Key Points
Question
What are the frequency and characteristics of neurological complications from anti–programmed death 1 (PD-1) antibody use?
Findings
Among 347 patients treated with anti–programmed death 1 (PD-1) antibody use (pembrolizumab or nivolumab), this cohort study supports a low frequency (2.9%) of neurological complications associated with anti–PD-1 therapy. The range and severity of complications are diverse, including necrotizing myopathy, various neuropathies, cerebellar ataxia, internuclear ophthalmoplegia, retinopathy, and headache; the median modified Rankin Scale score of 2.5 indicates mild to moderate disability.
Meaning
Subacute presentation of neurological symptoms in a patient receiving anti–PD-1 therapy should prompt consideration of an association and discontinuation of anti–PD-1 antibody use and possible treatment with corticosteroids or other immune treatment depending on the severity.
Abstract
Importance
Neurological complications are an increasingly recognized consequence of the use of anti–programmed death 1 (PD-1) antibodies in the treatment of solid-organ tumors, with an estimated frequency of 4.2%. To date, the clinical spectrum and optimum treatment approach are not established.
Objective
To investigate the frequency, clinical spectrum, and optimum treatment approach to neurological complications associated with anti–PD-1 therapy.
Design, Setting, and Participants
This single-center, retrospective cohort study was conducted from either September or December 2014 (the approval dates of the study drugs by the US Food and Drug Administration) to May 19, 2016. All patients receiving anti–PD-1 monoclonal antibodies were identified using the Mayo Cancer Pharmacy Database. Patients with development of neurological symptoms within 12 months of anti–PD-1 therapy were included. Patients with neurological complications directly attributable to metastatic disease or other concurrent cancer-related treatments were excluded.
Main Outcomes and Measures
Clinical and pathological characteristics, time to development of neurological symptoms, and modified Rankin Scale (mRS) score.
Results
Among 347 patients treated with anti–PD1 monoclonal antibodies (pembrolizumab or nivolumab), 10 (2.9%) developed subacute onset of neurological complications. Seven patients were receiving pembrolizumab, and 3 patients were receiving nivolumab. The patients included 8 men and 2 women. Their median age was 71 years (age range, 31-78 years). Neurological complications occurred after a median of 5.5 (range, 1-20) cycles of anti–PD-1 inhibitors. Complications included myopathy (n = 2), varied neuropathies (n = 4), cerebellar ataxia (n = 1), autoimmune retinopathy (n = 1), bilateral internuclear ophthalmoplegia (n = 1), and headache (n = 1). Peripheral neuropathies included axonal and demyelinating polyradiculoneuropathies (n = 2), length-dependent neuropathies (n = 1), and asymmetric vasculitic neuropathy (n = 1). The time to maximum symptom severity varied from 1 day to more than 3 months. The median mRS score was 2.5 (range, 1-5), indicating mild to moderate disability. Five patients experienced other systemic immune-mediated complications, including hypothyroidism (n = 3), colitis (n = 2), and hepatitis (n = 1). Treatment with anti–PD-1 antibodies was discontinued in 7 patients. Treatment included corticosteroids (n = 7), intravenous immunoglobulin (n = 3), and plasma exchange (n = 1). Nine patients improved, with a median mRS score of 2 (range, 0-6). One patient with severe necrotizing myopathy died.
Conclusions and Relevance
Neurological adverse events associated with anti–PD-1 therapy have a diverse phenotype, with more frequent neuromuscular complications. Although rare, they will likely be encountered with increasing frequency as anti–PD-1 therapy expands to other cancers. The time of onset is unpredictable, and evolution may be rapid and life-threatening. Prompt recognition and discontinuation of anti–PD-1 therapy is recommended. In some cases, immune rescue treatment may be required.
This cohort study investigates the frequency, clinical spectrum, and optimum treatment approach to neurological complications associated with anti–PD-1 inhibitor use.
Introduction
Neurological complications are an increasingly recognized consequence of the use of anti–programmed death 1 (PD-1) antibodies in the treatment of solid-organ tumors, with an estimated frequency of 4.2%.1 The major role of the human cell surface receptor PD-1 is to limit T-cell activity in peripheral tissues, which is important in self-tolerance and prevention of autoimmunity. When bound by its ligands PDL1 and PDL2, PD-1 inhibits T-cell activation and limits immune effector responses.2 Tumors can express PD-L1 as one mechanism of inhibiting antitumor T-cell–mediated responses in the tumor microenvironment. Therapeutic blockade of this pathway with the use of anti–PD-1 monoclonal antibodies, such as pembrolizumab and nivolumab, can thereby increase the immune response against tumor cells.2 Initially approved for the treatment of unresectable metastatic melanoma and non–small cell lung cancer, they are now increasingly used to treat a variety of solid-organ and hematological cancers.
Immune checkpoint inhibitors are generally thought to have a unique adverse effect profile in the form of immune-mediated adverse events, with disruption of immune checkpoint inhibition leading to imbalances in immune tolerance. However, the exact mechanism underpinning these adverse events largely remains unknown. Limited insight in favor of an immune mechanism comes from experience with cytotoxic T-lymphocyte antigen 4 (CTLA-4) inhibitors.3,4
From the series of KEYNOTE clinical trials with PD-1 inhibitors, a number of adverse events with an immune-mediated mechanism were identified as being of special interest, including thyroid dysfunction, pneumonitis, colitis, hepatitis, nephritis, hypophysitis, uveitis, type 1 diabetes, and myositis.5,6 With the exception of thyroid dysfunction, colitis, and hepatitis, most of these complications were rare, occurring in less than 1% of treated patients. Severe (grade 3-4) adverse events occur in approximately 7% to 12% of patients treated with PD-1 inhibitors,7 with the likelihood of adverse events rising to as high as 55% in those treated with the combination of a PD-1 inhibitor and a CTLA-4 inhibitor (ipilimumab).8
More recently, there has been an increase in the number of case reports of neurological complications associated with anti–PD-1 therapy. Neuromuscular complications appear to be the most common and include myasthenia gravis,1,9,10,11 necrotizing myopathy,12,13 vasculitic neuropathy,14 and polyradiculoneuropathy.15,16 Other neurological complications that have been described include focal seizures associated with inflammatory cerebral lesions on magnetic resonance imaging (MRI),17 limbic encephalitis,18 and retinopathy.19 However, the full spectrum of neurological complications relating to anti–PD-1 therapy and the clinical phenotype are not well characterized. The severity of these complications and the optimum approach for evaluation and treatment are also not well known to date. We aimed to define the frequency, phenotypes, and severity of neurological complications associated with anti–PD-1 therapy at a single center. Furthermore, we gathered information regarding prognosis and treatment and defined an approach to evaluation and treatment of these patients.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
Mayo Clinic Investigational Review Board approved the study. We included patients who had given written informed consent allowing their medical records to be used for research purposes.
Patient Selection
We searched the Mayo Cancer Pharmacy Database in Rochester, Minnesota, for patients receiving anti–PD-1 monoclonal antibodies (pembrolizumab or nivolumab) for the treatment of malignant melanoma or other solid-organ tumors from September and December 2014 (the approval dates of the study drugs by the US Food and Drug Administration) to May 19, 2016. We identified those patients who developed neurological disorders after treatment with these medications.
For inclusion, the neurological condition must have occurred within 12 months of anti–PD-1 antibody use. Any new neurological symptoms occurring during treatment were included. We excluded those patients with neurological symptoms that were found to be directly attributable to their metastatic disease or other concurrent cancer-related treatments. Clinical, laboratory, electrodiagnostic, radiological, and pathological information was extracted by retrospective medical record review. Clinical follow-up and management were reviewed in all cases.
Scoring of the Severity
The modified Rankin Scale (mRS) score was used to measure the degree of dependence in daily activities referable to the neurological complication.20 The scale ranges from 0 (no symptoms) to 6 (death).
Electrodiagnostic Testing
Nerve conduction studies and electromyography (EMG) were performed. These assessments used methods standard for the EMG laboratory at Mayo Clinic.
Statistical Analysis
Descriptive summaries are presented as frequencies and percentages for categorical variables. They are presented as the median and range for continuous variables.
Results
In total, 347 patients had received treatment with an anti–PD-1 antibody at the time of our study, with 204 patients receiving pembrolizumab and 142 patients receiving nivolumab. There was also one additional patient who initially received nivolumab and then was switched over to pembrolizumab.
We identified 10 patients of the 347 with neurological complications related to the treatment, giving a frequency of 2.9%. One case has been previously reported13 (patient 1 in Table 1). We excluded an additional 4 patients, including 1 patient with foot drop from peroneal neuropathy related to weight loss, 1 patient with peripheral neuropathy from the use of brentuximab, and 2 patients with subacute decline in mobility (1 with hydrocephalus on neuroimaging and 1 multifactorial).
Table 1. Clinical Features and Outcomes After Treatment.
| Patient No./Sexa | Cancer Diagnosis | Treatment | Cycles to Onset of Neurological Complication | Neurological Diagnosis | Modified Rankin Scale Score | Treatment Stopped | Additional Treatments | Neurological Outcome | Modified Rankin Scale Score After Treatment |
|---|---|---|---|---|---|---|---|---|---|
| 1/Male | Stage IV melanoma | Pembrolizumab | 2 | Severe necrotizing myopathy | 5 | Yes | Prednisone (80 mg daily) for 12 d, 3 sessions of plasmapheresis | Death | 6 |
| 2/Male | Stage IV melanoma | Pembrolizumab | 1 | Myopathy | 1 | Yes | Methylprednisolone (1-g single dose), prednisone (100 mg daily) for 3 d and then prednisone (60 mg daily), tapering weekly over 1 mo | Improved | 0 |
| 3/Male | Stage IV melanoma | Pembrolizumab | 10 | Axonal thoracolumbar polyradiculopathy | 4 | Yes | Prednisone (30 mg daily), weekly taper over 1 mo, IVIG (1 g/kg) for 2 doses | Improved | 2 |
| 4/Male | Stage IV melanoma | Pembrolizumab | 6 | Severe demyelinating length-dependent peripheral neuropathy with axonal loss | 3 | Yes | Prednisone (120 mg daily), followed by slow taper over 2.5 mo | Improved | 2 |
| 5/Male | Stage IV melanoma | Pembrolizumab | 20 | Facial diplegic variant of Guillain-Barré syndrome | 2 | Yes | IVIG (0.4 g/kg daily) for 5 d | Improved | 1 |
| 6/Male | Stage IV peritoneal mesothelioma | Nivolumab | 5 | Asymmetric vasculitic neuropathy | 3 | Yes | Methylprednisolone (1 g intravenously daily) for 5 d, prednisone (60 mg daily), tapering over 3 wk | Improved | 3 |
| 7/Male | Stage IV lung adenocarcinoma | Pembrolizumab | 11 | Cerebellar ataxia and dysarthria | 4 | Yes | None | Improved | 2 |
| 8/Male | Stage IV esophageal adenocarcinoma | Pembrolizumab | 3 | Autoimmune retinopathy | 2 | No | IVIG (0.4 g/kg) 3 consecutive days every 3 wk | Improved | 2 |
| 9/Female | Stage IV leiomyosarcoma | Nivolumab | 3 | Bilateral internuclear ophthalmoplegia | 2 | No | Corticosteroid (dose unknown) for 1 wk | Improved | 0 |
| 10/Female | Stage IV lung adenocarcinoma | Nivolumab | 14 | Headache | 2 | No | Dexamethasone (4 mg twice daily) for 1 wk | Improved | 0 |
Abbreviation: IVIG, intravenous immunoglobulin.
Ages range from 31-78 years.
The patients included 8 men and 2 women. Their median age was 71 years (age range, 31-78 years). Melanoma was the most common cancer (n = 5), followed by lung adenocarcinoma (n = 2), peritoneal mesothelioma (n = 1), esophageal adenocarcinoma (n = 1), and leiomyosarcoma (n = 1). All patients had stage IV metastatic disease. None of the patients had a history of autoimmune or neurological disease.
Seven patients had complications during pembrolizumab therapy, and 3 patients had complications during nivolumab therapy. Neuromuscular disorders were the most common neurological complications and included myopathy (n = 2) and neuropathy (n = 4). There were also single cases of cerebellar ataxia, autoimmune retinopathy, bilateral internuclear ophthalmoplegia, and headache. Neurological complications occurred after a median of 5.5 (range, 1-20) cycles of anti–PD-1 therapy. Each cycle was defined as the 3-week interval between treatments. The time of onset to maximum symptom severity varied considerably, ranging from 1 day to more than 3 months.
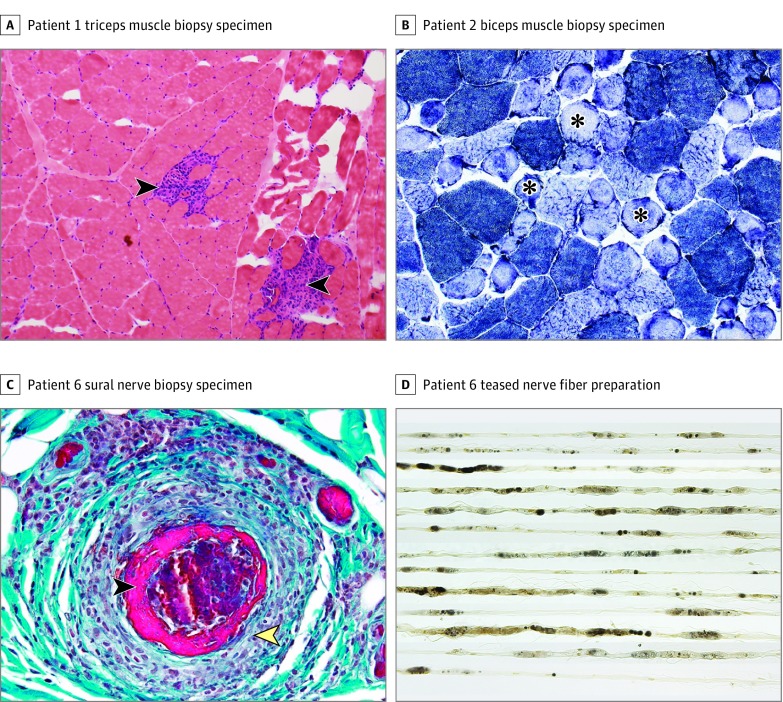
The cases of myopathy are summarized in Table 2. Patient 1 had an aggressive necrotizing myopathy with several unusual features, including severe extraocular, bulbar, and respiratory muscle weakness. Muscle biopsy of the right triceps showed confluent areas of muscle fiber necrosis (Figure, A). Patient 2 had a mild proximal myopathy clinically and on electrodiagnostic testing. Muscle biopsy of the right biceps showed only scattered necrotic fibers, along with some ring and lobulated fibers (Figure, B). There was absence of inflammatory cells in both patients on muscle biopsy. Patient 1 had anti-exosome (PM/Scl) antibody (35 U, with normal being <20 U). Neither patient had antibodies to 3-hydroxy-3-methylglutaryl–coenzyme A reductase, and antibodies to signal recognition protein were also negative in patient 1.
Table 2. Clinical, Laboratory, Imaging, and Pathological Features of Patients With Myopathy.
| Patient No. | Clinical Findings | Onset to Maximum Severity | CK | Electromyography | Magnetic Resonance Imaging | Histopathological Findings | Antibodies | Connective Tissue Markers | ESR/CRP |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Extraocular, bulbar, and proximal limb girdle weakness | 3 wk | 3.8 Times ULN | Proximal myopathy with fibrillation potentials, repetitive nerve stimulation normal | Increased T2-weighted signal and deep paraspinal musculature enhancement | Necrotizing myopathy (Figure, A) | HMGCR, SRP, PNP, and AChR antibody negative, striated muscle antibody 1:61440, anti–PM/Scl antibody positive (36 U) | ANA 0.2, SSA, SSB, Sm, RNP, Scl-70, Jo-1 negative | ESR 20 mm/h, CRP 3.9 mg/L |
| 2 | Mild proximal shoulder weakness | 9 d | 21 Times ULN | Proximal myopathy without fibrillation potentials, length-dependent peripheral neuropathy | NA | Myopathy: 3 necrotic fibers, many ring and lobulated fibers on oxidative enzyme staining (Figure, B) | HMGCR negative, PNP and AChR antibody negative | ANA 0.5 | NA |
Abbreviations: AChR, acetylcholine receptor; ANA, antinuclear antigen; CK, creatine kinase; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; HMGCR, 3-hydroxy-3-methylglutaryl–coenzyme A reductase; Jo-1, histidyl tRNA synthetase; NA, not applicable; PM/Scl, anti-exosome; PNP, paraneoplastic; RNP, ribonucleoprotein; Scl 70, anti–topoisomerase I; Sm, Smith; SRP, signal recognition particle; SSA, Sjögren syndrome–related antigen A; SSB, Sjögren syndrome–related antigen B; ULN, upper limit of normal.
SI conversion factor: To convert C-reactive protein level to nanomoles per liter, multiply by 9.524.
Figure. Pathological Features of Necrotizing Myopathy and Vasculitic Neuropathy Associated With Anti-Programmed Death 1 (PD-1) Therapy.
A, Hematoxylin-eosin stain demonstrates multifocal confluent areas of muscle fiber necrosis replaced by macrophages (arrowheads) (original magnification ×10). B, Nicotinamide adenine dinucleotide dehydrogenase–reacted section revealed numerous ring fibers (asterisks) (original magnification ×20). C, Transverse paraffin section with Gomori trichrome stain demonstrates a large collection of mononuclear cells (yellow arrowhead) invading and destroying an epineurial arteriole with fibrinoid necrosis (black arrowhead). Mononuclear cells reacted for CD-45 (leukocyte common antigen) preparation with predominantly CD-3 (T cell) positivity with scattered CD-20 (B cell) positivity (original magnification ×400). D, Teased fiber preparation shows all fibers undergoing axonal degeneration (original magnification ×160).
The 4 peripheral neuropathy cases are summarized in Table 3. These patients showed a variable phenotype. Patient 3 had an axonal thoracolumbar polyradiculopathy evolving over weeks to months. Patient 4 had a severe length-dependent peripheral neuropathy with mixed axonal and demyelinating features. Patient 5 had an acute inflammatory demyelinating polyradiculoneuropathy with prominent facial diplegia. Patient 6 had a severe subacute asymmetric neuropathy with peroneal predominance suggesting multiple mononeuropathies. Left sural nerve biopsy in this patient revealed necrotizing vasculitis (Figure, C and D).
Table 3. Clinical, Laboratory, Imaging, and Pathological Features of Patients With Peripheral Neuropathy.
| Patient No. | Clinical Findings | Onset to Maximum Severity | Electromyography | Magnetic Resonance Imaging | Cerebrospinal Fluid | Histopathological Findings | Antibodies | Connective-Tissue Markers | ESR/CRP |
|---|---|---|---|---|---|---|---|---|---|
| 3 | Asymmetric LE pain and weakness | Weeks to months | Axonal thoracolumbar polyradiculopathy | NA | Two-nucleated cell, protein, 0.071 g/dL; glucose, 71 mg/dL; OCB 0, cytology negative, paraneoplastic panel negative | NA | GM1 and GD1b negative, PNP antibody panel negative | ANA 0.1, SSA, SSB, RNP, Jo-1, Sm, and Scl-70 negative | CRP, 3 mg/L |
| 4 | Length-dependent UE and LE weakness and sensory loss | 3-4 wk | Severe length-dependent peripheral neuropathy with axonal and demyelinating features | C/T/L spine degenerative changes, no metastatic disease | NA | NA | NA | NA | CRP, 3.5 mg/L |
| 5 | Facial weakness, dyspnea, dysarthria | 1 wk | Demyelinating polyradiculoneuropathy | Enhancement of facial nerves | Twelve- nucleated cells; glucose, 95 mg/dL; protein, 0.192 g/dL; cytology “atypical” favoring reactive | NA | GQ1b, Ach, GM1, GD1b, and PNP antibody negative | NA | NA |
| 6 | Asymmetric distal UE and LE sensory loss and weakness | 2.5 mo | Severe length-dependent axonal sensorimotor neuropathy | NA | NA | Necrotizing vasculitis (Figure, C and D) | NA | ANCA, ANA, ENA, and RF negative | ESR, 38 mm/h |
Abbreviations: Ach, acetylcholine; ANA, antinuclear antigen; ANCA, antineutrophil cytoplasmic antibody; CRP, C-reactive protein; C/T/L, cervical, thoracic, lumbar; ESR, erythrocyte sedimentation rate; GD1b, ganglioside GD1b; GM1, ganglioside-monosialic acid; GQ1b, anti–ganglioside Q1b; Jo-1, histidyl tRNA synthetase; LE, lower extremity; NA, not applicable; OCB, oligoclonal bands; PM/Scl, anti-exosome; PNP, paraneoplastic; RF, rheumatoid factor; RNP, ribonucleoprotein; Scl 70, anti–topoisomerase I; Sm, Smith; SSA, Sjögren syndrome–related antigen A; SSB, Sjögren syndrome–related antigen B; UE, upper extremity.
SI conversion factors: To convert C-reactive protein level to nanomoles per liter, multiply by 9.524; glucose level to millimoles per liter, multiply by 0.0555; and protein level to grams per liter, multiply by 10.0.
In addition to the cases of myopathy and peripheral neuropathy, we identified 4 other cases of neurological complications from anti–PD-1 therapy (Table 1). Patient 7 developed progressive dysarthria, as well as truncal and gait ataxia. Magnetic resonance imaging showed no evidence of metastatic disease or abnormal contrast enhancement. There was spontaneous improvement after discontinuation of treatment with anti–PD-1 therapy, and no further testing was pursued. Patient 8 developed a dark spot in the periphery of his vision, and eye examination revealed a melanocytic choroidal lesion in the right eye, as well as bilateral diffuse uveal melanocytic proliferation. Patient 9 developed binocular diplopia with bilateral internuclear ophthalmoplegia on examination. Magnetic resonance imaging without contrast (owing to renal impairment) was unremarkable. Initial lumbar puncture was a traumatic tap, and follow-up cerebrospinal fluid examination was normal. Findings from electrophysiological testing, including 2-Hz repetitive nerve stimulation of the spinal accessory and facial nerves and single-fiber study of the frontalis muscle, were normal. Results of testing for acetylcholine receptor antibodies, striated muscle antibodies, and anti–ganglioside Q1b (GQ1b) were normal. Serum and cerebrospinal fluid paraneoplastic studies were remarkable only for a mildly elevated serum glutamic acid decarboxylase antibody titer of 0.13 nmol/L. Patient 10 had acute onset of severe headache the day after the 14th cycle of nivolumab requiring hospital admission. Brain MRI revealed no acute abnormalities, and the headache promptly improved with intravenous dexamethasone sodium phosphate therapy.
Five patients experienced other systemic PD-1–related immune-mediated complications, including hypothyroidism (in patients 3, 5, and 9), colitis (in patients 2 and 10), and hepatitis (in patient 5). The time to maximum symptom severity varied from 1 day to more than 3 months. The median mRS score was 2.5 (range, 1-5), indicating mild to moderate disability. Patient 1 required ventilatory and nutritional support. Four patients (patients 3, 4, 6, and 7) required an assist device for ambulation.
Anti–PD-1 therapy was discontinued in 7 patients (Table 1). The mean duration of symptoms until the initiation of immune treatment was 30 days (range, 1-70 days). Seven patients received corticosteroids as part of their treatment, which were typically administered as prednisone (1 mg/kg daily), tapering by 10 mg each week. The mean duration of treatment with corticosteroids was 27 days (range, 7-75 days). Also, 3 patients received courses of intravenous immunoglobulin (2 g/kg), 2 as monotherapy and the other in conjunction with prednisone, and 1 patient with myopathy received plasma exchange in addition to prednisone.
Nine patients improved, 1 spontaneously and 8 with immune rescue treatment, with a median mRS score of 2 (range, 0-6). Despite high-dose prednisone and plasma exchange, patient 1 died after withdrawal of ventilatory support 1 month after onset of symptoms. Of the 9 patients who survived, 3 had subsequent progression of their cancer and eventually died of their underlying disease, 2 patients remained in complete remission, and the other 4 patients were stable. Four of 10 patients received subsequent anti–PD-1 therapy. Patient 2 developed worsening liver function test results after 1 cycle, and the drug was discontinued again. Patients 8, 9, and 10 were able to tolerate further treatment, but 2 required maintenance intravenous immunoglobulin (patient 8) or dexamethasone (patient 10) for immunomodulation.
Discussion
In our series, we identified 10 cases of neurological adverse events among a total of 347 patients treated with anti–PD-1 monoclonal antibodies (pembrolizumab or nivolumab) (frequency, 2.9%). Therefore, a neurological complication from anti–PD-1 therapy still appears to be rare. This frequency is similar to the rate in a recent retrospective review by Zimmer et al1: from a total of 496 patients treated with anti–PD-1 therapy, they identified 6 patients with polyradiculitis or polyneuropathy, 2 patients with isolated cranial neuropathy, 1 patient with myasthenia gravis, 5 patients with myositis or muscle-related weakness, 3 patients with seizures (one of whom also had parkinsonism and bradykinesia), and 4 patients with uveitis or iritis. As in our series, neuromuscular complications were the most common neurological adverse events from anti–PD-1 therapy. Referral bias may also be a factor because our center sees complex oncology patients, often with multiple comorbidities, who may be at greater risk of treatment-related toxic effects. In addition, due to the retrospective nature of this cohort study, there is limited control over data collection, and existing data may be incomplete, inaccurate, or inconsistently measured among participants.
Our series expands the clinical phenotype of these disorders and provides important serological, electrodiagnostic, radiological, and pathological findings that, to our knowledge, have not been previously discussed in detail. Involvement of the peripheral nervous system appears to be more common in our study, which may be an incidental finding and needs to be replicated at other centers. The range of neuromuscular complications is diverse, without one specific phenotype. The spectrum of neuropathies suggests that there can be both axonal and demyelinating types. While there has been a previous report of a microvasculitis of nerve,14 our series demonstrates the first case of necrotizing vasculitis to date. The myopathy due to anti–PD-1 therapy seems to have a unique pathological profile with evidence of necrotizing myopathy, and the clinical phenotype can range from a mild proximal myopathy to a severe myopathy with prominent respiratory, bulbar, and extraocular involvement. We did not observe any disorder involving the neuromuscular junction in our series; however, the prominent bulbar involvement of the myopathy could be mistaken for a disorder of the neuromuscular junction. In addition, we found single cases of cerebellar ataxia and bilateral internuclear ophthalmoplegia that have not been described to date in association with anti–PD-1 therapy. There were also single cases of autoimmune retinopathy and headache. Autoimmune retinopathy has been previously reported,19 while headache is a commonly reported adverse event, with an estimated frequency between 12% and 24%.21 Our series demonstrates the importance of careful clinical evaluation and testing, as well as pathological confirmation, to understand these conditions and guide treatment.
The time from starting anti–PD-1 therapy to development of neurological complications was variable; therefore, the clinician needs to maintain a high suspicion for these disorders. This observation is consistent with the variability in published individual case reports. Furthermore, the timeline of these adverse events can also vary considerably, with some cases starting more insidiously and evolving over months but with other cases progressing rapidly over days to weeks.
The muscle and nerve biopsies provide additional information regarding the potential pathogenesis of adverse neurological events with anti–PD-1 antibodies. The patient with necrotizing vasculitis (patient 6) had large epineurial perivascular inflammatory collections composed of primarily T lymphocytes. The inflammatory reaction is likely due to the blockade of the PD-1 pathway, enhancing the activity of effector T cells in tissues.2 Patient 1 had no inflammation on muscle biopsy, which may be due to the broader mechanism of action of anti–PD-1 antibodies, which includes not only enhancing the activity of effector T cells but also enhancing natural killer cell activity, as well as promoting antibody production indirectly or through direct effects on PD1-positive B cells.2 This theory is further supported by the positive anti-PM/Scl antibody that can be found in patients with dermatomyositis, polymyositis, systemic sclerosis, and systemic autoimmune disease overlap syndromes.22
Limitations
Based on the small number of cases in our series, it is not possible to determine the optimum treatment regimen for these complications. Guidelines for the best treatment of these neurological adverse events remain sparse. Previously published recommendations include discontinuation of the medication in moderate to severe (grade 2-4) adverse reactions, plus a course of high-dose prednisone (0.5-2 mg/kg daily) tapered over at least 1 month, with additional immunosuppressive therapy reserved for those who worsen despite corticosteroid therapy.7 In our series, all but 3 patients had prompt discontinuation of treatment with anti–PD-1 antibodies. Most patients in our series also received high-dose prednisone (1 mg/kg), followed by a taper of 10 mg per week, with generally favorable outcomes. However, it was not clear if immune suppressant treatment was required in all of these patients, including 2 of the 3 patients continuing on therapy with anti–PD-1 antibodies who were given ongoing immune rescue treatment. The one clear exception was the patient with severe necrotizing myopathy, who did not respond to corticosteroids or the addition of plasma exchange. The severe bulbar weakness likely contributed to the poor outcome in that particular case. If a complication related to anti–PD-1 use is suspected, then prompt discontinuation of the anti–PD-1 antibody treatment is recommended, while evaluation is pursued. As part of the workup of neuromuscular complications, we recommend electrodiagnostic studies and consideration of muscle or nerve biopsy to better understand the pathophysiological mechanisms underlying these adverse events. If the clinical examination demonstrates severe clinical deficits at onset or worsens despite medication discontinuation, additional immune suppressant treatment should be considered. Corticosteroid treatment is the most common first-line agent, and a regimen of prednisone (1 mg/kg) with a taper over 1 month is recommended. The addition of intravenous immunoglobulin or plasma exchange can be considered if there is continued clinical worsening.
Conclusions
Patients receiving treatment with anti–PD-1 antibodies have metastatic cancer and are at risk of developing neurological complications related to their underlying disease. A thorough differential diagnosis and search for other potential causes must be performed in each case. As our series demonstrates, it is important to appreciate that new neurological symptoms in these patients could also herald onset of immune-mediated complications related to the treatment itself. The clinician must remain vigilant at all stages of treatment and even for a period after treatment has been completed. The neurological deficits can evolve rapidly and may be severe or life-threatening in some cases. However, with prompt recognition and intervention, the outcomes are generally favorable. Although neurological complications relating to anti–PD-1 antibody therapy appear to be rare, we will likely encounter more cases in the future as the use of these medications in the treatment of metastatic cancer continues to expand.
References
- 1.Zimmer L, Goldinger SM, Hofmann L, et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti–PD-1 therapy. Eur J Cancer. 2016;60:210-225. [DOI] [PubMed] [Google Scholar]
- 2.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med. 2014;6(230):230ra45. [DOI] [PubMed] [Google Scholar]
- 4.Tarhini AA, Zahoor H, Lin Y, et al. Baseline circulating IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J Immunother Cancer. 2015;3:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert C, Schachter J, Long GV, et al. ; KEYNOTE-006 Investigators . Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521-2532. [DOI] [PubMed] [Google Scholar]
- 6.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17(7):956-965. [DOI] [PubMed] [Google Scholar]
- 7.Naidoo J, Page DB, Li BT, et al. Toxicities of the anti–PD-1 and anti–PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marrone KA, Ying W, Naidoo J. Immune-related adverse events from immune checkpoint inhibitors. Clin Pharmacol Ther. 2016;100(3):242-251. [DOI] [PubMed] [Google Scholar]
- 9.Lau KH, Kumar A, Yang IH, Nowak RJ. Exacerbation of myasthenia gravis in a patient with melanoma treated with pembrolizumab. Muscle Nerve. 2016;54(1):157-161. [DOI] [PubMed] [Google Scholar]
- 10.Polat P, Donofrio PD. Myasthenia gravis induced by nivolumab therapy in a patient with non–small-cell lung cancer. Muscle Nerve. 2016;54(3):507. [DOI] [PubMed] [Google Scholar]
- 11.Sciacca G, Nicoletti A, Rampello L, Noto L, Parra HJ, Zappia M. Benign form of myasthenia gravis after nivolumab treatment. Muscle Nerve. 2016;54(3):507-509. [DOI] [PubMed] [Google Scholar]
- 12.Vallet H, Gaillet A, Weiss N, et al. Pembrolizumab-induced necrotic myositis in a patient with metastatic melanoma. Ann Oncol. 2016;27(7):1352-1353. [DOI] [PubMed] [Google Scholar]
- 13.Haddox CL, Shenoy N, Shah KK, et al. Pembrolizumab induced bulbar myopathy and respiratory failure with necrotizing myositis of the diaphragm. Ann Oncol. 2017;28(3):673-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aya F, Ruiz-Esquide V, Viladot M, et al. Vasculitic neuropathy induced by pembrolizumab. Ann Oncol. 2017;28(2):433-434. [DOI] [PubMed] [Google Scholar]
- 15.de Maleissye MF, Nicolas G, Saiag P. Pembrolizumab-induced demyelinating polyradiculoneuropathy. N Engl J Med. 2016;375(3):296-297. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka R, Maruyama H, Tomidokoro Y, et al. Nivolumab-induced chronic inflammatory demyelinating polyradiculoneuropathy mimicking rapid-onset Guillain-Barré syndrome: a case report. Jpn J Clin Oncol. 2016;46(9):875-878. [DOI] [PubMed] [Google Scholar]
- 17.Mandel JJ, Olar A, Aldape KD, Tremont-Lukats IW. Lambrolizumab induced central nervous system (CNS) toxicity. J Neurol Sci. 2014;344(1-2):229-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salam S, Lavin T, Turan A. Limbic encephalitis following immunotherapy against metastatic malignant melanoma. BMJ Case Rep. 2016;2016:bcr2016215012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts P, Fishman GA, Joshi K, Jampol LM. Chorioretinal lesions in a case of melanoma-associated retinopathy treated with pembrolizumab. JAMA Ophthalmol. 2016;134(10):1184-1188. [DOI] [PubMed] [Google Scholar]
- 20.Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin Scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38(3):1091-1096. [DOI] [PubMed] [Google Scholar]
- 21.Highlights of prescribing information. OPDIVO(R) (nivolumab) injection, for intravenous use. https://packageinserts.bms.com/pi/pi_opdivo.pdf. Initial US approval 2014. Accessed July 18, 2017.
- 22.D’Aoust J, Hudson M, Tatibouet S, et al. ; Canadian Scleroderma Research Group . Clinical and serologic correlates of anti-PM/Scl antibodies in systemic sclerosis: a multicenter study of 763 patients. Arthritis Rheumatol. 2014;66(6):1608-1615. [DOI] [PubMed] [Google Scholar]