This meta-analysis provides a quantitative synthesis of population-based studies on the relationship between sleep-disordered breathing and risk of cognitive impairment.
Key Points
Question
What are the effects of sleep-disordered breathing (SDB) on cognitive function and risk of cognitive impairment?
Findings
In this systematic review meta-analysis that included more than 4 million participants, those with SDB were 26% more likely to develop cognitive impairment than those without SDB. They also had slightly worse performance in executive function but not in global cognition or memory.
Meaning
Sleep-disordered breathing may be an important modifiable risk factor for dementia and other cognitive impairment; future studies are needed to examine if treatment of SDB might reduce risk of cognitive impairment.
Abstract
Importance
Growing evidence suggests an association between sleep-disordered breathing (SDB) and cognitive decline in elderly persons. However, results from population-based studies have been conflicting, possibly owing to different methods to assess SDB or cognitive domains, making it difficult to draw conclusions on this association.
Objective
To provide a quantitative synthesis of population-based studies on the relationship between SDB and risk of cognitive impairment.
Data Sources
PubMed, EMBASE, and PsychINFO were systematically searched to identify peer-reviewed articles published in English before January 2017 that reported on the association between SDB and cognitive function.
Study Selection
We included cross-sectional and prospective studies with at least 200 participants with a mean participant age of 40 years or older.
Data Extraction and Synthesis
Data were extracted independently by 2 investigators. We extracted and pooled adjusted risk ratios from prospective studies and standard mean differences from cross-sectional studies, using random-effect models. This meta-analysis followed the PRISMA guidelines and also adhered to the MOOSE guidelines.
Main Outcomes and Measures
Cognitive outcomes were based on standard tests or diagnosis of cognitive impairment. Sleep-disordered breathing was ascertained by apnea-hypopnea index or clinical diagnosis.
Results
We included 14 studies, 6 of which were prospective, covering a total of 4 288 419 men and women. Pooled analysis of the 6 prospective studies indicated that those with SDB were 26% (risk ratio, 1.26; 95% CI, 1.05-1.50) more likely to develop cognitive impairment, with no evidence of publication bias but significant heterogeneity between studies. After removing 1 study that introduced significant heterogeneity, the pooled risk ratio was 1.35 (95% CI, 1.11-1.65). Pooled analysis of the 7 cross-sectional studies suggested that those with SDB had slightly worse executive function (standard mean difference, −0.05; 95% CI, −0.09 to 0.00), with no evidence of heterogeneity or publication bias. Sleep-disordered breathing was not associated with global cognition or memory.
Conclusions and Relevance
Sleep-disordered breathing is associated with an increased risk of cognitive impairment and a small worsening in executive function. Further studies are required to determine the mechanisms linking these common conditions and whether treatment of SDB might reduce risk of cognitive impairment.
Introduction
Sleep-disordered breathing (SDB) is a very common but treatable condition in older adults. There has been growing interest in the relationship between SDB and adverse health consequences, including hypertension, diabetes, and cardiovascular diseases.1,2,3,4 While the association between SDB and health outcomes remains controversial, especially in older populations,5,6,7,8 recent evidence has suggested a link between SDB and cognitive decline in elderly persons.9,10,11 Notably, most early studies have examined the association between SDB and cognition in clinical populations, eg, among patients at sleep clinics.12,13,14 These studies usually consist of individuals with relatively severe SDB and were limited by small sample sizes and failure to account for confounding factors.
Over the past few years, an increasing number of population-based studies have been conducted on SDB and cognitive impairment.15,16,17 These community-dwelling samples often include individuals with milder SDB, as opposed to those examined in case-control studies. Some of these studies suggested that SDB was associated with increased risk of dementia or impairment across different cognitive domains,18,19,20 while others found no association.17,21 Owing to different study designs and methods to assess SDB, it is difficult to draw conclusions on the consistency of the associations. Moreover, because each study has reported on specific domains using different scales, it is unclear if SDB has differential effects on cognitive domains. Therefore, a meta-analytic approach is particularly useful for synthesizing these studies and elucidating pooled estimates for the effects of SDB on risk of cognitive impairment as well as effects across different cognitive domains. Given the high prevalence of cognitive impairment in elderly persons and its significant consequences,22,23 it is critical to explore the role of SDB as a modifiable risk factor.
Methods
Search Strategy and Study Selection
We searched for articles published before January 2017 using electronic databases, including PubMed, EMBASE, and PsychINFO, and hand searched the reference lists of identified articles. Studies were identified using the search terms “(sleep-disordered breathing OR sleep apnea OR obstructive sleep apnea) AND (cognition OR cognitive function OR cognitive decline OR dementia OR Alzheimer’s OR cognitive impairment).” The search was restricted to articles published in English. Studies were included if they (1) were original articles published in a peer-reviewed journal; (2) used a cross-sectional or prospective cohort design; (3) were conducted in population-based samples (N ≥ 200; mean age ≥40 years); (4) defined SDB by apnea-hypopnea index (AHI) or clinical diagnosis by International Classification of Diseases, Ninth Revision (ICD-9) codes; (5) incorporated outcomes on Alzheimer disease (AD), dementia risk, or cognitive impairment, as defined by validated cognitive tests; (6) reported effect estimates appropriate for the pooled analysis of effect sizes (eg, odds ratios [ORs], hazard ratios, mean differences, or standardized mean differences [SMDs] in cognitive test scores) or other types of estimates (eg, correlation or regression coefficient) that could be converted to the above forms; and (7) presented results adjusted for covariates (at least by age, sex, and education). Studies were excluded if they (1) were case reports, abstracts, reviews, or meta-analyses; (2) were conducted in clinical populations; (3) used case-control design; (4) used self-reported SDB; or (5) reported the prevalence of SDB rather than studying SDB as a risk factor.
Data Extraction and Cognitive Outcomes
The eligibility of the studies to be included in the analysis was determined and data were extracted independently by 2 investigators (Y.L. and C.T.M.). If multiple articles were published from the same cohort reporting on the same cognitive outcomes, we included only the one with the most complete details; if multiple articles from the same cohort had different study designs or if they reported on different cognitive outcomes, we included each of these articles separately in the analysis. Differences in data extraction between the extractors were resolved by consensus discussion and consultation with a third investigator (K.Y.).
Because most prospective studies reported the OR or hazard ratio for cognitive impairment,15,18,24,25,26 risk ratio estimates were extracted from these studies. To quantify the association between SDB and different cognitive domains, we extracted the adjusted cognitive test scores from the cross-sectional studies.17,27,28,29,30 We also obtained adjusted estimates from Nikodemova et al31 and Hrubos-Strøm et al32 by contacting the study authors. To be included in the pooled analysis, a cognitive domain has to be represented by more than 3 studies; thus, we focused on 3 cognitive domains: global function, delayed memory (unless the study only assessed immediate memory), and executive function. These domains are also particularly important in the context of aging and development of AD. The cognitive tests included from each study are summarized in Table 1 and Table 2.
Table 1. Summary of Prospective Studies on Sleep-Disordered Breathing and Risk of Cognitive Impairment.
| Source | Total No. (% Men) | Cohort; Country | Age (Baseline), y | Definition of Sleep Apnea | Cognitive Outcome | Adjusted Variables | NOS Score |
|---|---|---|---|---|---|---|---|
| Lutsey et al,17 2016 | 966 (48.6) | ARIC; United States | Mean (SD), 61.3 (5.0) | AHI4% (<5/5-14.9/15-29.9/≥30) | Decline in global cognition (average of scores in DWR, Word Fluency, and DSST) | Age, sex, field center, education level, alcohol intake, smoking, physical activity, presence of APOE4, BMI, CRP level, and CVD comorbidities | 8 |
| Blackwell et al,15 2015 | 2636 (100) | MrOS; United States | Mean (SD), 76.0 (5.3) | AHI3% (<15/>15) | Decline in global cognition (3MS score) | Age, site, race/ethnicity, BMI, education level, depressive symptoms, CVD comorbidities, Parkinson disease, IADL, benzodiazepine use, antidepressant use, self-reported health, physical activity, alcohol intake, and smoking | 8 |
| Martin et al,25 2015 | 559 (39.7) | Synapse; France | Mean (SD), 66.9 (0.9) | AHI3% (<15/15-30/>30) | Decline in attention (Trail Making Test A, Stroop Color-Word Test, and WAIS-III) | Age, sex, education level, follow-up length, BMI, ESS score, CVD comorbidities, anxiety, and depression | 9 |
| Yaffe et al,26 2015 | 200 000 (100) | VA health medical record; United States | ≥55 | Clinical diagnosis | Risk of dementia | Age, CVD comorbidities, obesity, depression, income, and education level | 9 |
| Chang et al,24 2013 | 8484 (59.3) | Longitudinal health insurance database; Taiwan | ≥40 | Clinical diagnosis | Risk of dementia | Age, sex, CVD comorbidities, urbanization level, and income | 9 |
| Yaffe et al,18 2011 | 298 (0) | SOF; United States | Mean (SD), 82.3 (3.2) | AHI3% (<15/≥15) | Risk of mild cognitive impairment or dementia | Age, race/ethnicity, BMI, education level, smoking, CVD comorbidities, antidepressant use, benzodiazepine use, non-benzodiazepine, and anxiolytics use | 9 |
Abbreviations: 3MS, Modified Mini-Mental State Examination; AHI, apnea-hypopnea index; ARIC, Atherosclerosis Risk in Communities Study; BMI, body mass index; CRP, C-reactive protein; CVD, cardiovascular diseases; DWR, delayed word recall; DSST, Digit Symbol Substitution test; ESS, Excessive Sleepiness Scale; IADL, instrumental activities of daily living; MrOS, Osteoporotic Fractures in Men Study; NOS, Newcastle-Ottawa Quality Scale; SOF, Study of Osteoporotic Fractures; WAIS, Wechsler Adult Intelligence Scale.
Table 2. Summary of Cross-sectional Studies on Sleep-Disordered Breathing and Performance in Different Cognitive Domains.
| Source | Total No. (% Men) | Cohort; Country | Age, y | Sleep Apnea | Global Function | Memory | Executive Function | Adjusted Variables | NOS Score |
|---|---|---|---|---|---|---|---|---|---|
| Lutsey et al,17 2016a | 966 (48.6) | ARIC; United States | Mean (SD), 61.3 (5.0) | AHI4% (<5/5-14.9/15-29.9/≥30) | MMSE | DWR/logical memory test | Word fluency/DSST/Trail Making Test B | Age, sex, field center, education level, alcohol intake, smoking, physical activity, presence of APOE4, BMI, CRP, and CVD comorbidities | 8 |
| Ramos et al,16 2015 | 8059 (37.6) | Hispanic/Latino population; United States | 45-74 | AHI3% (continuous) | B-SEVLT | SEVLT-recall | Word fluency/DSST | Age, sex, education level, CVD comorbidities, depressive symptoms, anxiety, smoking, BMI, and field center | 6 |
| Dlugaj et al,28 2014 | 1793 (51.3) | Heinz Nixdorf Recall Study; Germany | Mean (SD), 63.8 (7.5) | AHI (<15/15-29/≥30) | NA | Verbal memory | Problem solving/speed of processing | Age, sex, and education level | 6 |
| Nikodemova et al,31 2013 | 755 (59.1) | Wisconsin Sleep Cohort Study; United States | Mean, 53.9 | AHI4% (<5/5-14/≥15) | NA | AVLT | Trail Making Test B/symbol digit modalities test/COWT | Age, sex, education level, and BMI | 6 |
| Hrubos-Strøm et al,32 2012 | 290 (55.9) | Akershus Sleep Apnea Project; Norway | Mean (SD), 48.2 (11.2) | AHI4% (<15/≥15) | NA | AVLTb | Stroop test | Age, sex, and education level | 6 |
| Blackwell et al,27 2011 | 2909 (100) | MrOS; United States | Mean (SD), 76 (6) | AHI3% (<5/5-14/15-29/≥30) | 3MS | NA | Trail Making Test B | Age, race/ethnicity, clinic, BMI, IADL, CVD comorbidities, antidepressant use, benzodiazepine use, depression, education level, alcohol use, smoking, physical activity, and self-reported health | 7 |
| Spira et al,30 2008 | 448 (0) | SOF; United States | Mean (SD), 82.8 (3.4) | AHI3% (<30/≥30) | MMSE | NA | Trail Making Test B | Age, education level, and SSRI use | 6 |
| Sharafkhaneh et al,34 2005 | 4 060 504 (83.9) | VA health medical record; United States | Mean (SD), 59.0 (15.5) | Clinical diagnosis | Dementia prevalence | NA | NA | Age, sex, and race/ethnicity | 6 |
| Foley et al,29 2003 | 718 (100) | Honolulu-Asia Aging study; United States | 79-97 | AHI4% (<5/5-14/15-29/≥30) | MMSE | NA | NA | Age, education level, and marital status | 6 |
Abbreviations: AHI, apnea-hypopnea index; ARIC, Atherosclerosis Risk in Communities Study; AVLT, Auditory Verbal Listening Test; BMI, body mass index; B-SEVLT, Brief-Spanish English Verbal Learning Test; COWT, Controlled Oral Word Test; CRP, C-reactive protein; CVD, cardiovascular diseases; DWR, delayed word recall; DSST, Digit Symbol Substitution test; IADL, instrumental activities of daily living; MMSE, Mini-Mental State Examination; MrOS, Osteoporotic Fractures in Men Study; NA, not applicable; NOS, Newcastle-Ottawa Quality Scale; SOF, Study of Osteoporotic Fractures; SSRI, selective serotonin reuptake inhibitor.
The cross-sectional analysis from this prospective study is assessed.
Immediate memory; all other memory tests were based on delayed recall.
Definition of SDB
We included studies that used AHI or ICD-9 codes to define SDB. Apnea-hypopnea index is the average number of apnea and hypopnea events per hour of sleep. In general, hypopnea is defined as a discernible reduction in the airflow followed by at least a 4% reduction in oxyhemoglobin saturation (AHI4%), at least a 3% reduction (AHI3%), or an event-related arousal (AHI3a). Because there was a mixed use of these definitions in the included studies, we first presented narrative description of the definitions, and for the main analysis, we pooled the estimates by AHI less than 15 and 15 or greater to be consistent with the most commonly used cutoff33 and to provide a most generalizable categorization. For studies that have further broken down these 2 categories,17,25,27,28,29,32 we calculated a weighted mean for AHI less than 15 and 15 or greater. For other studies16,29,30 that did not provide sufficient information for analysis by this cutoff, we contacted study authors or used other literature to obtain necessary information for the calculation.
Statistical Analysis
We pooled risk ratios to summarize the prospective relationship between SDB and cognitive impairment. One prospective study17 reported the changes in z scores of 3 cognitive tests that were strongly associated with AD or vascular dementia; we converted the mean z scores of these tests into a single risk ratio using the online meta-analysis calculator (David B. Wilson, PhD; http://www.campbellcollaboration.org/escalc/html/EffectSizeCalculator-OR5.php).
For cross-sectional studies, we calculated SMD in cognitive test scores by SDB status. In cases where a higher score indicated worse cognition, we multiplied the score by −1 so that high scores indicated better performance across all tests. One study34 only reported the OR, and this was converted to Cohen d using the formula SMD = log(OR) × √3 / π. For one study29 where neither the SDs nor the 95% CIs were reported, we used the SD of the same test from another previous study.21 To estimate the pooled SMD for each cognitive domain, we first calculated a summary Hedges g estimate for each domain from each individual source study. This step ensures that multiple relevant tests within each source study were all considered in the analysis without being overrepresented in the pooled analysis. All effect estimates were then pooled using a weighted random-effects model.
We tested between-study heterogeneity using I2 statistics35 and used Egger test and funnel plot asymmetry to evaluate publication bias.36 All tests were 2-tailed. Sensitivity analysis was conducted by excluding the studies that introduced significant heterogeneity to the analysis on each cognitive outcome. The quality of the included studies was evaluated independently by 2 investigators (Y.L. and C.T.M.) using the Newcastle-Ottawa Quality Assessment Scale.37 Our study adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines38 and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) checklist.39 All statistical analysis was performed using Review Manager Software version 5 (Cochrane Collaboration). Significance was set at P < .05.
Results
Literature Search and Study Characteristics
We identified a total of 3527 articles, including 1347 articles from PubMed, 359 from PsychINFO, and 1821 from EMBASE. After screening titles and abstracts, we selected 134 articles for further evaluation, and after applying the inclusion and exclusion criteria, we retrieved 24 full-text articles for detailed review. After excluding studies with insufficient data for the meta-analysis, our analytic sample included 14 studies15,16,17,18,24,25,26,27,28,29,30,31,32,34 with 4 288 419 participants from 5 different countries (10 studies from the United States and 1 each from France, Germany, Norway, and Taiwan) (eFigure in the Supplement). Table 1 and Table 2 summarize the characteristics and Newcastle-Ottawa Quality Assessment Scale quality scores of included studies. Two studies of different designs were published from the Study of Osteoporotic Fractures,18,30 which recruited women only, and another 2 from the Osteoporotic Fractures in Men,15,27 focusing on men only. Moreover, 2 studies were based on the US Veterans Affairs health medical records and included primarily men.26,34 Three studies used clinically diagnosed SDB,24,26,34 while the rest presented results by AHI cutoffs15,17,18,21,25,27,28,29,30,31,32 or by continuous scores.16 Four studies17,29,31,32 used AHI4% for the definition of SDB, 6 studies15,16,18,25,27,30 used AHI3%, and 1 study28 did not specify the definition of AHI.
Prospective Studies of SDB and Risk of Cognitive Impairment
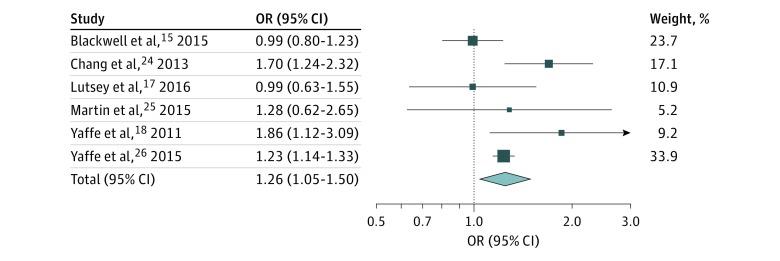
Pooled analysis of the 6 prospective studies (including 212 943 participants) indicated that those with SDB were 26% (risk ratio, 1.26; 95% CI, 1.05-1.50) more likely to develop cognitive impairment, defined by clinically relevant cognitive decline15,17,25 or risk of dementia,18,24,26 with no evidence of publication bias but significant between-study heterogeneity (Figure 1). The 3 prospective studies that showed positive findings all examined risk of mild cognitive impairment (MCI) or dementia as the outcome,18,24,26 and 2 of these used ICD-9 codes to define SDB.24,26 Sensitivity analysis shows that after removing the study by Blackwell et al15 from this pooled analysis, the heterogeneity disappeared (risk ratio, 1.35; 95% CI, 1.11-1.65).
Figure 1. Forest Plot of Prospective Studies on Association Between Sleep-Disordered Breathing and Risk of Cognitive Impairment.
All effect estimates were pooled using a weighted random-effects model. Heterogeneity: Τ2 = 0.02; χ2 = 11.40; df = 5; P = .04; I2 = 56%. Test for overall effect: z = 2.51; P = .01. Error bars indicate 95% CIs. OR indicates odds ratio.
Cross-sectional Studies
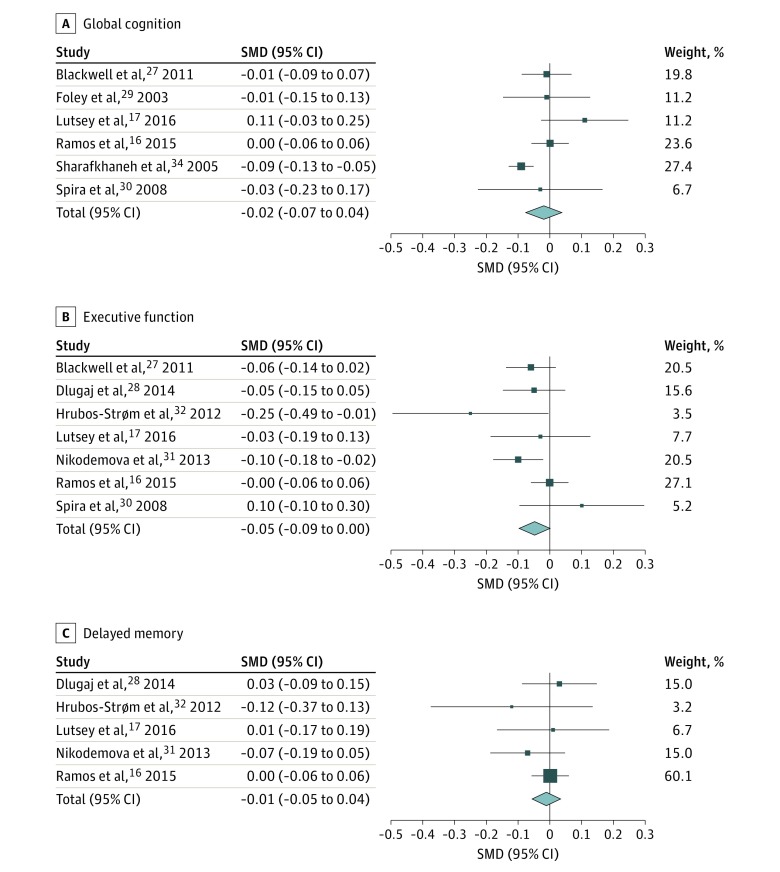
Global Cognition
Pooled analysis of 6 cross-sectional studies (including 4 073 604 participants) suggested that SDB was not significantly associated with global cognition (SMD, −0.02; 95% CI, −0.07 to 0.04) (Figure 2A). There was no evidence of publication bias but significant heterogeneity between studies. After removing the study by Sharafkhaneh et al,34 the heterogeneity disappeared (SMD, 0.01; 95% CI, −0.04 to 0.05).
Figure 2. Forest Plot of Cross-sectional Studies on Association Between Sleep-Disordered Breathing and Cognitive Domains.
All effect estimates were pooled using a weighted random-effects model. Error bars indicate 95% CIs. SMD indicates standard mean difference. A, Forest plot of association between sleep-disordered breathing and global cognition. Heterogeneity: Τ2 = 0.00; χ2 = 13.35; df = 5; P = .02; I2 = 63%. Test for overall effect: z = 0.59; P = .56. B, Forest plot of association between sleep-disordered breathing and executive function. Heterogeneity: Τ2 = 0.00; χ2 = 9.00; df = 6; P = .17; I2 = 33%. Test for overall effect: z = 1.92; P = .05. C, Forest plot of association between sleep-disordered breathing and delayed memory. Heterogeneity: Τ2 = 0.00; χ2 = 2.34; df = 4; P = .67; I2 = 0%. Test for overall effect: z = 0.37; P = .71.
Executive Function
Pooled analysis of 7 cross-sectional studies (including 15 220 participants) indicated that those with SDB had worse executive function compared with those without (SMD, −0.05; 95% CI, −0.09 to 0.00) (Figure 2B). There was no evidence of heterogeneity or publication bias.
Memory
Figure 2C shows the relationship between SDB and memory. One study only examined immediate memory,32 while the rest assessed delayed recall.16,17,28,31 Pooled analysis of 5 cross-sectional studies (including 11 863 participants) indicated an SMD of −0.01 (95% CI, −0.05 to 0.04) in memory score between those with and without SDB. There was no evidence of heterogeneity or publication bias.
Discussion
To our knowledge, this study is the first to provide a comprehensive systematic review and quantitative synthesis of population-based studies on SDB and cognitive function and to obtain pooled estimates of the effects of SDB on both the risk of cognitive impairment and on different domains of cognitive function. Our pooled analysis of more than 4 million adults showed that those with SDB were 26% (risk ratio, 1.26; 95% CI, 1.05-1.50) more likely to develop cognitive impairment and had slightly worse performance in executive function but not on global cognition or memory. Although some between-study heterogeneity was found, sensitivity analysis confirmed these associations, and there was no evidence of publication bias.
Our findings provide evidence that SDB may be an important modifiable risk factor for cognitive impairment in elderly persons. Notably, a 2017 study40 suggested that SDB might lead to early but possibly modifiable AD biomarker changes. Our results are also supported by a 2016 meta-analysis of case-control studies,41 which suggested that patients with AD were 5-fold more likely to have SDB compared with cognitively intact individuals of similar age. In our meta-analysis, the 3 prospective studies18,24,26 that examined the risk of MCI or dementia as the outcomes showed significant associations while the other 3 studies15,17,25 that did not find any association all defined cognitive impairment as a significant cognitive decline. This could be owing to the differences in these cognitive outcomes, given that the severity of cognitive impairment indicated by even a significant decline in cognitive scores might not be comparable with that of clinically diagnosed MCI or dementia. It is also possible that SDB might be particularly important through the course of conversion to MCI or dementia. However, to our knowledge, the underlying cause of this observation has yet to be determined. Furthermore, studies that found significant results mostly used clinical diagnosis of SDB rather than an AHI of 15 or greater.24,26 This could be because the more severe cases are more likely to be detected and less likely to be misclassified or because there exist mechanistic pathways through which severe SDB could particularly impair cognition. Sensitivity analysis showed similar results after removing the study15 that introduced significant heterogeneity to the pooled analysis of prospective studies. This heterogeneity might be because of specific characteristics of the study sample (all older men)15 or relatively short follow-up length compared with other similar studies.17,25
The pooled analysis of cross-sectional studies suggests that there was a modest association between SDB and worse executive function but not with global cognition or memory. This is in line with 2 previous meta-analyses of clinical studies,42,43 which indicated an effect of SDB on executive function, vigilance, and psychomotor speed, while mixed findings have been reported on the effects of SDB on memory.42,43,44 Notably, the included studies used cognitive tests that varied in the difficulty of administration. Moreover, not all studies examined the same cognitive domains, and even those that studied similar domains might have used different measures of that domain. This is particularly an issue for executive function, where certain components might be more vulnerable to the negative effects of SDB. Therefore, these findings should be interpreted with caution.
Several mechanisms have been proposed for the association between SDB and neurocognitive decline, including hypoxemia, daytime sleepiness, sleep fragmentation, and oxidative stress.45,46 To date, it remains controversial which is the most likely mechanism, especially in the absence of well-designed interventional studies to help disentangle the causal pathways. Notably, there has been growing attention on the important role that hypoxemia might play in the relationship between SDB and cognition.18,45,46,47 Findings from the HypnoLaus study,20 the Sleep Heart Health Study,48 the Apnea Positive Pressure Long-term Efficacy Study,49 and the Study of Osteoporotic Fractures14 all suggest that degree of hypoxemia or oxygen desaturation rather than sleep fragmentation might affect cognitive performance in middle-aged and elderly persons. Regular intermittent hypoxia may cause vascular dysfunction, kill neurons, and impair the blood-brain barrier, leading to long-term disruption of the brain’s microenvironment and synaptic plasticity.10,50 A few studies have suggested that measures of oxygen desaturation are associated with cognitive impairment.15,18,27,30 Because measures of oxygen desaturation were only reported in 2 studies included in this meta-analysis, we could not calculate pooled estimates for these measures. Future studies with consideration of different indices of SDB might provide more insights into the mechanisms.
Two included studies indicated that the effects of SDB on cognition were more pronounced in APOE4 carriers.30,31 While the exact mechanisms for the interaction between SDB and APOE4 on cognition are unclear, the presence of APOE4 genotype is believed to increase cellular vulnerability to oxidative damage and promote neuroinflammation.51,52 More studies are needed to investigate the combined effects of APOE4 and SDB on cognition and to help understand potential mechanisms of their interaction.
Limitations
A few factors limit the interpretation of our results. First, the limited amount of empirical data that is appropriate for inclusion in the meta-analysis has led to a few problems. For example, prospective studies that examined “clinically significant” cognitive decline and MCI or dementia were analyzed in the same model, despite the differences between these outcomes. However, sensitivity analysis showed similar results after removing the heterogeneity from the analysis. We focused on summarizing cross-sectional evidence on 3 broad cognitive domains owing to a limited number of studies that examined detailed components of the cognitive domain and the variations in the cognitive tests administered. Although some evidence was found on the relationship between SDB and executive function, the clinical relevance of these findings is unclear, given the modest effect sizes. Future studies should use comprehensive neuropsychological test batteries, and a greater number of studies is needed to examine the effects of SDB on specific cognitive abilities more carefully. The scarce information from existing studies has also limited our ability to perform subgroup analysis, eg, by age. Given the potential differences in SDB phenotypes among younger vs older populations,5,53,54 our analysis on the mixture of middle-aged and older populations might have led to underestimation of the overall association, and we were unable to differentiate the cognitive effects of SDB in different age groups. Besides, to control for confounding, we included only the most adjusted model within each study. However, the level of adjustment in each study was different, and many studies have failed to consider potentially important confounders, such as body mass index. Therefore, residual confounding remains a possibility. Finally, we used a clinical diagnosis or an AHI of 15 or greater to define SDB in the pooled analysis, as this is the cutoff used by most previous studies.33 This could have introduced misclassification that dilutes the association, especially given that the 3 different methods of scoring hypopneas could yield significantly different AHI values.55 Because there were not enough studies for meta-analysis if we were to perform the analysis separately for AHI3%, AHI3a, and AHI4%, we chose to use 1 cutoff in the analysis but have presented these methods narratively. This approach has also limited our ability to examine the association between the severity of SDB and cognitive outcomes. It is possible that while no association was found between certain cognitive domains and the dichotomized AHI, more severe SDB or other indicators of hypoxemia might be associated with significantly worse cognition. Future epidemiologic studies should consider continuous measures of AHI to minimize the possibility of misclassification and to reveal more information about the severity of SDB.
Conclusions
In summary, this meta-analysis of population-based studies suggests that individuals with SDB are 26% more likely to develop cognitive impairment. Given the high prevalence of both cognitive impairment and SDB, these findings could have significant clinical implications. Identification of SDB in elderly persons might help to predict future risk of cognitive impairment. Clinicians should closely follow patients who experience significant levels of SDB for the occurrence of cognitive dysfunction and might consider administering full neuropsychological batteries in some instances. This is potentially important for the early detection of dementia. Future studies are required to examine whether treatment of SDB could benefit cognition and to explore underlying mechanisms. Ultimately, this might open up new opportunities for the prevention of cognitive decline and dementia in elderly persons.
eFigure. Flowchart of literature search and inclusion studies.
References
- 1.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study: Sleep Heart Health Study. JAMA. 2000;283(14):1829-1836. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217-1239. [DOI] [PubMed] [Google Scholar]
- 3.Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: a bidirectional relationship. Circulation. 2012;126(12):1495-1510. [DOI] [PubMed] [Google Scholar]
- 4.Rajan P, Greenberg H. Obstructive sleep apnea as a risk factor for type 2 diabetes mellitus. Nat Sci Sleep. 2015;7:113-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haas DC, Foster GL, Nieto FJ, et al. Age-dependent associations between sleep-disordered breathing and hypertension: importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health Study. Circulation. 2005;111(5):614-621. [DOI] [PubMed] [Google Scholar]
- 6.Lavie P, Lavie L. Unexpected survival advantage in elderly people with moderate sleep apnoea. J Sleep Res. 2009;18(4):397-403. [DOI] [PubMed] [Google Scholar]
- 7.Kendzerska T, Mollayeva T, Gershon AS, Leung RS, Hawker G, Tomlinson G. Untreated obstructive sleep apnea and the risk for serious long-term adverse outcomes: a systematic review. Sleep Med Rev. 2014;18(1):49-59. [DOI] [PubMed] [Google Scholar]
- 8.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the Sleep Heart Health Study. Circulation. 2010;122(4):352-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayalon L, Ancoli-Israel S, Drummond SP. Obstructive sleep apnea and age: a double insult to brain function? Am J Respir Crit Care Med. 2010;182(3):413-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zimmerman ME, Aloia MS. Sleep-disordered breathing and cognition in older adults. Curr Neurol Neurosci Rep. 2012;12(5):537-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bliwise DL. Sleep apnea, APOE4 and Alzheimer’s disease 20 years and counting? J Psychosom Res. 2002;53(1):539-546. [DOI] [PubMed] [Google Scholar]
- 12.Saunamäki T, Himanen SL, Polo O, Jehkonen M. Executive dysfunction in patients with obstructive sleep apnea syndrome. Eur Neurol. 2009;62(4):237-242. [DOI] [PubMed] [Google Scholar]
- 13.Naëgelé B, Launois SH, Mazza S, Feuerstein C, Pépin JL, Lévy P. Which memory processes are affected in patients with obstructive sleep apnea? an evaluation of 3 types of memory. Sleep. 2006;29(4):533-544. [DOI] [PubMed] [Google Scholar]
- 14.Ju G, Yoon IY, Lee SD, Kim TH, Choe JY, Kim KW. Effects of sleep apnea syndrome on delayed memory and executive function in elderly adults. J Am Geriatr Soc. 2012;60(6):1099-1103. [DOI] [PubMed] [Google Scholar]
- 15.Blackwell T, Yaffe K, Laffan A, et al. ; Osteoporotic Fractures in Men Study Group . Associations between sleep-disordered breathing, nocturnal hypoxemia, and subsequent cognitive decline in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc. 2015;63(3):453-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramos AR, Tarraf W, Rundek T, et al. Obstructive sleep apnea and neurocognitive function in a Hispanic/Latino population. Neurology. 2015;84(4):391-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lutsey PL, Bengtson LG, Punjabi NM, et al. Obstructive sleep apnea and 15-year cognitive decline: the Atherosclerosis Risk in Communities (ARIC) Study. Sleep. 2016;39(2):309-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306(6):613-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim H, Dinges DF, Young T. Sleep-disordered breathing and psychomotor vigilance in a community-based sample. Sleep. 2007;30(10):1309-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haba-Rubio J, Marti-Soler H, Tobback N, et al. Sleep characteristics and cognitive impairment in the general population: the HypnoLaus study. Neurology. 2017;88(5):463-469. [DOI] [PubMed] [Google Scholar]
- 21.Sforza E, Roche F, Thomas-Anterion C, et al. Cognitive function and sleep related breathing disorders in a healthy elderly population: the SYNAPSE study. Sleep. 2010;33(4):515-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol. 2003;60(10):1385-1389. [DOI] [PubMed] [Google Scholar]
- 23.Evans DA, Funkenstein HH, Albert MS, et al. Prevalence of Alzheimer’s disease in a community population of older persons: higher than previously reported. JAMA. 1989;262(18):2551-2556. [PubMed] [Google Scholar]
- 24.Chang WP, Liu ME, Chang WC, et al. Sleep apnea and the risk of dementia: a population-based 5-year follow-up study in Taiwan. PLoS One. 2013;8(10):e78655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin MS, Sforza E, Roche F, Barthélémy JC, Thomas-Anterion C; PROOF study group . Sleep breathing disorders and cognitive function in the elderly: an 8-year follow-up study. the PROOF-synapse cohort. Sleep. 2015;38(2):179-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yaffe K, Nettiksimmons J, Yesavage J, Byers A. Sleep quality and risk of dementia among older male veterans. Am J Geriatr Psychiatry. 2015;23(6):651-654. [DOI] [PubMed] [Google Scholar]
- 27.Blackwell T, Yaffe K, Ancoli-Israel S, et al. ; Osteoporotic Fractures in Men Study Group . Associations between sleep architecture and sleep-disordered breathing and cognition in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc. 2011;59(12):2217-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dlugaj M, Weinreich G, Weimar C, et al. ; Heinz Nixdorf Recall Study Investigative Group . Sleep-disordered breathing, sleep quality, and mild cognitive impairment in the general population. J Alzheimers Dis. 2014;41(2):479-497. [DOI] [PubMed] [Google Scholar]
- 29.Foley DJ, Masaki K, White L, Larkin EK, Monjan A, Redline S. Sleep-disordered breathing and cognitive impairment in elderly Japanese-American men. Sleep. 2003;26(5):596-599. [DOI] [PubMed] [Google Scholar]
- 30.Spira AP, Blackwell T, Stone KL, et al. Sleep-disordered breathing and cognition in older women. J Am Geriatr Soc. 2008;56(1):45-50. [DOI] [PubMed] [Google Scholar]
- 31.Nikodemova M, Finn L, Mignot E, Salzieder N, Peppard PE. Association of sleep disordered breathing and cognitive deficit in APOE ε4 carriers. Sleep. 2013;36(6):873-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hrubos-Strøm H, Nordhus IH, Einvik G, et al. Obstructive sleep apnea, verbal memory, and executive function in a community-based high-risk population identified by the Berlin Questionnaire Akershus Sleep Apnea Project. Sleep Breath. 2012;16(1):223-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. the Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667-689. [PubMed] [Google Scholar]
- 34.Sharafkhaneh A, Giray N, Richardson P, Young T, Hirshkowitz M. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28(11):1405-1411. [DOI] [PubMed] [Google Scholar]
- 35.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed January 9, 2017.
- 38.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. [DOI] [PubMed] [Google Scholar]
- 40.Liguori C, Mercuri NB, Izzi F, et al. Obstructive sleep apnea is associated with early but possibly modifiable Alzheimer’s disease biomarkers changes. Sleep. 2017;40(5). [DOI] [PubMed] [Google Scholar]
- 41.Emamian F, Khazaie H, Tahmasian M, et al. The association between obstructive sleep apnea and Alzheimer’s disease: a meta-analysis perspective. Front Aging Neurosci. 2016;8:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beebe DW, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26(3):298-307. [DOI] [PubMed] [Google Scholar]
- 43.Stranks EK, Crowe SF. The cognitive effects of obstructive sleep apnea: an updated meta-analysis. Arch Clin Neuropsychol. 2016;31(2):186-193. [DOI] [PubMed] [Google Scholar]
- 44.Aloia MS, Arnedt JT, Davis JD, Riggs RL, Byrd D. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J Int Neuropsychol Soc. 2004;10(5):772-785. [DOI] [PubMed] [Google Scholar]
- 45.Engleman H, Joffe D. Neuropsychological function in obstructive sleep apnoea. Sleep Med Rev. 1999;3(1):59-78. [DOI] [PubMed] [Google Scholar]
- 46.Lim DC, Veasey SC. Neural injury in sleep apnea. Curr Neurol Neurosci Rep. 2010;10(1):47-52. [DOI] [PubMed] [Google Scholar]
- 47.Auerbach S, Yaffe K. The link between sleep-disordered breathing and cognition in the elderly: new opportunities? Neurology. 2017;88(5):424-425. [DOI] [PubMed] [Google Scholar]
- 48.Quan SF, Wright R, Baldwin CM, et al. Obstructive sleep apnea-hypopnea and neurocognitive functioning in the Sleep Heart Health Study. Sleep Med. 2006;7(6):498-507. [DOI] [PubMed] [Google Scholar]
- 49.Quan SF, Chan CS, Dement WC, et al. The association between obstructive sleep apnea and neurocognitive performance: the Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep. 2011;34(3):303-314B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim DC, Pack AI. Obstructive sleep apnea and cognitive impairment: addressing the blood-brain barrier. Sleep Med Rev. 2014;18(1):35-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nunomura A, Castellani RJ, Zhu X, Moreira PI, Perry G, Smith MA. Involvement of oxidative stress in Alzheimer disease. J Neuropathol Exp Neurol. 2006;65(7):631-641. [DOI] [PubMed] [Google Scholar]
- 52.Fazekas F, Enzinger C, Ropele S, Schmidt H, Schmidt R, Strasser-Fuchs S. The impact of our genes: consequences of the apolipoprotein E polymorphism in Alzheimer disease and multiple sclerosis. J Neurol Sci. 2006;245(1-2):35-39. [DOI] [PubMed] [Google Scholar]
- 53.McMillan A, Morrell MJ. Sleep disordered breathing at the extremes of age: the elderly. Breathe (Sheff). 2016;12(1):50-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bliwise DL. Epidemiology of age-dependence in sleep disordered breathing (Sdb) in old age: the Bay Area Sleep Cohort (Basc). Sleep Med Clin. 2009;4(1):57-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ho V, Crainiceanu CM, Punjabi NM, Redline S, Gottlieb DJ. Calibration model for apnea-hypopnea indices: impact of alternative criteria for hypopneas. Sleep. 2015;38(12):1887-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Flowchart of literature search and inclusion studies.