Key Points
Question
What are the associations between maternal epilepsy, antiepileptic drug use during pregnancy, and risks of pregnancy and perinatal outcomes?
Findings
In this population-based cohort study including more than 1.4 million singleton births, we found that antiepileptic drug use during pregnancy is generally not associated with adverse maternal and fetal or neonatal outcomes. However, a diagnosis of epilepsy still implies moderately increased risks of adverse pregnancy, delivery, and perinatal outcomes.
Meaning
Women with epilepsy should not be advised to discontinue their treatment, if this is clinically indicated. Preventive strategies aimed at mitigating the effect of maternal epilepsy on pregnancy and perinatal outcomes are warranted.
Abstract
Importance
To date, few attempts have been made to examine associations between exposure to maternal epilepsy with or without antiepileptic drug (AED) therapy and pregnancy and perinatal outcomes.
Objectives
To investigate associations between epilepsy in pregnancy and risks of pregnancy and perinatal outcomes as well as whether use of AEDs influenced risks.
Design, Setting, and Participants
A population-based cohort study was conducted on all singleton births at 22 or more completed gestational weeks in Sweden from 1997 through 2011; of these, 1 424 279 were included in the sample. Information on AED exposure was available in the subset of offspring from July 1, 2005, to December 31, 2011. Data analysis was performed from October 1, 2016, to February 15, 2017.
Main Outcomes and Measures
Pregnancy, delivery, and perinatal outcomes. Multivariable Poisson log-linear regression was used to estimate adjusted risk ratios (aRRs) and 95% CIs, after adjusting for maternal age, country of origin, educational level, cohabitation with a partner, height, early pregnancy body mass index, smoking, year of delivery, maternal pregestational diabetes, hypertension, and psychiatric disorders.
Results
Of the 1 429 652 births included in the sample, 5373 births were in 3586 women with epilepsy; mean (SD) age at first delivery of the epilepsy cohort was 30.54 (5.18) years. Compared with pregnancies of women without epilepsy, women with epilepsy were at increased risks of adverse pregnancy and delivery outcomes, including preeclampsia (aRR 1.24; 95% CI, 1.07-1.43), infection (aRR, 1.85; 95% CI, 1.43-2.29), placental abruption (aRR, 1.68; 95% CI, 1.18-2.38), induction (aRR, 1.31; 95% CI, 1.21-1.40), elective cesarean section (aRR, 1.58; 95% CI, 1.45-1.71), and emergency cesarean section (aRR, 1.09; 95% CI, 1.00-1.20). Infants of mothers with epilepsy were at increased risks of stillbirth (aRR, 1.55; 95% CI, 1.05-2.30), having both medically indicated (aRR, 1.24; 95% CI, 1.08-1.43) and spontaneous (aRR, 1.34; 95% CI, 1.20-1.53) preterm birth, being small for gestational age at birth (aRR, 1.25; 95% CI, 1.13-1.30), and having neonatal infections (aRR, 1.42; 95% CI, 1.17-1.73), any congenital malformation (aRR, 1.48; 95% CI, 1.35-1.62), major malformations (aRR, 1.61; 95% CI, 1.43-1.81), asphyxia-related complications (aRR, 1.75; 95% CI, 1.26-2.42), Apgar score of 4 to 6 at 5 minutes (aRR, 1.34; 95% CI, 1.03-1.76), Apgar score of 0 to 3 at 5 minutes (aRR, 2.42; 95% CI, 1.62-3.61), neonatal hypoglycemia (aRR, 1.53; 95% CI, 1.34-1.75), and respiratory distress syndrome (aRR, 1.48; 95% CI, 1.30-1.68) compared with infants of unaffected women. In women with epilepsy, using AEDs during pregnancy did not increase the risks of pregnancy and perinatal complications, except for a higher rate of induction of labor (aRR, 1.30; 95% CI, 1.10-1.55).
Conclusions and Relevance
Epilepsy during pregnancy is associated with increased risks of adverse pregnancy and perinatal outcomes. However, AED use during pregnancy is generally not associated with adverse outcomes.
This population-based cohort study examines the outcomes of epilepsy and use of antiepileptic drugs during pregnancy in women and infants.
Introduction
Between 0.3% and 0.5% of all pregnancies occur among women with epilepsy. To avoid the maternal and fetal risks associated with seizures, maternal antiepileptic drug (AED) therapy is often maintained during pregnancy, despite increased risk of congenital malformations and adverse cognitive development in the offspring of women receiving AEDs.
Follow-up studies of pregnant women with epilepsy have focused mainly on associations between exposure to AEDs and congenital malformations and cognition of the offspring. However, pregnancy and perinatal complications among women with epilepsy may extend beyond the effect of treatment with AEDs. Maternal mortality has been shown to be 10 times higher in women with epilepsy than in those without the disorder. Epilepsy in women could increase the risks of miscarriage, preterm delivery, cesarean section, preeclampsia, and gestational hypertension. A meta-analysis reported that, among women with epilepsy, exposure to AEDs during pregnancy may increase the risks of fetal growth restriction, induction of labor, postpartum hemorrhage, and admission to the neonatal intensive care unit compared with those who are not exposed to AEDs. Still, robust evidence from population-based studies is sparse on the association between maternal epilepsy and risks of adverse pregnancy outcomes and the contribution of AEDs to these outcomes.
In a population-based study including more than 1.4 million singleton-birth infants in Sweden, we investigated the associations between epilepsy in pregnancy and risks of pregnancy and perinatal outcomes. We also investigated whether AED use influenced the risks.
Methods
This retrospective, nationwide cohort study included all singleton births at 22 or more completed gestational weeks in Sweden from 1997 through 2011. Using the person-unique national registration numbers of mothers and their offspring, individual information was obtained from the Medical Birth Register, which contains information on antenatal, obstetric, and neonatal care that is prospectively recorded on standardized forms on more than 98% of all births in Sweden; the nationwide National Patient Register, which has provided diagnostic codes on hospital inpatient care since 1987 and hospital outpatient care from 2001; and the Prescribed Drug Registry, which stores data on all drugs prescribed in ambulatory care and dispensed at a Swedish pharmacy since July 1, 2005. Maternal educational level and country of origin were obtained from the Education Register and the Total Population Register. Diagnoses in these databases were coded using the Swedish version of the International Classification of Diseases, Ninth Revision (ICD-9) from 1987 through 1996, and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) from 1997 onward. Prescription medications were coded using the Drug Identification Numbers and the Anatomical Therapeutic Chemical (ATC) classification system. The study was approved by the Research Ethics Committee at Karolinska Institutet, Stockholm, Sweden. This study was based on encrypted data, for which the ethics committees do not require informed consent.
Maternal Epilepsy
Maternal epilepsy was identified if it met any of the following conditions: (1) an occurrence of 2 or more diagnostic codes for epilepsy (ICD-9 code 345 and ICD-10 code G40) on separate dates or (2) an occurrence of 1 or more diagnosis codes for convulsions (ICD-9 code 780.3 and ICD-10 code R56) and 1 or more diagnosis codes for epilepsy among separate medical encounters; diagnosis of convulsion had to precede that of epilepsy. The epilepsy cohort was restricted to individuals whose epilepsy onset occurred before their child’s birth and those with active epilepsy (ie, diagnosis code for epilepsy within 10 years prior to conception). We classified epilepsy as either focal (ICD-10 codes G40.0, G40.1, and G40.2), generalized (ICD-10 code G40.3), or nonspecific (if the women could not be assigned to either the generalized or focal groups).
AED Exposure
Using information from the Prescribed Drug Registry (starting July 1, 2005), we were able to define AED exposure in a subcohort of women as use of any redeemed medication belonging to ATC class N03A (antiepileptic) from July 1, 2005, to December 31, 2011. The exposure window was defined as 30 days before the estimated day of conception to the day of birth.
Pregnancy and Perinatal Outcomes
Pregnancy outcomes examined in this study were gestational diabetes, preeclampsia, chorioamnionitis, maternal infection, placental abruption, premature rupture of membranes, prolonged labor, induction of labor, mode of delivery, and postpartum hemorrhage (eTable 1 in the Supplement reports specific codes).
Perinatal outcomes included stillbirth, preterm birth, spontaneous and medically indicated preterm birth, small-for-gestational-age (SGA) live birth, neonatal infection, presence of congenital malformation detected during the first year of life (divided into 2 categories: all malformations and major malformations), asphyxia-related neonatal complications (including meconium aspiration, hypoxic ischemic encephalopathy and related conditions, and neonatal convulsions or seizures), 5-minute Apgar score, neonatal hypoglycemia, neonatal jaundice, and respiratory distress (eTable 1 and eTable 2 in the Supplement report specific ICD codes). The Birth Register includes live births from 22 completed gestational weeks onward. Information on stillbirths was available from 28 weeks onward from 1997 to July 1, 2008, and thereafter from 22 gestational weeks. In the present study, stillbirth was defined as a fetal death at 28 completed weeks or later.
Gestational age in completed weeks was estimated using the date of the early second trimester ultrasonography (which is offered to all women; 95% accept) in 87.7% of the women, the date of the last menstrual period in 7.4% of the women, or postnatal assessment in 4.9% of the women. Preterm birth was categorized as birth earlier than 37 completed weeks’ gestation. Medically indicated preterm birth was defined as being born preterm and having an induced onset of labor or a cesarean section before onset of labor. The SGA was defined using the current Swedish standard for normal fetal growth and categorized into less than the 10th percentile (SGA) and 10th percentile or higher (non-SGA). Induced abortion due to detected malformation at the 18 gestational weeks’ ultrasonography are legal until 21 gestational weeks in Sweden. These pregnancies are therefore not included in the Medical Birth Register.
Other Covariates
Maternal characteristics included age at delivery, country of origin, educational level, cohabitation with a partner, parity, height, early pregnancy body mass index (calculated as weight in kilograms divided by height in meters squared), smoking during early pregnancy, year of delivery, and maternal preexisting chronic conditions, such as pregestational diabetes, hypertension, and any psychiatric disorders. Maternal age at delivery was calculated as date of delivery minus mother’s birth date. Parity was defined as the number of births of each mother. Body mass index, categorized according to the World Health Organization recommendation, was calculated using weight measured at registration to antenatal care, wearing light indoor clothing, and self-reported height. Information on cohabitation with a partner was obtained at the first antenatal visit. Mothers who reported daily smoking at the first antenatal visit and/or at 30 to 32 gestational weeks were classified as smokers, whereas mothers who only stated that they were nonsmokers were classified as nonsmokers. Any psychiatric morbidity and substance abuse before the child’s birth were defined using inpatient and outpatient primary or secondary diagnoses of any psychiatric condition and substance abuse (eTable 1 in the Supplement reports specific codes).
Statistical Analysis
Maternal characteristics of women with and without epilepsy and those receiving and not receiving AEDs during pregnancy were compared using logistic regression. Multivariable Poisson log-linear regression models were used to estimate adjusted risk ratios (aRRs). For the computation of 95% CIs, generalized estimating equations, with an assumed unstructured correlation structure, were used to account for the correlations of sequential births to the same mother in the study. Models were adjusted for maternal age, country of origin, educational level, cohabitation with a partner, parity, height, body mass index, smoking, year of delivery, pregestational diabetes, hypertension, and psychiatric disorders. Pregnancy and neonatal events were rare in the relatively small AED-treated cohort. For all analyses that included the AED-treated cohort (all births from July 1, 2005, to December 31, 2011 only), we therefore used a propensity score approach to optimize adjustment for the above covariates. We calculated propensity scores using multivariable logistic regression, with exposure to AEDs as a dependent variable and all adjustment covariates as predictors. To obtain adjusted estimates, the resulting propensity score was entered into the Poisson log-linear regression models as a continuous variable. Data were analyzed with the use of SAS software, version 9.4 (SAS Institute). Two-sided P values less than <.05 were considered to indicate statistical significance. No adjustment was made for multiple comparisons. Data analysis was performed from October 1, 2016, to February 15, 2017.
Results
The final sample included 1 424 279 pregnancies of 869 947 mothers without epilepsy and 5373 pregnancies of 3586 mothers with epilepsy. Mean (SD) age at first delivery of the epilepsy cohort was 30.54 (5.18) years. During the period for which we had information on AED exposure (July 1, 2005, to December 31, 2011), there were 3231 offspring of mothers with epilepsy, of whom 42.2% (n = 1363) were exposed to AEDs 1 month before and/or during pregnancy. Lamotrigine (628 [46.1%]) and carbamazepine (418 [30.7%]) were the most commonly used AEDs, and 181 infants (13.3%) were exposed to polytherapy (eTable 3 in the Supplement).
Compared with women without epilepsy, women with epilepsy were younger at the time of delivery, primiparous, born in the Nordic countries, had a lower educational level, smoked, lived without a partner, were obese (body mass index, ≥30), and had a higher frequency of chronic conditions, such as pregestational diabetes, hypertension, psychiatric diagnosis, and substance abuse (Table). Among women with epilepsy, those receiving AEDs during pregnancy were older, more often primiparous, and born in non-Nordic countries.
Table. Maternal Characteristics of First Recorded Pregnancy According to Maternal Epilepsy and Maternal AED Use During Pregnancya.
| Maternal Characteristic | No. (%) | P Value | ||||
|---|---|---|---|---|---|---|
| No Epilepsy (n = 869 947) |
Epilepsy (n = 3586) |
P Value | Not Receiving AED (n = 1015) |
Receiving AED (n = 926)a |
||
| Age, y | ||||||
| ≤19 | 23 231 (2.7) | 117 (3.3) | .03 | 25 (2.5) | 46 (5.0) | .003 |
| 20-24 | 145 625 (16.7) | 655 (18.3) | 178 (17.5) | 189 (20.4) | ||
| 25-29 | 291 605 (33.5) | 1144 (31.9) | 304 (30.0) | 294 (31.8) | ||
| 30-34 | 268 613 (30.9) | 1090 (30.4) | 327 (32.2) | 252 (27.2) | ||
| ≥35 | 140 873 (16.2) | 580 (16.2) | 181 (17.8) | 145 (15.7) | ||
| Country of birth | ||||||
| Nordic | 708 737 (81.5) | 3122 (87.1) | <.001 | 855 (84.2) | 802 (86.6) | .33 |
| Non-Nordic | 160 342 (18.4) | 462 (12.9) | 159 (15.7) | 123 (13.3) | ||
| Data missing | 868 (0.1) | 2 (0.06) | 1 (0.1) | 1 (0.1) | ||
| Educational level, y | ||||||
| ≤9 | 82 308 (9.5) | 542 (15.1) | <.001 | 139 (13.7) | 159 (17.2) | .15 |
| 10-11 | 142 011 (16.3) | 654 (18.2) | 126 (12.4) | 108 (11.7) | ||
| 12 | 220 672 (25.4) | 976 (27.2) | 295 (29.1) | 289 (31.2) | ||
| 13-14 | 124 051 (14.3) | 458 (12.8) | 131 (12.9) | 114 (12.3) | ||
| ≥15 | 286 750 (33.9) | 892 (24.9) | 304 (30.0) | 243 (26.2) | ||
| Data missing | 14 155 (1.6) | 64 (1.8) | 20 (2.0) | 13 (1.4) | ||
| Cohabiting with partner | ||||||
| Yes | 765 720 (88.0) | 3051 (85.1) | <.001 | 875 (86.2) | 781 (84.3) | .05 |
| No | 57 110 (6.6) | 357 (10.0) | 109 (10.7) | 96 (10.4) | ||
| Data missing | 47 117 (5.4) | 178 (5.0) | 31 (3.0) | 49 (5.3) | ||
| Parity | ||||||
| 1 | 248 355 (28.6) | 1305 (36.4) | <.001 | 800 (78.8) | 743 (80.2) | <.001 |
| 2 | 402 479 (46.3) | 1521 (42.4) | 151 (14.9) | 103 (11.1) | ||
| 3 | 157 088 (18.1 | 540 (15.1) | 43 (4.2) | 58 (6.3) | ||
| ≥4 | 62 025 (7.1) | 220 (6.1) | 21 (2.1) | 22 (2.4) | ||
| Median (Q1-Q3) | 2 (1-3) | 2 (1-2) | 2 (1-2) | 1 (1-2) | ||
| Height, cm | ||||||
| ≤159 | 113 348 (13.0) | 529 (14.8) | .02 | 148 (14.6) | 132 (14.3) | .37 |
| 160-164 | 216 959 (24.9) | 910 (25.4) | 234 (23.1) | 248 (26.8) | ||
| 165-169 | 248 109 (28.5) | 970 (27.1) | 287 (28.3) | 245 (26.5) | ||
| ≥170 | 269 345 (31.0) | 1083 (30.2) | 315 (31.0) | 279 (30.1) | ||
| Data missing | 22 186 (2.6) | 94 (2.6) | 31 (3.1) | 22 (2.4) | ||
| Smoking | ||||||
| No | 736 252 (84.6) | 2911 (81.2) | <.001 | 879 (86.6) | 757 (81.7) | <.001 |
| Yes | 90 159 (10.4) | 504 (14.1) | 109 (10.7) | 107 (11.6) | ||
| Data missing | 43 536 (5.0) | 171 (4.8) | 27 (2.7) | 62 (6.7) | ||
| Year of delivery | ||||||
| 1997-1999 | 229 581 (26.4) | 561 (15.6) | <.001 | NA | NA | |
| 2000-2004 | 276 078 (31.7) | 1084 (30.2) | NA | NA | ||
| 2005-2008 | 204 935 (23.6) | 999 (27.9) | 458 (45.1) | 541 (58.4) | ||
| 2009-2011 | 159 353 (18.3) | 942 (26.3) | 557 (54.9) | 385 (41.6) | ||
| BMI | ||||||
| <18.5 | 20 758 (2.4) | 81 (2.3) | <.001 | 26 (2.6) | 22 (2.4) | .08 |
| 18.5-24.9 | 493 378 (56.7) | 1792 (50.0) | 491 (48.4) | 474 (51.2) | ||
| 25.0-29.9 | 182 556 (21.0) | 856 (23.9) | 281 (27.7) | 202 (21.8) | ||
| 30.0-34.9 | 54 540 (6.3) | 345 (9.6) | 96 (9.5) | 97 (10.5) | ||
| 35.0-39.9 | 15 822 (1.8) | 101 (2.8) | 32 (3.2) | 28 (3.0) | ||
| ≥40.0 | 5344 (0.6) | 38 (1.1) | 10 (.99) | 15 (1.6) | ||
| Data missing | 97 549 (11.2) | 373 (10.4) | 79 (7.9) | 88 (9.5) | ||
| Pregestational diabetes | ||||||
| No | 866 170 (99.6) | 3552 (99.1) | <.001 | 1003 (98.8) | 914 (98.7) | .82 |
| Yes | 3777 (0.4) | 34 (0.9) | 12 (1.2) | 12 (1.3) | ||
| Pregestational hypertension | ||||||
| No | 864 759 (99.4) | 3550 (99.0) | .002 | 1002 (98.7) | 915 (98.8) | .85 |
| Yes | 5188 (0.6) | 36 (1.0) | 13 (1.3) | 11 (1.2) | ||
| Any psychiatric diagnoses | ||||||
| No | 842 149 (96.8) | 3148 (87.8) | <.001 | 905 (89.2) | 812 (87.7) | .31 |
| Yes | 27 798 (3.2) | 438 (12.2) | 110 (10.8) | 114 (12.3) | ||
| Substance misuse | ||||||
| No | 867 095 (99.7) | 3505 (97.7) | <.001 | 991 (97.6) | 904 (97.6) | .98 |
| Yes | 2852 (0.3) | 81 (2.3) | 24 (2.4) | 22 (2.4) | ||
Abbreviations: AED, antiepileptic drug; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NA, not applicable.
Information on AED exposure was available only during the period from July 1, 2005, to December 31, 2011. Results determined with logistic regression.
Pregnancies in women with epilepsy were associated with elevated risks of preeclampsia (aRR, 1.24; 95% CI, 1.07-1.43), infection (aRR, 1.85; 95% CI, 1.43-2.29), placental abruption (aRR, 1.68; 95% CI, 1.18-2.38), induction (aRR, 1.31; 95% CI, 1.21-1.40), elective cesarean section (aRR, 1.58; 95% CI, 1.45-1.71), and emergency cesarean section (aRR, 1.09; 95% CI, 1.00-1.20) compared with pregnancies in women without epilepsy (Figure 1A). There was a higher frequency of postpartum hemorrhage in pregnancies of women with epilepsy compared with those without epilepsy; however, this association was of borderline significance (aRR, 1.11, 95% CI, 0.97-1.26). No increased risks of premature rupture of membranes and prolonged labor were observed in the epilepsy group.
Figure 1. Delivery, Pregnancy, and Perinatal Outcomes Among Women With and Women Without Epilepsy, Sweden, 1997-2011.
Pregnancy and delivery (A) and perinatal (B) outcomes determined using multivariable Poisson log-linear regression models adjusted for maternal age, country of origin, educational level, cohabitation with a partner, parity, height, early pregnancy body mass index, smoking during pregnancy, prepregnancy hypertension, prepregnancy diabetes, any psychiatric disorders, and year of delivery. Denominator for stillbirth was all births at 28 completed weeks or later and the denominator for the remaining variables in the figure was live births at 22 completed weeks or later. RR indicates risk ratio.
The frequency of stillbirth was higher in offspring of women with epilepsy compared with those without epilepsy (0.6% vs 0.3%) (Figure 1B). After adjustment for potential confounders, neonates of women with epilepsy had significantly higher risks of stillbirth, being born SGA, both medically indicated and spontaneous preterm births, any and major congenital malformations, neonatal infections, asphyxia-related complications, low 5-minute Apgar scores, and neonatal hypoglycemia and respiratory distress compared with neonates of unaffected women (Figure 1B).
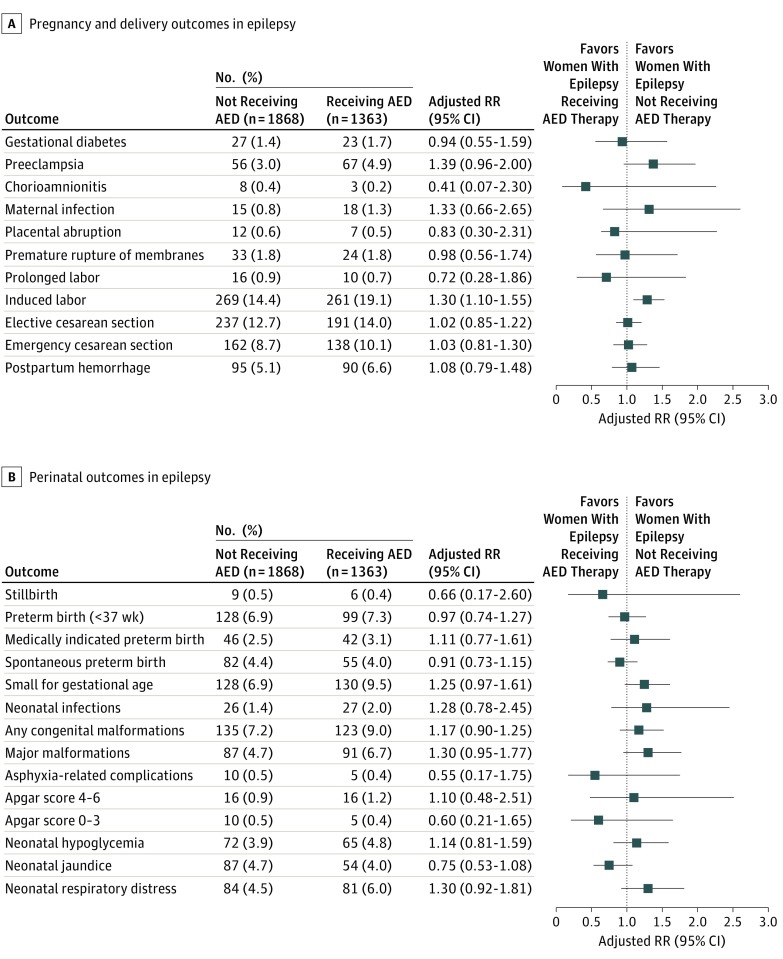
Among pregnancies in women with epilepsy who gave birth between July 2005 and December 2011, the rate of preeclampsia was higher in those receiving AEDs compared with pregnancies in women who did not receive AEDs (Figure 2A). However, in the propensity score–adjusted analyses, only induction of labor remained statistically significant (aRR, 1.30; 95% CI, 1.10-1.55) (Figure 2A).
Figure 2. Delivery, Pregnancy, and Perinatal Outcomes Among Women With Epilepsy by Antiepileptic Drug (AED) Use During Pregnancy, Sweden, 2005-2011.
Pregnancy and delivery (A) and perinatal (B) outcomes determined using propensity score approach to adjust for confounders, including maternal age, country of origin, educational level, cohabitation with a partner, parity, height, early pregnancy body mass index, smoking during pregnancy, prepregnancy hypertension, prepregnancy diabetes, and any psychiatric disorders. Denominator for stillbirth was all births at 28 completed weeks or later and the denominator for the remaining variables in the figure was live births at 22 completed weeks or later. RR indicates risk ratio.
Offspring of women exposed to AEDs had a higher frequency of major malformation (6.7% vs 4.7%), respiratory distress (6.0% vs 4.5%), and being SGA (9.5% vs 6.9%) at birth, compared with the nonexposed offspring (Figure 2B). The propensity score–adjusted analyses showed no statistically significant increased risk of adverse neonatal outcomes between the 2 groups for any of the neonatal outcomes.
Stratifying the analyses by type of AED monotherapy (1 AED type during pregnancy) and polytherapy (>1 AED type during pregnancy) and by gestational age (term ≥37 weeks vs preterm 22-36 weeks) revealed similar results, although statistical power was reduced. Among women receiving relatively common monotherapy, the highest rate of major malformations (10.6%) was obtained in pregnancies with fetal exposure to valproic acid (eTable 3 in the Supplement).
Discussion
In this nationwide cohort study, women with active epilepsy had higher risks of preeclampsia, maternal infection, placental abruption, induction of labor, and both emergency and elective cesarean section. Offspring of women with epilepsy were at higher risks of stillbirth, both medically indicated and spontaneous preterm births, SGA live birth, neonatal infection, any and major malformations, asphyxia-related neonatal complications, low 5-minute Apgar scores, and less severe but more prevalent neonatal complications, including neonatal hypoglycemia and respiratory distress. In women with epilepsy, using AEDs during pregnancy did not increase the risks of pregnancy and perinatal complications significantly, except for a higher rate of induction of labor.
Higher risks of pregnancy complications among women with epilepsy have previously been reported in some but not all studies. In our study, we further observed increased risks of placental abruption and maternal infection among women with epilepsy compared with the unaffected women. In addition, we confirmed the earlier described associations between maternal epilepsy and higher risks of preterm birth, SGA live birth, low Apgar score, and major malformation. The increased risk of stillbirth (55%) was also consistent with previous studies that had supported a significant, albeit smaller, risk increase. Moreover, our study showed that women with epilepsy were more likely than women without epilepsy to have an infant who experienced asphyxia-related neonatal complications, neonatal hypoglycemia, and neonatal respiratory distress. To date, few previous studies have explored the potential role of maternal epilepsy and the more prevalent neonatal complications. Our results are in line with those of one previous study observing a 2-fold increased risk of respiratory distress in offspring of women with epilepsy.
We found that women with epilepsy who used AEDs during pregnancy were not at greater risk of adverse pregnancy outcomes, with the exception of an increased risk of induction of labor and a nonsignificantly increased risk of preeclampsia. This finding is in contrast to previous findings of higher risks of antepartum and postpartum hemorrhage and cesarean section in women with epilepsy using AEDs. Furthermore, unlike previous studies, we found no significantly increased risk of adverse perinatal outcomes in offspring of women with epilepsy exposed to AEDs compared with nonexposed infants (although the rates for SGA live birth and major malformation were borderline significant). Our study differs from previous studies examining the effects of AED exposure on pregnancy outcomes in a number of ways. First, in this Swedish cohort, lamotrigine and carbamazepine accounted for approximately 77% of the treated pregnancies, whereas valproic acid and topiramate, known to be associated with increased risks of malformations, were used in only 19.2% and 4.0% of the pregnancies, respectively. Nevertheless, in line with other studies, among women receiving relatively common monotherapy, our data also suggest that valproic acid poses a greater risk for major malformation. Second, we utilized a previously validated case definition for epilepsy, rather than relying on a single ICD code to identify epilepsy. Third, unlike a previous Swedish study, that used self-reported data from the Medical Birth Registry, in our study, AED use in the most recent years, was captured from the Prescribed Drug Registry. Fourth, we included women with “active” epilepsy to ensure clinical relevance, given that epilepsy is considered to be “resolved” for individuals who have remained seizure-free for the past 10 years. Fifth, in our study, we made comparisons between outcomes of women with epilepsy who were receiving AEDs vs those with epilepsy who were not receiving AEDs, while most previous studies compared AED use in women with epilepsy with a large reference cohort of women without epilepsy, which introduces confounding by indication bias. Finally, we were able to account for data clustering arising from consecutive births of the same mother and adjust for several confounding variables not considered in previous studies, such as maternal preexisting chronic conditions.
The physiologic changes occurring in pregnancy significantly alter the volume of distribution and elimination of AEDs and consequently decrease the plasma concentration. This action could theoretically influence the potential adverse effects of AEDs on maternal and perinatal outcomes, unless dose adjustments are made. The clinical recommendation in Sweden is to monitor AED serum concentrations with dose adjustment throughout the pregnancy. In addition, women with epilepsy who are receiving AEDs during pregnancy may receive extra surveillance and monitoring from their clinicians that may have contributed to the comparable outcomes observed in our study. The teratogenicity of AEDs to the developing fetus has been of concern for women in whom discontinuation of AED therapy during pregnancy cannot be considered owing to the possibility of seizures. Our findings reveal that the increased risks of complications during pregnancy, labor, and the neonatal period might be due to pathologic factors related to epilepsy as a chronic disease more than being the effect of AEDs per se. Such epilepsy-related factors may be associated with the many comorbidities of epilepsy (eg, autoimmune disorders). Therefore, women with epilepsy should not be advised to discontinue clinically indicated treatment. Adverse effects of AED use have also been shown to be counterbalanced by the seizure control effect of AEDs.
Limitations
The main limitation of our study was that the Patient Drug Register only provides information on drugs that have been dispensed from pharmacies, and the adherence to treatment is unknown. However, previous research has shown high agreement between maternal reports of AED use during pregnancy and filled prescriptions for AEDs. We also lacked information about malformations subjected to induced abortions, which may have influenced the estimated association between exposure to AEDs and risk of major malformation in the offspring toward the null. In our AED-exposed cohort, we cannot rule out the possibility of false-negative results due to a lack of power to detect a meaningful difference. Finally, given our nonexperimental study design, the observed associations between exposure to maternal epilepsy and AEDs and pregnancy outcomes are not evidence of a causal relationship. The impact of other possible confounders, such as disease severity, seizure frequency during pregnancy, dosage of AED exposure, AED serum levels, or exposure to other potential teratogens, needs to be assessed in future studies.
Conclusions
Our findings provide reassurance to women with epilepsy that AED use during pregnancy is generally not associated with adverse maternal and fetal or neonatal outcomes, although it is important to be aware that AEDs differ in their teratogenic potential. However, a diagnosis of epilepsy still implies a moderately increased risk of adverse pregnancy, delivery, and perinatal outcomes. This information should improve counseling for women with epilepsy who contemplate discontinuing their treatment during pregnancy and provide useful information to their health care clinicians.
eTable 1. ICD-10 Codes for Maternal and Neonatal Diseases
eTable 2. ICD-10 Codes For Major Congenital Malformations According to the European Surveillance of Congenital Anomalies Classification
eTable 3. Clinical Characteristics of in Women With Epilepsy
References
- 1.Viinikainen K, Heinonen S, Eriksson K, Kälviäinen R. Community-based, prospective, controlled study of obstetric and neonatal outcome of 179 pregnancies in women with epilepsy. Epilepsia. 2006;47(1):186-192. [DOI] [PubMed] [Google Scholar]
- 2.Meador K, Reynolds MW, Crean S, Fahrbach K, Probst C. Pregnancy outcomes in women with epilepsy: a systematic review and meta-analysis of published pregnancy registries and cohorts. Epilepsy Res. 2008;81(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomson T, Battino D. Teratogenic effects of antiepileptic drugs. Lancet Neurol. 2012;11(9):803-813. [DOI] [PubMed] [Google Scholar]
- 4.Koo J, Zavras A. Antiepileptic drugs (AEDs) during pregnancy and risk of congenital jaw and oral malformation. Oral Dis. 2013;19(7):712-720. [DOI] [PubMed] [Google Scholar]
- 5.Christensen J, Grønborg TK, Sørensen MJ, et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA. 2013;309(16):1696-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacDonald SC, Bateman BT, McElrath TF, Hernández-Díaz S. Mortality and morbidity during delivery hospitalization among pregnant women with epilepsy in the United States. JAMA Neurol. 2015;72(9):981-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edey S, Moran N, Nashef L. SUDEP and epilepsy-related mortality in pregnancy. Epilepsia. 2014;55(7):e72-e74. [DOI] [PubMed] [Google Scholar]
- 8.Laganà AS, Triolo O, D’Amico V, et al. Management of women with epilepsy: from preconception to post-partum. Arch Gynecol Obstet. 2016;293(3):493-503. [DOI] [PubMed] [Google Scholar]
- 9.Viale L, Allotey J, Cheong-See F, et al. ; EBM CONNECT Collaboration . Epilepsy in pregnancy and reproductive outcomes: a systematic review and meta-analysis. Lancet. 2015;386(10006):1845-1852. [DOI] [PubMed] [Google Scholar]
- 10.Ludvigsson JF, Almqvist C, Bonamy AK, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;31(2):125-136. [DOI] [PubMed] [Google Scholar]
- 11.Swedish National Board of Health and Welfare The Swedish Medical Birth Register: a summary of content and quality. http://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/8306/2009-125-15_200912515_rev2.pdf. Published 2003. Accessed February 15, 2016.
- 12.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish National Inpatient Register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swedish National Board of Health and Welfare Quality and content in the Swedish Patient Register http://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/19490/2014-8-5.pdf. Published 2013. Accessed February 19, 2016.
- 14.Wettermark B, Hammar N, Fored CM, et al. The new Swedish Prescribed Drug Register—opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16(7):726-735. [DOI] [PubMed] [Google Scholar]
- 15.Statistics Sweden Evaluation of the Swedish register of education. http://www.scb.se/statistik/_publikationer/BE9999_2006A01_BR_BE96ST0604.pdf. Published 2006. Accessed February 15,2016.
- 16.Helmers SL, Thurman DJ, Durgin TL, Pai AK, Faught E. Descriptive epidemiology of epilepsy in the US population: a different approach. Epilepsia. 2015;56(6):942-948. [DOI] [PubMed] [Google Scholar]
- 17.Thurman DJ, Beghi E, Begley CE, et al. ; ILAE Commission on Epidemiology . Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia. 2011;52(suppl 7):2-26. [DOI] [PubMed] [Google Scholar]
- 18.Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475-482. [DOI] [PubMed] [Google Scholar]
- 19.Høgberg U, Larsson N. Early dating by ultrasound and perinatal outcome: a cohort study. Acta Obstet Gynecol Scand. 1997;76(10):907-912. [DOI] [PubMed] [Google Scholar]
- 20.Marsál K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996;85(7):843-848. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization Global Database on Body Mass Index: BMI Classification. http://apps.who.int/bmi/index.jsp. Published 2006. Accessed February 19, 2016.
- 22.Braitman LE, Rosenbaum PR. Rare outcomes, common treatments: analytic strategies using propensity scores. Ann Intern Med. 2002;137(8):693-695. [DOI] [PubMed] [Google Scholar]
- 23.Katz O, Levy A, Wiznitzer A, Sheiner E. Pregnancy and perinatal outcome in epileptic women: a population-based study. J Matern Fetal Neonatal Med. 2006;19(1):21-25. [DOI] [PubMed] [Google Scholar]
- 24.Borthen I, Eide MG, Veiby G, Daltveit AK, Gilhus NE. Complications during pregnancy in women with epilepsy: population-based cohort study. BJOG. 2009;116(13):1736-1742. [DOI] [PubMed] [Google Scholar]
- 25.Artama M, Gissler M, Malm H, Ritvanen A; Drug and Pregnancy Group . Effects of maternal epilepsy and antiepileptic drug use during pregnancy on perinatal health in offspring: nationwide, retrospective cohort study in Finland. Drug Saf. 2013;36(5):359-369. [DOI] [PubMed] [Google Scholar]
- 26.Veiby G, Daltveit AK, Engelsen BA, Gilhus NE. Pregnancy, delivery, and outcome for the child in maternal epilepsy. Epilepsia. 2009;50(9):2130-2139. [DOI] [PubMed] [Google Scholar]
- 27.Borthen I, Eide MG, Daltveit AK, Gilhus NE. Obstetric outcome in women with epilepsy: a hospital-based, retrospective study. BJOG. 2011;118(8):956-965. [DOI] [PubMed] [Google Scholar]
- 28.Battino D, Tomson T, Bonizzoni E, et al. ; EURAP Study Group . Seizure control and treatment changes in pregnancy: observations from the EURAP epilepsy pregnancy registry. Epilepsia. 2013;54(9):1621-1627. [DOI] [PubMed] [Google Scholar]
- 29.Keezer MR, Sisodiya SM, Sander JW. Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol. 2016;15(1):106-115. [DOI] [PubMed] [Google Scholar]
- 30.Wide K, Winbladh B, Källén B. Major malformations in infants exposed to antiepileptic drugs in utero, with emphasis on carbamazepine and valproic acid: a nation-wide, population-based register study. Acta Paediatr. 2004;93(2):174-176. [DOI] [PubMed] [Google Scholar]
- 31.Källén B. The problem of confounding in studies of the effect of maternal drug use on pregnancy outcome. Obstet Gynecol Int. 2012;2012:148616. doi: 10.1155/2012/148616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patsalos PN, Berry DJ, Bourgeois BF, et al. Antiepileptic drugs—best practice guidelines for therapeutic drug monitoring: a position paper by the Subcommission on Therapeutic Drug Monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia. 2008;49(7):1239-1276. [DOI] [PubMed] [Google Scholar]
- 33.Ong M-S, Kohane IS, Cai T, Gorman MP, Mandl KD. Population-level evidence for an autoimmune etiology of epilepsy. JAMA Neurol. 2014;71(5):569-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kilic D, Pedersen H, Kjaersgaard MIS, et al. Birth outcomes after prenatal exposure to antiepileptic drugs—a population-based study. Epilepsia. 2014;55(11):1714-1721. [DOI] [PubMed] [Google Scholar]
- 35.Tomson T, Battino D, Bonizzoni E, et al. ; EURAP Study Group . Withdrawal of valproic acid treatment during pregnancy and seizure outcome: observations from EURAP. Epilepsia. 2016;57(8):e173-e177. [DOI] [PubMed] [Google Scholar]
- 36.Olesen C, Søndergaard C, Thrane N, Nielsen GL, de Jong-van den Berg L, Olsen J; EuroMAP Group . Do pregnant women report use of dispensed medications? Epidemiology. 2001;12(5):497-501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. ICD-10 Codes for Maternal and Neonatal Diseases
eTable 2. ICD-10 Codes For Major Congenital Malformations According to the European Surveillance of Congenital Anomalies Classification
eTable 3. Clinical Characteristics of in Women With Epilepsy