This open-label, single-arm study evaluates the safety and explores the efficacy of conversion from tetrabenazine to deutetrabenazine in patients with chorea associated with Huntington disease.
Key Points
Question
Is overnight treatment conversion from tetrabenazine to deutetrabenazine safe in patients with Huntington disease?
Findings
In this open-label, single-arm study, 37 patients with chorea associated with Huntington disease switched overnight from a stable, therapeutic tetrabenazine regimen to deutetrabenazine. Deutetrabenazine was generally safe and well tolerated, with chorea scores maintained at the end of week 1 of deutetrabenazine therapy and improved at week 8.
Meaning
Chorea control may be safely maintained after an overnight switch from tetrabenazine to deutetrabenzine.
Abstract
Importance
Tetrabenazine is efficacious for chorea control; however, tolerability concerns exist. Deutetrabenazine, a novel molecule that reduces chorea, was well tolerated in a double-blind, placebo-controlled study.
Objectives
To evaluate the safety and explore the efficacy of conversion from tetrabenazine to deutetrabenazine in patients with chorea associated with Huntington disease (HD).
Design, Setting, and Participants
In this ongoing, open-label, single-arm study that started on December 21, 2013, 37 patients at 13 Huntington Study Group sites in the United States and Australia who were taking stable doses of tetrabenazine that provided a therapeutic benefit were switched overnight to deutetrabenazine therapy. After week 1, the deutetrabenazine dose was titrated on a weekly basis for optimal chorea control.
Interventions
Deutetrabenazine administration at a dosage thought to provide comparable systemic exposure to the active metabolites of the prior, stable tetrabenazine regimen.
Main Outcomes and Measures
Safety measures included adverse events (AEs), clinical laboratory tests, vital signs, electrocardiograms, and validated scales. Changes in the Unified Huntington’s Disease Rating Scale total maximal chorea score and total motor score were efficacy end points.
Results
Of the 53 patients with HD screened for the study, 37 ambulatory patients with manifest HD (mean [SD] age, 52.4 [11.5] years; 22 [59%] male and 15 [41%] female; 36 white [97.3%]) were enrolled. Deutetrabenazine was generally well tolerated, with low rates of neuropsychiatric AEs. Safety scales did not reveal subclinical toxicity with deutetrabenazine treatment. Rates of dose reduction or suspension attributable to AEs were also low. Chorea control, as measured by the total maximal chorea score, was maintained at week 1 and significantly improved at week 8 (mean [SD] change from baseline, 2.1 [3.2]; P < .001).
Conclusions and Relevance
In patients with chorea, overnight conversion to deutetrabenazine therapy provided a favorable safety profile and effectively maintained chorea control.
Introduction
Huntington disease (HD) is a hereditary, progressive, neurodegenerative disorder characterized by chorea and other motor symptoms, cognitive dysfunction, and psychiatric symptoms. Up to 90% of patients with HD have chorea that can interfere with daily function and may cause injury.1,2 Tetrabenazine was the first drug approved by the US Food and Drug Administration for chorea associated with HD.3 High peak plasma concentrations and large plasma fluctuations may contribute to the tolerability issues observed in some patients with tetrabenazine use.4 Deutetrabenazine, a novel molecule that contains 6 deuterium atoms in place of 6 hydrogen atoms in specific positions in the tetrabenazine molecule, was approved for treating chorea associated with HD in 2017.5 Clinical evaluation in the First-HD trial found that deutetrabenazine provides significant chorea and motor improvement while maintaining a favorable safety profile when compared with placebo.6 The efficacy and excellent safety profile were attributed to the unique pharmacokinetic profile of deutetrabenazine, which enables comparable systemic exposure at lower doses, lower peak concentrations, and reduced plasma fluctuations.5 The current open-label study evaluated the safety and explored the efficacy of an overnight conversion from tetrabenazine to deutetrabenazine therapy with subsequent optional dose adjustment in patients with HD receiving a stable tetrabenazine regimen for chorea.
Methods
Study Design
This is an ongoing, open-label, single-arm study that started on December 21, 2013, at 13 Huntington Study Group sites in the United States and Australia. In-person study visits were conducted at baseline (day 0), on the last day of tetrabenazine use, and at weeks 1, 4, and 8 after overnight conversion to deutetrabenazine therapy. Telephone consultations were made at weeks 2, 3, and 7. Written approvals from an independent ethics committee or institutional review board were received before the initiation of this study. Western Institutional Review Board was used at all sites except for the following individual institutional review boards: Western Sydney Local Health District Human Research Ethics Committee, University of Alabama Birmingham, Duke University Health System, The Cooper Health System, Vanderbilt University Medical Center, and Rocky Mountain Movement Disorders Center. Institutional review board approval was also obtained at the Chesapeake Insitutional Review Board and centrally at the Research Subjects Review Board at the University of Rochester. An independent, qualified health care professional evaluated all patients to determine patient capacity to consent to participate. If patients lacked the capacity to provide informed consent, a legally authorized representative provided written informed consent, in addition to the patients’ provided written assent.
Patients
Patients were eligible if they were ambulatory adults with manifest HD indicated by characteristic motor examination features and an expanded CAG repeat sequence (≥37) and were receiving a stable tetrabenazine regimen for 8 weeks or longer that provided a therapeutic benefit for chorea control. Patients had a total functional capacity score of 5 or higher at screening. The total functional capacity score is a 13-point standardized disease staging scale that assesses an individual’s ability to perform tasks in 5 functional areas: to perform chores, perform activities of daily living, work, manage finances, and live at home. Higher scores indicate earlier disease and better functional status. In early HD, the mean (SD) decline is 0.63 (0.75) points per year.7 Enrolled patients were required to have daily access to reliable caregiver support to oversee study drug administration, ensure attendance at study visits, and provide another level of oversight for safety. Patients with more severe functional impairment as indicated by a total functional capacity score of 5 to 7 at screening were required to have a live-in caregiver.
Patients with serious undertreated psychiatric illness were excluded; however, concomitant stable antidepressant therapy was permitted during the study. Patients with active or past suicidal ideation, thoughts, or behavior were excluded. Patients were excluded if they scored 11 or higher on the Hospital Anxiety and Depression Scale, 11 or higher on the Swallowing Disturbance Questionnaire, or 3 or higher on the dysarthria score of the Unified Parkinson’s Disease Rating Scale. Concomitant use of dopamine receptor antagonists, dopamine agonists, levodopa, reserpine, N-methyl-d-aspartate receptor antagonists, or monoamine oxidase inhibitors within 30 days of screening was also exclusionary.
Dosing
Patients were required to have been receiving a stable tetrabenazine regimen (≥8 weeks) to be eligible. Each patient’s individualized tetrabenazine treatment regimen was determined by his or her primary health care professional independently of this study and before study participation. Masked CYP2D6 (OMIM 124030) genotyping was conducted at screening and remained masked throughout the study. Therefore, subsequent dosing decisions were made without knowledge of the participants CYP2D6 metabolism status. The initial deutetrabenazine daily dose was approximately half the prior tetrabenazine daily dose determined to profile comparable systemic exposure (area under the curve) to active metabolites (eTable in the Supplement). One week after overnight conversion to deutetrabenazine, the investigator, in consultation with the patient and caregiver, could begin weekly dose adjustments of deutetrabenazine, if needed, to achieve optimal chorea control. Dose adjustments were made on the basis of assessment of tolerability and chorea control by the investigator. The maximum total daily dose of deutetrabenazine that could be used was 72 mg; however, if the patient was receiving a strong CYP2D6 inhibitor (eg, bupropion, paroxetine, fluoxetine), the maximum total daily dose permitted was 42 mg.
Assessments
Safety measures included assessment of adverse events (AEs), clinical laboratory tests, physical and neurologic examinations, vital signs, electrocardiograms, and the following scales: Unified Huntington’s Disease Rating Scale (UHDRS),8 Hospital Anxiety and Depression Scale,9 Columbia Suicide Severity Rating Scale,10 Barnes Akathisia Rating Scale,11 Swallowing Disturbance Questionnaire,12 Unified Parkinson’s Disease Rating Scale dysarthria item,13 Montreal Cognitive Assessment,14 and Epworth Sleepiness Scale.15
The changes from baseline in the UHDRS total maximal chorea (TMC) score and total motor score (TMS) were evaluated as exploratory efficacy end points. The TMC score is a subset of the TMS; for the TMC and TMS, higher scores indicate greater motor signs.
Safety
Adverse events were tabulated, and treatment-emergent AEs were defined as events that began after initiation of study drug treatment that were not present at baseline or, if present at baseline, that worsened in severity. Two-sided paired t tests were used for descriptive analysis of changes from baseline for the Epworth Sleepiness Scale, Swallowing Disturbance Questionnaire, Unified Parkinson’s Disease Rating Scale dysarthria, and Barnes Akathisia Rating Scale.
Efficacy
Descriptive statistics were conducted for the UHDRS TMC score and TMS for the actual data presented by visit and changes from baseline. Two-sided paired t tests were performed to analyze changes from baseline. Baseline values used for calculating change from baseline for TMC score and TMS were the mean of the values from the screening and day 0 baseline visits. Lower scores on the TMC and TMS indicate less severe impairment. Participants were receiving a stable tetrabenazine regimen for at least 8 weeks before the screening visit and continued this regimen through the day 0 baseline visit before the day 1 change to deutetrabenazine.
Statistical Analysis
Because this was an open-label safety study, statistical considerations did not determine sample size. All analyses include observed data, with no imputation of missing values. P < .05 (2-sided) was considered statistically significant. Statistical analyses were performed using SAS statistical software (SAS Institute Inc).
Results
Baseline Characteristics
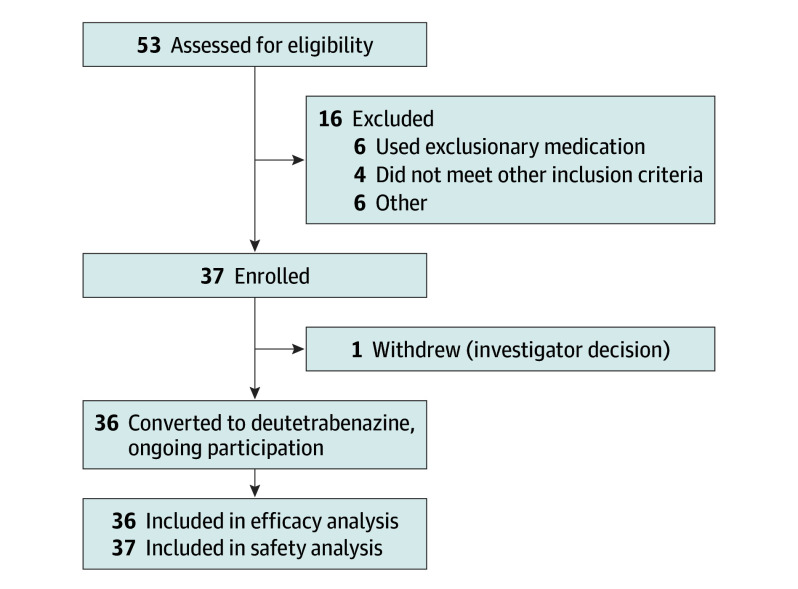
A total of 53 patients were assessed for eligibility in the study. Of these, 14 patients were excluded (6 were taking exclusionary medications, 4 did not meet inclusion criteria, 2 had severe suicidal ideation or depression, 1 had abnormal laboratory values, and 1 was excluded for other reasons) and 2 patients declined to participate in the study. Thus, 37 ambulatory patients with manifest HD (mean [SD] age, 52.4 [11.5] years; 22 [59%] male and 15 [41%] female; 36 white [97.3%]) were enrolled (Figure 1). Table 1 lists baseline characteristics, including the baseline TMC scores and TMSs. A total of 26 patients (70%) were being treated with antidepressants at baseline.
Figure 1. Flow of Study Patients.
After screening for eligibility, a total of 37 patients were enrolled. Of the 37 patients, 1 patient withdrew and all others continued in the ongoing study.
Table 1. Baseline Characteristics of the Study Patientsa.
| Characteristic | Finding (N = 37) |
|---|---|
| Patient demographic characteristics | |
| Age, y | 52.4 (11.5) |
| Female, No. (%) | 15 (41) |
| White, No. (%) | 36 (97) |
| Patient clinical characteristics | |
| CAG repeat length | 44.5 (3.3) |
| Body mass indexb | 23.9 (4.7) |
| Weight, kg | 71.4 (16.6) |
| UHDRS total functional capacity | 8.3 (2.1) |
| UHDRS total maximal chorea | 12.5 (5.2) |
| UHDRS total motor score | 38.5 (18.7) |
Abbreviation: UHDRS, Unified Huntington’s Disease Rating Scale.
Data are presented as mean (SD) unless otherwise indicated.
Calculated as weight in kilograms divided by height in meters squared.
Dosage
The initial median deutetrabenazine daily dose was 18.0 mg, approximately half the prior median tetrabenazine daily dose (37.5 mg). After dose adjustment, the median deutetrabenazine daily doses were 30.0 mg at week 4 (n = 37) and 36.0 mg at week 8 (n = 35). Eighteen of 37 participants (49%) were taking the same dose at the week 8 visit as they were taking or assigned at week 4.
Safety Outcomes
A total of 20 patients (54%) reported at least 1 treatment-emergent adverse event (Table 2). No AEs of worsening of chorea were reported in this study. Adverse events leading to dose reduction (4 [11%]) or dose suspension (1 [3%]) were observed, and no patient withdrew because of an AE. There were no clinically significant differences in laboratory values, vital signs, body weight, or electrocardiogram findings between baseline and week 8. Two patients (5%) experienced mild depression; the association with deutetrabenazine was rated as possible by the site investigators. There were 9 reported cases of somnolence, of which 6 (67%) were mild; among the remaining 3, 1 case required dose reduction and 1 required dose suspension. One patient (3%) experienced a serious AE of dehydration, possibly related to the study drug; the patient continued in the study without change in dose, and dehydration resolved. There were no deaths reported in this study. Dysphagia was not reported as an AE, and Swallowing Disturbance Questionnaire scores did not change significantly during the study.
Table 2. TEAEs Occurring in at Least 4% of Patients.
| TEAE | No. (%) of Patients (N = 37) |
|---|---|
| Any | 20 (54) |
| Somnolence | 9 (24) |
| Fall | 4 (11) |
| Nasopharyngitis | 3 (8) |
| Anxiety | 2 (5) |
| Diarrhea | 2 (5) |
| Constipation | 2 (5) |
| Dry mouth | 2 (5) |
| Depression | 2 (5) |
| Irritability | 2 (5) |
Abbreviation: TEAE, treatment-emergent adverse event.
Efficacy Outcomes
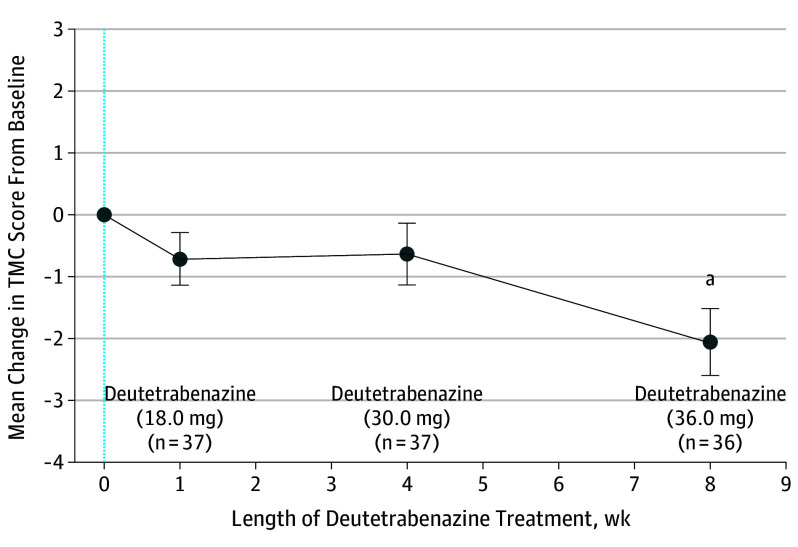
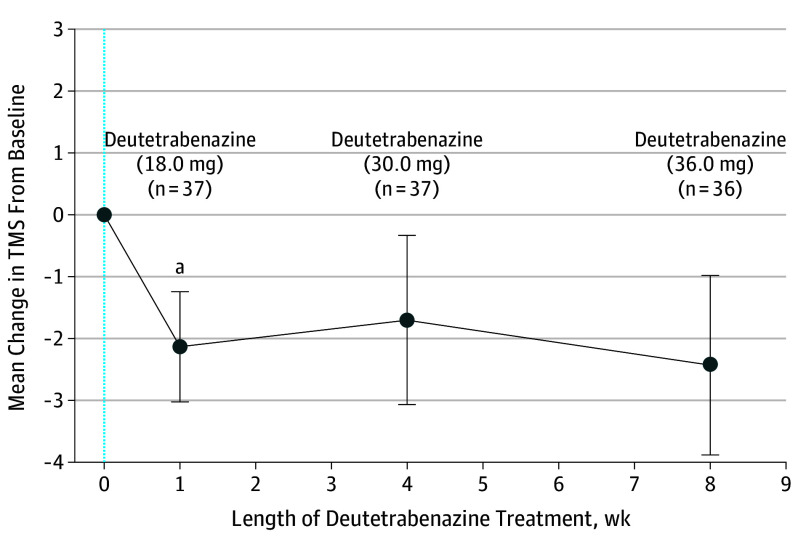
The mean (SD) TMS at baseline was 37.7 (18.6), and the mean (SD) TMC score was 12.5 (5.2). The mean (SD) reduction in TMC score from baseline to week 1 was 0.7 (2.6) UHDRS units (P = .10) (Figure 2). The mean (SD) reduction in TMS from baseline was 2.1 (5.4) UHDRS units (P = .02) at week 1 after dose conversion (Figure 3). The mean (SD) reduction in TMC score from baseline to week 4 was 0.6 (3.0) units (P = .21) (Figure 2). Chorea control improved compared with baseline at week 8 (2.1 [3.2]; P < .001). The mean (SD) TMS was unchanged compared with baseline at week 4 (1.7 [8.3]; P = .22) and week 8 (2.4 [8.7]; P = .10).
Figure 2. Mean Change in Total Maximal Chorea (TMC) Score.
The TMC score was assessed during 8 weeks. The TMC assessments at baseline (blue dotted line) and weeks 1, 4, and 8 are represented for the median daily doses of deutetrabenazine. Error bars represent SEM.
aP < .001.
Figure 3. Mean Change in Total Motor Score (TMS) After Switch.
The TMS was evaluated during 8 weeks. The TMS assessments at baseline (blue dotted line) and weeks 1, 4, and 8 are represented for the median daily doses of deutetrabenazine. Error bars represent SEM.
aP = .02.
Discussion
Overnight treatment conversion from tetrabenazine 3 times daily to deutetrabenazine twice daily can safely maintain chorea control in patients with HD. Deutetrabenazine therapy was generally well tolerated by patients after the overnight conversion from tetrabenazine. Adherence with a twice-daily medication was excellent and may be attributable to simplified medication regimens compared with existing therapies. The favorable safety profile observed in this study is reflective of the findings in the First-HD study, which supports the hypothesis that deuterium substitution–mediated attenuation of drug metabolism allows for maximal efficacy through dose adjustment while maintaining tolerability in patients with HD.6 The safety of deutetrabenazine is further highlighted by the low rates of depression and anxiety observed in participants up to week 8 after dose conversion. Although patients with active or undertreated psychiatric symptoms, such as depression, were excluded according to the study design, patients receiving stable antidepressant treatment were enrolled. There was a notable absence of suicidal ideation and parkinsonism during this 8-week study. Because this cohort is a part of an ongoing, long-term safety study, the safety profile of those patients with impaired CYP2D6 metabolism has not been included in this early safety analysis because CYP2D6 genetic variant status remains masked to investigators and participants. In addition, the results of this open-label study should be interpreted with caution because this study was not randomized or masked.
Limitations
This study was performed with an open-label design without a control group by unmasked site investigators determining chorea and TMSs in unmasked patients who had treatment converted from tetrabenazine to deutetrabenazine, factors that may impart treatment bias. Therefore, all efficacy findings reported in this study should be considered to be exploratory. Because the deutetrabenazine starting dose was thought to match the systemic active metabolite exposure achieved with the prior tetrabenazine dose, baseline levels of chorea were expected to be maintained but not necessarily improve after the switch to deutetrabenazine therapy. Worsening of chorea was not observed. With optional continued dose adjustments during 8 weeks, chorea control improved. There are a few explanations for continued dose adjustment after 4 weeks in half of the patients. Doses could be adjusted if site investigators and patients thought that the combination of chorea level, effect of chorea on the specific patient, and drug tolerance justified further dose adjustments, up or down, to maximize overall patient status. The TMC score improved at week 8 compared with baseline, suggesting that patients need to be reassessed for tolerability and chorea control after initial treatment conversion and any subsequent dose adjustments. The improvement in chorea raises the possibility that some patients were not able to tolerate higher doses of tetrabenazine or, despite being stable, may have had an incomplete response on tetrabenazine. Patients in either circumstance may be more likely to enroll in this type of study compared with other patients receiving a stable tetrabenazine regimen, creating an ascertainment bias. The starting dose for conversion may also have been too low in some patients.
Conclusions
The patients enrolled in this study were experiencing chorea and receiving tetrabenazine with clinical benefit before treatment conversion. This proof-of-concept study reveals that chorea control can be safely maintained when converting from tetrabenazine to deutetrabenazine, with a favorable safety profile. Change from a nondeuterated to a deuterium substituted compound can achieve treatment goals with fewer doses given across the day, lower total daily dose, and equivalent or potentially improved tolerance, all important considerations in populations with neurodegenerative disease.
eTable. Initial Dose Conversion
References
- 1.Burgunder J-M, Guttman M, Perlman S, Goodman N, van Kammen DP, Goodman L. An international survey-based algorithm for the pharmacological treatment of chorea in Huntington’s disease. PLoS Curr. 2011;3:RRN1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jankovic J, Roos RA. Chorea associated with Huntington’s disease: to treat or not to treat? Mov Disord. 2014;29(11):1414-1418. [DOI] [PubMed] [Google Scholar]
- 3.Kenney C, Hunter C, Jankovic J. Long-term tolerability of tetrabenazine in the treatment of hyperkinetic movement disorders. Mov Disord. 2007;22(2):193-197. [DOI] [PubMed] [Google Scholar]
- 4.Jankovic J. Dopamine depleters in the treatment of hyperkinetic movement disorders. Expert Opin Pharmacother. 2016;17(18):2461-2470. [DOI] [PubMed] [Google Scholar]
- 5.AustedoTM [package insert]. North Wales, PA: Teva Pharmaceuticals Inc; 2017.
- 6.Frank S, Testa CM, Stamler D, et al. ; Huntington Study Group . Effect of deutetrabenazine on chorea among patients with Huntington Disease: a randomized clinical trial. JAMA. 2016;316(1):40-50. [DOI] [PubMed] [Google Scholar]
- 7.Feigin A, Kieburtz K, Bordwell K, et al. Functional decline in Huntington’s disease. Mov Disord. 1995;10(2):211-214. [DOI] [PubMed] [Google Scholar]
- 8.Huntington Study Group . Unified Huntington’s Disease Rating Scale: reliability and consistency. Mov Disord. 1996;11(2):136-142. [DOI] [PubMed] [Google Scholar]
- 9.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361-370. [DOI] [PubMed] [Google Scholar]
- 10.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672-676. [DOI] [PubMed] [Google Scholar]
- 12.Manor Y, Giladi N, Cohen A, Fliss DM, Cohen JT. Validation of a swallowing disturbance questionnaire for detecting dysphagia in patients with Parkinson’s disease. Mov Disord. 2007;22(13):1917-1921. [DOI] [PubMed] [Google Scholar]
- 13.Fahn S, Elton RL; Members of the UDC . Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, eds. Recent Developments in Parkinson’s Disease. Vol 2. Florham Park, NJ: Macmillan Healthcare Information; 1987:153-163. [Google Scholar]
- 14.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. [DOI] [PubMed] [Google Scholar]
- 15.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Initial Dose Conversion