Abstract
Ventilator-associated pneumonia (VAP) is the most frequent life-threatening nosocomial infection in intensive care units. The diagnostic is difficult because radiological and clinical signs are inaccurate and could be associated with various respiratory diseases. The concept of infection-related ventilator-associated complication has been proposed as a surrogate of VAP to be used as a benchmark indicator of quality of care. Indeed, bundles of prevention measures are effective in decreasing the VAP rate. In case of VAP suspicion, respiratory secretions must be collected for bacteriological secretions before any new antimicrobials. Quantitative distal bacteriological exams may be preferable for a more reliable diagnosis and therefore a more appropriate use antimicrobials. To improve the prognosis, the treatment should be adequate as soon as possible but should avoid unnecessary broad-spectrum antimicrobials to limit antibiotic selection pressure. For empiric treatments, the selection of antimicrobials should consider the local prevalence of microorganisms along with their associated susceptibility profiles. Critically ill patients require high dosages of antimicrobials and more specifically continuous or prolonged infusions for beta-lactams. After patient stabilization, antimicrobials should be maintained for 7–8 days. The evaluation of VAP treatment based on 28-day mortality is being challenged by regulatory agencies, which are working on alternative surrogate endpoints and on trial design optimization.
Keywords: ventilator-associated pneumonia, VAP, nosocomial infection, antimicrobials
Introduction
Hospital-acquired pneumonia (HAP) is defined by an infection of the lung parenchyma that occurred at least 48 hours after hospital admission. Ventilator-associated pneumonia (VAP) develops in intensive care unit (ICU) patients mechanically ventilated for at least 48 hours 1, 2. In contrast, ventilator-associated tracheobronchitis (VAT) is characterized by signs of respiratory infection without new radiographic infiltrates in a patient mechanically ventilated for at least 48 hours 3– 5.
In the past 10 years, a great deal of progress has been made in understanding VAP. New concepts of infection-related ventilator-associated complications (IVACs) and ventilator-associated events (VAEs) have been proposed as outcome indicators for prevention strategies 6. In diagnostic strategies, criteria used to suspect a VAP have been challenged, as have optimal diagnostic tests used to confirm it 7. Traditional risk factors of VAP due to multidrug-resistant (MDR) bacteria (based on early-onset occurrence and previous antimicrobial therapy) are no longer sufficient. Proposed empirical therapy has been modified accordingly. The optimization of pharmacokinetic/pharmacodynamic parameters is now considered a key factor to ensure adequate and successful therapy. The use of adjunctive aerosolized therapy is also more and more debated. In addition, regulatory agencies are trying to find surrogate endpoints to replace 28-day mortality and to improve the design of randomized clinical trials in this field of investigation 8.
For VAP prevention, the concept of bundle of care was defined. It enabled great successes in VAP prevention; however, the insufficient compliance observed in clinical practice needs to be addressed in order to define easier-to-apply procedures.
This review aims to summarize the available knowledge on VAP, taking profit from the recent publication of North American 7 and European guidelines on VAP management and highlighting recent advances and remaining controversies of the new concepts.
Epidemiology
VAP is the second most common nosocomial infection and the leading cause of death from nosocomial infections in critically ill patients 9. Its incidence ranges from 5% to 67% depending on case mix and the diagnostic criteria used 10, and the highest rates are in immunocompromised, surgical, and elderly patients. In the US, the incidence of VAP ranges from 2 to 16 episodes per 1,000 ventilator-days 11. The estimated risk of VAP is 1.5% per day and decreases to less than 0.5% per day after the 14th day of mechanical ventilation 12. VAP increases the duration of hospitalization by 7 days and health-care costs by approximately $40,000 USD 13.
In published studies, the crude mortality of patients with VAP is highly variable according to case mix and definitions used 14– 16. The definition of attributable VAP mortality is the percentage of deaths that would not have occurred in the absence of the infection. Recent studies have reappraised the impact of VAP on mortality 17– 19. Specifically, given that the risk of VAP is time-dependent, this could potentially result in a significant time-dependent bias because mortality and ICU discharge both act as competing endpoints. Indeed, the most recent studies reported an attributable mortality below 10% with surgical patients 18 whereas those with mid-range illness severity presented the highest associated risk 18, 19.
Late-onset VAP is often reported to be associated with higher mortality rates than early-onset VAP 20– 22. Using a multistate model, we confirmed that the attributable mortality for early-onset VAP (5.8%) was considerably lower than for late-onset VAP (10.6%) 18.
Most studies showed that VAP is usually due to aerobic Enterobacteriaceae (25%), Staphylococcus aureus (20%), Pseudomonas aeruginosa (20%), Haemophilus influenza (10%), and streptococci 23. MDR pathogens are more common among late-onset cases. Trouillet et al. 24 found that prior use of broad-spectrum antibiotics and mechanical ventilation of more than 7 days were independent risk factors of infection caused by MDR pathogens. However, more recent reports 25– 29 have identified similar rates of etiologies in patients with early- versus late-onset VAP. This may be related to the worldwide rise in MDR pathogens; it emphasizes that the local ICU ecology 30 is the most important risk factor for acquiring MDR pathogens, irrespective of the length of intubation. In early-onset pneumonia, the initial VAP severity—that is, the presence of sepsis or septic shock (odds ratio [OR] = 3.7)—and pneumonia that developed in a center with a prevalence of resistant pathogens greater than 25% were independently associated with the presence of resistant pathogens (OR = 11.3) 26.
Risk factors of ventilator-associated pneumonia
VAP results from the microbial invasion of the normally sterile lower respiratory tract, which subsequently can overwhelm the host’s defense and establish infection. The major route for microbial invasion is microaspiration of oropharyngeal secretions contaminated by endogenous flora around the endotracheal tube cuff 31. VAP may also occur by other means 2, 32. In terms of potential reservoirs, it has been suggested that the stomach hosts bacteria that colonize the oropharynx. It has been postulated by some researchers that embolization into the alveoli during suctioning or bronchoscopy is caused by the colonization of the endotracheal tube with bacteria encased in a biofilm 33. Inhalation of pathogens from contaminated aerosols and direct inoculation are less common, and hematogenous spread from either infected intravascular catheters or bacterial translocation of the gastrointestinal tract lumen are rarer in occurrence.
Consequently, two groups of risk factors for VAP have been identified—namely ventilation-related factors (instrumentation of the airway with an endotracheal tube and subsequent microaspirations) and, less frequently, patient-related factors (for example, pre-existing pulmonary disease)—and only the former is accessible to prevention ( Table 1). As a result, VAP, unlike many other nosocomial infections, is difficult to prevent 34.
Table 1. Risk factors of ventilator-associated pneumonia.
| Host-related risk factors | Intervention-related risk factors |
|---|---|
| Medical history and underlying illness
Male gender Extreme age Prior central nervous system disorder Immunocompromised Acute underlying diseases Emergent surgery Neurosurgery Thoracic surgery Cardiac surgery Burns Re-intervention Acute severity factors Organ system failure index of at least 3 Acute renal failure Acute respiratory distress syndrome ECMO, intra-aortic support Ulcer disease |
Peri-operative transfusion of blood products
Duration of the mechanical ventilation Reintubation Supine head position in patients receiving enteral nutrition Antibiotic therapy a Enteral nutrition Absence of subglottic secretion drainage b Intra-hospital transports Continuous sedation, use of paralytic agents Nasogastric tubes Tracheostomy Frequent ventilator circuit changes Intracuff pressure of less than 20 cm H 2O |
Adapted from 2, 35– 38. aAntibiotic therapy protects from early-onset pneumonia due to susceptible bacteria but is a risk factor for late-onset pneumonia due to more resistant organisms. bProtective impact of subglottic secretion drainage is mainly demonstrated for cardiac surgery patients. ECMO, extra-corporeal membrane oxygenation.
Prevention
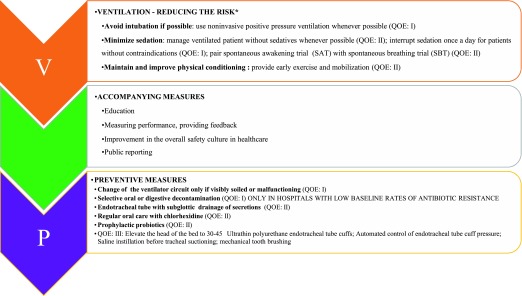
First of all, reducing the exposure to risk factors for VAP is the most efficient way to prevent VAP onset ( Figure 1). Therefore, intubation should be avoided whenever possible, and strategies such as non-invasive positive-pressure ventilation, sedation, and weaning protocols should be used to replace or shorten mechanical ventilation. In contrast, recent data suggest that the timing of the tracheotomy does not significantly change VAP incidence 40– 43.
Figure 1. Preventive measures of ventilator-associated pneumonia.
Patients at risk of VAP must be managed with a “bundle of preventive measures” ( Figure 1). Indeed, no single preventive strategy will efficiently prevent VAP. Bundles group together a small straightforward set of key interventions that are from evidence-based guidelines—generally three to five—and that are expected to result in a better outcome when performed collectively and reliably instead of individually. However, the ideal set of key preventive measures is unknown 44. Importantly, although studies demonstrated great success in reducing VAP rates using bundle of care in recent years 44– 48, meta-analyses showed that most of the preventive measures failed to demonstrate a sustained effect 49. This conclusion is in line with the absence of substantial improvement of VAP rates in the past decade 50. Figure 1 lists recommended preventive measures according to their level of evidence.
The sole preventive measures that positively impacted mortality are selective digestive decontamination (SDD) and selective oropharyngeal decontamination (SOD) 51. Compared with SOD, SDD was associated with a lower mortality, reduced length of stay, lower rates of ICU-acquired bacteremia and candidemia, and lower prevalence of rectal carriage of antibiotic-resistant Gram-negative bacteria but with a pronounced gradual increase in aminoglycoside-resistant Gram-negative bacteria 52. The main remaining question is the reproducibility of these results out of the Netherlands. Indeed, the antibiotic selection pressure induced by SOD or SDD may outweigh their benefits in countries with high levels of bacterial resistance.
Oral care with chlorhexidine is also debated. An updated meta-analysis focusing on double-blind studies in non-cardiac surgery patients showed that it had no impact on VAP rates or duration of mechanical ventilation or duration of ICU stay 53.
Ecological Effects of Decolonization Strategies in Intensive Care (RGNOSIS), a cluster-randomized study (ClinicalTrials.gov identifier NCT02208154) conducted in six European countries, is currently enrolling 10,800 patients into four arms: control, oral care with chlorhexidine, SOD, and SDD. The study’s new insights into these ongoing debates are awaited.
Many possible factors may explain why prevention measures did not result in reductions in mortality, duration of stay, or antibiotic consumption. First of all, the VAP definition may not be sufficiently accurate, especially when tested intervention could not be blinded. Second, in recent studies using modern statistics, the attributable mortality of VAP is only 3–4%, considerably smaller than previously reported 19. Both factors may induce a dramatic decrease of the power of the studies available.
Even if there is convincing evidence that specific interventions might prevent VAP, translating research into practice remains a challenge ( Figure 1). Two European surveys found that 37.0% of ICU physicians 54 and 22.3% of nurses 55 did not comply with the published recommendations for VAP prevention. Beyond the theoretical frame, a great deal of attention must be given to the factors that might facilitate a bundle implementation and allow a sustained compliance. An educational session alone, without an associated behavioral strategy, is unlikely to induce profound behavioral changes. It should be kept in mind that, to engage an individual in a particular behavior and improve compliance, we need to act on predisposing factors (knowledge, perceptions, and beliefs) to favor the access to new processes or technologies and to continually reinforce the behavior by feedback 56, 57.
Diagnosis
VAP, VAE, IVAC, and VAT: what do these abbreviations mean?
The diagnosis of VAP is traditionally based on clinical symptoms and radiographic criteria that require further bacteriological confirmation. However, it has been demonstrated that these criteria are inaccurate 14, 58, 59. Of note, VAP is now considered an indicator of performance in the US and some other countries. The National Healthcare Society Network reported a considerable decrease in the VAP incidence rate attributed to a multifaceted infection control program and its effective implementation 60. A 70% decrease of the incidence between 2006 and 2012 was reported by the Centers for Disease Control and Prevention (CDC). But in the same period, the Medicare Patient Safety Monitoring System reported an adjusted average annual change of 0% (95% confidence interval (CI) −0.05 to 0.07) in patients 65 years old or older 50. These findings emphasize a true discrepancy between rates reported in a quality monitoring program and rates observed in patients’ care program.
The discrepancies between results prompted the CDC to promote new objectives of the surveillance based on VAEs. Another important motivation of the CDC was to expand the purview of quality and safety surveillance to encompass multiple complications in mechanically ventilated patients instead of just pneumonia alone.
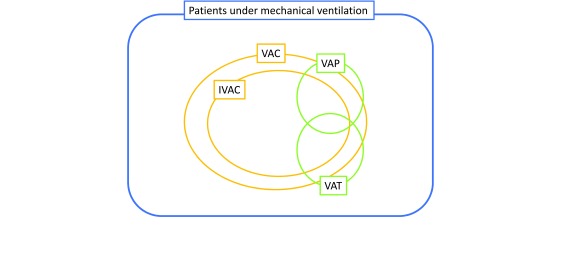
The VAE surveillance definition algorithm uses three new indicators: ventilator-associated complications (VACs), IVACs, and possible and probable VAP 61. VAC is the first step of VAE surveillance, with the aim of identifying any complication occurring in mechanically ventilated patients, regardless of the origin or mechanism. To meet the definition of VAC, a mechanically ventilated patient must have at least 2 days of stability or improvement of respiratory parameters—such as a stable or decreasing daily minimum positive end-expiratory pressure (PEEP) or fraction of inspired oxygen (FiO 2)—followed by at least 2 days of worsened oxygenation (diagnosed by an increase of the daily minimum PEEP (at least 3 cm H 2O) or FiO 2 (at least 20%)). The concept of IVAC aims to identify the subgroup of VACs that are potentially related to infection. A VAC associated with an abnormal white blood cell count or a modified temperature becomes an IVAC if the initiation of a new antimicrobial agent is maintained for at least 4 days. With evidence of purulent respiratory secretions or positive results of microbiological tests performed on respiratory tract specimens or both, an IVAC becomes a possible VAP. All of these definitions are summarized in Figure 2.
Figure 2. Ventilator-associated events, definitions, and nosology.
Ventilator-associated conditions (VACs): at least 2 calendar days of stable or decreasing daily minimum positive end-expiratory pressure (PEEP) or fraction of inspired oxygen (FiO 2) followed by rise in PEEP of at least 3 cm H 2O or rise in FiO 2 of at least 20 points sustained for at least 2 days. Infection-related ventilator-associated complications (IVACs): VAC plus: temperature of less than 36°C or more than 38°C OR white blood cell (WBC) count of not more than 4 or at least 12 × 10 3 cells/mm 3 AND at least one new antibiotics continued for at least 4 days WITHIN 2 days of VAC onset EXCLUDING first 2 days on the ventilator. Possible ventilator-associated pneumonia (VAP) (Centers for Disease Control and Prevention [CDC] definitions): IVAC plus: criterion 1: Positive culture meeting specific quantitative or semi-quantitative threshold; criterion 2: Purulent respiratory secretions AND identification of organisms NOT meeting the quantitative or semi-quantitative thresholds; criterion 3: Organisms identified from pleural fluid specimen, positive lung histopathology, and positive diagnostic test for Legionella species or selected respiratory viruses WITHIN 2 days of VAC onset EXCLUDING first 2 days on the ventilator. (The updated January 2017 definitions and comprehensive examples are detailed in the CDC National Healthcare Society Network website; https://www.cdc.gov/nhsn/pdfs/pscmanual/10-vae_final.pdf; accessed 23 October 2017.) VAP: radiographic criteria (new or progressive and persistent infiltrates or consolidation or cavitation); systemic criteria (temperature of less than 36°C or more than 38°C OR WBC count of not more than 4 or at least 12 × 10 3 cells/mm 3); pulmonary criteria (at least one of the following: (1) new onset or increase of purulent aspirates and (2) worsening gas exchange). Ventilator-associated tracheobronchitis (VAT): criteria for VAP but without radiographic criteria.
The VAE concept uses objective criteria and their collection can be automated for systematic recording. The VAP definition is still widely discussed, and a recent study showed that applying the various diagnostic criteria to the same patient population resulted in large differences in the incidence of VAP (that is, from 4% to 42%) 62. Furthermore, even distal quantitative samples are not 100% reproducible 63, 64.
This new approach might overcome the inaccuracy of the VAP definition, facilitate its electronic assessment, and make inter-ICU comparisons more relevant. Second, the association between VAE and antibiotic consumption (considering VAC rates and not only IVAC) was a point in favor of using VAC rate as one indicator of ICU quality of care for antimicrobial stewardship programs 12. Of note, VAE has very low sensitivity and specificity in diagnosing VAP 12. In our experience, VAP accounted for only 14.5% of the VAC episodes and 27.6% of the IVAC episodes 12; in addition, not all IVAC episodes were related to a nosocomial infection.
Furthermore, radiological criteria are not taken into account, so that the IVAC definition includes VAT and VAP ( Figure 2). Although VAP and VAT are both associated with an increased duration of mechanical ventilation, VAP impact on ICU mortality is higher than that of VAT 27. Finally, embedding VAP in the larger definition of IVAC may hamper the understanding of VAP pathophysiology and thus its prevention improvement.
Which bacteriological samples should be collected in case of suspicion of ventilator-associated pneumonia?
Great controversies persist about the bacteriological samples that should be used for diagnosing VAP. Of note, when bacteriological analyses are not immediately available, processing of a bacteriological specimen refrigerated after collection is a reliable alternative 65. Invasive techniques, such as bronchoalveolar lavage or protected specimen brush with quantitative culture, require qualified clinicians. Randomized studies that have evaluated their value as compared with proximal qualitative samples yielded contradictory results 66– 68. In one study with 413 patients 67, the invasive distal quantitative strategy was combined with an algorithm for treatment de-escalation and led to a significant increase in the number of antibiotic-free days at day 14 (5.0 ± 5.1 versus 2.2 ± 3.5) and day 28 (11.5 ± 9.0 versus 7.5 ± 7.6) in comparison with the strategy with non-invasive methods using qualitative cultures. In contrast, the Canadian Critical Care Trial Group reported no impact of distal quantitative samples on the day-28 antibiotic-free days or on survival 66. However, in that study, the research protocol may have facilitated appropriate discontinuation of antibiotics or targeted therapy in the two groups, thus minimizing the differences between them. Cohort studies confirmed the potential advantages of distal quantitative samples in narrowing antimicrobial therapy and limiting antibiotic selection pressure without adverse effects on mortality or length of stay 69– 71. Finally, an observational study in 89 patients with clinically suspected VAP and a negative quantitative bronchoalveolar lavage compared patients with early (within one day) and late antibiotic discontinuation. Early discontinuation was associated with a non-significant decrease in mortality and significantly lower risks of overall superinfections (22.5% versus 43%), respiratory superinfections (10% versus 29%), and superinfections due to MDR pathogens (7.5% versus 36%) 72.
Considering available literature, recent US guidelines recommend non-invasive sampling with semi-quantitative culture 7, whereas the European guidelines suggest obtaining distal samples with quantitative cultures to improve the accuracy of results 9. Despite this discrepancy, the two guidelines agreed that a bacteriological sample should be performed before any antibiotic treatment in order to reduce antibiotic exposure.
Treatment of ventilator-associated pneumonia
The initial treatment of VAP is based on empirical choices; however, an inappropriate initial antibiotic choice is associated with increased mortality 21, 73. In addition, the recovery of MDR bacteria is clearly associated with an increased risk of inappropriate therapy 74. As discussed earlier, the risk of MDR is conditioned by the local ecological data, previous colonization, and previous antibiotic therapy received by the patients. The increase in the risk of MDR in late-onset infections is challenged by recent studies. Regimens proposed by the North American guidelines are listed in Table 2 7. An algorithm for an empirical therapy strategy combining guidelines and practical rules is proposed in Figure 3.
Table 2. Empirical treatment of hospital-acquired pneumonia/ventilator-associated pneumonia.
| Not at high risk of mortality
and no risk factors a |
Not at high risk of mortality but with factors
increasing the likelihood of Gram-negative bacteria |
High risk of mortality or receipt of intravenous
antibiotics during the prior 90 days |
| One of the following:
Piperacillin-tazobactam 4.5 g IV q6h OR Cefepime 2 g IV q8h Levofloxacin 750 mg IV daily |
Piperacillin-tazobactam 4.5 g IV q6h
OR Cefepime or ceftazidime 2 g IV q8h OR Levofloxacin 750 mg IV daily Ciprofloxacin 400 mg IV q8h OR Imipenem 1g IV q8h Meropenem 1 g IV q6h |
Piperacillin-tazobactam 4.5 g IV q6h
OR Cefepime or ceftazidime 2 g IV q8h OR Levofloxacin 750 mg IV daily Ciprofloxacin 400 mg IV q8h OR Imipenem 1g IV q8h Meropenem 1 g IV q6h AND Amikacin 25 (30) mg/kg IV daily OR Gentamicin 5–7 mg/kg IV daily OR Tobramycin 5–7 mg/kg IV daily |
| Vancomycin 15 mg/kg IV q8–12h with goal to target
15–20 mg/mL trough level (consider a loading dose of 25–30 mg/kg × 1 for severe illness) OR Linezolid 600 mg IV q12h |
Vancomycin 15 mg/kg IV q8–12h with goal to
target 15–20 mg/mL trough level (consider a loading dose of 25–30 mg/kg × 1 for severe illness) OR Linezolid 600 mg IV q12h |
Adapted from Infectious Diseases Society of America/American Thoracic Society guidelines 7. aRisk factors of multidrug-resistant ventilator-associated pneumonia (VAP) are prior intravenous use within 90 days, septic shock at VAP onset, acute respiratory distress syndrome preceding VAP, five or more days of hospitalization prior to VAP onset, and acute renal replacement therapy prior to VAP onset. IV, intravenous; q, every.
Figure 3. Proposed strategy for empirical therapy.
*In areas with a risk of multidrug-resistant and carbapenemase-producing bacteria, the empirical choice should be decided on the basis of local ecology. 3rd GC, third-generation cephalosporin; ARDS, acute respiratory distress syndrome; ATB, antibiotics; GNB, Gram-negative bacteria; MDR, multidrug-resistant; MRSA, methicillin-resistant Staphylococcus aureus; PA, Pseudomonas aeruginosa; PIP/TAZ, piperacillin-tazobactam; R, Resistant; VAP, ventilator-associated pneumonia.
The challenge for the intensivist is to start an antimicrobial therapy that will be immediately effective while avoiding any overuse of extended-spectrum antimicrobials. New rapid diagnostic tests have been developed but their performances for VAP diagnosis remain to be evaluated 75, 76. Rapid nucleic acid amplification or mass spectrometry-based techniques provide rapid identification of targeted microorganisms. Some of these new tests are also able to detect resistance genes. However, the presence of genes detected by these techniques does not mean that the pathogens are alive or dead, nor does it provide information regarding phenotypic antimicrobial susceptibility. Rapid culture with semi-automated rapid antibiotic susceptibility tests are also in development. Fluorescence in situ hybridization-based microscopy identification and antibiotic susceptibility test (ID/AST) systems can evaluate antibiotic susceptibility from respiratory secretions on a previously defined panel of pathogens. A recent pilot study reported promising results: the technique was able to detect pathogens in bronchoalveolar lavage after 5 ± 7 hours of culture and 5 hours of analysis, and sensitivity and specificity were 100% and 97%, respectively 77. Technical developments with a better selection and quantification of pathogens and resistance patterns are warranted.
Beta-lactams remain a cornerstone antibiotic for the treatment of VAP. Critical care patients exhibit high clearance and distribution volume, which contribute to low blood levels of antimicrobials 78. Therefore, the doses that should be used to treat the most severe patients are frequently higher than the ones approved by regulatory agencies 79. For β-lactams, the best results seem to be associated with β-lactam plasma levels up to four times the minimal inhibitory concentration (MIC) of the involved pathogen and during 100% of the interval between each dose 80. For these agents, a loading dose followed by a continuous infusion may be a relevant method of administration to increase the antibiotic concentration in the blood and the lung lining fluid with a lower risk of neurological 81 or renal 82 toxicity. It is of special importance when bacteria are not fully susceptible or when MICs are high, as for P. aeruginosa and MDR Gram-negative bacteria 83.
Combination therapy with aminoglycosides increases the likelihood to immediately achieve an adequate therapy, especially for infection due to MDR Gram-negative bacteria 84. It is associated with an improved prognosis in the most severe patients 85. A dose as high as 25 mg/kg of amikacin is required to reach the optimal 60 mg/L peak concentration, even in the case of renal failure. Indeed, the distribution volume of aminoglycosides is not affected by renal dysfunction 86. However, renal impairment, present in almost 30% of ICU patients, will lead to prolonged intervals between doses, reducing the actual number of peak levels, thus possibly affecting the treatment efficiency.
Indeed, controversies still exist about advantages and disadvantages of aminoglycosides. Ong et al. compared empirical therapy of septic shock with or without gentamicin in two Dutch ICUs 87. One of the ICUs preferentially used aminoglycosides, whereas the other preferentially avoided them. After careful adjustment on patients’ characteristics, they found that gentamicin is an independent predictor of renal failure without affecting mortality. The main limitations of this study include (1) the absence of random allocation, (2) the absence of control on center effect, (3) the setting in two ICUs where the rate of MDR Gram-negative organisms is very low with a minimal risk of inadequate therapy, and (4) the absence of therapeutic drug monitoring for gentamicin. Nevertheless, the study by Ong et al. 87 emphasizes the importance of new well-conducted studies comparing antimicrobial therapy with or without aminoglycosides, given with careful therapeutic drug monitoring, for the most severe ICU patients.
Combination therapy with fluoroquinolones was associated with a decrease in the number of days alive without relapse or reinfection in a cohort of patients with VAP due to P. aeruginosa or Enterobacteriaceae and without any prior treatment with fluoroquinolones 88. However, the fluoroquinolones are inconstantly active against MDR Gram-negative bacteria and their use is associated with an important risk of emergence of MDR bacteria in the lung and gut microbiota 89.
Concerning extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBL-PE), carbapenems remain the first-line agents despite leading to the risk of emergence and spread of carbapenemase-producing Enterobacteriaceae 90. Other options such as piperacillin/tazobactam or a high dose of third-generation cephalosporins administered by continuous infusion could be considered, especially as step-down therapy for ESBL-PE with low MICs 30. New compounds such as ceftolozane/tazobactam and ceftazidime/avibactam have recently been released; however, against HAP/VAP, only the latter was non-inferior to meropenem in a recent randomized trial (REPROVE NCT01808092; results available on ClinicalTrials.gov, not yet published). Temocillin, a ticarcillin derivative that resists ESBL, can be used but only as a step-down therapy for pathogens with MICs below 8 mg/L 91. For carbapenem-resistant Gram-negative bacteria, colistin is a cornerstone of the treatment, although ceftazidime/avibactam association might be effective. A new association of meropenem and vaborbactam (M-V), recently approved for severe urinary tract infections in the US, has been compared with best available therapy (BAT) in severe infection presumably due to carbapenemase R Enterobacteriaceae, including nosocomial pneumonia (TANGO-2 NCT 02168946). The study was presented at the IDWeek convention (in San Diego, CA, in October 2017; abstract 1867) and enrolled 43 patients (more than 80% with Klebsiella pneumoniae carbapenemase). It has been stopped prematurely for significant superiority in terms of clinical failure, nephrotoxicity, and non-significant improvement of day-28 mortality of patients with HAP/VAP (M-V 4/16 versus BAT 4/9). Further studies are awaited to confirm these encouraging results and make formal recommendations on its use. An intravenous colistin regimen should be considered with a loading dose of 9 MU and with caution regarding its potential nephrotoxicity 92.
Inhaled antimicrobial therapy may be considered, as this route of administration enables very high concentrations of antimicrobials to be locally delivered 93, 94. However, there are no solutions specifically formulated for inhalation, and a limited number of devices are designed for the nebulization of antibiotics 95, 96. Of note, despite the possible advantages in terms of microbiological eradication and emergence of resistance 97, no impact on patient prognosis has been demonstrated 94, 97– 99.
In units with rates of methicillin-resistant Staphylococcus aureus (MRSA) around 10–20%, include vancomycin or linezolid in the empirical therapy 7. When the MIC to vancomycin is higher than 1.5 mg/L, the mortality of MRSA pneumonia is higher 100. Moreover, it is very difficult to reach pharmacokinetic targets using vancomycin 101 without any increase in renal toxicity. Consequently, linezolid should be preferred, particularly in patients with renal impairment or if the MRSA MIC to vancomycin is over 1.5 mg/L 102.
An 8-day antibiotic course appears safe in VAP. This duration can be shortened when a procalcitonin-guided algorithm is used 103 or when ventilator settings (PEEP ≤5 cm H 2O and FiO 2 ≤40%) are stable for 48 hours after antibiotic initiation 104. As procalcitonin levels above 1.5 ng/mL after three days of treatment seemed strongly associated with a poor outcome 105, re-evaluation of the accuracy of diagnosis and a search for drainable collections (for example, lung abscess or empyema) and revision of therapeutic antimicrobial regimens should be promptly revisited when procalcitonin levels remain high. However, definite data are lacking where Pseudomonas, Acinetobacter, Stenotrophomonas, and MRSA are concerned.
Abbreviations
BAT, best available therapy; CDC, Centers for Disease Control and Prevention; ESBL-PE, extended-spectrum beta-lactamase-producing Enterobacteriaceae; FiO 2, fraction of inspired oxygen; HAP, hospital-acquired pneumonia; ICU, intensive care unit; IVAC, infection-related ventilator-associated complication; MDR, multidrug-resistant; MIC, minimal inhibitory concentration; MRSA, methicillin-resistant Staphylococcus aureus; M-V, meropenem and vaborbactam; OR, odds ratio; PEEP, positive end expiratory pressure; SDD, selective digestive decontamination; SOD, selective oropharyngeal decontamination; VAC, ventilator-associated complication; VAE, ventilator-associated event; VAP, ventilator-associated pneumonia; VAT, ventilator-associated tracheobronchitis.
Acknowledgments
The authors thank Celine Feger (EMIBiotech) for her editorial assistance.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Michael Klompas, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, MA, USA; Department of Medicine, Brigham and Women's Hospital, Boston, MA, USA
Saad Nseir, ICU, Lille Univ Hosp, Lille, France
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 2 approved]
References
- 1. American Thoracic Society, Infectious Diseases Society of America: Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. 10.1164/rccm.200405-644ST [DOI] [PubMed] [Google Scholar]
- 2. Chastre J, Fagon JY: Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165(7):867–903. 10.1164/ajrccm.165.7.2105078 [DOI] [PubMed] [Google Scholar]
- 3. Craven DE, Hudcova J, Lei Y: Diagnosis of ventilator-associated respiratory infections (VARI): microbiologic clues for tracheobronchitis (VAT) and pneumonia (VAP). Clin Chest Med. 2011;32(3):547–57. 10.1016/j.ccm.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martin-Loeches I, Papiol E, Almansa R, et al. : Intubated patients developing tracheobronchitis or pneumonia have distinctive complement system gene expression signatures in the pre-infection period: a pilot study. Med Intensiva. 2012;36(4):257–63. 10.1016/j.medin.2011.10.009 [DOI] [PubMed] [Google Scholar]
- 5. Rello J, Riera J, Serrano R: What's new in ventilator-associated pneumonia? Intensive Care Med. 2015;41(11):1954–6. 10.1007/s00134-015-3909-8 [DOI] [PubMed] [Google Scholar]
- 6. Magill SS, Klompas M, Balk R, et al. : Developing a new, national approach to surveillance for ventilator-associated events: executive summary. Clin Infect Dis. 2013;57(12):1742–6. 10.1093/cid/cit577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kalil AC, Metersky ML, Klompas M, et al. : Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111. 10.1093/cid/ciw353 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. Timsit JF, de Kraker MEA, Sommer H, et al. : Appropriate endpoints for evaluation of new antibiotic therapies for severe infections: a perspective from COMBACTE's STAT-Net. Intensive Care Med. 2017;43(7):1002–1012. 10.1007/s00134-017-4802-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Torres A, Niederman MS, Chastre J, et al. : International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur Respir J. 2017;50(3): pii: 1700582. 10.1183/13993003.00582-2017 [DOI] [PubMed] [Google Scholar]
- 10. Barbier F, Andremont A, Wolff M, et al. : Hospital-acquired pneumonia and ventilator-associated pneumonia: recent advances in epidemiology and management. Curr Opin Pulm Med. 2013;19(3):216–28. 10.1097/MCP.0b013e32835f27be [DOI] [PubMed] [Google Scholar]
- 11. Rosenthal VD, Bijie H, Maki DG, et al. : International Nosocomial Infection Control Consortium (INICC) report, data summary of 36 countries, for 2004–2009. Am J Infect Control. 2012;40(5):396–407. 10.1016/j.ajic.2011.05.020 [DOI] [PubMed] [Google Scholar]
- 12. Bouadma L, Sonneville R, Garrouste-Orgeas M, et al. : Ventilator-Associated Events: Prevalence, Outcome, and Relationship With Ventilator-Associated Pneumonia. Crit Care Med. 2015;43(9):1798–806. 10.1097/CCM.0000000000001091 [DOI] [PubMed] [Google Scholar]
- 13. Warren DK, Shukla SJ, Olsen MA, et al. : Outcome and attributable cost of ventilator-associated pneumonia among intensive care unit patients in a suburban medical center. Crit Care Med. 2003;31(5):1312–7. 10.1097/01.CCM.0000063087.93157.06 [DOI] [PubMed] [Google Scholar]
- 14. Klompas M: Does this patient have ventilator-associated pneumonia? JAMA. 2007;297(14):1583–93. 10.1001/jama.297.14.1583 [DOI] [PubMed] [Google Scholar]
- 15. Safdar N, Dezfulian C, Collard HR, et al. : Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med. 2005;33(10):2184–93. 10.1097/01.CCM.0000181731.53912.D9 [DOI] [PubMed] [Google Scholar]
- 16. Timsit J, Zahar J, Chevret S: Attributable mortality of ventilator-associated pneumonia. Curr Opin Crit Care. 2011;17(5):464–71. 10.1097/MCC.0b013e32834a5ae9 [DOI] [PubMed] [Google Scholar]
- 17. Melsen WG, Rovers MM, Groenwold RHH, et al. : Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis. 2013;13(8):665–71. 10.1016/S1473-3099(13)70081-1 [DOI] [PubMed] [Google Scholar]
- 18. Nguile-Makao M, Zahar JR, Français A, et al. : Attributable mortality of ventilator-associated pneumonia: respective impact of main characteristics at ICU admission and VAP onset using conditional logistic regression and multi-state models. Intensive Care Med. 2010;36(5):781–9. 10.1007/s00134-010-1824-6 [DOI] [PubMed] [Google Scholar]
- 19. Bekaert M, Timsit JF, Vansteelandt S, et al. : Attributable mortality of ventilator-associated pneumonia: a reappraisal using causal analysis. Am J Respir Crit Care Med. 2011;184(10):1133–9. 10.1164/rccm.201105-0867OC [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Ibrahim EH, Ward S, Sherman G, et al. : A comparative analysis of patients with early-onset vs late-onset nosocomial pneumonia in the ICU setting. Chest. 2000;117(5):1434–42. 10.1378/chest.117.5.1434 [DOI] [PubMed] [Google Scholar]
- 21. Moine P, Timsit J, De Lassence A, et al. : Mortality associated with late-onset pneumonia in the intensive care unit: results of a multi-center cohort study. Intensive Care Med. 2002;28(2):154–63. 10.1007/s00134-001-1172-7 [DOI] [PubMed] [Google Scholar]
- 22. Vallés J, Pobo A, García-Esquirol O, et al. : Excess ICU mortality attributable to ventilator-associated pneumonia: the role of early vs late onset. Intensive Care Med. 2007;33(8):1363–8. 10.1007/s00134-007-0721-0 [DOI] [PubMed] [Google Scholar]
- 23. Park DR: The microbiology of ventilator-associated pneumonia. Respir Care. 2005;50(6):742–63; discussion 763–5. [PubMed] [Google Scholar]
- 24. Trouillet JL, Chastre J, Vuagnat A, et al. : Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am J Respir Crit Care Med. 1998;157(2):531–9. 10.1164/ajrccm.157.2.9705064 [DOI] [PubMed] [Google Scholar]
- 25. Ferrer M, Liapikou A, Valencia M, et al. : Validation of the American Thoracic Society-Infectious Diseases Society of America guidelines for hospital-acquired pneumonia in the intensive care unit. Clin Infect Dis. 2010;50(7):945–52. 10.1086/651075 [DOI] [PubMed] [Google Scholar]
- 26. Martin-Loeches I, Deja M, Koulenti D, et al. : Potentially resistant microorganisms in intubated patients with hospital-acquired pneumonia: the interaction of ecology, shock and risk factors. Intensive Care Med. 2013;39(4):672–81. 10.1007/s00134-012-2808-5 [DOI] [PubMed] [Google Scholar]
- 27. Martin-Loeches I, Povoa P, Rodríguez A, et al. : Incidence and prognosis of ventilator-associated tracheobronchitis (TAVeM): a multicentre, prospective, observational study. Lancet Respir Med. 2015;3(11):859–68. 10.1016/S2213-2600(15)00326-4 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Gastmeier P, Sohr D, Geffers C, et al. : Early- and late-onset pneumonia: is this still a useful classification? Antimicrob Agents Chemother. 2009;53(7):2714–8. 10.1128/AAC.01070-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Restrepo MI, Peterson J, Fernandez JF, et al. : Comparison of the bacterial etiology of early-onset and late-onset ventilator-associated pneumonia in subjects enrolled in 2 large clinical studies. Respir Care. 2013;58(7):1220–5. 10.4187/respcare.02173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Timsit JF, Pilmis B, Zahar JR: How Should We Treat Hospital-Acquired and Ventilator-Associated Pneumonia Caused by Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae? Semin Respir Crit Care Med. 2017;38(3):287–300. 10.1055/s-0037-1603112 [DOI] [PubMed] [Google Scholar]
- 31. Lacherade JC, de Jonghe B, Guezennec P, et al. : Intermittent subglottic secretion drainage and ventilator-associated pneumonia: a multicenter trial. Am J Respir Crit Care Med. 2010;182(7):910–7. 10.1164/rccm.200906-0838OC [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Bouadma L, Wolff M, Lucet JC: Ventilator-associated pneumonia and its prevention. Curr Opin Infect Dis. 2012;25(4):395–404. 10.1097/QCO.0b013e328355a835 [DOI] [PubMed] [Google Scholar]
- 33. Adair CG, Gorman SP, Feron BM, et al. : Implications of endotracheal tube biofilm for ventilator-associated pneumonia. Intensive Care Med. 1999;25(10):1072–6. 10.1007/s001340051014 [DOI] [PubMed] [Google Scholar]
- 34. Pronovost P, Needham D, Berenholtz S, et al. : An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725–32. 10.1056/NEJMoa061115 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Reignier J, Darmon M, Sonneville R, et al. : Impact of early nutrition and feeding route on outcomes of mechanically ventilated patients with shock: a post hoc marginal structural model study. Intensive Care Med. 2015;41(5):875–86. 10.1007/s00134-015-3730-4 [DOI] [PubMed] [Google Scholar]
- 36. Fitch ZW, Whitman GJ: Incidence, risk, and prevention of ventilator-associated pneumonia in adult cardiac surgical patients: a systematic review. J Card Surg. 2014;29(2):196–203. 10.1111/jocs.12260 [DOI] [PubMed] [Google Scholar]
- 37. Schwebel C, Clec'h C, Magne S, et al. : Safety of intrahospital transport in ventilated critically ill patients: a multicenter cohort study*. Crit Care Med. 2013;41(8):1919–28. 10.1097/CCM.0b013e31828a3bbd [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Bornstain C, Azoulay E, De Lassence A, et al. : Sedation, sucralfate, and antibiotic use are potential means for protection against early-onset ventilator-associated pneumonia. Clin Infect Dis. 2004;38(10):1401–8. 10.1086/386321 [DOI] [PubMed] [Google Scholar]
- 39. Klompas M, Branson R, Eichenwald EC, et al. : Strategies to prevent ventilator-associated pneumonia in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35 Suppl 2:S133–54. 10.1017/S0899823X00193894 [DOI] [PubMed] [Google Scholar]
- 40. Huang H, Li Y, Ariani F, et al. : Timing of tracheostomy in critically ill patients: a meta-analysis. PLoS One. 2014;9(3):e92981. 10.1371/journal.pone.0092981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Meng L, Wang C, Li J, et al. : Early vs late tracheostomy in critically ill patients: a systematic review and meta-analysis. Clin Respir J. 2016;10(6):684–92. 10.1111/crj.12286 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Szakmany T, Russell P, Wilkes AR, et al. : Effect of early tracheostomy on resource utilization and clinical outcomes in critically ill patients: meta-analysis of randomized controlled trials. Br J Anaesth. 2015;114(3):396–405. 10.1093/bja/aeu440 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Clec'h C, Alberti C, Vincent F, et al. : Tracheostomy does not improve the outcome of patients requiring prolonged mechanical ventilation: a propensity analysis. Crit Care Med. 2007;35(1):132–8. 10.1097/01.CCM.0000251134.96055.A6 [DOI] [PubMed] [Google Scholar]
- 44. Rello J, Lode H, Cornaglia G, et al. : A European care bundle for prevention of ventilator-associated pneumonia. Intensive Care Med. 2010;36(5):773–80. 10.1007/s00134-010-1841-5 [DOI] [PubMed] [Google Scholar]
- 45. Bouadma L, Deslandes E, Lolom I, et al. : Long-term impact of a multifaceted prevention program on ventilator-associated pneumonia in a medical intensive care unit. Clin Infect Dis. 2010;51(10):1115–22. 10.1086/656737 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Muscedere J, Sinuff T, Heyland DK, et al. : The clinical impact and preventability of ventilator-associated conditions in critically ill patients who are mechanically ventilated. Chest. 2013;144(5):1453–60. 10.1378/chest.13-0853 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Morris AC, Hay AW, Swann DG, et al. : Reducing ventilator-associated pneumonia in intensive care: impact of implementing a care bundle. Crit Care Med. 2011;39(10):2218–24. 10.1097/CCM.0b013e3182227d52 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Speck K, Rawat N, Weiner NC, et al. : A systematic approach for developing a ventilator-associated pneumonia prevention bundle. Am J Infect Control. 2016;44(6):652–6. 10.1016/j.ajic.2015.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Roquilly A, Marret E, Abraham E, et al. : Pneumonia prevention to decrease mortality in intensive care unit: a systematic review and meta-analysis. Clin Infect Dis. 2015;60(1):64–75. 10.1093/cid/ciu740 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Metersky ML, Wang Y, Klompas M, et al. : Trend in Ventilator-Associated Pneumonia Rates Between 2005 and 2013. JAMA. 2016;316(22):2427–9. 10.1001/jama.2016.16226 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. de Smet AM, Kluytmans JA, Cooper BS, et al. : Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med. 2009;360(1):20–31. 10.1056/NEJMoa0800394 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Oostdijk EAN, Kesecioglu J, Schultz MJ, et al. : Effects of decontamination of the oropharynx and intestinal tract on antibiotic resistance in ICUs: a randomized clinical trial. JAMA. 2014;312(14):1429–37. 10.1001/jama.2014.7247 [DOI] [PubMed] [Google Scholar]
- 53. Klompas M: Oropharyngeal Decontamination with Antiseptics to Prevent Ventilator-Associated Pneumonia: Rethinking the Benefits of Chlorhexidine. Semin Respir Crit Care Med. 2017;38(3):381–90. 10.1055/s-0037-1602584 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Rello J, Lorente C, Bodí M, et al. : Why do physicians not follow evidence-based guidelines for preventing ventilator-associated pneumonia?: a survey based on the opinions of an international panel of intensivists. Chest. 2002;122(2):656–61. 10.1378/chest.122.2.656 [DOI] [PubMed] [Google Scholar]
- 55. Ricart M, Lorente C, Diaz E, et al. : Nursing adherence with evidence-based guidelines for preventing ventilator-associated pneumonia. Crit Care Med. 2003;31(11):2693–6. 10.1097/01.CCM.0000094226.05094.AA [DOI] [PubMed] [Google Scholar]
- 56. Pittet D: The Lowbury lecture: behaviour in infection control. J Hosp Infect. 2004;58(1):1–13. 10.1016/j.jhin.2004.06.002 [DOI] [PubMed] [Google Scholar]
- 57. Bouadma L, Mourvillier B, Deiler V, et al. : Changes in knowledge, beliefs, and perceptions throughout a multifaceted behavioral program aimed at preventing ventilator-associated pneumonia. Intensive Care Med. 2010;36(8):1341–7. 10.1007/s00134-010-1890-9 [DOI] [PubMed] [Google Scholar]
- 58. Nair GB, Niederman MS: Ventilator-associated pneumonia: present understanding and ongoing debates. Intensive Care Med. 2015;41(1):34–48. 10.1007/s00134-014-3564-5 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 59. Tejerina E, Esteban A, Fernández-Segoviano P, et al. : Accuracy of clinical definitions of ventilator-associated pneumonia: comparison with autopsy findings. J Crit Care. 2010;25(1):62–8. 10.1016/j.jcrc.2009.05.008 [DOI] [PubMed] [Google Scholar]
- 60. Dudeck MA, Horan TC, Peterson KD, et al. : National Healthcare Safety Network (NHSN) Report, data summary for 2010, device-associated module. Am J Infect Control. 2011;39(10):798–816. 10.1016/j.ajic.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 61. Magill SS, Klompas M, Balk R, et al. : Developing a new, national approach to surveillance for ventilator-associated events*. Crit Care Med. 2013;41(11):2467–75. 10.1097/CCM.0b013e3182a262db [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ego A, Preiser JC, Vincent JL: Impact of diagnostic criteria on the incidence of ventilator-associated pneumonia. Chest. 2015;147(2):347–55. 10.1378/chest.14-0610 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Timsit JF, Misset B, Francoual S, et al. : Is protected specimen brush a reproducible method to diagnose ICU-acquired pneumonia? Chest. 1993;104(1):104–8. 10.1378/chest.104.1.104 [DOI] [PubMed] [Google Scholar]
- 64. Gerbeaux P, Ledoray V, Boussuges A, et al. : Diagnosis of nosocomial pneumonia in mechanically ventilated patients: repeatability of the bronchoalveolar lavage. Am J Respir Crit Care Med. 1998;157(1):76–80. 10.1164/ajrccm.157.1.9604070 [DOI] [PubMed] [Google Scholar]
- 65. de Lassence A, Joly-Guillou ML, Salah A, et al. : Accuracy of delayed (24 hours) processing of bronchoalveolar lavage for diagnosing bacterial pneumonia. Crit Care Med. 2004;32(3):680–5. 10.1097/01.CCM.0000114813.85853.EA [DOI] [PubMed] [Google Scholar]
- 66. Canadian Critical Care Trials Group: A randomized trial of diagnostic techniques for ventilator-associated pneumonia. N Engl J Med. 2006;355(25):2619–30. 10.1056/NEJMoa052904 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Fagon JY, Chastre J, Wolff M, et al. : Invasive and noninvasive strategies for management of suspected ventilator-associated pneumonia. A randomized trial. Ann Intern Med. 2000;132(8):621–30. 10.7326/0003-4819-132-8-200004180-00004 [DOI] [PubMed] [Google Scholar]
- 68. Solé Violán J, Fernández JA, Benítez AB, et al. : Impact of quantitative invasive diagnostic techniques in the management and outcome of mechanically ventilated patients with suspected pneumonia. Crit Care Med. 2000;28(8):2737–41. 10.1097/00003246-200008000-00009 [DOI] [PubMed] [Google Scholar]
- 69. Bonten MJ, Bergmans DC, Stobberingh EE, et al. : Implementation of bronchoscopic techniques in the diagnosis of ventilator-associated pneumonia to reduce antibiotic use. Am J Respir Crit Care Med. 1997;156(6):1820–4. 10.1164/ajrccm.156.6.9610117 [DOI] [PubMed] [Google Scholar]
- 70. Timsit JF, Cheval C, Gachot B, et al. : Usefulness of a strategy based on bronchoscopy with direct examination of bronchoalveolar lavage fluid in the initial antibiotic therapy of suspected ventilator-associated pneumonia. Intensive Care Med. 2001;27(4):640–7. 10.1007/s001340000840 [DOI] [PubMed] [Google Scholar]
- 71. Leone M, Bourgoin A, Cambon S, et al. : Empirical antimicrobial therapy of septic shock patients: adequacy and impact on the outcome. Crit Care Med. 2003;31(2):462–7. 10.1097/01.CCM.0000050298.59549.4A [DOI] [PubMed] [Google Scholar]
- 72. Raman K, Nailor MD, Nicolau DP, et al. : Early antibiotic discontinuation in patients with clinically suspected ventilator-associated pneumonia and negative quantitative bronchoscopy cultures. Crit Care Med. 2013;41(7):1656–63. 10.1097/CCM.0b013e318287f713 [DOI] [PubMed] [Google Scholar]
- 73. Zahar JR, Timsit JF, Garrouste-Orgeas M, et al. : Outcomes in severe sepsis and patients with septic shock: pathogen species and infection sites are not associated with mortality. Crit Care Med. 2011;39(8):1886–95. 10.1097/CCM.0b013e31821b827c [DOI] [PubMed] [Google Scholar]
- 74. Tabah A, Koulenti D, Laupland K, et al. : Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: the EUROBACT International Cohort Study. Intensive Care Med. 2012;38(12):1930–45. 10.1007/s00134-012-2695-9 [DOI] [PubMed] [Google Scholar]
- 75. Decousser JW, Poirel L, Nordmann P: Recent advances in biochemical and molecular diagnostics for the rapid detection of antibiotic-resistant Enterobacteriaceae: a focus on ß-lactam resistance. Expert Rev Mol Diagn. 2017;17(4):327–50. 10.1080/14737159.2017.1289087 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 76. Laupland KB, Paiva JA, Timsit JF: Focus on severe infections. Intensive Care Med. 2017;43(7):1033–6. 10.1007/s00134-017-4835-8 [DOI] [PubMed] [Google Scholar]
- 77. Douglas IS, Price CS, Overdier KH, et al. : Rapid automated microscopy for microbiological surveillance of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2015;191(5):566–73. 10.1164/rccm.201408-1468OC [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 78. Roberts JA, Taccone FS, Lipman J: Understanding PK/PD. Intensive Care Med. 2016;42(11):1797–1800. 10.1007/s00134-015-4032-6 [DOI] [PubMed] [Google Scholar]
- 79. Weiss E, Essaied W, Adrie C, et al. : Treatment of severe hospital-acquired and ventilator-associated pneumonia: a systematic review of inclusion and judgment criteria used in randomized controlled trials. Crit Care. 2017;21(1):162. 10.1186/s13054-017-1755-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. McKinnon PS, Paladino JA, Schentag JJ: Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration ( T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents. 2008;31(4):345–51. 10.1016/j.ijantimicag.2007.12.009 [DOI] [PubMed] [Google Scholar]
- 81. Chow KM, Hui AC, Szeto CC: Neurotoxicity induced by beta-lactam antibiotics: from bench to bedside. Eur J Clin Microbiol Infect Dis. 2005;24(10):649–53. 10.1007/s10096-005-0021-y [DOI] [PubMed] [Google Scholar]
- 82. Roberts JA, Lefrant JY, Lipman J: What's new in pharmacokinetics of antimicrobials in AKI and RRT? Intensive Care Med. 2017;43(6):904–6. 10.1007/s00134-017-4789-x [DOI] [PubMed] [Google Scholar]
- 83. Abdul-Aziz MH, Sulaiman H, Mat-Nor MB, et al. : Beta-Lactam Infusion in Severe Sepsis (BLISS): a prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med. 2016;42(10):1535–45. 10.1007/s00134-015-4188-0 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 84. Martínez JA, Cobos-Trigueros N, Soriano A, et al. : Influence of empiric therapy with a beta-lactam alone or combined with an aminoglycoside on prognosis of bacteremia due to gram-negative microorganisms. Antimicrob Agents Chemother. 2010;54(9):3590–6. 10.1128/AAC.00115-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kumar A, Zarychanski R, Light B, et al. : Early combination antibiotic therapy yields improved survival compared with monotherapy in septic shock: a propensity-matched analysis. Crit Care Med. 2010;38(9):1773–85. 10.1097/CCM.0b013e3181eb3ccd [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 86. Boyer A, Gruson D, Bouchet S, et al. : Aminoglycosides in septic shock: an overview, with specific consideration given to their nephrotoxic risk. Drug Saf. 2013;36(4):217–30. 10.1007/s40264-013-0031-0 [DOI] [PubMed] [Google Scholar]
- 87. Ong DSY, Frencken JF, Klein Klouwenberg PMC, et al. : Short-Course Adjunctive Gentamicin as Empirical Therapy in Patients With Severe Sepsis and Septic Shock: A Prospective Observational Cohort Study. Clin Infect Dis. 2017;64(12):1731–6. 10.1093/cid/cix186 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 88. Planquette B, Timsit JF, Misset BY, et al. : Pseudomonas aeruginosa ventilator-associated pneumonia. predictive factors of treatment failure. Am J Respir Crit Care Med. 2013;188(1):69–76. 10.1164/rccm.201210-1897OC [DOI] [PubMed] [Google Scholar]
- 89. de Lastours V, Chau F, Roy C, et al. : Emergence of quinolone resistance in the microbiota of hospitalized patients treated or not with a fluoroquinolone. J Antimicrob Chemother. 2014;69(12):3393–400. 10.1093/jac/dku283 [DOI] [PubMed] [Google Scholar]
- 90. Armand-Lefèvre L, Angebault C, Barbier F, et al. : Emergence of imipenem-resistant gram-negative bacilli in intestinal flora of intensive care patients. Antimicrob Agents Chemother. 2013;57(3):1488–95. 10.1128/AAC.01823-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Balakrishnan I, Awad-El-Kariem FM, Aali A, et al. : Temocillin use in England: clinical and microbiological efficacies in infections caused by extended-spectrum and/or derepressed AmpC β-lactamase-producing Enterobacteriaceae. J Antimicrob Chemother. 2011;66(11):2628–31. 10.1093/jac/dkr317 [DOI] [PubMed] [Google Scholar]
- 92. Vardakas KZ, Rellos K, Triarides NA, et al. : Colistin loading dose: evaluation of the published pharmacokinetic and clinical data. Int J Antimicrob Agents. 2016;48(5):475–84. 10.1016/j.ijantimicag.2016.08.009 [DOI] [PubMed] [Google Scholar]
- 93. Niederman MS, Chastre J, Corkery K, et al. : BAY41-6551 achieves bactericidal tracheal aspirate amikacin concentrations in mechanically ventilated patients with Gram-negative pneumonia. Intensive Care Med. 2012;38(2):263–71. 10.1007/s00134-011-2420-0 [DOI] [PubMed] [Google Scholar]
- 94. Kollef MH, Ricard JD, Roux D, et al. : A Randomized Trial of the Amikacin Fosfomycin Inhalation System for the Adjunctive Therapy of Gram-Negative Ventilator-Associated Pneumonia: IASIS Trial. Chest. 2017;151(6):1239–46. 10.1016/j.chest.2016.11.026 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 95. Kollef MH: COUNTERPOINT: Should Inhaled Antibiotic Therapy Be Used Routinely for the Treatment of Bacterial Lower Respiratory Tract Infections in the ICU Setting? No. Chest. 2017;151(4):740–3. 10.1016/j.chest.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 96. Bassetti M, Luyt CE, Nicolau DP, et al. : Characteristics of an ideal nebulized antibiotic for the treatment of pneumonia in the intubated patient. Ann Intensive Care. 2016;6(1):35. 10.1186/s13613-016-0140-x [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 97. Solé-Lleonart C, Rouby JJ, Blot S, et al. : Nebulization of Antiinfective Agents in Invasively Mechanically Ventilated Adults: A Systematic Review and Meta-analysis. Anesthesiology. 2017;126(5):890–908. 10.1097/ALN.0000000000001570 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 98. Zampieri FG, Nassar AP, Jr, Gusmao-Flores D, et al. : Nebulized antibiotics for ventilator-associated pneumonia: a systematic review and meta-analysis. Crit Care. 2015;19(1):150. 10.1186/s13054-015-0868-y [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 99. Rattanaumpawan P, Lorsutthitham J, Ungprasert P, et al. : Randomized controlled trial of nebulized colistimethate sodium as adjunctive therapy of ventilator-associated pneumonia caused by Gram-negative bacteria. J Antimicrob Chemother. 2010;65(12):2645–9. 10.1093/jac/dkq360 [DOI] [PubMed] [Google Scholar]
- 100. Choi EY, Huh JW, Lim CM, et al. : Relationship between the MIC of vancomycin and clinical outcome in patients with MRSA nosocomial pneumonia. Intensive Care Med. 2011;37(4):639–47. 10.1007/s00134-011-2130-7 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 101. Patel N, Pai MP, Rodvold KA, et al. : Vancomycin: we can't get there from here. Clin Infect Dis. 2011;52(8):969–74. 10.1093/cid/cir078 [DOI] [PubMed] [Google Scholar]
- 102. Wunderink RG, Niederman MS, Kollef MH, et al. : Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis. 2012;54(5):621–9. 10.1093/cid/cir895 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 103. Bouadma L, Luyt CE, Tubach F, et al. : Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375(9713):463–74. 10.1016/S0140-6736(09)61879-1 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 104. Klompas M, Li L, Menchaca JT, et al. : Ultra-Short-Course Antibiotics for Patients With Suspected Ventilator-Associated Pneumonia but Minimal and Stable Ventilator Settings. Clin Infect Dis. 2017;64(7):870–6. 10.1093/cid/ciw870 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 105. Luyt CE, Guérin V, Combes A, et al. : Procalcitonin kinetics as a prognostic marker of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2005;171(1):48–53. 10.1164/rccm.200406-746OC [DOI] [PubMed] [Google Scholar]