Key Points
Question
Are there different clinical phenotypes of atopic dermatitis depending on the onset and progression of the disease in childhood?
Findings
In this cohort study, we identified 2 phenotypes with early onset within the first 2 years of life (transient and persistent progression) and a late phenotype, with onset after the age of 2 years. Children with an early phenotype of atopic dermatitis, especially with persistent symptoms, were most at risk to develop respiratory allergy and food allergy.
Meaning
Children developing symptoms of atopic dermatitis in the first 2 years of life, especially those with food allergy, should require special attention for respiratory allergy prevention.
Abstract
Importance
Atopic dermatitis is an inflammatory, pruritic skin disease that often occurs in early infancy with a chronic course. However, a specific description of subtypes of atopic dermatitis depending on the timing of onset and progression of the disease in childhood is lacking.
Objective
To identify different phenotypes of atopic dermatitis using a definition based on symptoms before age 6 years and to determine whether some subtypes are more at risk for developing other allergic diseases.
Design, Setting, and Participants
The Protection Against Allergy Study in Rural Environments (PASTURE) is a European birth cohort where pregnant women were recruited between August 2002 and March 2005 and divided in 2 groups dependent on whether they lived on a farm. Children from this cohort with data on atopic dermatitis from birth to 6 years of age were included.
Exposures
Atopic dermatitis, defined as an itchy rash on typical locations from birth to 6 years.
Main Outcomes and Measures
The latent class analysis was used to identify subtypes of atopic dermatitis in childhood based on the course of symptoms. Multivariable logistic regressions were used to analyze the association between atopic dermatitis phenotypes and other allergic diseases.
Results
We included 1038 children; of these, 506 were girls. The latent class analysis model with the best fit to PASTURE data separated 4 phenotypes of atopic dermatitis in childhood: 2 early phenotypes with onset before age 2 years (early transient [n = 96; 9.2%] and early persistent [n = 67; 6.5%]), the late phenotype with onset at age 2 years or older (n = 50; 4.8%), and the never/infrequent phenotype (n = 825; 79.5%), defined as children with no atopic dermatitis. Children with both parents with history of allergies were 5 times more at risk to develop atopic dermatitis with an early-persistent phenotype compared with children with parents with no history of allergies. Both early phenotypes were strongly associated with food allergy. The risk of developing asthma was significantly increased among the early-persistent phenotype (adjusted odds ratio, 2.87; 95% CI, 1.31-6.31). The late phenotype was only positively associated with allergic rhinitis.
Conclusions and Relevance
Using latent class analysis, 4 phenotypes of atopic dermatitis were identified depending on the onset and course of the disease. The prevalence of asthma and food allergy by 6 years of age was strongly increased among children with early phenotypes (within age 2 years), especially with persistent symptoms. These findings are important for the development of strategies in allergy prevention.
This study identifies different phenotypes of atopic dermatitis using a definition based on symptoms before age 6 years and determines whether some subtypes are more at risk for developing other allergic diseases.
Introduction
More than 20% of children in industrialized countries have atopic dermatitis. In more than 60% of these children, the disease started within the first 2 years of life. Even though many children will outgrow the disease, in others it will persist. Atopic dermatitis is considered to be one of the first manifestations in the atopic march, which describes the typical progression of clinical symptoms of allergic diseases during childhood. Several studies have shown that atopic dermatitis is positively associated with asthma. The risk of developing asthma among children with atopic dermatitis was shown to be increased by 2 compared with children with no atopic dermatitis.
Although there is a strong association between atopic dermatitis and respiratory allergy, the hypothesis of the atopic march might be more complex. In 2015, it was suggested that instead of one atopic march, there are several subgroups of developmental profiles of allergic diseases depending on their onset and natural course. Therefore, it is important to define the different phenotypes of atopic dermatitis and to evaluate their association with the development of other allergic diseases to develop successful strategies in allergy prevention.
The birth cohort Protection Against Allergy Study in Rural Environments (PASTURE) offered the opportunity to prospectively investigate whether different phenotypes of atopic dermatitis could be identified during childhood based on the onset and natural course of the disease. In this study, we used the latent class analysis (LCA) to define different phenotypes of atopic dermatitis based on symptoms reported by the parents from birth to 6 years of age. We further examined whether subtypes of atopic dermatitis are differently associated with environmental exposures or familial allergy status and their association with the development of other allergic diseases.
Methods
Study Design and Population
The PASTURE study is a birth cohort involving children from rural areas in 5 European countries (Austria, Finland, France, Germany, and Switzerland) designed to evaluate risk and preventive factors for atopic diseases. Pregnant women were recruited during pregnancy between August 2002 and March 2005 and divided into 2 groups. Women who lived on family-run farms where livestock was kept composed the farm group. Women from the same rural areas not living on a farm were in the reference group. In total, 1133 children were included in the PASTURE birth cohort. For this analysis, 1038 children were included because for 90 children, data on symptoms of atopic dermatitis were missing at all times or reported at only 1 time. Additionally, 5 children could not be assigned to any classes defined by the LCA, as detailed in the Statistical Analysis subsection.
The study was approved by the local research ethics committees in each country (Ethikkommission St Gallen, Switzerland; Comité de Protection des Personnes, Besançon, France; The Research Ethics Committee of the Northern Savo Hospital District, Kuopio, Finland; Ethik-Kommission der Bayerischen Landesärztekammer, München, Germany; and Ethikkommission für das Bundesland Salzburg, Salzburg, Austria), and written informed consent was obtained from all parents.
Definitions
Questionnaires were administered in interviews or self-administered to the mothers within the third trimester of pregnancy, when children were aged 2 months, 12 months, 18 months, and 24 months, and then yearly until age 6 years. Feeding practices were reported by parents in monthly diaries in the first year of life.
Children were defined as having symptoms of atopic dermatitis when the parents reported an itchy rash at least once since the last questionnaire on at least 1 of the following specific locations: face, neck, elbow, behind the knees, hand, or feet. We used reports of atopic dermatitis symptoms at 7 points (at ages 1 year, 1.5 years, 2 years, 3 years, 4 years, 5 years, and 6 years).
Prenatal contact with farm animals was assumed if the mother reported contact at least several times per month in 1 of the pregnancy trimesters. Consumption of farm milk was defined as a consumption of at least a mean of 10 mL per day.
Children were defined as having asthma when parents reported at age 6 years that the child had either been diagnosed by a physician as having asthma at least once or been diagnosed by a physician as having at least 2 episodes of spastic, obstructive, or asthmatic bronchitis in the first 6 years of life. Obstructive bronchitis is commonly used to define the first occurrence of asthmatic symptoms. Additionally, we used a similar definition of asthma based on the same reported physician diagnosis but reported only in the 4-year, 5-year, or 6-year questionnaire, independently of the history of asthma in the first 3 years. Food allergy was defined when the parents reported a physician diagnosis of food allergy up to age 6 years. Another definition of food allergy was used, also based on physician diagnosis of food allergy but restricted to children with reported confirmation by an allergy test. Allergic rhinitis was defined by the presence of symptoms (itchy, runny, or blocked nose without a cold and associated with red itchy eyes) or a physician diagnosis of allergic rhinitis reported at 6 years. The Scoring Atopic Dermatitis (SCORAD) score was assessed during the medical examination at 1 year and 6 years of age. Allergen-specific IgE antibodies (Dermatophagoides pteronyssinus, Dermatophagoides farinae, alder, birch, hazel, grass pollen, rye, mugwort, plantain, cat, horse, dog, alternaria, hen’s egg, cow’s milk, peanut, hazelnut, carrot, and wheat flour) were measured in blood among children at age 1 year and 6 years using the Allergy Screen Test Panel (Mediwiss Analytic), as described previously. Sensitization was defined as specific IgE level of 0.7 IU/mL or more. Positive parental history of allergies was defined as ever having asthma, allergic rhinitis, or atopic dermatitis.
Statistical Analysis
Longitudinal LCA was used to identify subtypes of atopic dermatitis symptoms over time. Children having no data on symptoms of atopic dermatitis or reported at only 1 time from birth to 6 years of age were excluded (n = 90). The Akaike information criteria were used to define the number of classes with the best fit to the data, with the smallest value representing the most optimal model. The probability for an individual to belong to each class is estimated based on conditional probabilities of atopic dermatitis at each time given a class membership. Individuals were assigned to the class for which they had a probability of at least 0.5. Five children were not assigned to any classes because all probabilities were less than 0.5 at each time. Therefore, in this study, 1038 children were included. As sensitivity analysis, LCA was performed among children with information on atopic dermatitis symptoms at all 7 points up to 6 years (n = 636).
Multinomial logistic regression for 4-level outcome was used to investigate the association between exposures and phenotypes of atopic dermatitis. Logistic regression models were used to compare the odds of allergic outcomes across groups defined by atopic dermatitis phenotype. For the association between exposures and atopic dermatitis phenotypes, we stratified the analyses by parental history of allergy, which is a well-known predictive factor for allergic diseases. Multivariable models were further adjusted for this variable and the following potential confounders: sex and breastfeeding because some associations with phenotypes of atopic dermatitis and other allergic diseases were found; center; and farming status as used in the selection of the study population.
Data analysis was conducted using SAS software, version 9.4 (SAS Institute Inc). Statistical significance was taken as a 2-sided P value < .05.
Results
Characteristics of the Study Population
One thousand thirty-eight children were included in this study. The proportion of farmers’ children was 47.7% (n = 495), and 53.4% (n = 551) had at least 1 allergic parent (Table 1). Six hundred thirty-six children had complete data, with information on atopic dermatitis symptoms reported at all 7 times up to 6 years. No significant differences were observed between children with complete data and the total study population (eTable 1 in the Supplement).
Table 1. Characteristics of Study Population.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Total Study Population (n = 1038) |
Farmer (n = 495 [47.7%]) |
Nonfarmer (n = 543 [52.3%]) |
|
| Center | |||
| Austria | 205 (19.8) | 96 (19.4) | 109 (20.1) |
| Switzerland | 220 (21.2) | 97 (19.6) | 123 (22.6) |
| France | 188 (18.1) | 91 (18.4) | 97 (17.9) |
| Germany | 230 (22.2) | 109 (22) | 121 (22.3) |
| Finland | 195 (18.8) | 102 (20.6) | 93 (17.1) |
| Sex | |||
| Female | 506 (48.8) | 242 (48.9) | 264 (48.8) |
| Parents with allergy history | |||
| 0 | 481 (46.6) | 278 (56.3) | 203 (37.7) |
| 1 | 433 (42.0) | 179 (36.2) | 254 (47.2) |
| 2 | 118 (11.4) | 37 (7.5) | 81 (15.1) |
| Breastfeeding, mo | |||
| 0 | 98 (9.6) | 49 (9.5) | 52 (9.8) |
| >0-2 | 166 (16.3) | 71 (14.6) | 95 (17.8) |
| 3-6 | 282 (27.7) | 153 (31.4) | 129 (24.2) |
| ≥7 | 474 (46.5) | 217 (44.5) | 257 (48.2) |
Latent Class Analysis With Atopic Dermatitis Symptoms
We used LCA to define different phenotypes of atopic dermatitis based on symptoms from birth to 6 years of age. Four classes were identified as the best model fitting the PASTURE study data, using Akaike information criteria, with the smallest value representing the most optimal model (with 3 classes, 216.41; with 4 classes, 174.78; with 5 classes, 176.71; and with 6 classes, 177.39). We could define 4 different phenotypes of atopic dermatitis symptoms: the early-transient phenotype (n = 96; 9.2%), with onset of atopic dermatitis within age 2 years and no further symptoms after age 4 years; the early-persistent phenotype (n = 67; 6.5%), with onset within age 2 years and the persistence of symptoms until age 6 years; the late phenotype (n = 50; 4.8%), with onset of the disease after 2 years of age; and the never/infrequent phenotype (n = 825; 79.5%) (Figure 1). Latent class analysis, performed among the subgroup of children with a complete data set (n = 636), identified the same phenotypes of atopic dermatitis (eFigure 1 in the Supplement). The point prevalence of atopic dermatitis symptoms was similar at the different times from birth up to 6 years of age: between 11.4% and 16.9% (eTable 2 in the Supplement).
Figure 1. Estimated Probabilities of Atopic Dermatitis Symptoms at Each Time Point From Birth to 6 Years of Age for Each Atopic Dermatitis Phenotype in the 4-Class Model.
The prevalences of the phenotypes are 9.2% for early transient (n = 96), 6.5% for early persistent (n = 67), 4.8% for later (n = 50), and 79.5% for never/infrequent (n = 825).
Associations between SCORAD at 1 year and 6 years and atopic dermatitis phenotypes showed that the early-transient phenotype was positively associated with a positive SCORAD (score >0) only at the age of 1 year and the late phenotype only at the age of 6 years, while the early-persistent phenotype was strongly associated with a positive SCORAD at both times (eTable 3 in the Supplement). The distribution of SCORAD scores showed an increased proportion of children with higher score among children with early-persistent phenotype compared to other phenotypes (eFigure 2 in the Supplement).
Association Between Exposures and Phenotypes of Atopic Dermatitis Symptoms
Next, we investigated whether exposures during pregnancy or first year of life were associated with atopic dermatitis phenotypes identified by LCA. Parental allergic status was strongly associated with early phenotypes, especially the early-persistent phenotype (eFigure 3 in the Supplement). Children having both parents with history of allergy had a nearly 6-fold increased risk of developing early-persistent atopic dermatitis compared with children with parents with no history of allergy (Table 2 and eTable 4 in the Supplement).
Table 2. Association Between Exposures and Atopic Dermatitis Phenotypes.
| Variable | ORa (95% CI) | ||
|---|---|---|---|
| Early Transient (n = 96) |
Early Persistent (n = 67) |
Late (n = 50) |
|
| Girls vs boys | 1.30 (0.85-2.00) | 1.84 (1.09-3.10) | 1.45 (0.81-2.59) |
| Allergic parent | |||
| 2 | 2.46 (1.27-4.76) | 5.35 (2.52-11.36) | 2.41 (0.95-6.09) |
| 1 | 1.36 (0.84-2.20) | 2.15 (1.15-4.03) | 1.58 (0.83-3.03) |
| 0 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Farmer vs nonfarmer | 0.86 (0.56-1.34) | 1.61 (0.96-2.72) | 0.98 (0.55-1.77) |
| Prenatal exposures | |||
| Farm milk during pregnancy vs no farm milk | 0.82 (0.47-1.45) | 1.38 (0.72-2.64) | 1.38 (0.65-2.94) |
| Unboiled farm milk during pregnancy vs no farm milk | 0.73 (0.42-1.28) | 1.22 (0.66-2.24) | 1.08 (0.52-2.23) |
| Work/stay in stable during pregnancy vs no exposure | 0.99 (0.53-1.85) | 0.84 (0.39-1.82) | 0.87 (0.37-2.02) |
| Work/stay in barn during pregnancy vs no exposure | 1.26 (0.71-2.22) | 1.19 (0.62-2.27) | 1.57 (0.74-3.34) |
| Animal species, during pregnancy, species | |||
| 3-4 | 0.55 (0.23-1.35) | 0.46 (0.14-1.47) | 0.16 (0.02-1.35) |
| 1-2 | 0.74 (0.43-1.30) | 0.71 (0.34-1.47) | 0.87 (0.40-1.91) |
| 0 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 4 Animal species, continuous | 0.83 (0.64-1.07) | 0.85 (0.61-1.19) | 0.95 (0.66-1.37) |
| Contact with pets (dogs/cats) vs no contact | 0.78 (0.49-1.27) | 0.44 (0.24-0.81) | 0.77 (0.40-1.50) |
| With cats | 1.36 (0.84-2.21) | 0.59 (0.32-1.09) | 0.95 (0.48-1.86) |
| With dogs | 0.64 (0.38-1.08) | 0.66 (0.36-1.18) | 1.03 (0.53-1.98) |
| Postnatal exposures | |||
| Breastfeeding, mo | |||
| 0 | 0.36 (0.08-1.70) | NA | NA |
| >0-2 | 0.53 (0.17-1.63) | 0.29 (0.04-2.33) | 0.90 (0.35-2.34) |
| 3-6 | 1.30 (0.63-2.64) | 0.56 (0.18-1.76) | 1.23 (0.59-2.53) |
| ≥7 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Yogurt introduced before 1 y vs no yogurt | 1.04 (0.42-2.52) | 0.35 (0.12-1.04) | 1.25 (0.49-3.18) |
Abbreviations: NA, not applicable; OR, odds ratio.
Adjusted for farmer, center, sex, and parents with history of allergy and with postnatal exposures; exclusion of children with symptoms of atopic dermatitis in the first year of life.
Prenatal contact to an increased number of different farm animal species showed a tendency of a negative association with all phenotypes of atopic dermatitis. Prenatal exposures to pets (dog or cat) were negatively associated with the early-persistent phenotype of atopic dermatitis. This protective effect was observed specifically among the children with at least 1 parent with history of allergy (eTable 5 in the Supplement).
After adjustment and exclusion of children with atopic dermatitis symptoms in the first year of life, no association was observed between breastfeeding and atopic dermatitis phenotypes. The risk of early-persistent atopic dermatitis was decreased when yogurt was introduced in the first year of life (eTable 4 in the Supplement). After adjustment and exclusion of children with atopic dermatitis symptoms in the first year of life, we still observed a negative association (Table 2). Introduction of other food items was not associated with atopic dermatitis phenotypes (data not shown).
Association Between Phenotypes of Atopic Dermatitis Symptoms and Other Allergic Diseases
Up to 6 years of age, the prevalence of asthma was 8.5% (n = 78), the prevalence of food allergy was 9.0% (n = 78), and the prevalence of allergic rhinitis was 7.9% (n = 73) (Table 3).
Table 3. Association Between Atopic Dermatitis Phenotypes and Other Allergic Diseases up to 6 Years of Age (Asthma, Food Allergy, and Allergic Rhinitis) and Sensitization to Food and Inhalant Allergens at 6 Years (Cutoff: 0.7 IU/mL).
| Variable | No./Total No. (%) | OR (95% CI) | ORa (95% CI) |
|---|---|---|---|
| Asthma | 78/923 (8.5) | NA | NA |
| Early transient | 10/86 (11.6) | 1.62 (0.79-3.315) | 1.60 (0.77-3.305) |
| Early persistent | 10/57 (17.5) | 2.62 (1.26-5.475) | 2.87 (1.31 -6.315) |
| Late | 3/48 (6.3) | 0.82 (0.25-2.735) | 0.83 (0.25-2.805) |
| Never/infrequent | 55/732 (7.5) | 1 [Reference] | 1 [Reference] |
| Food allergy | 78/864 (9.0) | NA | NA |
| Early transient | 16/80 (20.0) | 3.8 (2.02-7.13) | 3.69 (1.93-7.035) |
| Early persistent | 19/56 (33.9) | 7.8 (4.13-14.72) | 7.08 (3.59-13.975) |
| Late | 1/48 (2.1) | 0.32 (0.04-2.4) | 0.32 (0.04-2.395) |
| Never/infrequent | 42/680 (6.2) | 1 [Reference] | 1 [Reference] |
| Allergic rhinitis | 73/921 (7.9) | NA | NA |
| Early transient | 9/86 (10.5) | 1.82 (0.86-3.88) | 1.90 (0.88-4.115) |
| Early persistent | 12/57 (21.0) | 4.16 (2.05-8.42) | 4.04 (1.82-8.955) |
| Late | 8/48 (16.7) | 3.12 (1.38-7.07) | 3.23 (1.37-7.615) |
| Never/infrequent | 44/730 (6.0) | 1 [Reference] | 1 [Reference] |
| Sensitization to food allergens at 6 y | 220/741 (29.7) | NA | NA |
| Early transient | 13/64 (20.3) | 0.62 (0.33-1.17) | 0.70 (0.36-1.365) |
| Early persistent | 18/46 (39.1) | 1.57 (0.85-2.91) | 1.48 (0.76-2.865) |
| Late | 18/43 (41.9) | 1.76 (0.93-3.30) | 2.06 (1.05 -4.035) |
| Never/infrequent | 171/588 (29.1) | 1 [Reference] | 1 [Reference] |
| Sensitization to inhalant allergens at 6 y | 240/741 (32.4) | NA | NA |
| Early transient | 20/64 (31.3) | 1.05 (0.60-1.83) | 1.12 (0.63-1.975) |
| Early persistent | 26/46 (56.5) | 2.99 (1.63-5.51) | 3.36 (1.78-6.355) |
| Late | 216/43 (37.2) | 1.37 (0.72-2.60) | 1.51 (0.79-2.915) |
| Never/infrequent | 178/588 (30.3) | 1 [Reference] | 1 [Reference] |
Abbreviations: NA, not applicable; OR, odds ratio.
Adjusted for farmer, center, sex, and breastfeeding.
Asthma
Both early phenotypes of atopic dermatitis showed a tendency of an increased risk of developing asthma, although the association was stronger with the early-persistent phenotype (Table 3). The proportion of children having asthma was 17.5% among children with early-persistent phenotype (n = 10) compared with 7.5% among those with never/infrequent atopic dermatitis (n = 55). Additional analyses with a definition of asthma based on reported physician diagnosis of asthma or obstructive bronchitis only between 4 and 6 years, independently of the history of asthma in the first 3 years, showed also an increased risk of developing asthma (adjusted OR, 2.55; 95% CI, 1.10-5.93). No association was observed with the late phenotype of atopic dermatitis.
Food Allergy
Early phenotypes of atopic dermatitis were strongly associated with food allergy, but late phenotypes were not (Table 3). Additionally, analyses were performed with a definition of food allergy based on physician diagnosis of food allergy, with reported confirmation by an allergy test (48 of 834; 5.8%). Using this definition, we also observed a strongly positive association with early phenotypes (adjusted OR for food allergy: with early transient, 3.71; 95% CI, 1.66-8.26; and with early persistent, 7.79; 95% CI, 3.42-17.73).
Allergic Rhinitis
Children with early-persistent phenotype and those with late phenotype had an increased risk of developing allergic rhinitis (Table 3). No significant association was observed with the early-transient phenotype.
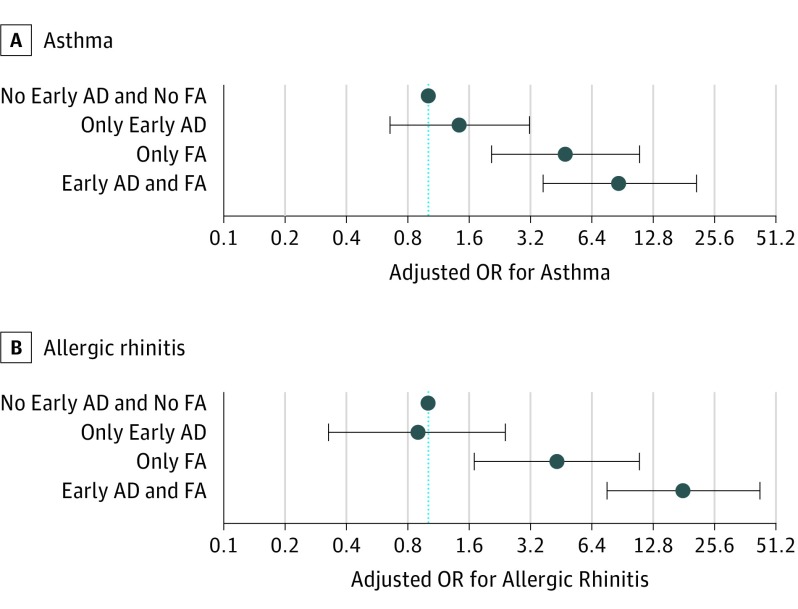
To evaluate the effect of early phenotypes and food allergy separately and combined on asthma and allergic rhinitis, we used a variable with 4 categories: children having an early phenotype (transient or persistent) of atopic dermatitis or not and having food allergy or not (Figure 2). The children with only early atopic dermatitis (n = 101) did not have an increased risk of respiratory allergy. On the other hand, children with only food allergy (n = 38) had an increased risk of respiratory allergy. The highest risk to develop respiratory allergy was among children with both (n = 30) (adjusted OR for asthma, 8.61; 95% CI, 3.68-20.18; and OR for allergic rhinitis, 18.03; 95% CI, 7.60-42.77).
Figure 2. Association Between Early Phenotypes of Atopic Dermatitis (AD) Combined With Food Allergy (FA) and Asthma and Allergic Rhinitis.
A, Asthma. B, Allergic rhinitis. Odds ratios adjusted for farmer, center, sex, and breastfeeding. Early AD indicates both early phenotypes combined, early-transient and early-persistent.
Atopic Sensitization
Sensitization measured at 6 years showed positive associations between the early-persistent phenotype and sensitization to inhalant allergens and between the late phenotype and sensitization to food allergens (Table 3).
We additionally investigated whether sensitization at the age of 1 year could predict the phenotypes of atopic dermatitis and found a strong association between sensitization to food allergens and the early-persistent phenotype (eTable 6 in the Supplement). No associations were found between sensitization to inhalant allergens at 1 year and the phenotypes of atopic dermatitis.
Discussion
In this study, we identified 4 different phenotypes of atopic dermatitis using the LCA with atopic dermatitis symptoms at 7 different times in childhood. These phenotypes are characterized by the age of onset of first symptoms and natural course of the disease from birth to 6 years of age. We define 2 phenotypes with early onset within age 2 years, with transient and persistent progression; a late phenotype, with onset after the age of 2 years; and a phenotype including children mainly free of symptoms.
Although early manifestation of atopic dermatitis was previously described, to our knowledge, this is one of the first studies using the LCA method with data from a large birth cohort study to define atopic dermatitis phenotypes. This method allows us to confirm that different phenotypes of atopic dermatitis could be defined by the timing of onset but also by their progression during childhood.
The major findings of this study are that only children with early phenotypes of atopic dermatitis determined by LCA have an increased risk for developing asthma and food allergy. Early-transient and early-persistent phenotypes are similar regarding their positive associations with other allergic outcomes, although stronger with the early-persistent phenotype. The late phenotype seems to be different, being only associated with allergic rhinitis and not with asthma or food allergy.
There are many factors associated with the development of asthma. Genetic factors are clearly important, but the presence of atopic dermatitis is also recognized as a risk factor and is a major criterion of validated asthma predictive index. Consistent with our results, it was also observed that children with an early onset of atopic dermatitis had an increased risk to develop asthma, in contrast to children with later onset.
Latent class analysis is a well-known statistic method and was already used in several birth cohorts to define phenotypes of wheeze in childhood. In 2016, LCA method was also used to identify phenotypes of atopic sensitization. To our knowledge, this is one of the first studies using the LCA method to evaluate different subtypes of atopic dermatitis in childhood. The strengths of this study are the large population design of the PASTURE study and the repeated data on atopic dermatitis symptoms, prospectively collected from birth to school age, which give the opportunity to use this method. Our findings indicate that both genetic and environmental factors influence the course of atopic dermatitis differently depending on the phenotype. The family history of allergy shows a positive association with all phenotypes of atopic dermatitis determined by LCA but stronger with the early-persistent phenotype. In the same study population, we already reported an increased prevalence of early-onset physician diagnosis of atopic dermatitis among children at high risk and not of atopic dermatitis with onset after age 1 year. Consistently, another birth cohort showed that parental atopy was positively associated with atopic dermatitis with onset within the first 2 years of age.
We found only weak associations between environmental exposures and phenotypes of atopic dermatitis. Previously, we reported that prenatal farm animal contact was associated with a lower risk of physician diagnosis of atopic dermatitis. In this study, prenatal exposure to an increased number of farm animal species had a tendency to be protective against all atopic dermatitis phenotypes. A protective effect of prenatal exposure to pets was shown only on the early-persistent phenotype and specifically among children with allergic parents.
Previously, we have shown a potential protective effect of yogurt’s consumption in the first year of life on physician diagnosis of atopic dermatitis and other allergic diseases. In this study, we could show that this protective effect of yogurt in the first year of life was only on the early-persistent phenotype of atopic dermatitis.
Our results showed that children with an early-persistent phenotype are most at risk to develop respiratory allergy. Therefore, children developing symptoms of atopic dermatitis before age 2 years with a positive food sensitization, being associated with persistent atopic dermatitis, should be a focus group for respiratory allergy prevention. One simple strategy could be based on the diet, such as recommendation of introduction of yogurt in first year of life, which we showed here to be a protective factor on the early-persistent phenotype.
Moreover, we observed that children having both an early phenotype of atopic dermatitis and food allergy had a very high risk of developing asthma or allergic rhinitis. This finding is of great importance because atopic dermatitis and food allergy are diseases appearing often in early childhood, with the hypothesis that children with atopic dermatitis are more prone to develop sensitization to food allergens owing to a defect of skin barrier among those children. Children developing those diseases in early life might require special attention for prevention strategies of respiratory allergy. Moreover, it would be important to further find immunologic markers for these clinical phenotypes of atopic dermatitis because it was suggested that among atopic children, there are different immunological phenotypes.
Limitations
Although LCA is a robust statistical method, misclassification of atopic dermatitis between the classes might be considered. Nevertheless, objective measurements, such as the evaluation of SCORAD score, performed during medical examination at 1 year and 6 years of age, showed consistency with our classification of atopic dermatitis phenotypes. Definition of asthma based on reported physician diagnosis might lead to an underestimation of the prevalence of these diseases and therefore might bias the association with atopic dermatitis phenotypes toward the null. However, we found a strong positive association between early-persistent phenotype and asthma. Definition of food allergy based on reported physician diagnosis might include food intolerance or delayed reaction, which most likely leads to an overestimation of the prevalence. Therefore, we used a second definition also based on physician diagnosis of food allergy but only including children with reported confirmation by allergy test and the results were very similar with both definitions. Owing to the limitation of the definition of food allergy, one explanation of the strong positive association between the early-persistent phenotype and food allergy might be that some children with this phenotype of atopic dermatitis were sensitized to food allergens without clinical significance.
Conclusions
In conclusion, among a birth cohort study, using the LCA method, we identified 4 different phenotypes of atopic dermatitis symptoms in childhood, depending on the age of onset and natural course of the disease. Children with early phenotypes, especially with persistent symptoms, have an increased risk of developing other allergic diseases, and having additional food allergy substantially increases this risk. These results might give hints for future development of prevention strategies such as using precision medicine approaches.
eTable 1. Characteristics of Study Population With Complete Data (Information on Atopic Dermatitis Symptoms at All 7 Time Points Up to 6 Years)
eTable 2. Point Prevalence of Atopic Dermatitis Symptoms Up to 6 Years
eTable 3. Association Between a Positive SCORAD at 1 and 6 Years of Age and Atopic Dermatitis Phenotypes
eTable 4. Association Between Exposures and Atopic Dermatitis Phenotypes (Unadjusted)
eTable 5. Association Between Prenatal Exposures and Atopic Dermatitis Phenotypes, Stratified by Allergic Status of the Parents
eTable 6. Association Between Atopic Dermatitis Phenotypes and Sensitization to Food and Inhalant Allergens at 1 Year of Age (Cutoff: 0.7 IU/mL)
eFigure 1. Probability of Atopic Dermatitis Symptoms at Each Time Point From Birth to 6 Years of Age for Each Atopic Dermatitis Phenotype in the 4-Class Model. With No Missing Data.
eFigure 2. SCORAD Scores at 1 and 6 Years of Age and Atopic Dermatitis Phenotypes (Only With Children With SCORAD Score >0)
eFigure 3. The Prevalences of Atopic Dermatitis Phenotypes Stratified By the Parental Allergic Status.
References
- 1.The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet. 1998;351(9111):1225-1232. [PubMed] [Google Scholar]
- 2.Asher MI, Montefort S, Björkstén B, et al. ; ISAAC Phase Three Study Group . Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368(9537):733-743. [DOI] [PubMed] [Google Scholar]
- 3.Williams H, Flohr C. How epidemiology has challenged 3 prevailing concepts about atopic dermatitis. J Allergy Clin Immunol. 2006;118(1):209-213. [DOI] [PubMed] [Google Scholar]
- 4.DaVeiga SP. Epidemiology of atopic dermatitis: a review. Allergy Asthma Proc. 2012;33(3):227-234. [DOI] [PubMed] [Google Scholar]
- 5.Kay J, Gawkrodger DJ, Mortimer MJ, Jaron AG. The prevalence of childhood atopic eczema in a general population. J Am Acad Dermatol. 1994;30(1):35-39. [DOI] [PubMed] [Google Scholar]
- 6.Garmhausen D, Hagemann T, Bieber T, et al. . Characterization of different courses of atopic dermatitis in adolescent and adult patients. Allergy. 2013;68(4):498-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saunes M, Øien T, Dotterud CK, et al. . Early eczema and the risk of childhood asthma: a prospective, population-based study. BMC Pediatr. 2012;12:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinart M, Benet M, Annesi-Maesano I, et al. . Comorbidity of eczema, rhinitis, and asthma in IgE-sensitised and non-IgE-sensitised children in MeDALL: a population-based cohort study. Lancet Respir Med. 2014;2(2):131-140. [DOI] [PubMed] [Google Scholar]
- 9.Illi S, von Mutius E, Lau S, et al. ; Multicenter Allergy Study Group . The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J Allergy Clin Immunol. 2004;113(5):925-931. [DOI] [PubMed] [Google Scholar]
- 10.van der Hulst AE, Klip H, Brand PL. Risk of developing asthma in young children with atopic eczema: a systematic review. J Allergy Clin Immunol. 2007;120(3):565-569. [DOI] [PubMed] [Google Scholar]
- 11.Belgrave DC, Simpson A, Buchan IE, Custovic A. Atopic dermatitis and respiratory allergy: what is the link. Curr Dermatol Rep. 2015;4(4):221-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amat F, Saint-Pierre P, Bourrat E, et al. . Early-onset atopic dermatitis in children: which are the phenotypes at risk of asthma? results from the ORCA cohort. PLoS One. 2015;10(6):e0131369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Mutius E, Schmid S; PASTURE Study Group . The PASTURE project: EU support for the improvement of knowledge about risk factors and preventive factors for atopy in Europe. Allergy. 2006;61(4):407-413. [DOI] [PubMed] [Google Scholar]
- 14.Herzum I, Blümer N, Kersten W, Renz H. Diagnostic and analytical performance of a screening panel for allergy. Clin Chem Lab Med. 2005;43(9):963-966. [DOI] [PubMed] [Google Scholar]
- 15.Castro-Rodríguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162(4, pt 1):1403-1406. [DOI] [PubMed] [Google Scholar]
- 16.Gustafsson D, Sjöberg O, Foucard T. Development of allergies and asthma in infants and young children with atopic dermatitis: a prospective follow-up to 7 years of age. Allergy. 2000;55(3):240-245. [DOI] [PubMed] [Google Scholar]
- 17.Almqvist C, Li Q, Britton WJ, et al. ; CAPS team . Early predictors for developing allergic disease and asthma: examining separate steps in the “allergic march.” Clin Exp Allergy. 2007;37(9):1296-1302. [DOI] [PubMed] [Google Scholar]
- 18.Hopper JL, Bui QM, Erbas B, et al. . Does eczema in infancy cause hay fever, asthma, or both in childhood? insights from a novel regression model of sibling data. J Allergy Clin Immunol. 2012;130(5):1117-1122.e1. [DOI] [PubMed] [Google Scholar]
- 19.Henderson J, Granell R, Heron J, et al. . Associations of wheezing phenotypes in the first 6 years of life with atopy, lung function and airway responsiveness in mid-childhood. Thorax. 2008;63(11):974-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savenije OE, Granell R, Caudri D, et al. . Comparison of childhood wheezing phenotypes in 2 birth cohorts: ALSPAC and PIAMA. J Allergy Clin Immunol. 2011;127(6):1505-12.e14. [DOI] [PubMed] [Google Scholar]
- 21.Depner M, Fuchs O, Genuneit J, et al. ; PASTURE Study Group . Clinical and epidemiologic phenotypes of childhood asthma. Am J Respir Crit Care Med. 2014;189(2):129-138. [DOI] [PubMed] [Google Scholar]
- 22.Herr M, Just J, Nikasinovic L, et al. . Risk factors and characteristics of respiratory and allergic phenotypes in early childhood. J Allergy Clin Immunol. 2012;130(2):389-96.e4. [DOI] [PubMed] [Google Scholar]
- 23.Havstad S, Johnson CC, Kim H, et al. . Atopic phenotypes identified with latent class analyses at age 2 years. J Allergy Clin Immunol. 2014;134(3):722-727.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hose AJ, Depner M, Illi S, et al. ; MAS; PASTURE study groups . Latent class analysis reveals clinically relevant atopy phenotypes in 2 birth cohorts. J Allergy Clin Immunol. 2016;S0091-6749(16)31135-6. [DOI] [PubMed] [Google Scholar]
- 25.Roduit C, Frei R, Loss G, et al. ; Protection Against Allergy–Study in Rural Environments study group . Development of atopic dermatitis according to age of onset and association with early-life exposures. J Allergy Clin Immunol. 2012;130(1):130-6.e5. [DOI] [PubMed] [Google Scholar]
- 26.Roduit C, Wohlgensinger J, Frei R, et al. ; PASTURE Study Group . Prenatal animal contact and gene expression of innate immunity receptors at birth are associated with atopic dermatitis. J Allergy Clin Immunol. 2011;127(1):179-185, 185.e1. [DOI] [PubMed] [Google Scholar]
- 27.Roduit C, Frei R, Depner M, et al. ; PASTURE study group . Increased food diversity in the first year of life is inversely associated with allergic diseases. J Allergy Clin Immunol. 2014;133(4):1056-1064. [DOI] [PubMed] [Google Scholar]
- 28.Du Toit G, Roberts G, Sayre PH, et al. ; LEAP Study Team . Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372(9):803-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.du Toit G, Tsakok T, Lack S, Lack G. Prevention of food allergy. J Allergy Clin Immunol. 2016;137(4):998-1010. [DOI] [PubMed] [Google Scholar]
- 30.Renz H, mutius Ev, Illi S, Wolkers F, Hirsch T, Weiland SK. T(H)1/T(H)2 immune response profiles differ between atopic children in eastern and western Germany. J Allergy Clin Immunol. 2002;109(2):338-342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Characteristics of Study Population With Complete Data (Information on Atopic Dermatitis Symptoms at All 7 Time Points Up to 6 Years)
eTable 2. Point Prevalence of Atopic Dermatitis Symptoms Up to 6 Years
eTable 3. Association Between a Positive SCORAD at 1 and 6 Years of Age and Atopic Dermatitis Phenotypes
eTable 4. Association Between Exposures and Atopic Dermatitis Phenotypes (Unadjusted)
eTable 5. Association Between Prenatal Exposures and Atopic Dermatitis Phenotypes, Stratified by Allergic Status of the Parents
eTable 6. Association Between Atopic Dermatitis Phenotypes and Sensitization to Food and Inhalant Allergens at 1 Year of Age (Cutoff: 0.7 IU/mL)
eFigure 1. Probability of Atopic Dermatitis Symptoms at Each Time Point From Birth to 6 Years of Age for Each Atopic Dermatitis Phenotype in the 4-Class Model. With No Missing Data.
eFigure 2. SCORAD Scores at 1 and 6 Years of Age and Atopic Dermatitis Phenotypes (Only With Children With SCORAD Score >0)
eFigure 3. The Prevalences of Atopic Dermatitis Phenotypes Stratified By the Parental Allergic Status.