Stockton RA, Slaughter MM. B-wave of the electroretinogram: a reflection of ON bipolar cell activity. J Gen Physiol. 1989;93(1):101-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robson JG, Frishman LJ. Dissecting the dark-adapted electroretinogram. Doc Ophthalmol. 1998-1999;95(3-4):187-215. [DOI] [PubMed] [Google Scholar]
- 26.Åkerblom H, Andréasson S, Larsson E, Holmström G. Photoreceptor function in school-aged children is affected by preterm birth. Transl Vis Sci Technol. 2014;3(6):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fulton AB, Hansen RM, Petersen RA, Vanderveen DK. The rod photoreceptors in retinopathy of prematurity: an electroretinographic study. Arch Ophthalmol. 2001;119(4):499-505. [DOI] [PubMed] [Google Scholar]
- 28.Harris ME, Moskowitz A, Fulton AB, Hansen RM. Long-term effects of retinopathy of prematurity (ROP) on rod and rod-driven function. Doc Ophthalmol. 2011;122(1):19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamilton R, Bradnam MS, Dudgeon J, Mactier H. Maturation of rod function in preterm infants with and without retinopathy of prematurity. J Pediatr. 2008;153(5):605-611. [DOI] [PubMed] [Google Scholar]
- 30.Ecsedy M, Varsányi B, Szigeti A, Szrnka G, Németh J, Récsán Z. Cone function in children with a history of preterm birth. Doc Ophthalmol. 2011;122(3):141-148. [DOI] [PubMed] [Google Scholar]
- 31.Fulton AB, Hansen RM, Moskowitz A, Akula JD. The neurovascular retina in retinopathy of prematurity. Prog Retin Eye Res. 2009;28(6):452-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fulton AB, Hansen RM, Moskowitz A. The cone electroretinogram in retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2008;49(2):814-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raffa LH, Nilsson J, Dahlgren J, Grönlund MA. Electrophysiological changes in 12-year-old children born MLP: reduced VEP amplitude in MLP children [published online January 18, 2017]. Br J Ophthalmol. doi: 10.1136/bjophthalmol-2016-309536 [DOI] [PubMed] [Google Scholar]
- 34.Young RW. Cell differentiation in the retina of the mouse. Anat Rec. 1985;212(2):199-205. [DOI] [PubMed] [Google Scholar]
- 35.Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D. Cell fate determination in the vertebrate retina. Proc Natl Acad Sci U S A. 1996;93(2):589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hendrickson A. Development of retinal layers in prenatal human retina. Am J Ophthalmol. 2016;161:29-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Provis JM. Development of the primate retinal vasculature. Prog Retin Eye Res. 2001;20(6):799-821. [DOI] [PubMed] [Google Scholar]
- 38.Hughes S, Yang H, Chan-Ling T. Vascularization of the human fetal retina: roles of vasculogenesis and angiogenesis. Invest Ophthalmol Vis Sci. 2000;41(5):1217-1228. [PubMed] [Google Scholar]
- 39.Provis JM, Hendrickson AE. The foveal avascular region of developing human retina. Arch Ophthalmol. 2008;126(4):507-511. [DOI] [PubMed] [Google Scholar]
- 40.Fulton AB, Reynaud X, Hansen RM, Lemere CA, Parker C, Williams TP. Rod photoreceptors in infant rats with a history of oxygen exposure. Invest Ophthalmol Vis Sci. 1999;40(1):168-174. [PubMed] [Google Scholar]
- 41.Reynaud X, Hansen RM, Fulton AB. Effect of prior oxygen exposure on the electroretinographic responses of infant rats. Invest Ophthalmol Vis Sci. 1995;36(10):2071-2079. [PubMed] [Google Scholar]
- 42.Åkerblom H, Andréasson S, Holmström G. Macular function in preterm children at school age. Doc Ophthalmol. 2016;133(3):151-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michalczuk M, Urban B, Chrzanowska-Grenda B, Oziębło-Kupczyk M, Bakunowicz-Łazarczyk A, Krętowska M. The assessment of multifocal ERG responses in school-age children with history of prematurity. Doc Ophthalmol. 2016;132(1):47-55. [DOI] [PubMed] [Google Scholar]
- 44.O’Connor AR, Stephenson T, Johnson A, et al. Long-term ophthalmic outcome of low birth weight children with and without retinopathy of prematurity. Pediatrics. 2002;109(1):12-18. [DOI] [PubMed] [Google Scholar]
- 45.Holmström G, Larsson E. Long-term follow-up of visual functions in prematurely born children—a prospective population-based study up to 10 years of age. J AAPOS. 2008;12(2):157-162. [DOI] [PubMed] [Google Scholar]
- 46.Perlman I, Meyer E, Haim T, Zonis S. Retinal function in high refractive error assessed electroretinographically. Br J Ophthalmol. 1984;68(2):79-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fulton AB, Hansen RM. Electroretinogram responses and refractive errors in patients with a history of retinopathy prematurity. Doc Ophthalmol. 1995-1996;91(2):87-100. [DOI] [PubMed] [Google Scholar]
- 48.Westall CA, Dhaliwal HS, Panton CM, et al. Values of electroretinogram responses according to axial length. Doc Ophthalmol. 2001;102(2):115-130. [DOI] [PubMed] [Google Scholar]
- 49.Flitcroft DI, Adams GG, Robson AG, Holder GE. Retinal dysfunction and refractive errors: an electrophysiological study of children. Br J Ophthalmol. 2005;89(4):484-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leaf AA, Green CR, Esack A, Costeloe KL, Prior PF. Maturation of electroretinograms and visual evoked potentials in preterm infants. Dev Med Child Neurol. 1995;37(9):814-826. [DOI] [PubMed] [Google Scholar]
- 51.Berezovsky A, Moraes NS, Nusinowitz S, Salomão SR. Standard full-field electroretinography in healthy preterm infants. Doc Ophthalmol. 2003;107(3):243-249. [DOI] [PubMed] [Google Scholar]
- 52.Molnar AE, Rosén RM, Nilsson M, Larsson EK, Holmström GE, Hellgren KM. Central macular thickness in 6.5-year-old children born extremely preterm is strongly associated with gestational age even when adjusted for risk factors [published online January 16, 2017]. Retina. doi: 10.1097/IAE.0000000000001469 [DOI] [PubMed] [Google Scholar]
- 53.Åkerblom H, Larsson E, Eriksson U, Holmström G. Central macular thickness is correlated with gestational age at birth in prematurely born children. Br J Ophthalmol. 2011;95(6):799-803. [DOI] [PubMed] [Google Scholar]
- 54.Maldonado RS, O’Connell RV, Sarin N, et al. Dynamics of human foveal development after premature birth. Ophthalmology. 2011;118(12):2315-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yanni SE, Wang J, Chan M, et al. Foveal avascular zone and foveal pit formation after preterm birth. Br J Ophthalmol. 2012;96(7):961-966. [DOI] [PubMed] [Google Scholar]
- 56.Gränse L, Ponjavic V, Andréasson S. Full-field ERG, multifocal ERG and multifocal VEP in patients with retinitis pigmentosa and residual central visual fields. Acta Ophthalmol Scand. 2004;82(6):701-706. [DOI] [PubMed] [Google Scholar]
- 57.Michaelides M, Hunt DM, Moore AT. The cone dysfunction syndromes. Br J Ophthalmol. 2004;88(2):291-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368(9549):1795-1809. [DOI] [PubMed] [Google Scholar]