Key Points
Question
Does a resuscitation protocol with administration of intravenous fluids, vasopressors, and blood transfusion implemented early after presentation to the emergency department improve in-hospital mortality among Zambian adults with sepsis and hypotension compared with usual care?
Findings
In this randomized clinical trial that included 209 adults with sepsis and hypotension presenting to an emergency department in Zambia, a 6-hour sepsis protocol emphasizing administration of intravenous fluids, vasopressors, and blood transfusion significantly increased in-hospital mortality compared with usual care (48.1% vs 33.0%, respectively).
Meaning
In resource-limited settings, an early resuscitation protocol with administration of intravenous fluids, vasopressors, and blood transfusion for adults with sepsis may increase mortality compared with usual care.
Abstract
Importance
The effect of an early resuscitation protocol on sepsis outcomes in developing countries remains unknown.
Objective
To determine whether an early resuscitation protocol with administration of intravenous fluids, vasopressors, and blood transfusion decreases mortality among Zambian adults with sepsis and hypotension compared with usual care.
Design, Setting, and Participants
Randomized clinical trial of 212 adults with sepsis (suspected infection plus ≥2 systemic inflammatory response syndrome criteria) and hypotension (systolic blood pressure ≤90 mm Hg or mean arterial pressure ≤65 mm Hg) presenting to the emergency department at a 1500-bed referral hospital in Zambia between October 22, 2012, and November 11, 2013. Data collection concluded December 9, 2013.
Interventions
Patients were randomized 1:1 to either (1) an early resuscitation protocol for sepsis (n = 107) that included intravenous fluid bolus administration with monitoring of jugular venous pressure, respiratory rate, and arterial oxygen saturation and treatment with vasopressors targeting mean arterial pressure (≥65 mm Hg) and blood transfusion (for patients with a hemoglobin level <7 g/dL) or (2) usual care (n = 105) in which treating clinicians determined hemodynamic management.
Main Outcomes and Measures
The primary outcome was in-hospital mortality and the secondary outcomes included the volume of intravenous fluid received and receipt of vasopressors.
Results
Among 212 patients randomized to receive either the sepsis protocol or usual care, 3 were ineligible and the remaining 209 completed the study and were included in the analysis (mean [SD] age, 36.7 [12.4] years; 117 men [56.0%]; 187 [89.5%] positive for the human immunodeficiency virus). The primary outcome of in-hospital mortality occurred in 51 of 106 patients (48.1%) in the sepsis protocol group compared with 34 of 103 patients (33.0%) in the usual care group (between-group difference, 15.1% [95% CI, 2.0%-28.3%]; relative risk, 1.46 [95% CI, 1.04-2.05]; P = .03). In the 6 hours after presentation to the emergency department, patients in the sepsis protocol group received a median of 3.5 L (interquartile range, 2.7-4.0 L) of intravenous fluid compared with 2.0 L (interquartile range, 1.0-2.5 L) in the usual care group (mean difference, 1.2 L [95% CI, 1.0-1.5 L]; P < .001). Fifteen patients (14.2%) in the sepsis protocol group and 2 patients (1.9%) in the usual care group received vasopressors (between-group difference, 12.3% [95% CI, 5.1%-19.4%]; P < .001).
Conclusions and Relevance
Among adults with sepsis and hypotension, most of whom were positive for HIV, in a resource-limited setting, a protocol for early resuscitation with administration of intravenous fluids and vasopressors increased in-hospital mortality compared with usual care. Further studies are needed to understand the effects of administration of intravenous fluid boluses and vasopressors in patients with sepsis across different low- and middle-income clinical settings and patient populations.
Trial Registration
clinicaltrials.gov Identifier: NCT01663701
This randomized clinical trial compares the effects of an early resuscitation sepsis protocol (administration of intravenous fluids, vasopressors, and blood transfusion) vs usual care for prevention of in-hospital mortality among adults with sepsis and hypotension in Zambia.
Introduction
Sepsis mortality in the developed world steadily declined between 2000 and 2012. Part of this improvement has been attributed to the implementation of sepsis protocols emphasizing early resuscitation with intravenous fluid boluses and vasopressors to achieve hemodynamic targets.
In contrast, mortality from sepsis in low- and middle-income countries remains high and current usual care frequently does not include early resuscitation with intravenous fluid boluses or vasopressors. Whether an early resuscitation protocol could improve sepsis outcomes in resource-limited settings remains uncertain.
Three studies compared early resuscitation with usual care among African patients with severe infection and yielded conflicting results. A before-after study in Uganda suggested decreased mortality with a multicomponent intervention including intravenous fluid boluses among adults with sepsis. A randomized clinical trial (RCT) in Zambia observed no mortality benefit with a protocol of early intravenous fluid and vasopressor administration among adults with sepsis; however, the trial was stopped early for possible harm in the subgroup of patients with hypoxemia and tachypnea. An RCT conducted among children with severe febrile illness in Kenya, Uganda, and Tanzania demonstrated increased mortality with intravenous fluid bolus administration.
However, each of these studies had important limitations. In particular, both RCTs included patients with nonspecific markers of tissue hypoperfusion rather than only patients with sepsis and overt hypotension, for whom the benefit of early intravenous fluid bolus and vasopressor administration may be greatest.
The primary objective of this RCT was to determine whether a sepsis protocol with early administration of intravenous fluid boluses, vasopressors, and blood transfusion would decrease in-hospital mortality compared with usual care among African adults with sepsis and hypotension.
Methods
The Simplified Severe Sepsis Protocol 2 trial was a parallel-group, nonblinded RCT conducted at a 1500-bed national referral university hospital in Zambia. The University of Zambia biomedical research ethics committee and the Vanderbilt University institutional review board granted ethical approval and the trial was overseen by an independent data and safety monitoring board. Written informed consent was obtained from patients or their legally authorized representatives prior to study enrollment. The trial protocol appears in Supplement 1.
From October 22, 2012, through November 11, 2013, we screened all patients presenting to the emergency department (ED) between 8 am on Monday and 12 pm on Friday. Patients aged 18 years or older were eligible if they had (1) sepsis (defined as suspected infection plus ≥2 systemic inflammatory response syndrome criteria) and (2) hypotension (defined as systolic blood pressure ≤90 mm Hg or mean arterial pressure ≤65 mm Hg). Based on the results of a prior trial in the same setting, we excluded patients with hypoxemia and severe tachypnea (defined as arterial oxygen saturation <90% and respiratory rate >40 breaths per minute). Additional exclusion criteria included gastrointestinal bleeding in the absence of fever, congestive heart failure exacerbation, end-stage renal disease, elevated jugular venous pressure (JVP), incarceration, or the need for immediate surgery. Enrollment occurred within 4 hours of the first eligible blood pressure measurement and within 24 hours of ED registration.
Study group assignment was generated using computerized randomization in permuted block sizes of 2, 4, and 6. Allocation slips were placed in sealed opaque envelopes, which were opened after informed consent was obtained. Patients, treating clinicians, and clinical study personnel were aware of group assignment after enrollment. Study personnel responsible for outcomes assessment and data analysis were blinded to group assignment.
For patients in both groups, treating clinicians determined the location of care (intensive care unit or medical ward) and antibiotic selection (including use of empirical antituberculous and antimalarial therapy). In addition, a dedicated study nurse measured heart rate, systolic and diastolic blood pressure, respiratory rate, and oxygen saturation hourly for the 6 hours after enrollment and supervised the administration of all ordered fluids and medications.
Sepsis Protocol Group
Patients randomized to the sepsis protocol received hemodynamic management for the first 6 hours after enrollment. An initial 2-L bolus of intravenous isotonic crystalloid was administered within 1 hour of enrollment, followed by an additional 2 L over the subsequent 4 hours. After each liter of intravenous fluid was administered, an investigator or study nurse measured arterial oxygen saturation, respiratory rate, and JVP (details appear in the eMethods in Supplement 2). If the arterial oxygen saturation decreased by 3%, the respiratory rate increased by 5 breaths per minute, or JVP reached 3 cm or greater above the sternal angle, fluid infusion was discontinued. The sepsis protocol limited intravenous fluid administration to a total of 4 L, including any fluid given in the ED prior to enrollment.
If mean arterial pressure remained less than 65 mm Hg after completion of the initial 2-L fluid bolus, a dopamine infusion (vasopressor) was initiated via a peripheral intravenous line starting at 10 μg/kg/min and titrated to reach a mean arterial pressure of 65 mm Hg or greater. The sepsis protocol recommended blood transfusion for patients with a hemoglobin level of less than 7 g/dL or with severe pallor.
Usual Care Group
For patients randomized to usual care, treating clinicians determined intravenous fluid administration, vasopressor use, and blood transfusion. During usual care for sepsis in the study setting, the volume of intravenous fluid administered in the first 6 hours averages less than 2 L, less than half of patients receive any intravenous fluid bolus, less than 2% of patients receive a vasopressor, and less than 20% of patients receive a blood transfusion (additional details appear in the eMethods in Supplement 2).
Data Collection
Because most patients were not ambulatory, we measured upper arm circumference in lieu of weight to assess nutritional status. Study personnel recorded the volume of intravenous fluid administered between ED registration and 6 hours after enrollment, 6 to 24 hours after enrollment, and 24 to 72 hours after enrollment. Patients were followed up until death or 28 days after enrollment. For patients discharged from the hospital, blinded study personnel called the patient or next-of-kin to ascertain vital status at 28 days. Additional details regarding data collection appear in the eMethods in Supplement 2.
Outcomes
The primary outcome was in-hospital mortality. Secondary efficacy outcomes included 28-day mortality and time to death. Secondary safety outcomes included the incidence of worsening hypoxemia or tachypnea (decrease in arterial oxygen saturation of ≥3% or an increase in respiratory rate of ≥5 breaths per minute). Process measures included volume of intravenous fluid administered within 6, 24, and 72 hours; reasons for intravenous fluid discontinuation; and receipt of antibiotics, dopamine, and a blood transfusion. Prospectively collected adverse events included dopamine extravasation, tissue ischemia or necrosis, iatrogenic pulmonary edema, and reaction to blood transfusion.
Statistical Analysis
Based on an in-hospital mortality rate of 65% in a prior trial in the same setting, we calculated that enrolling 212 patients would provide statistical power of 80% at an α level of .05 to detect an absolute risk reduction in mortality of 20% (equivalent to a relative risk [RR] reduction of 30.8% and similar to the RR reduction reported in a prior trial). One interim analysis was planned, performed, and reviewed by the data and safety monitoring board after enrollment of 50% of the patients using a conservative Haybittle-Peto boundary (P < .001) to allow performance of the final analysis using an unchanged 2-sided level of significance (P = .05).
All analyses were conducted in a modified intention-to-treat fashion, analyzing patients by the group to which they were assigned and excluding those who were recognized as ineligible immediately after enrollment and who did not receive study interventions (Figure 1). Continuous variables were reported as mean and standard deviation or median and interquartile range (IQR) and categorical variables as frequencies and proportions. Between-group differences were analyzed using the t test for parametric continuous variables, the Mann-Whitney test for nonparametric continuous variables, the χ2 test for categorical variables, and the log-rank test for survival analysis.
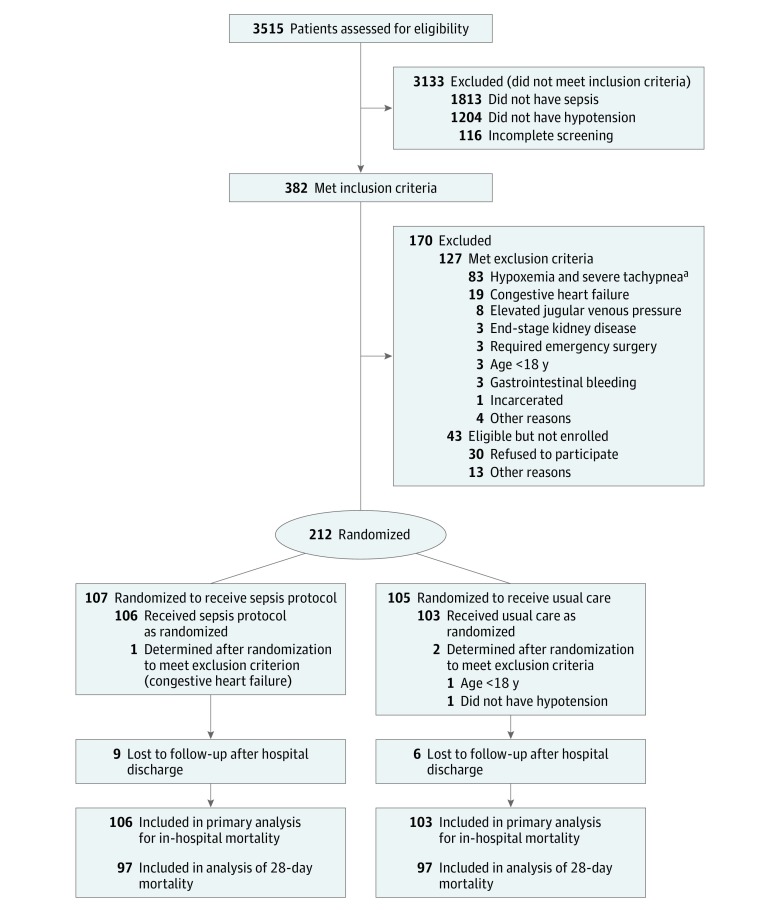
Figure 1. Screening, Randomization, and Follow-up of Patients Through the Trial.
aDefined as a noninvasively measured arterial oxygen saturation of less than 90% and a respiratory rate greater than 40 breaths per minute.
The primary analysis compared in-hospital mortality between the sepsis protocol group and the usual care group using the χ2 test. In secondary analyses, we compared the sepsis protocol group with the usual care group after adjusting for baseline Simplified Acute Physiology Score 3 (SAPS-3) and in prespecified subgroups defined by the presence of human immunodeficiency virus infection, Glasgow Coma Scale score at presentation, baseline hemoglobin level, baseline lactate level, baseline SAPS-3, and baseline JVP. Subgroup analyses used the Mantel-Haenszel test for heterogeneity to assess for subgroup × study group interaction effects on the risk of in-hospital mortality. Analyses were performed using Stata version 12.1 (StataCorp).
Results
Of 382 patients with sepsis and hypotension, 212 met eligibility criteria, provided consent to participate, and were randomized. Immediately after randomization, it was recognized that 3 patients should have been excluded (age <18 years, absence of hypotension, and presence of congestive heart failure), leaving 209 patients who received the study interventions, completed follow-up, and were included in the primary analysis (Figure 1). Data collection concluded December 9, 2013.
Patients assigned to the sepsis protocol (n = 106) and usual care (n = 103) were similar at baseline (Table 1). Overall, patients were young (mean age, 36.7 years [SD, 12.4 years]) with high human immunodeficiency virus prevalence (89.5%) and low CD4 lymphocyte counts (median, 66/μL [IQR, 21-143/μL] among patients with human immunodeficiency virus). Median albumin level was 2.2 g/dL (IQR, 1.8-2.7 g/dL) and most patients were malnourished, with a median upper arm circumference of 20.1 cm (IQR, 18.4-22.9 cm). Median systolic blood pressure was 83 mm Hg (IQR, 76-87 mm Hg) with a median lactate level of 4.3 mmol/L (IQR, 2.8-7.7 mmol/L).
Table 1. Baseline Characteristics of the Patients.
| Sepsis Protocol (n = 106) |
Usual Care (n = 103) |
|
|---|---|---|
| Age, mean (SD), y | 37.5 (12.9) | 35.8 (11.9) |
| Male sex, No. (%) | 62 (58.5) | 55 (53.4) |
| Positive diagnosis for HIV, No. (%)a | 94 (88.7) | 93 (90.3) |
| Time since HIV diagnosis, median (IQR), d | 90 (30-1095) | 75 (14-730) |
| CD4 lymphocyte count, median (IQR), /μL | 72 (22-143) | 65 (20-159) |
| Receiving antiretroviral therapy, No. (%) | 56 (52.8) | 51 (49.5) |
| Duration of antiretroviral therapy, median (IQR), d | 105 (42-1460) | 195 (30-730) |
| History of Mycobacterium tuberculosis infection, No. (%) | 49 (46.2) | 46 (44.7) |
| Receiving treatment for M tuberculosis at presentation, No. (%) | 26 (24.5) | 24 (23.3) |
| Physiological variables, median (IQR) | ||
| Temperature, °C | 36.9 (35.5-38.5) | 37.7 (35.4-38.5) |
| Heart rate, beats/min | 115 (104-129) | 115 (103-130) |
| Systolic blood pressure, mm Hg | 83 (77-87) | 83 (75-87) |
| Diastolic blood pressure, mm Hg | 50 (42-54) | 48 (42-53) |
| Respiratory rate, breaths/min | 30 (28-38) | 32 (28-39) |
| Glasgow Coma Scale score <15, No. (%)b | 32 (30.2) | 42 (40.8) |
| Jugular venous pressure by proximity to the sternal angle, No. (%)c | ||
| ≥4 cm below | 34 (32.1) | 42 (40.8) |
| 1-3 cm below | 30 (28.3) | 22 (21.4) |
| At the sternal angle | 20 (18.9) | 28 (27.2) |
| ≥1 cm above | 22 (20.8) | 11 (10.7) |
| Upper arm circumference, median (IQR), cmd | 20.1 (18.0-22.8) | 20.1 (18.9-23.0) |
| Inability to ambulate, No. (%) | 66 (62.3) | 67 (65.0) |
| Duration of inability to ambulate, median (IQR), d | 16.5 (10-35) | 10 (7-21) |
| SAPS-3 score, median (IQR)e | 55 (50-65) | 57 (50-66) |
| Laboratory values | ||
| Whole blood lactate, median (IQR), mmol/L | 4.7 (2.8-8.7) | 4.0 (2.6-7.0) |
| Serum creatinine, median (IQR), mg/dL | 1.3 (1.0-2.4) | 1.3 (0.9-2.7) |
| Hemoglobin, mean (SD), g/dL | 7.8 (0.3) | 7.8 (0.3) |
| Serum albumin, median (IQR), g/dL | 2.1 (1.7-2.6) | 2.3 (1.9-2.8) |
| Time from ED registration to enrollment, median (IQR), minf | 71 (5-205) | 76 (0-240) |
Abbreviations: ED, emergency department; IQR, interquartile range; SAPS-3, Simplified Acute Physiology Score 3.
SI conversion factors: To convert creatinine to μmol/L, multiply by 88.4; lactate to mg/dL, divide by 0.111.
Of 187 patients, 162 (86.6%) had AIDS as defined by a CD4 lymphocyte count of less than 200/μL or tuberculosis infection.
Objective assessment of the level of consciousness (range, 3 [deep unconsciousness] to 15 [normal level of consciousness]).
Measured above the clavicle with the patient positioned at 45°.
A measure of 22.5 cm or less correlates with a body mass index (calculated as weight in kilograms divided by height in meters squared) of less than 18.5.
A severity score and mortality estimation tool (range, 0-217; higher values indicate higher risk of in-hospital mortality).
Calculated as time from ED registration to signed informed consent. Because the study nurse began screening for eligible patients in the waiting room prior to ED registration, enrollment in the study occurred simultaneously with ED registration for some patients, producing a value of 0 minutes for the time from ED registration to study enrollment.
Diagnosis and Treatment of Infection
The most common admitting diagnoses were pneumonia (49.3%) and suspected tuberculosis (62.7%), with 80 patients (38.3%) having both. Forty-three patients (20.6%) had positive tuberculosis blood cultures. Details of admitting diagnoses and microbiological data appear in eTables 1 and 2 in Supplement 2. The median time between ED registration and the first dose of intravenous antimicrobial therapy was similar in the sepsis protocol group and in the usual care group (2.0 vs 1.5 hours, respectively; P = .15).
Hemodynamic Interventions
In the 6 hours after presentation to the emergency department, patients in the sepsis protocol group received a median of 3.5 L (IQR, 2.7-4.0 L) of intravenous fluid compared with 2.0 L (IQR, 1.0-2.5 L) in the usual care group (mean difference, 1.2 L [95% CI, 1.0-1.5 L]; P < .001). A total of 41 patients (38.7%) in the sepsis protocol group received 4 L or greater of intravenous fluid between ED registration and 6 hours after enrollment. Among the remaining 65 patients (61.3%) in the sepsis protocol group, intravenous fluids were discontinued prior to a total volume of 4 L due to an increase in respiratory rate or a decrease in arterial oxygen saturation (32 patients [30.2%]), JVP of 3 cm or greater (9 patients [8.5%]), blood transfusion through an intravenous line (5 patients [4.7%]), and other reasons (4 patients [3.8%]). In the usual care group, only 50 patients (48.3%) received any intravenous fluid bolus and the most common fluid order was for the administration of 3 L of intravenous fluid over 24 hours (eTable 3 in Supplement 2).
Blood pressure generally increased over the first 6 hours of treatment in both study groups (Table 2). A total of 15 patients (14.2%) received a dopamine infusion (vasopressor) in the 6 hours after enrollment in the sepsis protocol group compared with 2 patients (1.9%) in the usual care group (between-group difference, 12.3% [95% CI, 5.1% to 19.4%]; P < .001). The decrease in lactic acid concentration from baseline to 6 hours was greater in the sepsis protocol group (median, −1.2 mmol/L; IQR, −3.4 to 0.3 mmol/L) than in the usual care group (median, −0.5 mmol/L; IQR, 2.2 to 1.1 mmol/L) (mean difference, 1.45 mmol/L [95% CI, 0.4 to 2.5 mmol/L]; P = .02).
Table 2. Elements of Sepsis Resuscitation.
| Sepsis Protocol (n = 106) |
Usual Care (n = 103) |
P Value | |
|---|---|---|---|
| Intravenous fluid administration, median (IQR), La | |||
| 6 h | 3.5 (2.7 to 4.0) | 2.0 (1.0 to 2.5) | <.001 |
| 24 h | 4.0 (3.0 to 5.0) | 3.0 (2.0 to 4.3) | <.001 |
| 72 h | 5.0 (3.5 to 6.5) | 4.0 (3.0 to 6.0) | .33 |
| Dopamine (vasopressor) administration, No. (%) | |||
| During first 6 h | 15 (14.2) | 2 (1.9) | .001 |
| During hospitalization | 22 (20.8) | 7 (6.8) | .004 |
| Blood transfusion, No. (%) | |||
| During first 6 h | 17 (16.0) | 13 (12.6) | .48 |
| During hospitalization | 37 (34.9) | 31 (30.1) | .46 |
| Time to antibiotics, median (IQR), h | 2.0 (0.7 to 4.1) | 1.5 (0.5 to 2.8) | .15 |
| Physiological variables, median (IQR) | |||
| Systolic blood pressure 2 h after enrollment, mm Hg | 89 (85 to 95) | 88 (83 to 92) | .09 |
| Diastolic blood pressure 2 h after enrollment, mm Hg | 55 (48 to 59) | 54 (47 to 61) | .99 |
| Systolic blood pressure 6 h after enrollment, mm Hg | 95 (90 to 104) | 96 (90 to 105) | .95 |
| Diastolic blood pressure 6 h after enrollment, mm Hg | 61 (55 to 67) | 61 (55 to 65) | .82 |
| Whole blood lactate, median (IQR), mmol/L | 3.3 (2.1 to 5.4) | 3.9 (2.1 to 6.6) | .25 |
| Change in lactic acid concentration from baseline to 6 h after enrollment, mmol/L | −1.2 (−3.4 to 0.3) | −0.5 (−2.2 to 1.1) | .02 |
| Respiratory rate increased by ≥5 breaths/min or SpO2 decreased by ≥3%, No. (%)b | 38 (35.8) | 23 (22.3) | .03 |
| Resolved by 6 h after enrollment | 20 (18.9) | 8 (7.8) | .02 |
| Persistent beyond 6 h after enrollment | 18 (17.0) | 15 (14.6) | .63 |
Abbreviations: IQR, interquartile range; SpO2, oxygen saturation by pulse oximeter.
SI conversion factor: To convert lactate to mg/dL, divide by 0.111.
Represents the cumulative volume of crystalloid solutions administered between emergency department registration and the 3 time points.
Respiratory compromise was prospectively defined as an increase in respiratory rate of at least 5 breaths per minute vs baseline or a decrease in oxygen saturation of more than 3% from baseline. Classification of respiratory compromise as either resolving by 6 hours after enrollment or persisting beyond 6 hours after enrollment was performed post hoc.
Due to limited intensive care unit capacity, 208 of the 209 patients (99.5%) were cared for on regular medical wards without the availability of mechanical ventilation. More patients in the sepsis protocol group (35.8%) than in the usual care group (22.3%) experienced a decrease in oxygen saturation of 3% or greater or an increase in respiratory rate of 5 breaths or more per minute (between-group difference, 13.5% [95% CI, 1.4%-25.7%]; P = .03) (Table 2).
Clinical Outcomes
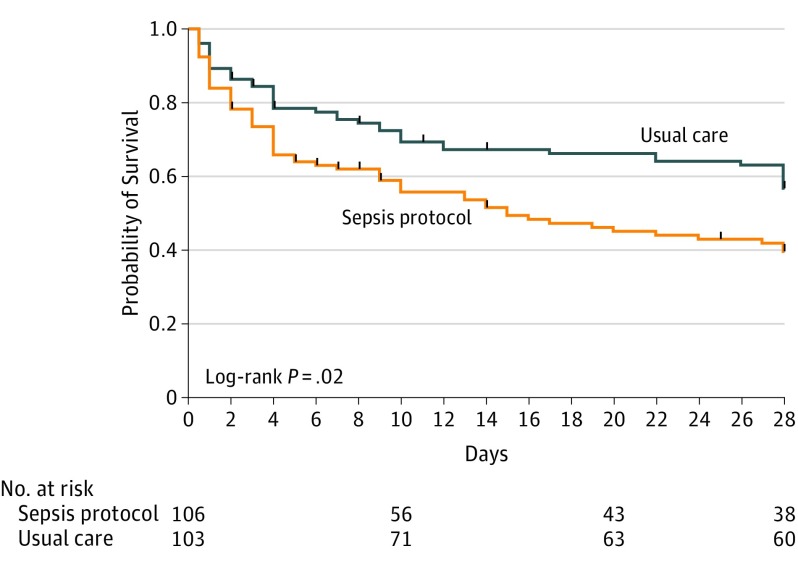
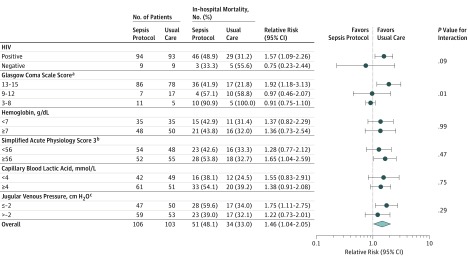
The primary outcome of in-hospital mortality occurred in 51 of 106 patients (48.1%) in the sepsis protocol group vs 34 of 103 patients (33.0%) in the usual care group (between-group difference, 15.1% [95% CI, 2.0%-28.3%]; RR, 1.46 [95% CI, 1.04-2.05]; P = .03). Vital status after hospital discharge at day 28 was known for 97 patients in each study group; 28-day mortality was 67.0% with the sepsis protocol vs 45.3% with usual care (between-group difference, 21.6% [95% CI, 8.0%-35.3%]; RR, 1.48 [95% CI, 1.14-1.91]; P = .002). In the multivariable analysis adjusting for SAPS-3 at enrollment, risk of in-hospital mortality (RR, 1.45 [95% CI, 1.04-2.02]; P = .03) and 28-day mortality (RR, 1.41 [95% CI, 1.08-1.84]; P = .01) was greater in the sepsis protocol group than in the usual care group. In the time-to-event analysis, the probability of survival was lower in the sepsis protocol group than in the usual care group (P = .02) (Figure 2 and eTable 4 in Supplement 2). Increased in-hospital mortality among patients assigned to the sepsis protocol was consistent across prespecified patient subgroups (Figure 3). Median hospital length of stay was 5 days (IQR, 3-8 days) in the sepsis protocol group vs 7 days (IQR, 4-12 days) in the usual care group (P = .01). Rates of adverse events were similar between groups (eTable 5 in Supplement 2).
Figure 2. Kaplan-Meier Plot of the Probability of Survival Until Day 28 After Enrollment.
Vital status was known through study day 28 for 194 patients (94.2%). The median duration of follow-up was 28 days (interquartile range, 28-28 days) in both study groups. Vertical ticks on the curves indicate censoring due to loss to follow-up after hospital discharge.
Figure 3. Risk of In-hospital Mortality by Subgroup for Patients Treated With the Sepsis Protocol vs Usual Care.
The sepsis protocol increased the overall absolute risk of in-hospital mortality by 15.1% (95% CI, 2.0%-28.3%) compared with usual care.
aObjective assessment of the level of consciousness (range, 3 [deep unconsciousness] to 15 [normal level of consciousness]).
bA severity score and mortality estimation tool (range, 0-217; higher values indicate higher risk of in-hospital mortality).
cMeasured above the clavicle with the patient positioned at 45°. For example, because most patients in the trial had depleted volume levels, the median jugular venous pressure was around 2 cm H2O below the clavicle or −2 cm H2O.
Discussion
This RCT among Zambian adults with sepsis and hypotension, most of whom had been diagnosed with HIV, found that a protocol for early resuscitation with intravenous fluid boluses and vasopressors increased mortality compared with usual care. The sepsis protocol resulted in greater intravenous fluid administration, vasopressor use, and lactate clearance but caused more frequent worsening of hypoxemia and tachypnea and higher rates of in-hospital and 28-day mortality. These findings may have important implications for the clinical care of patients with sepsis in low- and middle-income countries and for future research in early sepsis management across settings.
Despite international recommendations for fluid bolus and vasopressor administration in the treatment of sepsis-induced hypotension, supportive data are scarce. The Early Goal-Directed Therapy (EGDT) trial, which involved 263 adults with sepsis at a single ED in the United States, reported reduced mortality with administration of intravenous fluid boluses per protocol to achieve central venous pressure of 8 mm Hg to 12 mm Hg, vasopressors to achieve mean arterial pressure of 65 mm Hg to 90 mm Hg, and blood transfusion or administration of inotropes to achieve central venous oxygen saturation of 70% or greater.
Three recent multicenter trials found no difference between EGDT and usual care, but may have achieved smaller between-group differences in fluid administration due to incorporation of early fluid administration into usual sepsis care in resource-intense settings. In contrast, the current study is the third RCT to suggest that early resuscitation for African patients with infection and hypoperfusion may increase mortality compared with usual care.
Several potential explanations exist for the discordance in findings between the EGDT trial and the more recent African trials. Similar to the Fluid Expansion As Supportive Therapy (FEAST) trial and the prior Simplified Severe Sepsis Protocol trial, patients in the current study were predominantly young, malnourished individuals at risk for tuberculosis and malaria. In this patient population, rapid administration of intravenous fluid boluses may predispose to pulmonary edema and respiratory failure, conferring high mortality in the absence of ventilator support.
Despite excluding 20% of otherwise eligible patients for hypoxemia and severe tachypnea, nearly one-third of patients in the sepsis protocol group required discontinuation of intravenous fluids due to decreased oxygen saturation or increased respiratory rate. Nearly all patients in the current trial were cared for on the medical ward without access to mechanical ventilation compared with the 30% to 70% of patients who received mechanical ventilation in the EGDT trials, which were performed in high-income countries.
Resource limitations mandated that the resuscitation targets in the sepsis protocol in the current trial differ from the EGDT algorithm. In the absence of access to central venous catheterization, the protocol in the current study prescribed an initial 2-L intravenous fluid bolus during the first hour and used JVP measurement and respiratory examination to determine when fluid administration should be discontinued. It is possible that JVP did not serve as a reliable surrogate measure of central venous pressure or that central venous pressure itself was an inaccurate indicator of developing volume overload. The fact that more than 30% of patients treated with the sepsis protocol developed worsening respiratory function but less than 10% developed JVP elevation suggests that JVP cannot be safely used as an end point for fluid administration in this context.
A before-after study in Uganda reported the safety and efficacy of fluid boluses guided by blood pressure measurement rather than JVP, but the before-after design and the presence of a dedicated medical officer for the intervention group make comparison with the current study challenging. The only vasopressor available in the setting of the current study was dopamine. Recent studies have demonstrated better clinical outcomes with norepinephrine than with dopamine and increased dopamine administration in the sepsis protocol group may have contributed to increased mortality.
This study has several important strengths. The design included randomization to balance baseline confounders, concealed allocation to prevent selection bias, monitoring by an independent data and safety monitoring board, and collection of clinical outcomes by blinded study personnel. Unlike recent trials in high-income countries, usual care in the current study setting involved limited early fluid or vasopressor administration. As a result, the differences in the volume of intravenous fluid received and receipt of vasopressors between patients in the sepsis protocol group and the usual care group were greater than in any prior sepsis resuscitation trial, strengthening causal inferences between study group and clinical outcomes.
Limitations
This study also has several limitations. First, moderate size and conduct at a single center may exaggerate the observed treatment effect. However, any baseline imbalances between groups that occurred despite randomization appeared to be relatively small (eg, between-group difference in baseline lactic acid concentration of 0.7 mmol/L), and likely do not explain the between-group differences in clinical outcomes. Second, although study enrollment occurred shortly after arrival in the ED, the onset of infection for many patients may have occurred days to weeks before presentation. Third, patients, treating clinicians, and clinical study personnel were not blinded to group assignment. Fourth, the sepsis protocol relied on determination of JVP, a semireproducible skill, and data were not collected on the concordance of JVP measurement between study personnel. Fifth, secondary multivariable analyses relied on the SAPS-3, which has not been validated as a marker for severity of illness in the study setting. Sixth, only 1 of 209 patients was cared for in an intensive care unit. Although this reflects the reality of medical care in most hospitals in sub-Saharan Africa, it limits the generalizability to more resource-intense settings.
Coupled with the results of the FEAST trial and the prior Simplified Severe Sepsis Protocol trial, the findings of the current study suggest that in settings without routine access to mechanical ventilation, the risks of intravenous fluid bolus administration for patients acutely ill from infection may outweigh the benefits. These findings also increase the uncertainty regarding the ideal approach to intravenous fluid administration during early sepsis management in high-income settings. Further trials carefully examining the hemodynamic, cellular, and clinical effects of intravenous fluid bolus and vasopressor administration in sepsis are needed.
Conclusions
Among adults with sepsis and hypotension, most of whom were positive for HIV, in a resource-limited setting, a protocol for early resuscitation with administration of intravenous fluids and vasopressors increased in-hospital mortality compared with usual care. Further studies are needed to understand the effects of administration of intravenous fluid boluses and vasopressors in patients with sepsis across different low- and middle-income clinical settings and patient populations.
Trial protocol
eMethods
eTable 1. Suspected diagnoses at the time of hospital admission for enrolled patients
eTable 2. Microbial pathogens
eTable 3. Initial intravenous fluid order for each patient assigned to the usual care group
eTable 4. Sensitivity analyses
eTable 5. Adverse events
Section Editor: Derek C. Angus, MD, MPH, Associate Editor, JAMA (angusdc@upmc.edu).
References
- 1.Kaukonen K-M, Bailey M, Suzuki S, et al. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311(13):1308-1316. [DOI] [PubMed] [Google Scholar]
- 2.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368-1377. [DOI] [PubMed] [Google Scholar]
- 3.Levy MM, Rhodes A, Phillips GS, et al. Surviving Sepsis Campaign. Crit Care Med. 2015;43(1):3-12. [DOI] [PubMed] [Google Scholar]
- 4.Andrews B, Muchemwa L, Kelly P, et al. Simplified Severe Sepsis Protocol. Crit Care Med. 2014;42(11):2315-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacob ST, Banura P, Baeten JM, et al. The impact of early monitored management on survival in hospitalized adult Ugandan patients with severe sepsis. Crit Care Med. 2012;40(7):2050-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maitland K, Kiguli S, Opoka RO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364(26):2483-2495. [DOI] [PubMed] [Google Scholar]
- 7.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101(6):1644-1655. [DOI] [PubMed] [Google Scholar]
- 8.Tang AM, Dong K, Deitchler M, et al. Use of cutoffs for mid-upper arm circumference as an indicator or predictor of nutritional and health related outcomes in adolescents and adults: a systematic review. https://www.fantaproject.org/sites/default/files/resources/MUAC%20Systematic%20Review%20_Nov%2019.pdf. Accessed January 2, 2017.
- 9.Benítez Brito N, Suárez Llanos JP, Fuentes Ferrer M, et al. Relationship between mid-upper arm circumference and body mass index in inpatients. PLoS One. 2016;11(8):e0160480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreno RP, Metnitz PGH, Almeida E, et al. SAPS 3—from evaluation of the patient to evaluation of the intensive care unit: part 2. Intensive Care Med. 2005;31(10):1345-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock [published correction appears in Crit Care Med. 2004;32(6):1448]. Crit Care Med. 2004;32(3):858-873. [DOI] [PubMed] [Google Scholar]
- 12.Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peake SL, Delaney A, Bailey M, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16):1496-1506. [DOI] [PubMed] [Google Scholar]
- 14.Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372(14):1301-1311. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen HB, Jaehne AK, Jayaprakash N, et al. Early goal-directed therapy in severe sepsis and septic shock. Crit Care. 2016;20(1):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook DJ. Clinical assessment of central venous pressure in the critically ill. Am J Med Sci. 1990;299(3):175-178. [DOI] [PubMed] [Google Scholar]
- 17.Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness?. Crit Care Med. 2013;41(7):1774-1781. [DOI] [PubMed] [Google Scholar]
- 18.Ventura AMC, Shieh HH, Bousso A, et al. Double-blind prospective randomized controlled trial of dopamine versus epinephrine as first-line vasoactive drugs in pediatric septic shock. Crit Care Med. 2015;43(11):2292-2302. [DOI] [PubMed] [Google Scholar]
- 19.De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362(9):779-789. [DOI] [PubMed] [Google Scholar]
- 20.De Backer D, Aldecoa C, Njimi H, Vincent J-L. Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis*. Crit Care Med. 2012;40(3):725-730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eMethods
eTable 1. Suspected diagnoses at the time of hospital admission for enrolled patients
eTable 2. Microbial pathogens
eTable 3. Initial intravenous fluid order for each patient assigned to the usual care group
eTable 4. Sensitivity analyses
eTable 5. Adverse events