Abstract
Creutzfeldt-Jakob disease (CJD) is a rare prion disorder that has been the subject of both professional and public interest following the identification of variant CJD as a zoonotic disorder. There have been recent advances in diagnostic techniques, including real-time quaking-induced conversion and magnetic resonance imaging brain scan, that have allowed more accurate case recognition in all forms of CJD. Although the epidemic of variant CJD is clearly in decline, prevalence studies suggest that it may be premature to be complacent about concerns for public health.
Keywords: Creutzfeldt-Jakob, prion disorder, variant CJD, diagnosis
Introduction
Creutzfeldt-Jakob disease (CJD) belongs to a family of fatal degenerative disorders of the nervous system known as transmissible spongiform encephalopathies or prion diseases, which affect both animals and humans 1. The term prion, derived from proteinaceous infectious particle, was coined by Stanley Prusiner after the identification of the disease-associated protein 2. The normal prion protein, PrP C, is present in all mammalian species and is encoded by the prion gene ( PRNP) on human chromosome 20. The function of prion protein has not been established. Prion diseases are characterised by the deposition of PrP Sc, an abnormally misfolded isoform of the native prion protein, within the nervous system. The mechanism for triggering this conformational change is not known, but the accumulation of this abnormal prion protein leads to neuronal degeneration, astrocytic gliosis, and spongiform change, resulting in a uniformly fatal neurological disorder 3.
The human prion disorders are heterogeneous with different phenotypes, epidemiology, and pathogenesis. Sporadic CJD (sCJD) is the commonest human prion disease, accounting for around 85% of cases; 10–15% are associated with mutations of PRNP and 1% are iatrogenic, most frequently associated with prior treatment with human pituitary-derived hormones or human dura mater grafts. Variant CJD (vCJD) is a novel human prion disease which occurs predominantly in the UK and has been linked to the consumption of beef products contaminated with the agent of the cattle disease, bovine spongiform encephalopathy (BSE) 4– 6.
sCJD has a very rapid disease course; mean survival is six months. Indeed, over 90% of patients die within a year of symptom onset. The peak incidence is in the seventh decade, and younger (20–40s) or older (>80) cases are less common 7. The favoured hypothesis is that sCJD is a spontaneous neurodegenerative disease, resulting from either a somatic PRNP gene mutation or a random structural change in the PrP protein causing the formation of PrP Sc 2. An environmental source for the disease is not supported by epidemiological studies.
There have been recent developments in diagnostic investigations in CJD, and, although the vCJD outbreak is in decline, there are continuing concerns for public health in relation to the prevalence of infection in the normal population. These issues and the potential relevance of prion diseases to other neurodegenerative disorders are the main topics of this article.
Diagnosis
There is considerable variability in the way in which CJD can present clinically, which can make the initial diagnosis difficult. This heterogeneity of clinical presentation is linked, at least in part, to variations in the genotype at codon 129 of the prion protein gene and the type of PrP Sc deposited in the brain. The genotype at codon 129 can be methionine homozygous (MM), valine homozygous (VV), or heterozygous (MV), and in the UK population, the normal codon 129 distribution has been reported as 39% MM, 50% MV, and 11% VV 8. Two biochemically distinct forms of PrP Sc, type 1 and type 2, can be deposited in the brain.
The classic clinical features of sCJD are rapid cognitive decline, ataxia, and myoclonus terminating in an akinetic mute state. The diagnosis of CJD is dependent upon assessment of clinical features together with specialist investigations. There has been an evolution in the diagnosis of CJD in recent years with the identification of new diagnostic tests, and this has been reflected in changes to the formal diagnostic criteria for sCJD used in the European Union (EU).
Magnetic resonance imaging
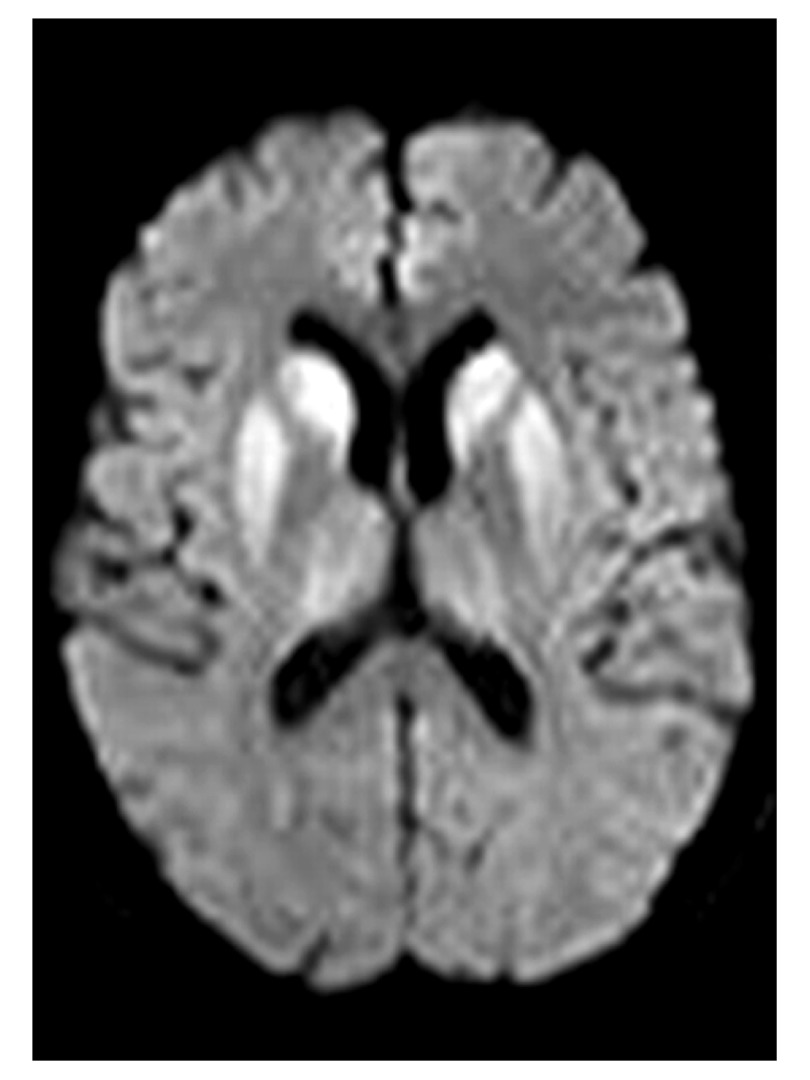
Magnetic resonance imaging in sporadic Creutzfeldt-Jakob disease. Magnetic resonance imaging (MRI) is the most useful investigation in sCJD, as it is highly sensitive and specific as well as widely available and relatively non-invasive 9. The classic MRI findings in sCJD, including high signal in the caudate, putamen, or cortex (or a combination of these) on diffusion-weighted imaging (DWI) and fluid-attenuated inversion recovery (FLAIR) sequences, are present in about 80% of cases 9. DWI is more sensitive at detecting early cortical and subcortical changes ( Figure 1).
Figure 1. Magnetic resonance imaging of sporadic Creutzfeldt-Jakob disease.
Diffusion-weighted image at the level of the basal ganglia demonstrates marked symmetrical hyperintensity in the caudate head and putamen with less marked affection of the thalami. Image courtesy of David Summers, Western General Hospital, Edinburgh, UK.
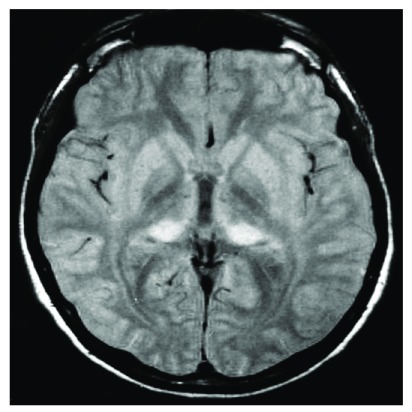
Magnetic resonance imaging in variant Creutzfeldt-Jakob disease. Symmetrical hyperintensity in the posterior thalamus, relative to the anterior putamen, on T2-weighted or FLAIR MRI is characteristic of vCJD and is known as the pulvinar sign ( Figure 2) 10. This finding is very rare in other types of prion disease 11 and is reported to have a sensitivity of 78–90% and a specificity of 100% for vCJD in the right clinical setting 5. The mediodorsal thalamic nucleus may also be involved and in combination with pulvinar hyperintensity produces an appearance coined the ‘hockey stick sign’. In 86 neuropathologically confirmed cases, involvement of the caudate nucleus on FLAIR MRI was shown in 40%, the putamen in 23.3%, and the periaqueductal grey matter in 83.3% ( Figure 2) 10.
Figure 2. Pulvinar sign of variant Creutzfeldt-Jakob disease.
Axial fluid-attenuated inversion recovery image demonstrates symmetrical hyperintensity of the posterior thalamic nuclei. Image courtesy of David Summers, Western General Hospital, Edinburgh, UK.
Until recently, all definite cases of vCJD have been MM at codon 129, which may account for the observed similarity of MRI findings in these cases when compared with other human prion diseases. This concept has been challenged, however, with the recent description of a neuropathologically confirmed case of vCJD with a heterozygous genotype at codon 129 12. The MRI findings in this case demonstrated restricted diffusion in the basal ganglia, hypothalamus, insular cortexes, and medial thalami but not in the pulvinar nuclei.
Real-time quaking-induced conversion
A number of cerebrospinal fluid (CSF) biomarkers, including 14-3-3, S100b, and tau, have been found to be elevated in patients with CJD. These proteins have limited specificity but can be diagnostically useful in the appropriate clinical context 13.
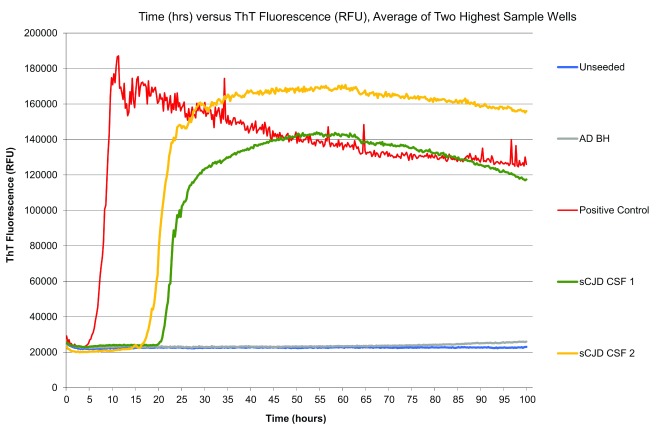
Real-time quaking-induced conversion (RT-QuIC) is a relatively new CSF test which has shown remarkably high sensitivity and specificity in recent studies (sensitivity of 85.7% and specificity of 100%) in sCJD 14. The technique involves using recombinant PrP as a substrate which is then induced to aggregate by adding CSF containing PrP Sc. Thioflavin T then binds to the aggregated PrP Sc, causing a change in the thioflavin T emission spectrum and enabling the reaction to be monitored in real time ( Figure 3). It is also a useful investigation for some genetic forms of CJD 15.
Figure 3. Real-time quaking-induced conversion (RT-QuIC) responses from reactions seeded with cerebrospinal fluid (CSF) from two sporadic Creutzfeldt-Jakob disease (sCJD) cases and a positive sCJD CSF control.
The RT-QuIC from an unseeded reaction and a reaction seeded with brain homogenate from Alzheimer’s disease are also shown. Image courtesy of Neil McKenzie, University of Edinburgh, Edinburgh, UK. AD BH, Alzheimer’s disease brain homogenate; RFU, relative fluorescence units; ThT, thioflavin T.
Real-time quaking-induced conversion olfactory mucosa and cerebrospinal fluid. The use of olfactory mucosa (OM) nasal brushings combined with RT-QuIC has been shown to improve the diagnosis of sCJD 16. In a recent study 17, the combined utilisation of CSF and OM RT-QuIC produced an overall specificity and sensitivity of 100% for the diagnosis of sCJD. OM RT-QuIC is limited, however, by the technical expertise required to obtain appropriate specimens. Although RT-QuIC is clearly a useful test for sCJD, the assay in its current form does not amplify the PrP Sc in vCJD 18.
Blood and urine tests in variant Creutzfeldt-Jakob disease
In an attempt to develop a better pre-mortem test for vCJD, a number of studies have attempted to detect the abnormal PrP Sc in vCJD in blood and urine by using protein misfolding cyclic amplification (PMCA). In 2014, Moda et al. successfully detected PrP Sc in the urine of patients with vCJD 19. The study looked at 238 urine samples from patients with vCJD, sCJD, or genetic prion diseases and healthy controls in a blinded study. Using PMCA, they were able to amplify the PrP Sc to detectable amounts with a sensitivity of 93% and a specificity of 100% in vCJD. All the sCJD and control samples were negative.
Similar studies using PMCA have led to the detection of PrP Sc in plasma from clinical vCJD as well as in pre-clinical blood samples taken from two vCJD patients who had donated blood prior to the onset of symptoms 20, 21. This is the first time that a test has successfully identified PrP Sc in pre-clinical samples, and it may have the potential to be applied as a screening tool in transfusion medicine, although further validation is necessary.
Electroencephalogram
The electroencephalogram (EEG) is now a less useful investigation given the advent of MRI and CSF tests. Nonetheless, it remains an important, non-invasive surrogate marker for sCJD. The typical EEG appearances in sCJD are periodic, triphasic sharp wave complexes. These changes in the right clinical setting have a 90% specificity for CJD 9 but are known to occur in other conditions, such as end-stage Alzheimer’s disease, Lewy body dementia, and metabolic encephalopathies 22, 23.
Diagnostic criteria for sCJD
The current diagnostic criteria used in the EU have been updated to include the cortical changes on MRI and RT-QuIC and are shown in Table 1.
Table 1. Diagnostic criteria for surveillance of sporadic Creutzfeldt-Jakob disease from 1 January 2017.
| 1.1 DEFINITE:
Progressive neurological syndrome AND Neuropathologically or immunohistochemically or biochemically confirmed |
| 1.2 PROBABLE:
1.2.1 I + two of II and typical electroencephalogram a OR 1.2.2 I + two of II and typical magnetic resonance imaging brain scan b OR 1.2.3 I + two of II and positive cerebrospinal fluid (CSF) 14-3-3 OR 1.2.4 Progressive neurological syndrome and positive real-time quaking-induced conversion in CSF or other tissues |
| 1.3 POSSIBLE:
I + two of II + duration <2 years |
| I Rapidly progressive cognitive impairment |
| II A Myoclonus
B Visual or cerebellar problems C Pyramidal or extrapyramidal features D Akinetic mutism |
aGeneralised periodic complexes. bHigh signal in caudate/putamen on magnetic resonance imaging brain scan or at least two cortical regions (temporal, parietal, occipital) on either diffusion-weighted imaging or fluid-attenuated inversion recovery.
Epidemiology
The incidence of sCJD is frequently quoted as one per million population per annum. However, there has been a consistent gradual increase in mortality rates in the UK and in many other countries in which systematic surveillance is undertaken. This may relate to changes in population demographics, with an increase in the numbers of individuals in the age cohorts with a high incidence of sCJD, and improved case ascertainment as a result of increased awareness and more sensitive diagnostic investigations. Mortality rates of 1.5–2 cases per million may be more realistic on current evidence.
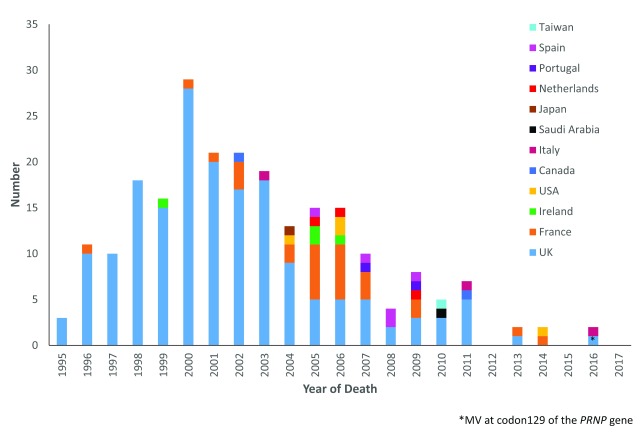
The vCJD epidemic is in marked decline both in the UK and internationally ( Figure 4). Until recently, all tested cases of definite and probable vCJD had been MM at codon 129 of PRNP, but in 2015 a confirmed case with an MV genotype was identified 12. This raises the possibility of a further outbreak, although it is likely that should this occur the number of cases will be relatively limited and probably lower than those of the primary epidemic in the MM population. The UK population were exposed to a significant level of BSE infectivity in the food chain in the 1980s and early 1990s, and the relatively small scale of the vCJD epidemic may imply a significant barrier to infection between bovines and humans 24.
Figure 4. Variant Creutzfeldt-Jakob disease cases by year and country.
MV, methionine valine heterozygous; PRNP, prion gene.
Secondary transmission of vCJD through blood transfusion is now established with three clinical cases of vCJD linked to the transfusion of non-leucodepleted red blood cells derived from individuals who themselves went on to develop vCJD 25. In addition, a recipient of an implicated transfusion who died of a non-neurological disorder was found to have PrP Sc positivity in the spleen, suggesting pre-clinical infection 26. This individual was a codon 129 heterozygote, and laboratory transmission studies demonstrated prion infectivity in the spleen with characteristics similar to those of primary cases.
The prevalence of vCJD infection in the general UK population has been studied by examining routine appendicectomy samples by immunocytochemistry. Positive samples were identified in 19 out of 33,115 appendices, leading to an estimated prevalence of 1 in 4,000 in the general population 27. This is a matter of concern, as there is no clinical evidence of infection prior to neuro-invasion in prion diseases and the mean incubation period in vCJD has been estimated to be 15 years. A more recent study examined appendix samples from before 1980, prior to the presumed start of the BSE epidemic, and in those born after 1996, after which human exposure to BSE was thought to be minimal. Surprisingly, 7 out of 15,959 of these samples were positive, suggesting either a more extended period of human exposure to BSE or perhaps that positivity may not imply infection with vCJD 28.
Other misfolding protein disorders
In addition to prion diseases, there are a number of diseases associated with protein misfolding. These conditions are associated with the accumulation of oligomers of A-beta, tau, and alpha-synuclein and include Alzheimer’s disease, Parkinson’s disease (PD), and Huntington’s 29. The identification of alpha-synuclein deposits in a foetal graft in patients with PD 30, 31 led to the hypothesis that there may be the potential of seeding of protein from abnormal to normal tissue and subsequently there have been extensive studies in laboratory animals that suggest that this may occur in many protein misfolding disorders 32. The presence of beta-amyloid deposits in the brains of human growth hormone recipients and not in age-matched controls has added to concerns about parallels between prion diseases and other neurodegenerative diseases 33, 34. Whether this is an important issue for public health is uncertain and there may be a difference between seeding of protein and the actual transmission of a disease. One epidemiological study, for example, has shown no evidence of transfusion transmission of a number of neurodegenerative disorders, including Alzheimer’s disease 35.
Conclusions
The diagnosis of CJD has improved in recent years with the advent of improved brain imaging and the development of specific CSF tests in sCJD and the potential for diagnostic tests in plasma and urine in vCJD. The public health concerns raised by the BSE epidemic have clearly decreased as new cases of vCJD have become a rarity in both the UK and other countries. However, the potential prevalence of infection in the general population remains a matter of concern and the parallels between prion diseases and other protein misfolding disorders are an area of active and continued research.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Gianluigi Zanusso, Department of Neurosciences, Biomedicine and Movement Sciences, Section of Neurology, University of Verona, Verona, Italy
Brian Appleby, Departments of Neurology, Psychiatry, and Pathology, University Hospitals-Cleveland Medical Center, Cleveland, Ohio, USA
Funding Statement
This report is based on independent research commissioned and funded by the Department of Health Policy Research Programme (National CJD Research and Surveillance PR-ST-061400008). The views expressed in this publication are those of the authors and not necessarily those of the Department of Health, ‘arms’ length bodies, or other government departments.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
References
- 1. World Health Organisation: WHO manual for surveillance of human transmissible spongiform encephalopathies, including variant CJD. Geneva.2003. Reference Source [Google Scholar]
- 2. Prusiner SB: Prions. Proc Natl Acad Sci U S A. 1998;95(23):13363–83. 10.1073/pnas.95.23.13363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ironside JW, Ghetti B, Head MW, et al. : Prion diseases. In: Greenfield’s neuropathology, 8th ed., Eds: Love S, Louis DN, Ellison DW. Chapter 16, Hodder Arnold, London.2008;1197–1273. [Google Scholar]
- 4. Will RG: Acquired prion disease: iatrogenic CJD, variant CJD, kuru. Br Med Bull. 2003;66:255–65. 10.1093/bmb/66.1.255 [DOI] [PubMed] [Google Scholar]
- 5. Will RG, Zeidler M, Stewart GE, et al. : Diagnosis of new variant Creutzfeldt-Jakob disease. Ann Neurol. 2000;47(5):575–82. [DOI] [PubMed] [Google Scholar]
- 6. Ward HJ, Everington D, Cousens SN, et al. : Risk factors for variant Creutzfeldt-Jakob disease: a case-control study. Ann Neurol. 2006;59(1):111–20. 10.1002/ana.20708 [DOI] [PubMed] [Google Scholar]
- 7. Ladogana A, Puopolo M, Croes EA, et al. : Mortality from Creutzfeldt-Jakob disease and related disorders in Europe, Australia, and Canada. Neurology. 2005;64(9):1586–91. 10.1212/01.WNL.0000160117.56690.B2 [DOI] [PubMed] [Google Scholar]
- 8. Bishop MT, Pennington C, Heath CA, et al. : PRNP variation in UK sporadic and variant Creutzfeldt Jakob disease highlights genetic risk factors and a novel non-synonymous polymorphism. BMC Med Genet. 2009;10:146. 10.1186/1471-2350-10-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zerr I, Kallenberg K, Summers DM, et al. : Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain. 2009;132(Pt 10):2659–68. 10.1093/brain/awp191 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Collie DA, Summers DM, Sellar RJ, et al. : Diagnosing variant Creutzfeldt-Jakob disease with the pulvinar sign: MR imaging findings in 86 neuropathologically confirmed cases. AJNR Am J Neuroradiol. 2003;24(8):1560–9. [PMC free article] [PubMed] [Google Scholar]
- 11. Petzold GC, Westner I, Bohner G, et al. : False-positive pulvinar sign on MRI in sporadic Creutzfeldt-Jakob disease. Neurology. 2004;62(7):1235–6. 10.1212/01.WNL.0000123265.91365.AF [DOI] [PubMed] [Google Scholar]
- 12. Mok T, Jaunmuktane Z, Joiner S, et al. : Variant Creutzfeldt-Jakob Disease in a Patient with Heterozygosity at PRNP Codon 129. N Engl J Med. 2017;376(3):292–4. 10.1056/NEJMc1610003 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. Green A, Sanchez-Juan P, Ladogana A, et al. : CSF analysis in patients with sporadic CJD and other transmissible spongiform encephalopathies. Eur J Neurol. 2007;14(2):121–4. 10.1111/j.1468-1331.2006.01630.x [DOI] [PubMed] [Google Scholar]
- 14. McGuire LI, Poleggi A, Poggiolini I, et al. : Cerebrospinal fluid real-time quaking-induced conversion is a robust and reliable test for sporadic creutzfeldt-jakob disease: An international study. Ann Neurol. 2016;80(1):160–5. 10.1002/ana.24679 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Sano K, Satoh K, Atarashi R, et al. : Early detection of abnormal prion protein in genetic human prion diseases now possible using real-time QUIC assay. PLoS One. 2013;8(1):e54915. 10.1371/journal.pone.0054915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Orrú CD, Bongianni M, Tonoli G, et al. : A test for Creutzfeldt-Jakob disease using nasal brushings. N Engl J Med. 2014;371(6):519–29. 10.1056/NEJMoa1315200 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Bongianni M, Orrù C, Groveman BR, et al. : Diagnosis of Human Prion Disease Using Real-Time Quaking-Induced Conversion Testing of Olfactory Mucosa and Cerebrospinal Fluid Samples. JAMA Neurol. 2017;74(2):155–62. 10.1001/jamaneurol.2016.4614 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Peden AH, McGuire LI, Appleford NE, et al. : Sensitive and specific detection of sporadic Creutzfeldt-Jakob disease brain prion protein using real-time quaking-induced conversion. J Gen Virol. 2012;93(Pt 2):438–49. 10.1099/vir.0.033365-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moda F, Gambetti P, Notari S, et al. : Prions in the urine of patients with variant Creutzfeldt-Jakob disease. N Engl J Med. 2014;371(6):530–9. 10.1056/NEJMoa1404401 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Concha-Marambio L, Pritzkow S, Moda F, et al. : Detection of prions in blood from patients with variant Creutzfeldt-Jakob disease. Sci Transl Med. 2016;8(370):370ra183. 10.1126/scitranslmed.aaf6188 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Bougard D, Brandel JP, Bélondrade M, et al. : Detection of prions in the plasma of presymptomatic and symptomatic patients with variant Creutzfeldt-Jakob disease. Sci Transl Med. 2016;8(370):370ra182. 10.1126/scitranslmed.aag1257 [DOI] [PubMed] [Google Scholar]
- 22. Zeidler M, Meslin F: WHO Manual for strengthening diagnosis and surveillance of CJD. Geneva,1998. Reference Source [Google Scholar]
- 23. Smith SJ: EEG in neurological conditions other than epilepsy: when does it help, what does it add? J Neurol Neurosurg Psychiatry. 2005;76 Suppl 2:ii8–12. 10.1136/jnnp.2005.068486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen CC, Wang YH: Estimation of the exposure of the UK population to the bovine spongiform encephalopathy agent through dietary intake during the period 1980 to 1996. PLoS One. 2014;9(4):e94020. 10.1371/journal.pone.0094020 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Urwin PJ, Mackenzie JM, Llewelyn CA, et al. : Creutzfeldt-Jakob disease and blood transfusion: updated results of the UK Transfusion Medicine Epidemiology Review Study. Vox Sang. 2016;110(4):310–6. 10.1111/vox.12371 [DOI] [PubMed] [Google Scholar]
- 26. Peden AH, Head MW, Ritchie DL, et al. : Preclinical vCJD after blood transfusion in a PRNP codon 129 heterozygous patient. Lancet. 2004;364(9433):527–9. 10.1016/S0140-6736(04)16811-6 [DOI] [PubMed] [Google Scholar]
- 27. Gill ON, Spencer Y, Richard-Loendt A, et al. : Prevalent abnormal prion protein in human appendixes after bovine spongiform encephalopathy epizootic: large scale survey. BMJ. 2013;347:f5675. 10.1136/bmj.f5675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Public Health England: Summary results of the third national survey of abnormal prion prevalence in archived appendix specimens. Health Protection Weekly Report, Infection Report.2016;10(26). Reference Source [Google Scholar]
- 29. Frost B, Diamond MI: The expanding realm of prion phenomena in neurodegenerative disease. Prion. 2009;3(2):74–7. 10.4161/pri.3.2.8754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li JY, Englund E, Holton JL, et al. : Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat Med. 2008;14(5):501–3. 10.1038/nm1746 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Kordower JH, Chu Y, Hauser RA, et al. : Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med. 2008;14(5):504–6. 10.1038/nm1747 [DOI] [PubMed] [Google Scholar]
- 32. Jucker M, Walker LC: Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501(7465):45–51. 10.1038/nature12481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jaunmuktane Z, Mead S, Ellis M, et al. : Evidence for human transmission of amyloid-β pathology and cerebral amyloid angiopathy. Nature. 2015;525(7568):247–50. 10.1038/nature15369 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Ritchie DL, Adlard P, Peden AH, et al. : Amyloid-β accumulation in the CNS in human growth hormone recipients in the UK. Acta Neuropathol. 2017;134(2):221–40. 10.1007/s00401-017-1703-0 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Edgren G, Hjalgrim H, Rostgaard K, et al. : Transmission of Neurodegenerative Disorders Through Blood Transfusion: A Cohort Study. Ann Intern Med. 2016;165(5):316–24. 10.7326/M15-2421 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation