This cohort study aims to elucidate the clinical features of myositis in patients with antisynthetase syndrome.
Key Points
Question
What are the features of myositis in patients with antisynthetase syndrome?
Findings
In this cohort study of 460 patients with idiopathic inflammatory myopathies, 51 (11.1%) had anti-aminoacyl transfer RNA synthetase antibodies. Autoantibody detection was consistent between RNA immunoprecipitation and line blot assay except for anti-OJ antibodies, which were associated with severe muscle involvement.
Meaning
Antisynthetase syndrome is a clinical and histological subset among idiopathic inflammatory myopathies.
Abstract
Importance
Antisynthetase syndrome, characterized by myositis, interstitial lung disease, skin rash, arthropathy, and Raynaud phenomenon, is a clinical entity based on the presence of aminoacyl transfer RNA synthetase (ARS) antibodies in patients’ serum. However, antisynthetase syndrome is not included in the histological subsets of idiopathic inflammatory myopathies.
Objective
To elucidate the clinical features of myositis in patients with antisynthetase syndrome.
Design, Setting, and Participants
In this cohort study, muscle biopsy and blood samples were collected from 460 patients with idiopathic inflammatory myositis from various regional referral centers throughout Japan between October 2010 and December 2014. Data were analyzed in March 2016.
Exposures
Six different anti-ARS antibodies were detected in serum by RNA immunoprecipitation. Line blot assay and protein immunoprecipitation were also performed. HLA-DRB1 alleles were genotyped.
Main Outcomes and Measures
The main outcomes were muscle manifestations and histological findings. Predisposing factors, extramuscular symptoms, and follow-up information were also studied.
Results
Of 460 patients with idiopathic inflammatory myopathies, 51 (11.1%) had anti-ARS antibodies. Of this subset, 31 (61%) were women, with a mean (SD) age at disease onset of 60.2 (16.1) years. Among 6 different anti-ARS antibodies, only 1—the anti-OJ antibody—was not detected by line blot assay but by RNA immunoprecipitation. There were no significant HLA-DRB1 alleles associated with anti-ARS antibodies. All 51 patients presented with muscle limb weakness; 14 (27%) had severe limb weakness, 17 (33%) had neck muscle weakness, 15 (29%) had dysphagia, and 15 (29%) had muscle atrophy. Although patients with anti-OJ antibodies showed severe muscle weakness, the clinical presentations of antisynthetase syndrome were relatively homogeneous. In histology, perifascicular necrosis, the characteristic finding of antisynthetase syndrome, was found in 24 patients (47%). Myositis with anti-ARS antibodies responded to the combination of immunosuppressive therapy with favorable outcomes. Interstitial lung disease, found in 41 patients (80%), was more closely associated with mortality than myositis.
Conclusions and Relevance
Although clinical presentations of antisynthetase syndrome were relatively homogeneous, anti-OJ antibodies were associated with severe muscle involvement. Antisynthetase syndrome is a clinical and histological subset among idiopathic inflammatory myopathies.
Introduction
Aminoacyl transfer RNA synthetase (ARS) is a cytoplasmic protein synthetase that catalyzes the binding of amino acids to their corresponding transfer RNA in an energy-dependent manner. Eight autoantibodies reacting with different ARSs have been recognized: anti-Jo-1 (histidyl), anti-PL-7 (threonyl), anti-PL-12 (alanyl), anti-EJ (glycyl), anti-OJ (isoleucyl), anti-KS (asparaginyl), anti-Zo (phenylalanyl), and anti-Ha (tyrosyl). Antisynthetase syndrome, characterized by myositis, interstitial lung disease, skin rash, arthropathy, and Raynaud phenomenon, is a clinical entity based on the presence of 1 of the ARS antibodies in patient’s serum. Because anti-Jo-1 antibodies are preferentially screened in patients with myositis, to our knowledge, muscle involvement of antisynthetase syndrome has not been fully elucidated.
Some researchers have reported the pathological features of patients with anti-Jo-1, anti-PL-7, and anti-PL-12 antibodies. Based on the current consensus of histological diagnosis, idiopathic inflammatory myopathies are divided into inclusion body myositis, polymyositis, dermatomyositis, immune-mediated necrotizing myopathy, and nonspecific myositis. By contrast, antisynthetase syndrome is not listed in the pathological classification. The histological diagnoses of patients with antisynthetase syndrome include dermatomyositis, immune-mediated necrotizing myopathy, and nonspecific myositis. This inconsistency between clinical and histological concepts prohibits a proper understanding of antisynthetase syndrome.
Methods
Patients
Between October 2010 and December 2014, we received frozen muscle biopsy blocks and blood samples of Japanese patients with tentative diagnoses of inflammatory myopathies from all over Japan. Patients were included if (1) the patient was available for a muscle biopsy and could provide blood samples, accompanied by full clinical information; (2) the patient exhibited objective limb muscle weakness supported by electromyography and/or muscle magnetic resonance imaging; (3) a diagnosis of inflammatory myopathy was made after a comprehensive histological examination; and (4) the patient signed an informed consent agreement. Consequently, we enrolled 460 patients with idiopathic inflammatory myopathies, being careful to exclude patients with other disorders, such as muscular dystrophies. This study was approved by the institutional review boards of the National Center of Neurology and Psychiatry, Keio University, and Tokai University.
Clinical Features
Patients’ clinical information was provided by their referring physician, who completed detailed medical records, including clinical course, neurological examination, and laboratory findings. We additionally obtained follow-up information. Neurological outcomes were assessed using the modified Rankin Scale.
Muscle Histology
All of the clinical materials used in this study for diagnostic purposes were obtained with written informed consent. We processed the skeletal muscle tissue for pathological analysis at the National Center of Neurology and Psychiatry. We stained serial 10-μm-thick cryosections using a battery of histochemical methods. We further analyzed expression of major histocompatibility complex (MHC) class I and II and membrane attack complex. Additionally, to exclude major muscular dystrophies, we performed immunohistochemistry by using antibodies toward dystrophin, α-sarcoglycans to δ-sarcoglycans, α-dystroglycans and β-dystroglycans, dysferlin, caveolin-3, emerin, laminin α2, and collagen VI as well as mini multiplex Western blotting using antidystrophin, antidysferlin, anticalpain 3, anti–α-sarcoglycan, and antitelethonin. Gene screening for muscular dystrophies was not performed for all participants.
RNA and Protein Immunoprecipitation
Ten-microliter aliquots of serum were mixed with 2 mg of protein A-Sepharose CL-4B (Pharmacia Biotech) in 500 μL of immunoprecipitation buffer and incubated for 2 hours. After washing 3 times with immunoprecipitation buffer, antigen-bound Sepharose beads were mixed with 100 μL of HeLa cell extract (6 × 106 cell equivalents per sample) for 2 hours and then 30 μL of 3M sodium acetate, 30 μL of 10% sodium dodecyl sulfate, and 300 μL of phenol:chloroform:isoamyl alcohol (50:50:1, containing 0.1% 8-hydroxyquinoline) were added to extract the bound RNA. After ethanol precipitation, the RNA was resolved using a 7M urea-8% polyacrylamide gel, and the gel was silver-stained (Bio-Rad).
The protein components of the antigens were recognized by immunoprecipitation assay using 35S-methionine–labeled HeLa cellular extracts. The immunoprecipitated material was resolved by electrophoresis on sodium dodecyl sulfate 7.5% polyacrylamide gels, which were subsequently treated with 0.5M sodium salicylate to enhance the radioactivity and evaluated by autoradiography using a BAS-5000 system (Fuji Film).
Human Leukocyte Antigen Genotyping
Genomic DNA was extracted from peripheral blood using standard methods. HLA-DRB1 typing was performed using the Luminex assay system the WAKFlow Human Leukocyte Antigen Typing Kit (Wakunaga). We also studied 460 unrelated healthy Japanese control patients.
Statistical Analyses
Analyses were performed using SPSS version 20 (IBM) or R version R-3.2.3 software (The R Foundation). Comparisons of relative frequencies were tested for significance using the χ2 test for 2 × 2 tables. Continuous variables were compared using the Mann-Whitney U test. P < .05 was considered significant.
Results
Autoantibodies Detection
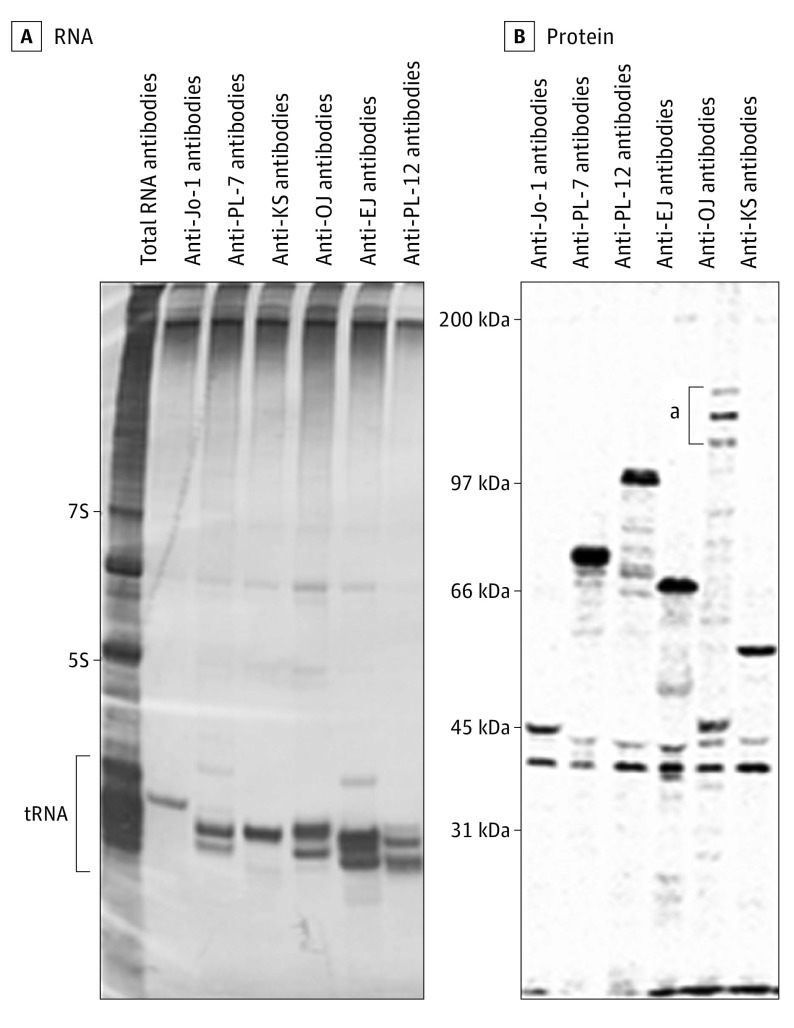
Among 657 patients who had been registered in the project, we enrolled 460 patients (70.0%) with idiopathic inflammatory myopathies confirmed by muscle biopsy. Then, we screened various autoantibodies. RNA immunoprecipitation revealed autoantigens in the transfer RNA region in 51 of 460 patients (11.1%) (Figure 1A). Based on the electrophoresis profiles of prototype sera, we detected anti-Jo-1 antibodies in 15 patients (29%), anti-OJ in 14 (27%), anti-PL-7 in 12 (24%), anti-EJ in 5 (10%), anti-PL-12 in 4 (8%), and anti-KS in 1 (2%). Anti-Zo and anti-Ha antibodies were not found.
Figure 1. RNA and Protein Immunoprecipitation.
A, Total RNA, with 7S, 5S, and transfer RNA (tRNA) regions indicated. B, Molecular weights are indicated.
aThe 3 characteristic bands of anti-OJ antibodies.
We compared these results with line blot assay using commercially available Euroline Myositis profile 3 (Euroimmune). Five of the 6 subtypes of ARS antibodies were included in the kit (anti-KS was not included). We obtained the same results in the 36 sera samples containing anti-Jo-1, anti-PL-7, anti-EJ, and anti-PL-12 antibodies between RNA immunoprecipitation and line blot assay. However, the 14 sera samples with anti-OJ antibodies by RNA immunoprecipitation showed no response in the line blot assay.
To evaluate the discrepancy of results, we also performed protein immunoprecipitation using 35S-methionine-labeled HeLa cellular extracts. We could clearly detect the 3 characteristic protein bands at the molecular weight of 140 kDa, 150 kDa, and 160 kDa in the sera of anti-OJ antibodies (Figure 1B). Because all anti-OJ–positive sera showed similar results, we confirmed the accuracy of RNA immunoprecipitation. Taken together, we concluded that the results of anti-OJ antibodies detection using the line blot assay were false-negatives.
Demographic Features and Predisposing Factors
Demographic and clinical features in the 51 patients with antisynthetase syndrome are summarized in the Table. The study included 31 females (61%) and 20 males (39%). The mean (SD) age at disease onset was 60.2 (16.1) years (range, 13-85 years). Pediatric disease onset at 16 years or younger was found in 2 patients (4%). Disease progression within 4 months (subacute onset) was found in 46 patients (90%). In contrast, 5 patients (10%) had long-term progression for more than 1 year before the first examination.
Table. Demographic and Clinical Features of 51 Patients With Antisynthetase Syndrome.
| Characteristic | Aminoacyl Transfer RNA Synthetase, No. (%) (n = 51) |
|---|---|
| Female | 31 (61) |
| Age at disease onset, mean (SD) [range], y | 60.2 (16.1) [13-85] |
| Disease progression | |
| Subacute | 46 (90) |
| Long-term | 5 (10) |
| Predisposing factors | |
| Antecedent infection | 10 (20) |
| Statin exposure | 1 (2) |
| Cancer | 6 (12) |
| Systemic autoimmune disease | 8 (16) |
| Muscle weakness | |
| Limb muscle weakness | 51 (100) |
| Legs dominant | 34 (67) |
| Severe limb muscle weakness | 14 (27) |
| Asymmetry | 10 (20) |
| Distal limb muscle dominant | 1 (2) |
| Neck muscle weakness | 17 (33) |
| Dysphagia | 15 (29) |
| Facial muscle involvement | 2 (4) |
| Cardiac muscle involvement | 1 (2) |
| Respiratory muscle involvement | 4 (8) |
| Muscle atrophy | 15 (29) |
| Decreased deep tendon reflex | 8 (16) |
| Myalgia | 23 (45) |
| Extramuscular symptoms | |
| Fever | 20 (39) |
| Skin rash | 34 (67) |
| Arthropathy | 21 (41) |
| Raynaud phenomenon | 4 (8) |
| Interstitial lung disease | 41 (80) |
| Blood Examination | |
| Creatine kinase level, mean (SD) [range], U/L | 4288 (5030) [31-22820] |
| Elevated C-reactive protein levels | 31 (61) |
| Antinuclear antibody positivity | 6 (12) |
SI conversion factor: To convert creatinine kinase to microkatals per liter, multiply by 0.0167.
Nonspecific antecedent infection, such as upper respiratory tract infection, was reported in 10 patients (20%). Statin exposure preceded myositis in only 1 patient (2%). Six patients (12%) had cancers within 3 years compared with the diagnosis of antisynthetase syndrome, including lung cancer in 4 patients (8%), prostate cancer in 1 (2%), and uterus cancer in 1 (2%). Systemic autoimmune diseases included Sjögren syndrome in 3 patients (6%) and rheumatic arthritis in 5 patients (10%).
We next evaluated the immunogenetic risk factors using HLA-DRB1 genotyping. HLA-DRB1 alleles were compared between all patients with antisynthetase syndrome and healthy controls (eTable 1 in the Supplement). However, we found no statistical differences between patients and healthy controls. To evaluate differences in individual ARS antibodies, we examined HLA-DRB1 alleles in subgroups stratified by anti-Jo-1, anti-OJ, or anti-PL-7 antibodies. However, we did not find any alleles associated with the different antisynthetase syndrome subtypes.
Clinical Features
Limb muscle weakness showed a limb-girdle distribution. Asymmetry of limb muscle weakness was reported in 10 patients (20%). Severe limb muscle weakness with a grade of 3 or greater assessed by manual muscle strength (Medical Research Council scale grade) was seen in 14 patients (27%). Legs were more severely affected than arms.
Although 17 patients (33%) showed neck muscle weakness, there were no patients with marked weakness presenting as continuous dropped head. Dysphagia was observed in 15 patients (29%). Facial and cardiac muscle involvement was infrequent. Although it was difficult to discriminate respiratory muscle involvement from interstitial lung disease, respiratory muscles were affected in 4 patients (8%) based on the clinical course and evaluation using pulmonary function test and/or blood gas analysis. Neurological examination revealed muscle atrophy in 15 patients (29%). Deep tendon reflexes were decreased or absent in 8 patients (16%). Myalgia preceded muscle weakness in 23 patients (45%).
With regard to extramuscular manifestations, skin rash and interstitial lung disease were commonly observed. Mechanics hands, the characteristic findings of antisynthetase syndrome, was seen in 16 patients (31%), although Gottron sign/papule and heliotrope rash were also reported by the attending physicians (Gottron, 14 [27%]; heliotrope, 7 [14%]; both, 3 [6%]). Chest computed tomography showed interstitial lung disease in 41 patients (80%). Among them, 23 patients (45%) had respiratory symptoms, and the other 18 patients (35%) were asymptomatic at the diagnosis of myositis. Fever and arthropathy were occasionally present, but Raynaud phenomenon was relatively rare. Five patients (10%) with anticitrullinated peptide/protein antibodies tended to have severe arthritis compared with patients without them.
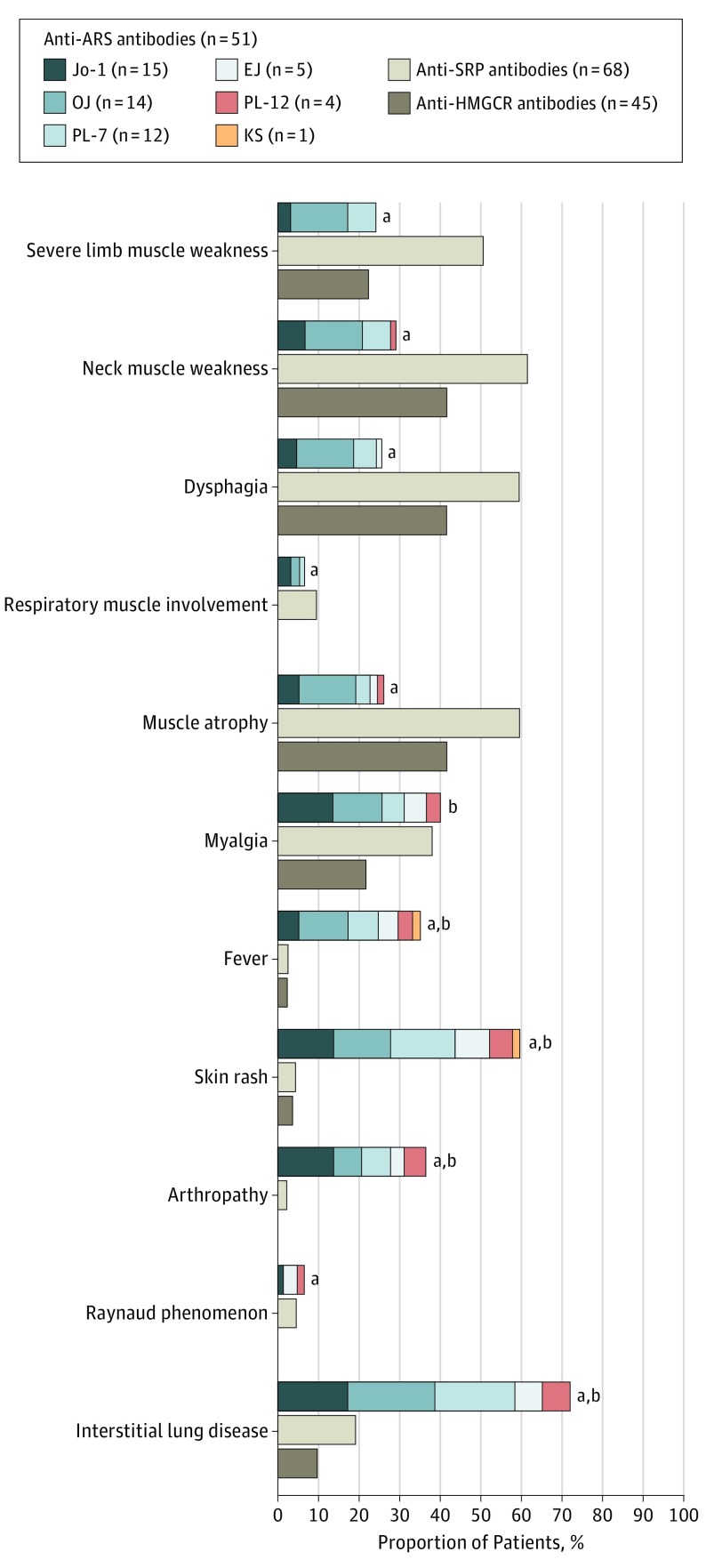
We also assessed clinical manifestations stratified by 6 different anti-ARS antibodies (Figure 2). The presence of muscular and extramuscular symptoms in patients with antisynthetase syndrome was relatively homogeneous. However, severe muscle involvement was especially prominent in patients with anti-OJ antibodies. We compared clinical features between patients with and without anti-OJ antibodies (eTable 2 in the Supplement). The frequencies of severe limb muscle weakness, neck muscle weakness, dysphagia, and muscle atrophy were significantly higher in patients with anti-OJ antibodies than in those without them.
Figure 2. Clinical Manifestations Stratified by 6 Different Aminoacyl Transfer RNA Synthetase (ARS) Antibodies.
Proportions of patients were compared with anti-signal recognition particle (SRP) antibodies or anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) antibodies.
aP < .05 vs anti-SRP antibodies.
bP < .05 vs anti-HMGCR antibodies.
Next, we compared clinical features between patients with anti-ARS antibodies and those with anti-signal recognition particle (SRP) antibodies or anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR). Clinical features of 68 patients with anti-SRP antibodies and 45 patients with anti-HMGCR antibodies were obtained from our previous study with the same cohort. The frequencies of severe limb muscle weakness, neck muscle weakness, dysphagia, and muscle atrophy were significantly higher in patients with anti-SRP antibodies than in patients with antisynthetase syndrome. In contrast, the frequencies of fever, skin rash, arthropathy, and interstitial lung disease were significantly higher in patients with antisynthetase syndrome than in patients with anti-SRP antibodies or anti-HMGCR antibodies.
The mean peak serum creatine kinase activity was 4288 IU/L. Of 51 patients, 35 (69%) had creatine kinase activity greater than 1000 IU/L. Creatine kinase activity tended to be higher in patients with anti-OJ antibodies than in patients without these antibodies (6207 IU/L vs 3561 IU/L). Elevation of C-reactive protein (≥1 mg/dL) was seen in 31 patients (61%). Positivity for antinuclear antibodies (≥1:160) was detected in only 6 patients (12%).
Histological Evaluation
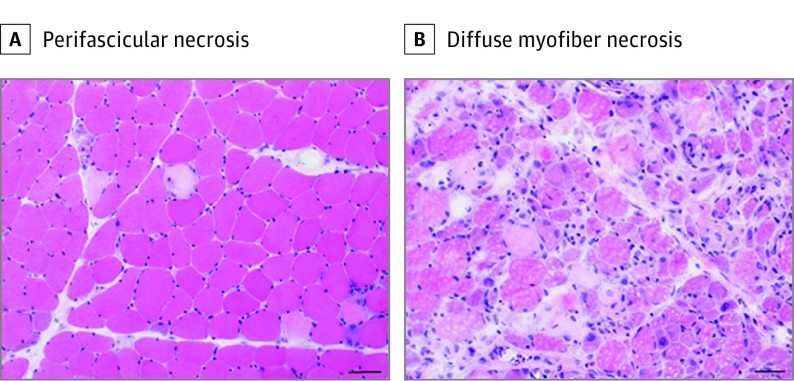
Histological findings were evaluated in 50 patients with antisynthetase syndrome, excluding 1 of the patients whose muscle specimens were in poor condition. Myofiber necrosis in the perifascicular region (perifascicular necrosis) was observed in 24 patients (48%) (Figure 3A). In addition, 8 patients, including 5 (10%) with anti-OJ antibodies, 2 (4%) with anti-PL-7 antibodies, and 1 (2%) with anti-Jo-1 antibodies, showed diffusely distributed necrotic and regenerating fibers (myofiber necrosis was not restricted to perifascicular area) (Figure 3B). In the other 18 patients (36%), few necrotic and regenerating fibers with perimysial (usually perivascular) mononuclear cellular infiltration and/or MHC class I and II expression on the cytoplasm and sarcolemma of nonnecrotic and nonregenerating fibers were observed. No endomysial mononuclear infiltration surrounding or invading into nonnecrotic fibers, as typically observed in polymyositis and inclusion body myositis, was seen. Perimysial connective tissue was highlighted by alkaline phosphatase in 19 patients.
Figure 3. Muscle Histology.
A, Myofiber necrosis in the perifascicular region (hematoxylin-eosin). B, Diffusely distributed necrotic and regenerating fibers (hematoxylin-eosin). Scale bar: 50 μm.
Increased expression of MHC class I and class II in the cytoplasm and sarcolemma fibers was observed in 41 patients (82%) and 21 patients (42%), respectively. The distribution of MHC class I and class II expression tended to be predominant in the perifascicular region. Sarcolemmal membrane attack complex deposition in the perifascicular region was seen in 17 patients (34%). No dense deposition of membrane attack complex on capillaries was observed.
Finally, we compared histological findings between patients with and without anti-OJ antibodies (eTable 2 in the Supplement). The frequency of diffuse myofiber necrosis was higher in patients with anti-OJ antibodies than those without.
Follow-up Data
We further assessed the follow-up data of 40 patients with antisynthetase syndrome. The mean (SD) follow-up period was 39.6 (13.8) months. All except 1 of 40 patients (3%) were treated with oral prednisolone. The initial dose of oral prednisolone was 1 mg/kg/d. Six patients (15%) received corticosteroids only, while 33 patients (83%) required additional immunotherapy, including 11 (28%) with intravenous immunoglobulin, 18 (45%) with intravenous methyl-prednisolone plus therapy, 15 (38%) with tacrolimus, 4 (10%) with methotrexate, 6 (15%) with azathioprine, 7 (18%) with cyclosporine, 4 (10%) with intravenous cyclophosphamide, or 1 (3%) with plasma exchange.
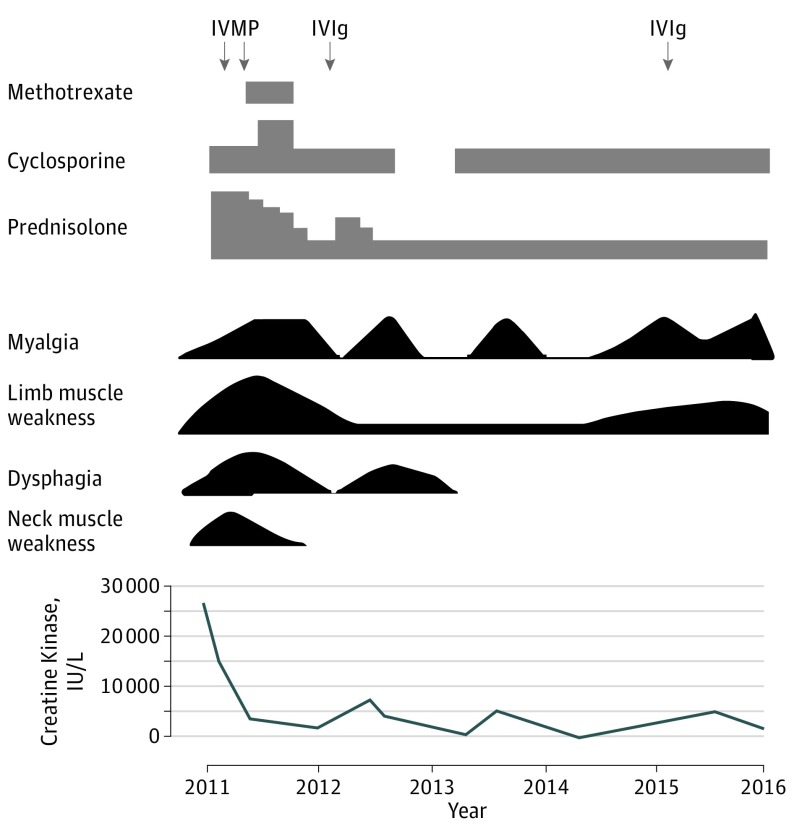
Immunotherapy was generally effective for muscle weakness. Among the 40 patients with antisynthetase syndrome, 26 patients (65%) responded to immunotherapy with improvement in muscle strength and reduced creatine kinase levels. However, a relapse of myositis occurred in 7 patients (3 [8%] with anti-PL-7 antibodies, 2 [5%] with anti-OJ antibodies, 1 [3%] with anti-Jo-1 antibodies, and 1 [3%] with anti-EJ antibodies). Figure 4 shows the clinical course of a man aged 37 years with anti-OJ antibodies. He had severe limb muscle weakness, neck muscle weakness, dysphagia, and muscle atrophy with marked elevation of serum creatine kinase activity. He experienced 3 relapses of myositis and received various regimens of immunotherapy.
Figure 4. Clinical Course of a Man Aged 37 Years With Anti-OJ Antibodies.
The timescale is indicated at the bottom of the figure. IVIg indicates intravenous immunoglobulin; IVMP, intravenous methyl-prednisolone pulse therapy.
The patients’ neurological outcomes were assessed using a modified Rankin Scale. Good recovery in muscle weakness, defined as a modified Rankin Scale score of 0 to 2, was observed in 26 patients (65%). Among them, 7 patients (18%) were in remission with minimal doses of corticosteroids. In contrast, 8 (20%) had difficulties in their daily living and had modified Rankin Scale scores of 3 to 5. Although we could not evaluate the detailed follow-up data, patients with unfavorable Rankin Scale scores tended to experience severe muscle weakness at disease onset. We speculate that the poor outcomes were because of permanent muscle injury rather than disease activity. There were no differences in treatment regimens and neurological outcomes between patients with and without anti-OJ antibodies.
In contrast, 7 patients (18%) had severe involvement of interstitial lung disease, although their myositis was well controlled. We thought that functional disability of patients assessed using a modified Rankin Scale was partially related to interstitial lung disease. We confirmed that 6 patients (15%) died during the follow-up. Causes of death included interstitial lung disease in 4 patients (10%), lung cancer in 1 (3%), and pneumonia in 1 (3%). A woman in her 60s with anti-PL-7 antibodies died of adenocarcinoma 2 years after the diagnosis of antisynthetase syndrome.
Discussion
Our cohort of 51 patients with antisynthetase syndrome can be summarized as follows: (1) the presence of autoantibodies did not differ between RNA immunoprecipitation and line blot assay for anti-Jo-1, anti-PL-7, anti-EJ, anti-PL-12, but this was not the case for anti-OJ antibodies; and (2) overall, skeletal muscle symptoms and clinical course were similar among antisynthetase syndrome subsets, but manifestations of anti-OJ patients tended to be more severe, at least in the early stage.
The previous large cohort of patients with 6 major anti-ARS antibodies detected by RNA immunoprecipitation showed that anti-OJ antibodies were least frequent in both US patients (5 of 202 [2.5%]) and Japanese patients (8 of 166 [4.8%]). These studies also showed that the frequencies of anti-Jo-1 antibodies in these populations were 60% and 36%, respectively, and of anti-PL-7 antibodies were 12% and 18%. In contrast, we found that anti-OJ antibodies were relatively frequent in the present muscle biopsy-oriented study (14 of 51 [27%]). This discrepancy may be attributed to differences in entry criteria between our study and prior work, with our patients representing a broader variety of inflammatory myopathies.
Because the structural confirmation of the complex may be necessary for recognition by anti-OJ antibodies, it is difficult to detect anti-OJ antibodies by line blot assay. In contrast, RNA and protein immunoprecipitation are original and accurate methods for the detection and discrimination of each anti-ARS antibody. With a few exceptions, each patient has only one of these antibodies when the serum sample is analyzed by immunoprecipitation assay.
Because the production mechanism of anti-ARS antibodies was unclear, we tried to identify the factors that predisposed patients to developing myositis. However, our analysis revealed no clear tendency in antecedent infection, statin exposure, cancer, and systemic autoimmune disease in patients with antisynthetase syndrome, in contrast to other inflammatory myopathies (eg, viral infection in inclusion body myositis, statin exposure in HMGCR antibody-positive necrotizing myopathy, and cancer in dermatomyositis and immune-mediated necrotizing myopathies). A large population of European American patients with myositis showed that DRB1*03:01 was frequently detected in patients with antisynthetase syndrome compared with healthy controls (73% vs 23%). In contrast, the significant HLA alleles associated with antisynthetase syndrome were not identified in Asian populations. We did not observe a significant prevalence of HLA-DRB1 alleles in patients with anti-ARS antibodies, although a larger sample size of patients may be necessary to draw a conclusion.
While antisynthetase syndrome shares common clinical features, different ARS antibodies have been reported to have different severities. For instance, with respect to muscle involvement, patients with anti-PL-7 antibodies were reported to have lower serum muscle enzyme levels and milder muscle weakness compared with patients with anti-Jo-1 antibodies. Another group’s study revealed that anti-PL-7 and anti-PL-12 phenotypes were distinct from the anti-Jo-1 phenotype and characterized by mild involvement of myositis. In this regard, we found that 4 patients with anti-PL-12 antibodies showed mild muscle weakness without marked elevation of serum creatine kinase activity. In contrast, we demonstrated that anti-OJ antibodies were associated with severe muscle involvement. Clinicians should consider the possibility of anti-OJ–positive myositis as a differential diagnosis of patients with severe subacute muscular symptoms, even if screening autoantibodies are negative on line blot assay, which can be false-negative for anti-OJ antibodies.
Prior reports from French groups, German groups, and our group show that perifascicular necrosis is the most distinctive hallmark of antisynthetase syndrome in muscle pathology. In addition, histological features, such as myofiber HLA-DR expression with a perifascicular distribution and myonuclear actin filaments with rod formation, are also associated with antisynthetase syndrome. The frequencies of myofiber necrosis/regeneration on muscle biopsy were comparable among patients with anti-Jo-1 antibodies, PL-7 antibodies, and PL-12 antibodies. The previous and present studies suggest that anti-ARS–associated myositis is a distinctive pathological subset among idiopathic inflammatory myopathies.
Patients with antisynthetase syndrome were reported to have dermatomyositis skin rash by the attending physicians. Recent reports from different groups have suggested antisynthetase syndrome and dermatomyositis are different subsets in terms of muscle histology and autoantibody profiles. Notably, myxovirus resistance A expression in the cytoplasm of myofibers and myositis-specific autoantibodies detection can differentiate well between antisynthetase syndrome and dermatomyositis. However, it may be difficult to recognize clear differences of skin manifestations between these subsets, although further investigation would be necessary.
There is no established treatment strategy for myositis with anti-ARS antibodies. Considering the results of our prior study regarding immune-mediated necrotizing myopathies, the method of which was similar to that of the present study, patients with anti-ARS antibodies seemed to show better prognosis than those with anti-SRP antibodies. In contrast, intestinal lung disease was likely to be more critical for patients with antisynthetase syndrome. A study at a single regional health center in the United States over a 24-year period clearly showed that the most common cause of death was pulmonary fibrosis (32 of 76 patients [42%]), the frequency of which was similar for patients with Jo-1 and non-Jo-1 antibodies. With regard to survival, 2 independent studies showed that patients with non-Jo-1 antibodies (especially PL-7 and PL-12 antibodies) had a less favorable prognosis than patients with anti-Jo-1 antibodies. We found no differences in the prognosis among subtypes of anti-ARS antibodies; however, the selection of immunotherapy may depend on interstitial lung disease rather than myositis.
Limitations
We are aware of 2 major limitations in this study. First, selection bias may have influenced our results. We used a muscle biopsy–oriented cohort. While this is our study’s biggest strength in delineating skeletal muscle manifestations of antisynthetase syndrome, patients do not always possess clinically apparent muscle involvement. Prior studies have shown that patients with anti-Jo-1 antibodies are more likely to have muscle involvement than those with anti-PL-7 or anti-PL-12 antibodies. In fact, patients with anti-PL-7 and anti-PL-12 antibodies are more likely to have interstitial lung disease. In this study, differences in the prevalence of muscle involvement cannot not be evaluated because muscle involvement was essentially a prerequisite for study inclusion. Second, among 6 different ARS subtypes, only anti-Jo-1 antibodies are familiar to clinicians and selectively measured for patients with suspected myositis in clinical settings. As the positivity of anti-Jo-1 antibodies can strongly support the diagnosis of myositis, muscle biopsy tends to be avoided in patients with anti-Jo-1 antibodies. Accordingly, patients with anti-Jo-1 myositis may have been incidentally excluded from this cohort.
Conclusions
For the diagnosis of antisynthetase syndrome, appropriate screening for autoantibodies as well as identification of the clinical syndrome and histological diagnosis are indispensable.
eTable 1. HLA-DRB1 alleles in patients with antisynthetase syndrome and healthy individuals.
eTable 2. Comparison between patients with and without anti-OJ antibodies.
References
- 1.Targoff IN. Autoantibodies in polymyositis. Rheum Dis Clin North Am. 1992;18(2):455-482. [PubMed] [Google Scholar]
- 2.Mozaffar T, Pestronk A. Myopathy with anti-Jo-1 antibodies: pathology in perimysium and neighbouring muscle fibres. J Neurol Neurosurg Psychiatry. 2000;68(4):472-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stenzel W, Preuße C, Allenbach Y, et al. . Nuclear actin aggregation is a hallmark of anti-synthetase syndrome-induced dysimmune myopathy. Neurology. 2015;84(13):1346-1354. [DOI] [PubMed] [Google Scholar]
- 4.Aouizerate J, De Antonio M, Bassez G, et al. . Myofiber HLA-DR expression is a distinctive biomarker for antisynthetase-associated myopathy. Acta Neuropathol Commun. 2014;2:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mescam-Mancini L, Allenbach Y, Hervier B, et al. . Anti-Jo-1 antibody-positive patients show a characteristic necrotizing perifascicular myositis. Brain. 2015;138(pt 9):2485-2492. [DOI] [PubMed] [Google Scholar]
- 6.Dalakas MC. Inflammatory muscle diseases. N Engl J Med. 2015;373(4):393-394. [DOI] [PubMed] [Google Scholar]
- 7.De Bleecker JL, De Paepe B, Aronica E, et al. ; ENMC Myositis Muscle Biopsy Study Group . 205th ENMC International Workshop: pathology diagnosis of idiopathic inflammatory myopathies part II 28-30 March 2014, Naarden, The Netherlands. Neuromuscul Disord. 2015;25(3):268-272. [DOI] [PubMed] [Google Scholar]
- 8.Stenzel W, Goebel HH, Aronica E. Review: immune-mediated necrotizing myopathies: a heterogeneous group of diseases with specific myopathological features. Neuropathol Appl Neurobiol. 2012;38(7):632-646. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki S, Yonekawa T, Kuwana M, et al. . Clinical and histological findings associated with autoantibodies detected by RNA immunoprecipitation in inflammatory myopathies. J Neuroimmunol. 2014;274(1-2):202-208. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe Y, Uruha A, Suzuki S, et al. . Clinical features and prognosis in anti-SRP and anti-HMGCR necrotising myopathy. J Neurol Neurosurg Psychiatry. 2016;87(10):1038-1044. [DOI] [PubMed] [Google Scholar]
- 11.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604-607. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki S, Hayashi YK, Kuwana M, Tsuburaya R, Suzuki N, Nishino I. Myopathy associated with antibodies to signal recognition particle: disease progression and neurological outcome. Arch Neurol. 2012;69(6):728-732. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki S, Satoh T, Yasuoka H, et al. . Novel autoantibodies to a voltage-gated potassium channel Kv1.4 in a severe form of myasthenia gravis. J Neuroimmunol. 2005;170(1-2):141-149. [DOI] [PubMed] [Google Scholar]
- 14.Ohnuki Y, Suzuki S, Shiina T, et al. . HLA-DRB1 alleles in immune-mediated necrotizing myopathy. Neurology. 2016;87(18):1954-1955. [DOI] [PubMed] [Google Scholar]
- 15.Targoff IN, Trieu EP, Miller FW. Reaction of anti-OJ autoantibodies with components of the multi-enzyme complex of aminoacyl-tRNA synthetases in addition to isoleucyl-tRNA synthetase. J Clin Invest. 1993;91(6):2556-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer A, Lefevre G, Bierry G, et al. ; Club Rhumatismes et Inflammation . In antisynthetase syndrome, ACPA are associated with severe and erosive arthritis: an overlapping rheumatoid arthritis and antisynthetase syndrome. Medicine (Baltimore). 2015;94(20):e523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uruha A, Suzuki S, Suzuki N, Nishino I. Perifascicular necrosis in anti-synthetase syndrome beyond anti-Jo-1. Brain. 2016;139(pt 9):e50. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal R, Cassidy E, Fertig N, et al. . Patients with non-Jo-1 anti-tRNA-synthetase autoantibodies have worse survival than Jo-1 positive patients. Ann Rheum Dis. 2014;73(1):227-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamaguchi Y, Fujimoto M, Matsushita T, et al. . Common and distinct clinical features in adult patients with anti-aminoacyl-tRNA synthetase antibodies: heterogeneity within the syndrome. PLoS One. 2013;8(4):e60442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahler M, Miller FW, Fritzler MJ. Idiopathic inflammatory myopathies and the anti-synthetase syndrome: a comprehensive review. Autoimmun Rev. 2014;13(4-5):367-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakashima R, Imura Y, Hosono Y, et al. . The multicenter study of a new assay for simultaneous detection of multiple anti-aminoacyl-tRNA synthetases in myositis and interstitial pneumonia. PLoS One. 2014;9(1):e85062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uruha A, Noguchi S, Hayashi YK, et al. . Hepatitis C virus infection in inclusion body myositis: a case-control study. Neurology. 2016;86(3):211-217. [DOI] [PubMed] [Google Scholar]
- 23.Hida A, Yamashita T, Hosono Y, et al. . Anti-TIF1-γ antibody and cancer-associated myositis: a clinicohistopathologic study. Neurology. 2016;87(3):299-308. [DOI] [PubMed] [Google Scholar]
- 24.Allenbach Y, Keraen J, Bouvier AM, et al. . High risk of cancer in autoimmune necrotizing myopathies: usefulness of myositis specific antibody. Brain. 2016;139(pt 8):2131-2135. [DOI] [PubMed] [Google Scholar]
- 25.O’Hanlon TP, Carrick DM, Targoff IN, et al. . Immunogenetic risk and protective factors for the idiopathic inflammatory myopathies: distinct HLA-A, -B, -Cw, -DRB1, and -DQA1 allelic profiles distinguish European American patients with different myositis autoantibodies. Medicine (Baltimore). 2006;85(2):111-127. [DOI] [PubMed] [Google Scholar]
- 26.Hervier B, Devilliers H, Stanciu R, et al. . Hierarchical cluster and survival analyses of antisynthetase syndrome: phenotype and outcome are correlated with anti-tRNA synthetase antibody specificity. Autoimmun Rev. 2012;12(2):210-217. [DOI] [PubMed] [Google Scholar]
- 27.Yamasaki Y, Yamada H, Nozaki T, et al. . Unusually high frequency of autoantibodies to PL-7 associated with milder muscle disease in Japanese patients with polymyositis/dermatomyositis. Arthritis Rheum. 2006;54(6):2004-2009. [DOI] [PubMed] [Google Scholar]
- 28.Uruha A, Nishikawa A, Tsuburaya RS, et al. . Sarcoplasmic MxA expression: a valuable marker of dermatomyositis. Neurology. 2017;88(5):493-500. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki S, Nishikawa A, Kuwana M, et al. . Inflammatory myopathy with anti-signal recognition particle antibodies: case series of 100 patients. Orphanet J Rare Dis. 2015;10:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. HLA-DRB1 alleles in patients with antisynthetase syndrome and healthy individuals.
eTable 2. Comparison between patients with and without anti-OJ antibodies.