Abstract
Importance
Medical treatment of levodopa-induced dyskinesia (LID) in Parkinson disease (PD) is an unmet need.
Objective
To evaluate the efficacy and safety of ADS-5102 (amantadine) extended-release 274-mg capsules for treatment of LID in patients with PD.
Design, Setting, and Participants
A randomized, double-blind, placebo-controlled clinical trial was conducted between May 7, 2014, and July 22, 2015, at 44 North American sites among patients with PD treated with levodopa who experienced at least 1 hour of troublesome dyskinesia per day with at least mild functional impact.
Interventions
Patients were randomized to receive placebo or 274 mg of ADS-5102 administered orally at bedtime for up to 25 weeks.
Main Outcomes and Measures
The primary efficacy analysis was the change from baseline to week 12 in the Unified Dyskinesia Rating Scale total score for ADS-5102 vs placebo in the modified intent-to-treat population. OFF time (amount of time the PD medication is not controlling motor symptoms) was a key secondary end point. Safety analyses included all patients who received the study drug (ADS-5102 or placebo).
Results
A total of 189 patients were screened, and 126 were randomized; the modified intent-to-treat population included 121 patients (51 women and 70 men; mean [SD] age, 64.7 [9.1] years). At week 12, the least-squares mean (SE) change in the Unified Dyskinesia Rating Scale score was –15.9 (1.6) for ADS-5102 (n = 63) and –8.0 (1.6) for placebo (n = 58) (treatment difference, –7.9; 95% CI, –12.5 to –3.3; P < .001). OFF time decreased by a mean (SE) of 0.6 (0.3) hours for ADS-5102 and increased by 0.3 (0.3) hours for placebo (treatment difference, –0.9 hours; 95% CI, –1.6 to –0.2; P = .02). Common adverse events for ADS-5102 vs placebo included visual hallucinations (15 [23.8%] vs 1 [1.7%]), peripheral edema (15 [23.8%] vs 0), and dizziness (14 [22.2%] vs 0). Adverse events led to treatment discontinuation for 13 patients receiving ADS-5102 (20.6%) vs 4 patients receiving placebo (6.9%).
Conclusions and Relevance
ADS-5102, 274 mg at bedtime, may be an effective treatment for LID. An additional benefit is reduced OFF time. To our knowledge, this is the first demonstration of an oral treatment reducing both LID and OFF time in patients with PD with dyskinesia.
Trial Registration
clinicaltrials.gov Identifier: NCT02136914
This randomized, double-blind, placebo-controlled clinical trial evaluates the efficacy and safety of extended-release ADS-5102 (amantadine) 274-mg capsules for treatment of levodopa-induced dyskinesia in patients with Parkinson disease.
Key Points
Question
Are 274-mg ADS-5102 (amantadine) extended-release capsules safe and effective for the treatment of levodopa-induced dyskinesia in Parkinson disease?
Findings
In this randomized clinical trial, ADS-5102 was associated with a significantly greater reduction in the duration, severity, and impact of dyskinesia at 12 weeks compared with placebo, as measured by the least-squares mean change in the Unified Dyskinesia Rating Scale score (treatment difference, –7.9); this reduction in the duration, severity, and impact of dyskinesia was maintained through week 24 (treatment difference, –9.3). Adverse events led to treatment discontinuation for 13 of 63 patients receiving ADS-5102 (20.6%) vs 4 of 58 patients receiving placebo (6.9%).
Meaning
ADS-5102 may be an effective treatment for levodopa-induced dyskinesia.
Introduction
The medical treatment of levodopa-induced dyskinesia (LID) in Parkinson disease (PD) is an unmet need. There is no approved drug therapy for LID, which impairs activities of daily living, decreases quality of life, increases caregiver burden and health care use, and is associated with a higher risk for falls. Levodopa remains the most efficacious treatment for motor symptoms of PD; however, long-term treatment with levodopa often leads to the development of dyskinesia. For patients treated with levodopa, dyskinesia can develop early and affects nearly 90% of patients within approximately 10 years of treatment.
Immediate-release amantadine hydrochloride (amantadine IR) was originally approved for influenza, with an additional indication for parkinsonism. Several small studies suggest that amantadine IR may have an antidyskinetic effect; however, the drug has not been extensively studied in well-controlled clinical trials, and the durability of its effect has been disputed. The safety profile of amantadine IR has been well characterized. Although most patients with PD can tolerate amantadine IR doses of 81 to 161 mg daily (equivalent to 100 to 200 mg daily of amantadine hydrochloride), the higher doses that may produce an antidyskinetic effect are associated with increased frequency of central nervous system adverse events (AEs).
ADS-5102 (amantadine) extended-release capsules are in development for the treatment of LID. ADS-5102 is administered at bedtime and is specifically formulated such that the shape of the resulting plasma concentration–time curve is characterized by an initially slow increase during sleep, peak concentrations in the morning, and sustained concentrations throughout waking hours, when patients most need relief from dyskinesia. In the dose-finding study of ADS-5102, the 274-mg dose (340 mg of amantadine hydrochloride) provided the best benefit and risk profile. The present study was designed to evaluate the efficacy and safety of 274 mg of ADS-5102 once daily at bedtime for the treatment of LID in patients with PD.
Methods
Study Design and Participants
A phase 3, randomized, double-blind, placebo-controlled clinical trial (ADS-5102 Extended Release Capsules for the Treatment of Levodopa Induced Dyskinesia [EASE LID] Study, Adamas Pharmaceuticals Inc, ADS-AMT-PD301) was conducted at 44 North American sites. The complete trial protocol can be found in Supplement 1. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. Before initiating the study, all participating sites received institutional review board approval. Written informed consent was obtained from all study participants before any study-related procedures were performed.
Key inclusion criteria were as follows: patients with PD taking levodopa; age between 30 and 85 years; diagnosis of PD based on the UK Parkinson Disease Society Brain Bank Clinical Diagnostic Criteria; at least a mild functional impact of dyskinesia (score of 2, on a scale of 0-4, where 0 is no impact and 4 is severe impact to the point that the patient does not perform most activities) based on the Movement Disorder Society–Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), part IV, item 4.2, at screening and day 1 (baseline); and 2 or more half-hour periods between 9 am and 4 pm of ON time (periods when PD medications provide good benefit for motor symptoms) with troublesome dyskinesia, as recorded in a 24-hour PD patient diary on each of 2 consecutive days just prior to day 1. Antiparkinsonian medications, including levodopa preparations, were to be unchanged for at least 30 days before screening and during study participation. Levodopa preparations had to be administered at least 3 times daily.
Key exclusion criteria included the following: history of dyskinesia that was exclusively diphasic, OFF state, myoclonic, dystonic, or akathetic without peak-dose dyskinesia; neurosurgical intervention related to PD; atypical parkinsonism; levodopa- or dopamine agonist–induced psychosis; cognitive impairment as evidenced by a Mini-Mental State Examination score of less than 24 during screening; an estimated glomerular filtration rate of less than 50 mL/min/1.73 m2; use of amantadine within 30 days before screening; documented inability to tolerate amantadine treatment or lack of dyskinesia response to prior amantadine treatment; current treatment with apomorphine hydrochloride or dopamine receptor blocking agents; current treatment with medications that prolong the QT interval and have a known risk of torsades de pointes; clinically significant abnormalities based on results of electrocardiogram; use of rimantadine hydrochloride; or history of hypersensitivity or allergic reaction to amantadine, rimantadine hydrochloride, or memantine hydrochloride.
Randomization and Blinding
Between May 7, 2014, and July 22, 2015, eligible patients were randomized on day 1 in a 1:1 ratio to receive placebo or ADS-5102. ADS-5102 and placebo capsules and packaging were identical in appearance. The randomization list was generated and validated by PharmaStat, LLC. Randomization was accomplished through an interactive web-based response system managed by Endpoint Clinical, which allowed for unblinding if necessary for patient safety. All patients, study site personnel, raters, the sponsor, and contract research organization staff were blinded to group assignment.
Procedures
Prequalified raters completed training using the MDS teaching modules for the Unified Dyskinesia Rating Scale (UDysRS) and the MDS-UPDRS. Each assessment was at least 30 minutes after the patient’s regularly scheduled levodopa dose, during ON time when the patient was experiencing typical dyskinesia. There were 11 scheduled visits (screening; baseline [day 1]; weeks 1, 2, 4, 8, 12, 18, 24, and 25; and a safety follow-up).
The study was originally designed as a 13-week trial. Based on a regulatory health authority recommendation, the protocol was amended to increase the treatment duration to 25 weeks to characterize the durability of effect.
During the screening visit (up to 3 weeks before baseline), written informed consent was obtained and study eligibility criteria were assessed. Patient training in filling out the PD home diary was completed, and diary concordance testing (between patient and rater) was performed. Two 24-hour PD home diaries were distributed for completion just before the scheduled baseline visit. During the baseline visit, eligibility was confirmed and patients were randomized to receive ADS-5102 or placebo. In addition, the following assessments associated with efficacy were performed: completion of the UDysRS and MDS-UPDRS, review of completed PD home diaries, and recording of investigator notes relevant to the Clinician’s Global Impression of Change (CGIC) scale.
During the first week of treatment, patients randomized to receive ADS-5102 received a daily ADS-5102 dose of 137 mg. To maintain blinding, patients took 1 capsule containing ADS-5102 and 1 capsule containing placebo. During weeks 2 through 24, the daily ADS-5102 dose was increased to 274 mg, administered as two 137-mg capsules. During the last week of dosing, the dosage was reduced back to 137 mg daily. Patients randomized to receive placebo were given 2 placebo capsules for 25 weeks.
The UDysRS, MDS-UPDRS, CGIC, and standard safety assessments were performed at weeks 2, 8, 12, 18, and 24. The PD home diaries were completed before each of these visits. A final safety follow-up visit occurred approximately 7 days following treatment completion, unless a patient elected to enroll directly into a companion open-label safety study (ClinicalTrials.gov identifier: NCT02202551).
Outcomes
The primary outcome measure was the change from baseline in the UDysRS total score at 12 weeks. Key secondary outcome measures included the change from baseline in the UDysRS total score at 24 weeks, ON time without troublesome dyskinesia (ON time without dyskinesia plus ON time with nontroublesome dyskinesia) at 12 and 24 weeks, and OFF time (amount of time the PD medication is not controlling motor symptoms) at 12 and 24 weeks. Other secondary measures included the change from baseline at 12 and 24 weeks in the MDS-UPDRS score, ON time with troublesome dyskinesia, total ON time with dyskinesia (nontroublesome plus troublesome), and the CGIC score. Safety assessments included AEs, reasons for discontinuation, physical examinations, vital signs, and clinical laboratory testing.
Statistical Analysis
Based on the previous phase 2/3 study, 46 patients per group were expected to provide 90% power to detect a mean (SD) treatment difference of 9.5 (14.0) units between the ADS-5102 and placebo groups, with α = .05. To account for a potential dropout rate of up to 20% at 12 weeks, 120 patients (60 per group) were planned for randomization.
The primary efficacy analysis compared the active (274 mg of ADS-5102) group with the placebo group at week 12 using a linear mixed model with repeated measures, with the changes from baseline in the UDysRS total score at weeks 2, 8, and 12 as the dependent variable. The model included categorical effects for treatment group, visit, and the interaction between treatment group and visit, and baseline UDysRS total score as a covariate. Estimates for the least-squares mean change were provided with 95% CIs.
The key secondary analyses were conducted using a fixed-sequence hierarchical procedure to control the overall level of significance. A specified comparison was considered confirmatory only if the primary efficacy analysis and all previously conducted key secondary analyses were statistically significant at P < .05.
The modified intent-to-treat population was the prespecified efficacy analysis population and included randomized patients who received treatment and provided 1 or more postbaseline assessments of the UDysRS. The safety population included randomized patients who received 1 or more doses of the study drug. A synchronized time profile analysis was generated displaying the percentages of patients reporting a given PD home diary state. Software package SAS, version 9.4 (SAS Institute Inc), was used for analysis. A data monitoring committee was not used for this trial.
This study was stopped early by the sponsor to accelerate the availability of primary efficacy data to permit timely submission of these data to the US Food and Drug Administration. All randomized patients had the opportunity to complete their week 12 study visit. A priori study power was preserved for the primary analysis because 103 patients contributed week 12 efficacy data. At the time the study was stopped, consideration was also given to having a sufficient number of patients contribute week 24 efficacy data (n = 84) to allow adequate statistical power (at least 80%) for treatment comparisons at this point to characterize the durability of effect beyond 12 weeks. The decision to stop the study early was not based on any safety finding or premature unblinding of the data.
Results
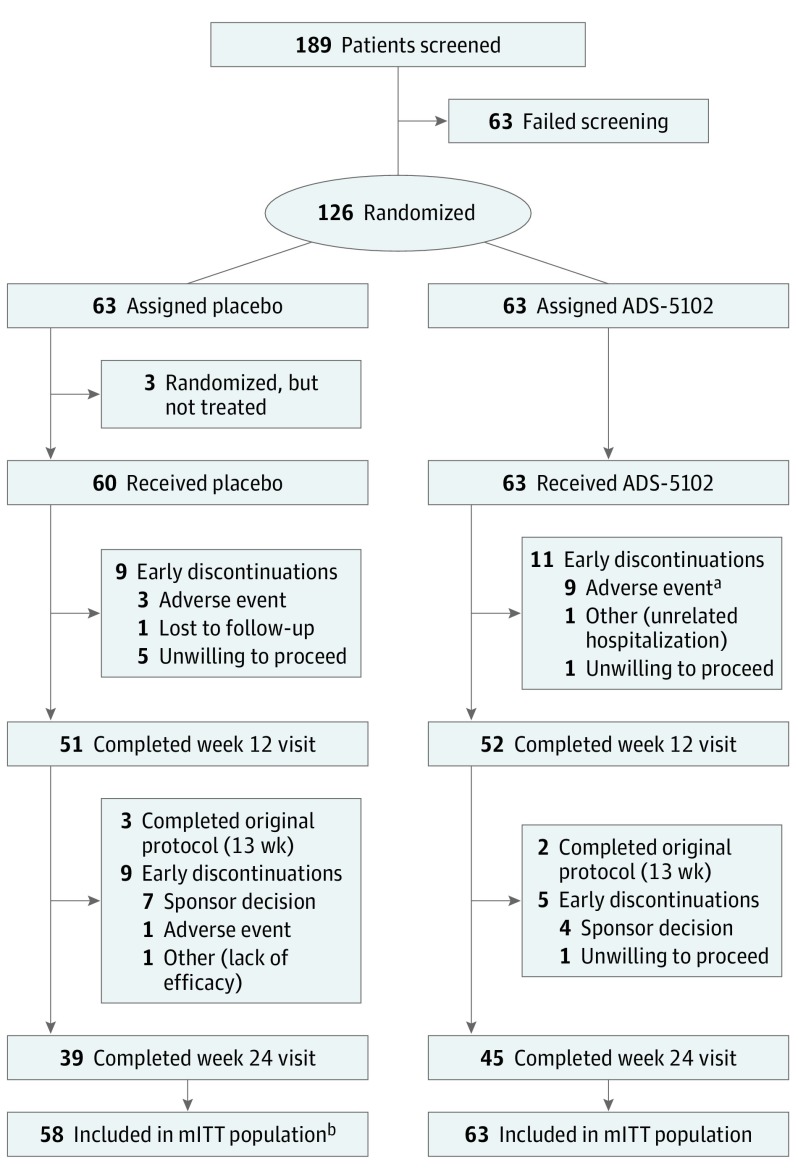
A total of 189 patients were screened, and 126 patients were randomized (Figure 1). The most common reason for failure to be randomized (24 of 63 total) was that the patient did not report at least 2 half-hour ON time periods with troublesome dyskinesia at baseline. The modified intent-to-treat population included 121 patients; 5 patients receiving placebo were excluded according to predefined criteria. Three of these 5 patients were randomized but never received a dose, and 2 patients did not have a postbaseline UDysRS assessment. The safety population included 123 patients. A week 12 visit (primary efficacy time point) was completed by 103 of 126 randomized patients (81.7%). The most common reason for study drug discontinuation was AEs in the ADS-5102–treated group and unwillingness to proceed in the placebo group (Figure 1). No unblinding of treatment assignment occurred during the study.
Figure 1. Trial Flowchart.
aFour additional patients discontinued the study drug owing to adverse events but continued the study.
bTwo patients did not have a postbaseline Unified Dyskinesia Rating Scale assessment (predefined criterion) and thus were excluded from the modified intent-to-treat (mITT) population.
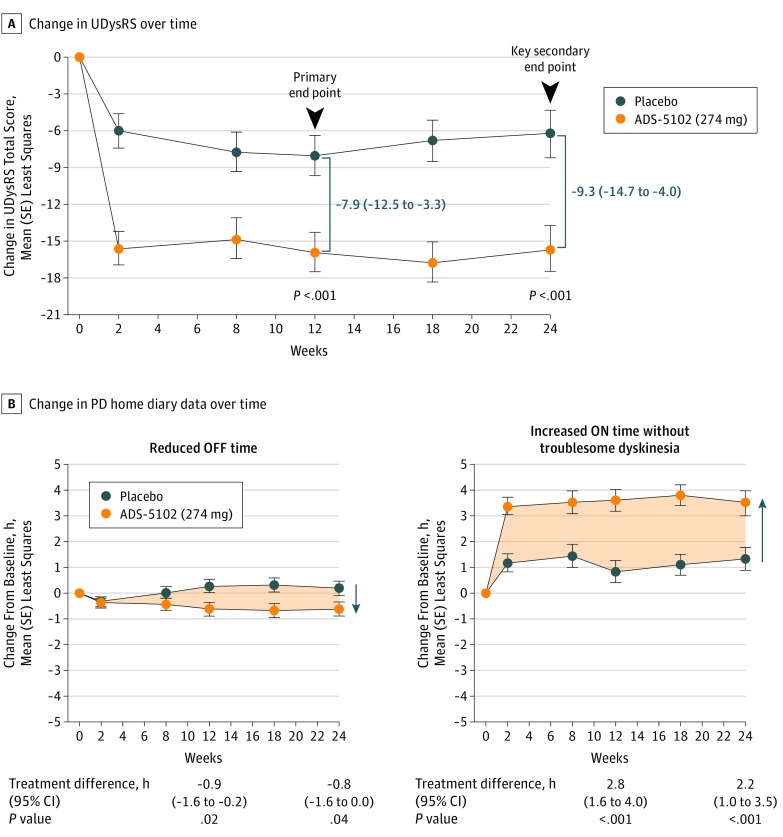
Demographics and baseline characteristics of the modified intent-to-treat population are shown in Table 1. The primary efficacy analysis demonstrated a significantly greater decrease in UDysRS total score (reduction in duration, severity, and impact of dyskinesia) in the ADS-5102 group compared with the placebo group at week 12 (Figure 2A; least-squares mean treatment difference, –7.9; 95% CI, –12.5 to –3.3; P < .001). Similarly, at week 24, a significantly greater decrease in UDysRS total score was observed in the ADS-5102 group compared with the placebo group (least-squares mean treatment difference, –9.3; 95% CI, –14.7 to –4.0; P < .001). The treatment effect of ADS-5102 on the UDysRS total score was consistent across the following subgroups: sex, age, body mass index, renal clearance (estimated glomerular filtration rate), and dyskinesia severity (eFigure 1 in Supplement 2). The historical (patient-reported duration and effect) and objective (rater assessment of impairment and disability) UDysRS scores showed a significantly greater mean (SE) reduction in the ADS-5102 group compared with the placebo group at 12 and 24 weeks (total historical UDysRS score: at 12 weeks, –9.9 [1.0] vs –5.4 [1.0] and at 24 weeks, –8.4 [1.2] vs –4.2 [1.3]; total objective UDyrRS score: at 12 weeks, –6.1 [0.9] vs –2.7 [1.0] and at 24 weeks, –7.4 [1.0] vs –2.1 [1.1]) (Table 2).
Table 1. Baseline Demographics and PD Characteristics of the Modified Intent-to-Treat Population.
| Characteristic | Mean (SD) | |
|---|---|---|
| Placebo (n = 58) |
ADS-5102 (n = 63) |
|
| Age, y | 65.5 (8.7) | 63.9 (9.4) |
| Male sex, No. (%) | 35 (60.3) | 35 (55.6) |
| White race, No. (%) | 51 (87.9) | 60 (95.2) |
| Baseline levodopa dose (any preparation), mg | 813.8 (513.5) | 905.6 (482.2) |
| Duration of LID, y | 3.3 (2.5) | 4.1 (3.1) |
| Time since PD diagnosis, y | 9.0 (3.9) | 9.5 (4.4) |
| Mini-Mental State Examination score | 28.8 (1.2) | 28.7 (1.5) |
| Hoehn and Yahr score | 2.3 (0.6) | 2.2 (0.5) |
| UDysRS score, total | 38.5 (11.2) | 40.9 (13.3) |
| PD home diary | ||
| ON time with troublesome dyskinesia, ha | 4.5 (2.0) | 4.7 (2.5) |
| ON time without troublesome dyskinesia, h | 8.5 (2.8) | 8.3 (3.5) |
| OFF time, hb | 3.0 (2.1) | 3.2 (2.4) |
| Time asleep, h | 8.1 (1.5) | 7.8 (1.7) |
| Patients with OFF time at baseline, No. (%) | 52 (89.7) | 57 (91.5) |
| MDS-UPDRS score | ||
| Part I (nonmotor experiences of daily living) | 11.4 (4.0)c | 12.5 (6.2) |
| Part II (motor experiences of daily living) | 15.7 (5.9) | 15.7 (6.8) |
| Part III (motor examination) | 24.8 (12.2) | 25.9 (14.5) |
| Combined score (parts I-III) | 51.9 (17.0)c | 54.2 (20.4) |
| Part IV (motor complications) | 11.3 (2.4) | 11.8 (3.0) |
| Part IV, item 4.1 (time spent with dyskinesias) | 2.4 (0.8) | 2.6 (0.9) |
| Part IV, item 4.2 (functional impact of dyskinesias) | 2.5 (0.5) | 2.6 (0.6) |
| Concomitant medication use at baseline, No. (%)d | ||
| Dopamine agonist | 34 (56.7) | 29 (46.0) |
| MAO inhibitor | 24 (40.0) | 26 (41.3) |
| COMT inhibitor | 9 (15.0) | 7 (11.1) |
| Anticholinergic | 3 (5.0) | 2 (3.2) |
Abbreviations: COMT, catechol-O-methyltransferase; LID, levodopa-induced dyskinesia; MAO, monoamine oxidase; MDS-UPDRS, Movement Disorder Society–Unified Parkinson’s Disease Rating Scale; PD, Parkinson disease; UDysRS, Unified Dyskinesia Rating Scale.
ON time indicates amount of time the PD medication provided good benefit for motor symptoms.
OFF time indicates amount of time the PD medication was not controlling motor symptoms.
For 57 patients.
For the safety population.
Figure 2. Change in Primary Efficacy Analysis and Key Secondary End Points.
A, Change in Unified Dyskinesia Rating Scale (UDysRS) score over time in the modified intent-to-treat population. Ranges in parentheses indicate 95% CIs. B, Change in Parkinson disease (PD) home diary data over time in the modified intent-to-treat population. Least-squares mean changes in PD diary data from baseline through week 24 are summarized for the modified intent-to-treat population. The shaded areas represent the difference (in hours) between placebo and ADS-5102 across time for amount of time the PD medication was not controlling motor symptoms (OFF time) and amount of time the PD medication provided good benefit for motor symptoms (ON time) without troublesome dyskinesia. Error bars indicate SE.
Table 2. Efficacy Results of the Modified Intent-to-Treat Population.
| Characteristic | LS Mean (SE) Change From Baseline | Treatment Difference (95% CI) | P Value | |
|---|---|---|---|---|
| Placebo (n = 58) |
ADS-5102 (n = 63) |
|||
| Primary End Point | ||||
| UDysRS total score | ||||
| Week 12 | –8.0 (1.6) | –15.9 (1.6) | –7.9 (–12.5 to –3.3) | <.001 |
| Key Secondary End Points | ||||
| UDysRS total score | ||||
| Week 24 | –6.3 (1.9) | –15.6 (1.9) | –9.3 (–14.7 to –4.0) | <.001 |
| ON time without troublesome dyskinesiaa | ||||
| Week 12 | 0.9 (0.4) | 3.6 (0.4) | 2.8 (1.6 to 4.0) | <.001 |
| Week 24 | 1.4 (0.5) | 3.6 (0.4) | 2.2 (1.0 to 3.5) | <.001 |
| OFF timeb | ||||
| Week 12 | 0.3 (0.3) | –0.6 (0.3) | –0.9 (–1.6 to –0.2) | .02 |
| Week 24 | 0.2 (0.3) | –0.6 (0.3) | –0.8 (–1.6 to –0.0) | .04 |
| Other Secondary End Points | ||||
| ON time with troublesome dyskinesia | ||||
| Week 12 | –1.6 (0.4) | –3.2 (0.4) | –1.6 (–2.6 to –0.6) | .003 |
| Week 24 | –1.9 (0.4) | –3.3 (0.4) | –1.5 (–2.5 to –0.4) | .007 |
| ASLEEP time, mean (SD) | ||||
| Week 12 | 8.4 (1.7) | 7.9 (1.3) | NA | NA |
| Week 24 | 8.4 (1.5) | 7.9 (1.8) | NA | NA |
| UDysRS historical score (parts I and II) | ||||
| Week 12 | –5.4 (1.0) | –9.9 (1.0) | –4.5 (–7.4 to –1.6) | .003 |
| Week 24 | –4.2 (1.3) | –8.4 (1.2) | –4.2 (–7.8 to –0.7) | .02 |
| UDysRS objective score (parts III and IV) | ||||
| Week 12 | –2.7 (1.0) | –6.1 (0.9) | –3.4 (–6.0 to –0.7) | .01 |
| Week 24 | –2.1 (1.1) | –7.4 (1.0) | –5.3 (–8.3 to –2.3) | <.001 |
| MDS-UPDRS score, part IV (motor complications) | ||||
| Week 12 | –2.5 (0.4) | –4.4 (0.4) | –1.9 (–2.9 to –0.9) | <.001 |
| Week 24 | –2.0 (0.5) | –4.2 (0.5) | –2.2 (–3.5 to –0.9) | .001 |
| MDS-UPDRS score, part IV, item 4.1 (time spent with dyskinesia) | ||||
| Week 12 | –0.6 (0.1) | –1.1 (0.1) | –0.6 (–0.9 to –0.2) | .002 |
| Week 24 | –0.6 (0.1) | –0.8 (0.1) | –0.2 (–0.6 to 0.2) | .24 |
| MDS-UPDRS score, part IV, item 4.2 (functional impact of dyskinesia) | ||||
| Week 12 | –0.9 (0.1) | –1.6 (0.1) | –0.7 (–1.1 to –0.3) | .001 |
| Week 24 | –0.9 (0.2) | –1.6 (0.2) | –0.7 (–1.2 to –0.3) | .003 |
| MDS-UPDRS score, combined parts I-III | ||||
| Week 12 | –4.0 (2.0) | –5.2 (1.9) | –1.1 (–6.6 to 4.3) | .68 |
| Week 24 | –3.5 (2.6) | –1.3 (2.5) | 2.1 (–5.0 to 9.2) | .56 |
| Mean (SD) change in daily levodopa dose (any preparation) from baseline, mg | ||||
| Week 12 | 0 | 0.0 (53.9) | NA | NA |
| Week 24 | 0 | –4.0 (136.3) | NA | NA |
Abbreviations: LS, least-squares; MDS-UPDRS, Movement Disorder Society–Unified Parkinson’s Disease Rating Scale; NA, not applicable; UDysRS, Unified Dyskinesia Rating Scale.
ON time indicates amount of time the PD medication provided good benefit for motor symptoms.
OFF time indicates amount of time the PD medication was not controlling motor symptoms.
Key secondary PD diary end points (mean [SD] ON time without troublesome dyskinesia and mean [SD] OFF time) showed significant improvements in the ADS-5102 group compared with the placebo group (ON time without troublesome dyskinesia: at 12 weeks, 3.6 [0.4] vs 0.9 [0.4] hours and at 24 weeks, 3.6 [0.4] vs 1.4 [0.5] hours; OFF time: at 12 weeks, –0.6 [0.3] vs 0.3 [0.3] hours and at 24 weeks, –0.6 [0.3] vs 0.2 [0.3] hours) (Table 2). Mean changes in PD diary end points at all study visits for the ADS-5102 and placebo groups are shown in Figure 2B. A summary of diary states across waking hours showed a greater increase in ON time without troublesome dyskinesia due to a decrease in OFF and ON times with troublesome dyskinesia for the ADS-5102–treated patients compared with the patients receiving placebo (eFigures 2 and 3 in Supplement 2).
At weeks 12 and 24, there were no differences between the ADS-5102 and placebo groups in MDS-UPDRS (in the ON state) combined or individual scores (parts I, II, and III), suggesting that ADS-5102 does not worsen PD motor function (Table 2). The CGIC results showed that 51 of the 63 patients (81.0%) in the ADS-5102 group and 21 of 58 patients (36.2%) in the placebo group were assessed as improved in overall PD symptoms, including dyskinesia, at week 12 (P < .001 for overall distribution). Furthermore, 43 patients (68.3%) in the ADS-5102 group and 27 of 58 patients (46.6%) in the placebo group were assessed as improved from baseline at week 24 (P = .11 for overall distribution).
Overall, AEs were reported for 56 of 63 patients (88.9%) in the ADS-5102 group and 36 of 60 patients (60.0%) in the placebo group (Table 3). Most patients in both groups reported AEs that were mild to moderate in intensity (43 [68.3%] in the ADS-5102 group and 32 [53.3%] in the placebo group). The most common AEs (≥5% in the active arm) included visual hallucinations, peripheral edema, dizziness, dry mouth, and constipation (Table 3). Other AEs occurring in less than 5% of patients in the ADS-5102 group included nausea (3 [4.8%]), confusion (2 [3.2%]), and orthostatic hypotension (1 [1.6%]). There were no reports of impulse control disorder in the ADS-5102 group. No serious AEs associated with the study drug were reported. There was 1 death in the ADS-5102 group; the investigator reported this as not associated with study drug and attributed the event to advanced PD. Adverse events broken down by time of onset are listed in the eTable in Supplement 2.
Table 3. Adverse Events in Patients Receiving ADS-5102 and Placebo (Safety Population).
| Characteristic | No. (%) | |
|---|---|---|
| Placebo (n = 60) |
ADS-5102 (n = 63) |
|
| Patients with any AEs | 36 (60.0) | 56 (88.9) |
| Patients with any study drug–related AEs | 7 (11.7) | 40 (63.5) |
| Patients with any serious AEs | 3 (5.0) | 7 (11.1) |
| Patients with any study drug–related serious AEs | 0 | 0 |
| Patients who permanently discontinued treatment owing to any AEs | 4 (6.7) | 13 (20.6) |
| Patients who permanently discontinued treatment owing to any study drug–related AEs | 4 (6.7) | 12 (19.0) |
| Most common AEsa | ||
| Visual hallucinations | 1 (1.7) | 15 (23.8) |
| Peripheral edema | 0 | 15 (23.8) |
| Dizziness | 0 | 14 (22.2) |
| Dry mouth | 0 | 11 (17.5) |
| Constipation | 3 (5.0) | 10 (15.9) |
| Fall | 5 (8.3) | 10 (15.9) |
| Anxiety | 1 (1.7) | 6 (9.5) |
| Livedo reticularis | 0 | 6 (9.5) |
| Auditory hallucinations | 0 | 5 (7.9) |
| Abnormal dreams | 2 (3.3) | 4 (6.3) |
| Depression | 1 (1.7) | 4 (6.3) |
Abbreviation: AEs, adverse events.
At least 5% in the active arm and greater than in the placebo arm.
Fifteen patients (23.8%) in the ADS-5102 group and 1 patient in the placebo group reported visual hallucinations. For 10 of these 15 patients in the ADS-5102 group, visual hallucinations were mild (defined as easily tolerated, causing minimal discomfort, and not interfering with a person’s normal daily functions). One patient reported a severe visual hallucination (defined as producing significant impairment of functioning or incapacitation and a definite hazard to the individual’s health), and no patients reported visual hallucinations that met the criteria for a serious AE. Five patients reported visual hallucinations within the first months of treatment, but no visual hallucinations were reported within the first week of dosing, while patients were receiving the 137-mg dose. Of the 15 patients who reported visual hallucinations, 5 discontinued treatment, 3 had a dose interruption or reduction (to 137 mg), and 7 continued treatment at 274 mg (4 of these 7 patients experienced spontaneous resolution). Overall, for 8 of these 15 patients, the visual hallucinations resolved within 2 weeks of onset, and none of the patients required hospitalization or treatment with an antipsychotic medication. Two of these 15 patients experienced auditory hallucinations, while 3 additional patients experienced only auditory hallucinations. In total, 18 patients (28.6%) in the ADS-5102 group experienced a hallucination of any type.
Thirteen patients (20.6%) in the ADS-5102 group and 4 patients (6.7%) in the placebo group discontinued the study drug because of AEs. The most common AEs leading to treatment discontinuation in the ADS-5102 group were visual hallucinations (5 [7.9%]), peripheral edema (3 [4.8%]), and dry mouth (3 [4.8%]). Within the ADS-5102 group, 10 of the 13 patients who discontinued treatment because of AEs did so during the first month of treatment. For 3 patients, a dose reduction to 137 mg at bedtime was allowed because of AEs. In general, vital signs and laboratory test results remained consistent with baseline values and were similar between treatment groups throughout the study.
Discussion
ADS-5102 is a high-dose amantadine administered once daily at bedtime with a slow initial increase in amantadine concentrations and a prolonged time to reach the maximum concentration. ADS-5102 provides continuous coverage throughout the day to alleviate LID, with high plasma concentrations (approximately 1500 ng/mL) that cannot be achieved with conventional dosing (100 mg 2 or 3 times daily) with amantadine IR.
This study demonstrates that bedtime administration of 274 mg of ADS-5102 reduces the duration, severity, and impact of LID, as assessed by multiple outcome measures (UDysRS, MDS-UPDRS, PD home diary, and CGIC). ADS-5102–associated reduction in LID was evident at the first UDysRS assessment at week 2 and was maintained through week 24. More important, this reduction in the duration, severity, and implact of LID was achieved without worsening of underlying control of PD, as assessed by the MDS-UPDRS combined score (parts I, II, and III). The significant decrease in mean ON time with troublesome dyskinesia (as assessed by patient-completed PD diaries) supports the clinical relevance of the mean change in the UDysRS total score for a reduction in the duration, severity, and impact of LID. Diary assessments also demonstrated a reduction of almost 1 hour in OFF time at week 12 in the ADS-5102 group compared with the placebo group. The reduction in ON time with troublesome dyskinesia and OFF time resulted in a significant increase in ON time without troublesome dyskinesia. More important, the benefit of ADS-5102 was achieved without affecting the duration of sleep.
The most common AEs with ADS-5102 treatment were largely consistent with its N-methyl-d-aspartate receptor antagonist activity (eg, hallucinations and dizziness) and anticholinergic activity (eg, dry mouth and constipation). Hallucinations, the most commonly observed clinically relevant AEs, were mostly mild and reversible and did not require intervention or lead to study drug discontinuation. Therefore, these events did not constitute irreversible morbidity. Other risk factors for hallucinations in PD include disease progression, advancing age, cognitive impairment, comorbidities, daytime somnolence, sleep disorders, decreased visual acuity, and concomitant medications. Patients treated with ADS-5102 should be observed for the occurrence of hallucinations throughout treatment, especially at initiation and after dose increases. The use of ADS-5102 for patients with a history of clinically significant hallucinations has not been studied, to our knowledge.
Limitations
One limitation of this study was the decision to stop the study early, decreasing the number of patients contributing to the secondary end-point assessments at week 24. However, this decision had no effect on the primary efficacy analyses at week 12, and despite the smaller sample size, a significant treatment effect on dyskinesia and OFF time was still observed at week 24. Also, both placebo and ADS-5102 treatment groups were equally affected by this decision. Last, any conclusions regarding the relative efficacy and safety of ADS-5102 and amantadine IR require a direct comparison of these agents in a future randomized clinical trial.
Conclusions
The effective management of LID in patients with PD is challenging because there is currently no medication approved by the US Food and Drug Administration for its treatment. Within the past decade, several investigational agents have failed in development for the treatment of dyskinesia (ie, sarizotan hydrochloride, fipamezole, and mavoglurant). The results of the present study indicate that ADS-5102 administered once daily at bedtime may be an effective treatment of LID. In addition, ADS-5102 is the only investigational agent that also demonstrated a clinically meaningful, secondary benefit on OFF time. The baseline OFF time in this study was 3 hours, compared with a baseline OFF time of 6 hours in other PD studies. Taken together, these data support the use of ADS-5102 for patients with PD with LID, irrespective of its severity and duration, as well as OFF time. ADS-5102, 274 mg once daily at bedtime, should therefore be considered for the primary treatment of LID in patients with PD.
Trial Protocol
eFigure 1. Change in UDysRS Total Score—Subgroups at Week 12 (mITT Population)
eFigure 2. Synchronized Time Profiles of Patient-Reported Diary States at 12 Weeks
eFigure 3. Synchronized Time Profiles of Patient-Reported Diary States at 24 Weeks
eTable. Summary of Most Common Adverse Events by Time to Onset
Section Editor: Ira Shoulson, MD.
References
- 1.Suh DC, Pahwa R, Mallya U. Treatment patterns and associated costs with Parkinson’s disease levodopa induced dyskinesia. J Neurol Sci. 2012;319(1-2):24-31. [DOI] [PubMed] [Google Scholar]
- 2.Khlebtovsky A, Rigbi A, Melamed E, et al. . Patient and caregiver perceptions of the social impact of advanced Parkinson’s disease and dyskinesias. J Neural Transm (Vienna). 2012;119(11):1367-1371. [DOI] [PubMed] [Google Scholar]
- 3.Hechtner MC, Vogt T, Zöllner Y, et al. . Quality of life in Parkinson’s disease patients with motor fluctuations and dyskinesias in five European countries. Parkinsonism Relat Disord. 2014;20(9):969-974. [DOI] [PubMed] [Google Scholar]
- 4.Rascol O, Perez-Lloret S, Damier P, et al. . Falls in ambulatory non-demented patients with Parkinson’s disease. J Neural Transm (Vienna). 2015;122(10):1447-1455. [DOI] [PubMed] [Google Scholar]
- 5.Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16(3):448-458. [DOI] [PubMed] [Google Scholar]
- 6.Metman LV, Del Dotto P, LePoole K, Konitsiotis S, Fang J, Chase TN. Amantadine for levodopa-induced dyskinesias: a 1-year follow-up study. Arch Neurol. 1999;56(11):1383-1386. [DOI] [PubMed] [Google Scholar]
- 7.Verhagen Metman L, Del Dotto P, van den Munckhof P, Fang J, Mouradian MM, Chase TN. Amantadine as treatment for dyskinesias and motor fluctuations in Parkinson’s disease. Neurology. 1998;50(5):1323-1326. [DOI] [PubMed] [Google Scholar]
- 8.Wolf E, Seppi K, Katzenschlager R, et al. . Long-term antidyskinetic efficacy of amantadine in Parkinson’s disease. Mov Disord. 2010;25(10):1357-1363. [DOI] [PubMed] [Google Scholar]
- 9.Schwab RS, England AC Jr, Poskanzer DC, Young RR. Amantadine in the treatment of Parkinson’s disease. JAMA. 1969;208(7):1168-1170. [PubMed] [Google Scholar]
- 10.Silver DE, Sahs AL. Double blind study using amantadine hydrochloride in the therapy of Parkinson’s disease. Trans Am Neurol Assoc. 1971;96:307-308. [PubMed] [Google Scholar]
- 11.Ory-Magne F, Corvol JC, Azulay JP, et al. ; NS-Park CIC Network . Withdrawing amantadine in dyskinetic patients with Parkinson disease: the AMANDYSK trial. Neurology. 2014;82(4):300-307. [DOI] [PubMed] [Google Scholar]
- 12.Goetz CG, Stebbins GT, Chung KA, et al. . Which dyskinesia scale best detects treatment response? Mov Disord. 2013;28(3):341-346. [DOI] [PubMed] [Google Scholar]
- 13.Thomas A, Iacono D, Luciano AL, Armellino K, Di Iorio A, Onofrj M. Duration of amantadine benefit on dyskinesia of severe Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75(1):141-143. [PMC free article] [PubMed] [Google Scholar]
- 14.Factor SA, Molho ES. Transient benefit of amantadine in Parkinson’s disease: the facts about the myth. Mov Disord. 1999;14(3):515-517. [DOI] [PubMed] [Google Scholar]
- 15.Hayden FG, Gwaltney JM Jr, Van de Castle RL, Adams KF, Giordani B. Comparative toxicity of amantadine hydrochloride and rimantadine hydrochloride in healthy adults. Antimicrob Agents Chemother. 1981;19(2):226-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Symmetrel (amantadine hydrochloride, USP) [package insert]. Chadds Ford, PA: Endo Pharmaceuticals Inc; 2009.
- 17.Pahwa R, Tanner CM, Hauser RA, et al. . Amantadine extended release for levodopa-induced dyskinesia in Parkinson’s disease (EASED Study). Mov Disord. 2015;30(6):788-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 19.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauser RA, Friedlander J, Zesiewicz TA, et al. . A home diary to assess functional status in patients with Parkinson’s disease with motor fluctuations and dyskinesia. Clin Neuropharmacol. 2000;23(2):75-81. [DOI] [PubMed] [Google Scholar]
- 21.Folstein M, Folstein S. Mini-Mental State Examination. Lutz, FL: Psychological Assessment Resources, Inc; 2001. [Google Scholar]
- 22.Guy W, ed. Clinical Global Impressions. In: ECDEU Assessment Manual for Psychopharmacology Rockville, MD: US Dept of Health, Education, and Welfare; Public Health Service, Alcohol; Drug Abuse, and Mental Health Administration; National Institute of Mental Health; Psychopharmacology Research Branch; Division of Extramural Research Programs; DHEW publication (ADM) 76-338. 1976:218-222.
- 23.Diederich NJ, Fénelon G, Stebbins G, Goetz CG. Hallucinations in Parkinson disease. Nat Rev Neurol. 2009;5(6):331-342. [DOI] [PubMed] [Google Scholar]
- 24.Goetz CG, Laska E, Hicking C, et al. . Placebo influences on dyskinesia in Parkinson’s disease. Mov Disord. 2008;23(5):700-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewitt PA, Hauser RA, Lu M, et al. . Randomized clinical trial of fipamezole for dyskinesia in Parkinson disease (FJORD study). Neurology. 2012;79(2):163-169. [DOI] [PubMed] [Google Scholar]
- 26.Kumar R, Hauser RA, Mostillo J, et al. . Mavoglurant (AFQ056) in combination with increased levodopa dosages in Parkinson’s disease patients. Int J Neurosci. 2016;126(1):20-24. [DOI] [PubMed] [Google Scholar]
- 27.Hauser RA, Auinger P; Parkinson Study Group . Determination of minimal clinically important change in early and advanced Parkinson’s disease. Mov Disord. 2011;26(5):813-818. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. Change in UDysRS Total Score—Subgroups at Week 12 (mITT Population)
eFigure 2. Synchronized Time Profiles of Patient-Reported Diary States at 12 Weeks
eFigure 3. Synchronized Time Profiles of Patient-Reported Diary States at 24 Weeks
eTable. Summary of Most Common Adverse Events by Time to Onset