This population-based cohort study explores the associations between antenatal corticosteroid administration-to-birth interval and survival and morbidity among very preterm infants.
Key Points
Question
What is the shortest administration-to-birth interval of antenatal corticosteroids that promotes survival and decreases neonatal morbidity in very preterm infants?
Findings
In a population-based cohort of 4594 European infants born before 32 weeks’ gestation, we found that in-hospital mortality was significantly reduced when antenatal corticosteroids had been administered only a few hours prior to delivery.
Meaning
Encouraging administration of antenatal corticosteroids when delivery is very imminent could result in substantial survival and health gains for very preterm infants.
Abstract
Importance
Administration-to-birth intervals of antenatal corticosteroids (ANS) vary. The significance of this variation is unclear. Specifically, to our knowledge, the shortest effective administration-to-birth interval is unknown.
Objective
To explore the associations between ANS administration-to-birth interval and survival and morbidity among very preterm infants.
Design, Setting, and Participants
The Effective Perinatal Intensive Care in Europe (EPICE) study, a population-based prospective cohort study, gathered data from 19 regions in 11 European countries in 2011 and 2012 on 4594 singleton infants with gestational ages between 24 and 31 weeks, without severe anomalies and unexposed to repeated courses of ANS. Data were analyzed November 2016.
Exposure
Time from first injection of ANS to delivery in hours and days.
Main Outcomes and Measures
Three outcomes were studied: in-hospital mortality; a composite of mortality or severe neonatal morbidity, defined as an intraventricular hemorrhage grade of 3 or greater, cystic periventricular leukomalacia, surgical necrotizing enterocolitis, or stage 3 or greater retinopathy of prematurity; and severe neonatal brain injury, defined as an intraventricular hemorrhage grade of 3 or greater or cystic periventricular leukomalacia.
Results
Of the 4594 infants included in the cohort, 2496 infants (54.3%) were boys, and the mean (SD) gestational age was 28.5 (2.2) weeks and mean (SD) birth weight was 1213 (400) g. Mortality for the 662 infants (14.4%) unexposed to ANS was 20.6% (136 of 661). Administration of ANS was associated with an immediate and rapid decline in mortality, reaching a plateau with more than 50% risk reduction after an administration-to-birth interval of 18 to 36 hours. A similar pattern for timing was seen for the composite mortality or morbidity outcome, whereas a significant risk reduction of severe neonatal brain injury was associated with longer administration-to-birth intervals (greater than 48 hours). For all outcomes, the risk reduction associated with ANS was transient, with increasing mortality and risk for severe neonatal brain injury associated with administration-to-birth intervals exceeding 1 week. Under the assumption of a causal relationship between timing of ANS and mortality, a simulation of ANS administered 3 hours before delivery to infants who did not receive ANS showed that their estimated decline in mortality would be 26%.
Conclusions and Relevance
Antenatal corticosteroids may be effective even if given only hours before delivery. Therefore, the infants of pregnant women at risk of imminent preterm delivery may benefit from its use.
Introduction
Antenatal corticosteroids (ANS) reduce morbidity and mortality in preterm infants and are recommended for women at risk of delivery before 34 weeks’ gestation. Antenatal corticosteroids have been estimated to reduce infant mortality by 31%, respiratory distress syndrome by 34%, intraventricular hemorrhage by 46%, and necrotizing enterocolitis by 54%.
While there seems to be agreement that neonatal benefits are maximized when ANS are administered 24 hours to 7 days before delivery, less is known about other and narrower administration-to-birth intervals. In particular, to our knowledge, the latest point at which ANS can be effectively used is not known. Given favorable pharmacokinetics and rapid circulatory effects, ANS may be effective even hours before birth. At the other end of the administration-to-birth interval, some but not all studies suggest declining benefits from ANS if administered more than 7 days before preterm birth. Limitations in statistical power, varying exposures, and outcomes may contribute to such diverse results and interpretations.
Because the problem of timely prediction of preterm delivery remains unresolved, administration of ANS will continue to occur hours, days, or weeks before delivery. Therefore, a more detailed understanding of the importance of the administration-to-birth interval of ANS for infant outcome is needed. The objective of this study was to investigate associations between different administration-to-birth intervals of ANS in hours and morbidity and mortality in very preterm infants.
Methods
The Effective Perinatal Intensive Care in Europe (EPICE) cohort study is a population-based study on the use of evidence-based practices in very preterm births with less than 32 weeks’ gestation in 19 regions across 11 European countries, conducted in 2011 and 2012 (http://www.epiceproject.eu). Geographic and organizational diversity, feasibility, and sample size were accounted for when selecting regions. Inclusions occurred over a 12-month period except in France (6-month period). Ethics approval was obtained in each region, as required by national legislation. The European study was also approved by the French Advisory Committee on Use of Health Data in Medical Research (CCTIRS) and the French Commission for Data Protection and Liberties (CNIL). Informed parental consent (active or passive, depending on each participating country’s national legislations) was obtained; in some regions, consent requirements were waived by ethics boards for parents not included in the follow-up cohort (ie, stillbirths and neonatal deaths), as data were deidentified and from routine sources.
Study Population
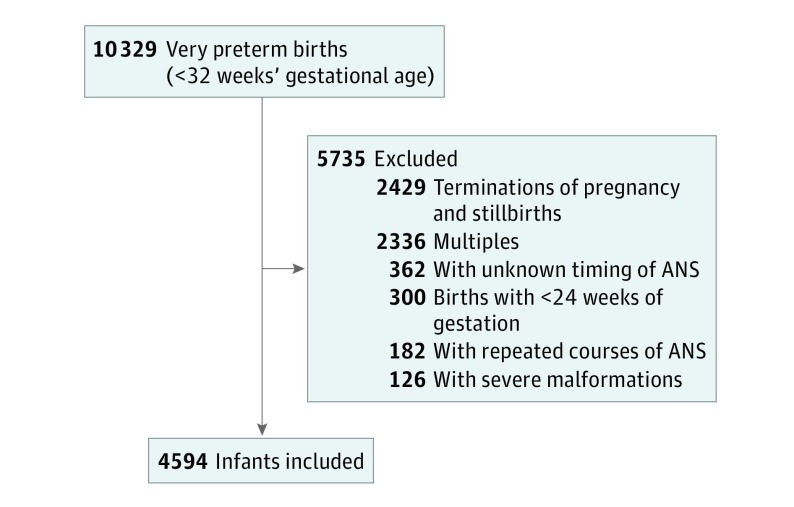
From a total of 10 329 births, we excluded 2429 terminations of pregnancy and stillbirths, 126 infants with severe congenital anomalies, 2336 multiples, 300 infants born at 22 and 23 weeks’ gestational age (GA), 362 infants of mothers with missing data on date and time of ANS administration, and 182 infants of mothers who had received more than 1 course of ANS. A repeated course was defined as an interval between ANS injections exceeding 1 week. The final sample consisted of 4594 singleton live-births at 24 to 31 weeks’ GA (Figure 1). Administration of corticosteroids during pregnancy for indications other than preterm birth was not included in the study protocol.
Figure 1. Flowchart for Inclusions.
ANS indicates antenatal corticosteroids.
Exposure
For each infant, information was collected on dates and times of the first ANS injection as well as the total number of injections. Questionnaires were also sent to the heads of the maternity units regularly managing 10 or more very preterm admissions annually. Most maternity units (123 of 135 [91.1%]) responded, and protocols for ANS, including type of drug, doses, and number and intervals of injections, were collected.
We defined our exposure as time from the first injection of ANS to delivery. We classified ANS exposure in 4 categories (no ANS, first injection <24 hours, first injection between 24 hours and 7 days, and first injection >7 days before birth) for descriptive analyses of the characteristics of women and newborns and for comparison with other studies. For more detailed analyses of associations between timing of ANS and outcome, we used administration-to-birth intervals as a continuous variable. We did not include information on 1 or several doses of ANS because of high collinearity between timing of ANS and number of doses; 866 of 1111 women (77.9%) receiving ANS less than 24 hours prior to delivery received only 1 dose.
For some women, we only had information on the date of ANS administration but not the time. This caused problems for our classifications when administration was within 2 days of delivery (204 of 1522 women [13.4%] delivering within 2 days). For a solution, we used multiple imputations chained equations to impute these data based on all variables in the study in addition to the time of delivery, date of administration, and number of injections received. We used 100 imputed data sets. Results are presented using imputed data.
Outcomes
Based on the evidence of the effects of ANS on preterm infants, the outcomes selected were in-hospital mortality, a composite of mortality or severe neonatal morbidity or both, or severe neonatal brain injury. In-hospital mortality was defined as death before discharge home. Severe neonatal morbidity was defined as an intraventricular hemorrhage grade of 3 or 4, cystic periventricular leukomalacia, stage 3, 4, or 5 retinopathy of prematurity, or severe necrotizing enterocolitis (assessed as need for surgery or peritoneal drainage; Bell stages were not routinely recorded in all regions) among infants discharged alive. Severe neonatal brain injury was defined as an intraventricular hemorrhage grade of 3 or 4, cystic periventricular leukomalacia, or both. We did not include bronchopulmonary dysplasia into our composite outcome because of regional variability in respiratory management and oxygen saturation targets have major effects on rates of bronchopulmonary dysplasia.
Covariables
Covariables that could be potential confounders of the associations between timing of ANS and infant outcome were selected based on the literature and clinical knowledge. We included variables reflecting case mix (maternal age; parity; pregnancy complications, including preeclampsia, eclampsia, and hemolysis, elevated liver enzyme levels, low platelet count syndrome; preterm prelabor rupture of membranes; GA [defined as best assessment based on last menstrual period or antenatal ultrasonography, part of routine obstetric care in all regions]; smallness for gestational age [defined as birth weight less than the third and between the third and 10th centiles, based on customized intrauterine growth curves]; and infant sex) and variables related to management of the delivery (birth at a level III neonatal unit; mode of delivery; and delivery on same day as admission to hospital). Preeclampsia was defined as hypertension after 20 weeks’ GA (blood pressure of 140/90 mm Hg or higher) and proteinuria with a protein level of 0.3 g/L or greater, and eclampsia was defined as hypertension associated with convulsions or coma. Preterm prelabor rupture of membranes was defined as spontaneous rupture of membranes 12 hours or more before onset of contractions.
Statistical Analyses
We first compared perinatal characteristics in our study population by timing of ANS administration using 4 categories. We then modeled the associations between ANS administration-to-birth intervals and the 3 outcomes using the 4-category variable and a continuous variable in hours. For the latter analysis, we used restricted cubic splines with 5 knots located at the fifth, 20th, 28th, 40th, and 95th percentiles (knots placed at 0, 1, 6, 24, and 520 hours) for mortality and the composite mortality and morbidity outcome. We selected 3 knots close to each other because of strong nonlinearity in the first 24 hours after ANS. For severe neonatal brain injury, we used the default percentiles (knots placed at 0, 6, 49, 122, and 525 hours) used by Harrell. Generalized linear models, assuming a Poisson distribution, were used to estimate risk ratios across exposures for all analyses. Adjusted models included population case mix and pregnancy management variables, as described above. Region was included as a fixed effect, and we accounted for clustering between observations within regions.
Finally, we assessed the effect on in-hospital mortality if all infants without ANS had been administered ANS less than 3 hours, 3 to 5 hours, and 6 to 12 hours before delivery by rerunning our final 4-category model and subdividing the less-than-24-hours category into these subgroups; we then predicted mortality after setting the population with no ANS sequentially to the first, second, and third subgroups.
M.N., A.P., and J.Z. analyzed the data. Analyses were carried out using STATA version 14.0 SE (StataCorp).
Results
The most common ANS protocol was two 12-mg injections 24 hours apart (performed in 105 of 123 units [85.4%]), followed by two 12-mg injections 12 hours apart (performed in 13 units [10.6%]). Another 4 units (3.3%) used other protocols. Betamethasone was used in 97 units (78.9%), dexamethasone in 6 units (4.9%), and both in 19 units (15.4%) (eTable 1 in the Supplement).
Distribution of Administration-to-Birth Intervals
Fewer than half of included women (1871 of 4594 [40.7%]) received ANS 24 hours to 7 days before delivery, ie, by current standards, the administration-to-birth interval considered to be optimal. Many women received ANS closer to delivery (1111 [24.2%]) or did not receive ANS (662 [14.4%]) (Table 1). The cumulative distribution of administration-to-birth intervals are presented in eFigure 1 in the Supplement. Administration and timing of ANS varied across the 19 European regions (eFigure 2 in the Supplement).
Table 1. Characteristics of Very Preterm Infants by 4 ANS Administration-to-Birth Intervals.
| Characteristic | ANS Administration-to-Birth Interval, No. (%) | P Value | |||
|---|---|---|---|---|---|
| No ANS (n = 662 [14.4%]) | <24 h (n = 1111 [24.2%]) | 24 h-7 d (n = 1871 [40.7%]) | >7 d (n = 950 [20.7%]) | ||
| Infant | |||||
| Gestational age, mean (SD), wk | 28.3 (2.3) | 28.5 (2.2) | 28.4 (2.2) | 28.8 (1.9) | <.001 |
| SGA, birth weight centiles | <.001 | ||||
| <3 | 104 (15.7) | 204 (18.4) | 487 (26) | 224 (23.6) | |
| 3-10 | 62 (9.4) | 103 (9.3) | 221 (11.8) | 79 (8.3) | |
| >10 | 496 (74.9) | 804 (72.4) | 1162 (62.1) | 647 (68.1) | |
| Sex | .09 | ||||
| Male | 383 (57.9) | 608 (54.8) | 980 (52.4) | 525 (55.3) | |
| Female | 279 (42.2) | 503 (45.2) | 890 (47.6) | 425 (44.7) | |
| Surfactant <2 h after birth | <.001 | ||||
| No | 282 (47.6) | 560 (51.6) | 1134 (61.9) | 534 (58.4) | |
| Yes | 310 (52.4) | 524 (48.4) | 700 (38.2) | 381 (41.6) | |
| Maternal | |||||
| Age, mean (SD), y | 28.9 (6.4) | 29.6 (6.2) | 30.4 (6.1) | 30.5 (6.2) | <.001 |
| Parity | <.001 | ||||
| Primiparous | 330 (50.7) | 625 (56.7) | 1073 (57.7) | 441 (46.9) | |
| Multiparous | 321 (49.3) | 477 (43.3) | 787 (42.3) | 500 (53.1) | |
| Preeclampsia/eclampsia/HELLP | <.001 | ||||
| No | 565 (89.4) | 888 (80.9) | 1381 (74) | 808 (85.9) | |
| Yes | 67 (10.6) | 209 (19.1) | 484 (26) | 133 (14.1) | |
| PPROM | <.001 | ||||
| No | 580 (91.5) | 972 (88.4) | 1265 (67.8) | 540 (57.4) | |
| Yes | 54 (8.5) | 127 (11.6) | 600 (32.2) | 401 (42.6) | |
| Mode of delivery onset | <.001 | ||||
| Spontaneous or induced | 414 (63.6) | 662 (60.1) | 918 (49.5) | 507 (53.9) | |
| Cesarean section | 237 (36.4) | 439 (39.9) | 936 (50.5) | 433 (46.1) | |
| Mode of delivery | <.001 | ||||
| Vaginal | 312 (48.1) | 450 (40.9) | 596 (32.1) | 276 (29.3) | |
| Cesarean section | 337 (51.9) | 652 (59.2) | 1263 (67.9) | 667 (70.7) | |
| Delivery at level III unita | <.001 | ||||
| No | 283 (42.9) | 379 (34.1) | 311 (16.6) | 133 (14) | |
| Yes | 376 (57.1) | 731 (65.9) | 1559 (83.4) | 817 (86) | |
| Delivery on day of admission | <.001 | ||||
| No | 203 (32.6) | 605 (56.3) | 1725 (94.9) | 802 (88.2) | |
| Yes | 419 (67.4) | 469 (43.7) | 94 (5.2) | 107 (11.8) | |
Abbreviations: ANS, antenatal corticosteroids; HELLP, hemolysis, elevated liver enzyme levels, low platelet count syndrome; PPROM, preterm prelabor rupture of membranes; SGA, smallness for gestational age.
Level III neonatal units were defined using local definitions of level of care.
Perinatal Characteristics by Timing of ANS
Variables reflecting population case mix and management of preterm delivery differed between ANS categories, with the exception of infant sex (Table 1). In particular, women receiving ANS more than 7 days before delivery were more likely to have preterm prelabor rupture of membranes and a higher GA and to deliver in a level III unit. Of patients with missing data, 235 of 362 (64.9%) came from 3 of the 19 regions, reflecting difficulties in data collection. After adjustment on region, infants with missing information were more often born to mothers with preeclampsia, more often delivered with cesarean section, and less often born at level III units than infants in the study population (eTable 2 in the Supplement).
Timing of ANS and Outcome
Using 4 categories of ANS timing, any ANS—irrespective of the administration-to-birth interval—was associated with lower infant mortality than no ANS, with the largest risk reduction for ANS administered 24 hours to 7 days before delivery (adjusted risk ratio, 0.5; 95% CI, 0.4-0.6). Antenatal corticosteroids administered 24 hours to 7 days before delivery was also associated with lower risks of mortality or severe neonatal morbidity and of lower risk for severe neonatal brain injury than no ANS (Table 2).
Table 2. Risk Ratios for In-Hospital Infant Mortality, a Composite of Mortality or Severe Neonatal Morbidity, and Neonatal Brain Injury Associated With ANS Administration-to-Birth Intervals Among Singleton Infants Born Before 32 Weeks’ Gestational Age.
| ANS Administration-to-Birth Interval | In-Hospital Mortality | In-Hospital Mortality or Severe Neonatal Morbiditya | Severe Neonatal Brain Injuryb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No./Total No. (%) | RR (95% CI) | No./Total No. (%) | RR (95% CI) | No./Total No. (%) | RR (95% CI) | ||||
| Crude | Adjustedc | Crude | Adjustedc | Crude | Adjustedc | ||||
| No ANS | 136/661 (20.6) | 1 [Reference] | 1 [Reference] | 205/654 (31.3) | 1 [Reference] | 1 [Reference] | 81/601 (13.5) | 1 [Reference] | 1 [Reference] |
| <24 h | 117/1110 (10.5) | 0.5 (0.4-0.6) | 0.6 (0.5-0.7) | 235/1082 (21.7) | 0.7 (0.6-0.8) | 0.7 (0.6-0.9) | 122/1067 (11.4) | 0.9 (0.7-1.1) | 0.9 (0.7-1.1) |
| 24 h-7 d | 171/1871 (9.1) | 0.4 (0.4-0.5) | 0.5 (0.4-0.6) | 356/1837 (19.4) | 0.6 (0.5-0.8) | 0.7 (0.6-0.8) | 129/1816 (7.1) | 0.5 (0.4-0.7) | 0.6 (0.5-0.9) |
| >7 d | 89/950 (9.4) | 0.5 (0.4-0.6) | 0.7 (0.6-0.9) | 171/931 (18.4) | 0.6 (0.5-0.7) | 0.8 (0.7-1.0) | 75/924 (8.1) | 0.6 (0.4-0.9) | 0.8 (0.6-1.2) |
Abbreviations: ANS, antenatal corticosteroids; RR, risk ratio.
Severe neonatal morbidity includes intraventricular hemorrhage grade 3 or more, cystic periventricular leukomalacia, surgical necrotizing enterocolitis, or stage 3 or more retinopathy of prematurity.
Severe neonatal brain injury includes intraventricular hemorrhage grade 3 or more, cystic periventricular leukomalacia, or both.
Adjusted for patient case mix (maternal age; parity; pregnancy complications, including preeclampsia, eclampsia, and hemolysis, elevated liver enzyme levels, low platelet count syndrome; preterm prelabor rupture of membranes; gestational age; small for gestational age; and infant sex) and factors related to management of the delivery (birth at a level III neonatal unit; mode of delivery; and delivery on same day as admission to hospital).
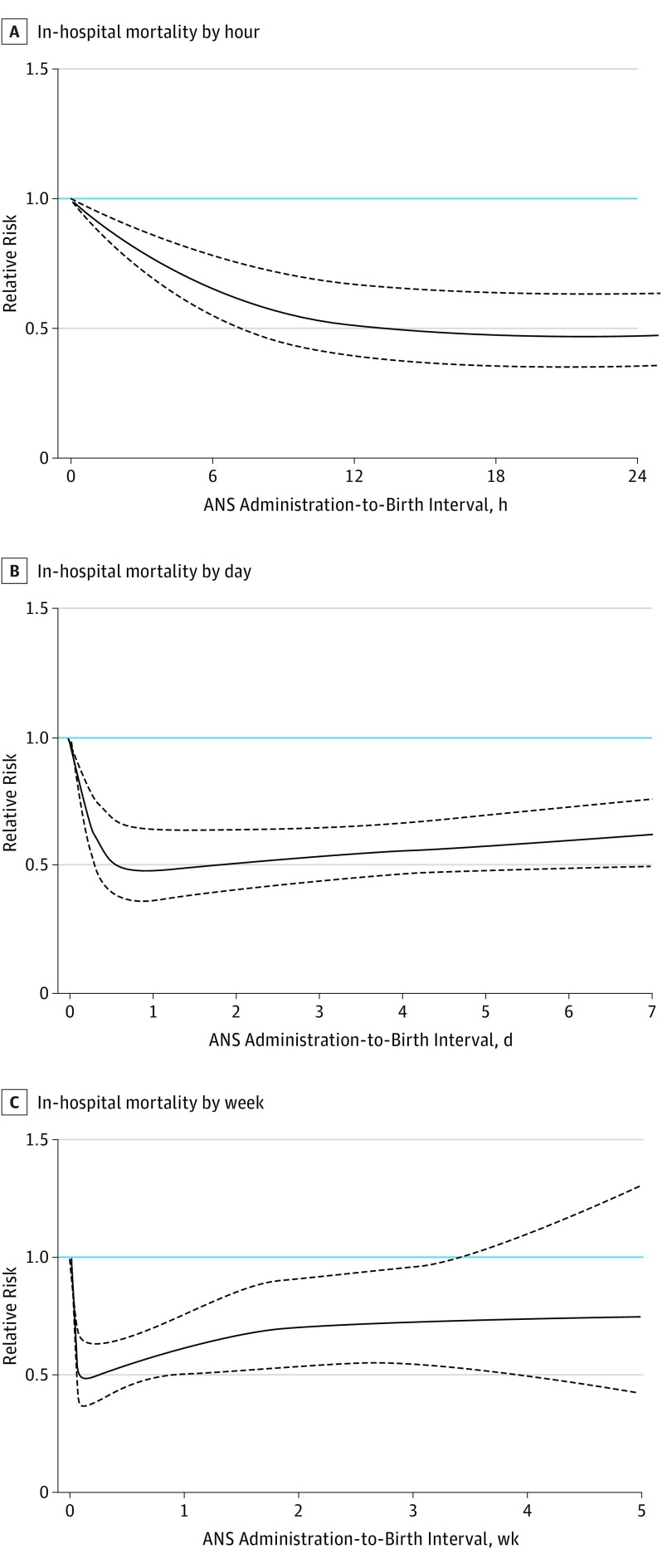
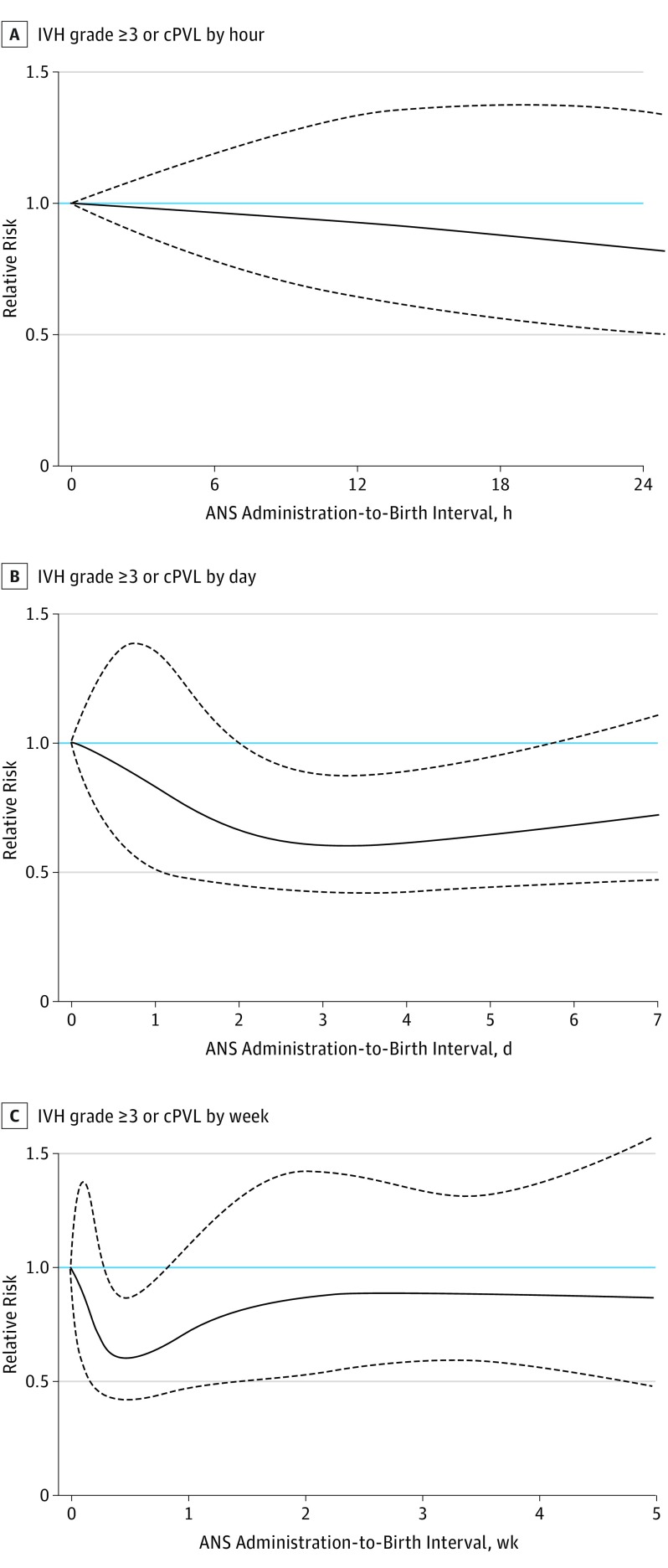
Increasing the resolution of the analyses by expressing the risk for our 3 outcomes as a continuous function of the administration-to-birth interval, ANS less than 12 hours before birth was associated with an immediate and rapid decline in mortality, followed by a slower risk reduction and reaching a plateau with more than 50% risk reduction after an administration-to-birth interval of 18 to 36 hours (Figure 2). A similar pattern for timing of ANS was seen for the composite outcome of mortality or severe neonatal morbidity (eFigure 3 in the Supplement), whereas a significant risk reduction of severe neonatal brain injury was associated with longer administration-to-birth intervals (Figure 3). For all outcomes, the risk reduction associated with ANS was transient, with increasing mortality and risk for severe neonatal brain injury associated with ANS administration-to-birth intervals of 5 to 7 days or more.
Figure 2. Association Between Timing of Antenatal Corticosteroids (ANS) and In-Hospital Mortality in 4594 Very Preterm Infants.
The reference relative risk represents very preterm infants who were not exposed to ANS before birth. The curves for adjusted relative risks represent means and 95% CI bands.
Figure 3. Association Between Timing of Antenatal Corticosteroids (ANS) and Severe Neonatal Brain Injury in 4594 Very Preterm Infants.
The reference relative risk represents very preterm infants who were not exposed to ANS before birth. The curves for adjusted relative risks represent means and 95% CI bands. cPVL indicates cystic periventricular leukomalacia; IVH, intraventricular hemorrhage.
In a simulation of providing ANS for the 661 infants in the sample who did not receive ANS, our model predicted a 26% decrease in mortality if these infants received ANS at least 3 hours before delivery, a 37% decrease if they received ANS 3 to 5 hours before delivery, and a 51% decrease if they received ANS 6 to 12 hours before delivery (eTable 3 in the Supplement).
Discussion
Our findings suggest that infant mortality was rapidly and significantly reduced when ANS were administered only a few hours before delivery. Infant birth 12 hours following ANS administration was associated with similar mortality risk as those exposed to ANS 18 to 48 hours before delivery. Under the assumption of a causal relationship between timing of ANS and mortality, our simulations showed that if infants with no ANS prior to delivery received ANS 6 to 12 hours before delivery, their mortality was estimated to have been reduced by 51%. Furthermore, the administration of ANS was associated with a significant reduction in severe neonatal morbidity.
Our findings challenge current thinking about the optimal timing of ANS. Previously, ANS administered less than 24 hours before birth have been referred to as suboptimal, partial, or incomplete, suggesting poorer effectiveness at administration-to-birth intervals less than 24 hours. However, the categories used in previous studies appear to have hidden clinically important effects of ANS occurring within this period. In addition, given that most (77.9%) pregnant women in our study delivering within 24 hours of ANS only received 1 injection, significant effects may be achieved by a single ANS injection. In this context, we note with interest the recent statement by the National Institute for Health and Care Excellence Guideline Development Committee; taking account of the pharmacological mechanism of action of ANS, the committee suspects that any benefits from ANS would likely be transferred even if there was only a limited amount of time between administration and time of birth.
The underlying mechanisms for the rapid actions of ANS suggested herein are not fully understood. Cord blood concentrations of ANS peak 1 hour after administration to the mother and become undetectable 2 days after the last dose. While induction of many corticosteroid effects involve genomic interactions and therefore take time, nongenomic effects of corticosteroids have in more recent years also been discovered. These effects can be very rapid and occur within minutes. Nongenomic effects may contribute to findings in preterm lambs, demonstrating beneficial corticosteroid effects on pulmonary edema and blood pressure within 8 hours after administration and significant lung function improvement within 15 hours. In humans, fetal movements and heart variability increased significantly within 8 hours after ANS, possibly indicating fetal well-being or fetal stress enhancing maturation.
Although the risk reduction was slower than that associated with survival—suggesting competing outcomes (mortality and morbidity) or that the underlying mechanisms may be different—an ANS administration-to-birth interval of 48 hours to 1 week was associated with a significantly reduced risk for severe neonatal brain injury. A lower risk of severe neonatal brain injury after ANS has previously been reported for patients at highest risk, ie, those born extremely preterm. A lower risk after a partial course of ANS has also been demonstrated. In these patients, the ANS-associated reduction in severe neonatal brain injury mediated protection against later neurodevelopmental impairment.
We observed a 40% increased mortality in infants with an ANS administration-to-birth interval greater than 7 days compared with an interval of 1 to 7 days, a group representing 18.8% of all infants in our study. In 2015, Melamed et al reported similar findings using the Canadian Neonatal Network. Repeated courses of corticosteroids (betamethasone was the only drug tested) have been associated with a reduction in the incidence of respiratory distress syndrome and possibly also severity of neonatal lung disease. At 2-year to 3-year follow-up, there was no evidence of either significant benefit or harm with a repeated course of ANS in terms of rates of neurosensory disabilities, developmental delay, or growth failure. Follow-up in midchildhood demonstrated long-term safety.
Strengths and Limitations
The strengths of our study include its prospective design, large size, and heterogeneous population including both tertiary and nontertiary centers all over Europe. This ensures generalizability to a wide range of settings. Case mix, mortality, and morbidity rates are within the same ranges as previously reported from other high-income countries. We had power and data in sufficient detail to perform adjusted analyses on timing of ANS and outcome at very short administration-to-birth intervals. The outcomes—infant survival and major neonatal morbidities—were robust and clinically highly relevant.
Our study also has limitations. Even though we excluded infants with malformations and infants born before 24 weeks’ GA, and even after adjusting for group differences presented in Table 1, residual confounding may have occurred, as the populations of women who delivered at various points after ANS administration may have been different. We investigated several outcomes, and multiple confidence intervals are reported in this article; caution should be applied when evaluating their joint statistical level. The EPICE database does not contain information on time of admission, so we could not determine admission-to-administration intervals. In addition, reasons for not treating pregnant women with ANS were not available, and we cannot exclude that withholding treatment may have occurred in some cases, especially at the lower end of GAs. However, we were able to adjust for a wide range of perinatal characteristics as well as factors reflecting management.
In our cohort, 7.0% of women received ANS but had no data on timing of administration; comparison of their characteristics suggests that these missing data may represent emergencies with suboptimal documentation. Some women had incomplete data on timing; for these patients, we used multiple imputation, which yielded results similar to complete case analysis. Our study was descriptive with no attempts in the study framework to standardize treatment, and therefore, we were unable to investigate whether one corticosteroid (or one particular dosing regimen) has advantages over another.
Conclusions
In conclusion, our results suggest significant health-promoting effects of ANS beginning just hours before delivery. This new knowledge encourages a more proactive management of women at risk for imminent preterm birth, which may help reduce infant mortality and severe neonatal brain injury. Our study also highlights that a large proportion of women remain at risk for very preterm birth more than 7 days after ANS and that their infants have increased morbidity and mortality. We suggest that future research areas should include testing optimal dosing (1 vs 2 doses) and dosing intervals of ANS. Given the suggestion of very rapid action, immediate postnatal corticosteroid rescue for very preterm infants unexposed to ANS could also be a topic for future research.
eTable 1. Maternity unit protocols for the first course of antenatal corticosteroids.
eTable 2. Characteristics of included infants and those excluded because of missing information on timing of antenatal corticosteroids (ANS).
eTable 3. Simulation of in-hospital mortality if providing ANS hours before delivery to infants who did not receive ANS (n = 661).
eFigure 1. Cumulative distribution of administration-to-birth intervals of antenatal corticosteroids (ANS) for very preterm birth (delivered livebirths only).
eFigure 2. Administration of antenatal corticosteroids and administration-to-birth intervals (colored) by European region.
eFigure 3. Association between timing of antenatal corticosteroids (ANS) and a composite of in-hospital mortality or severe neonatal morbidity.
References
- 1.NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes Effect of corticosteroids for fetal maturation on perinatal outcomes. JAMA. 1995;273(5):413-418. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization WHO Recommendations on Interventions to Improve Preterm Birth Outcomes. Geneva, Switzerland: WHO Press; 2015. [PubMed] [Google Scholar]
- 3.National Institute for Health and Care Excellence National Institute for Health and Care Excellence Clinical Guidelines. London, England: Preterm Labour and Birth; 2015. [Google Scholar]
- 4.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;3(3):CD004454. [DOI] [PubMed] [Google Scholar]
- 5.Brownfoot FC, Gagliardi DI, Bain E, Middleton P, Crowther CA. Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2013;(8):CD006764. [DOI] [PubMed] [Google Scholar]
- 6.Melamed N, Shah J, Soraisham A, et al. . Association between antenatal corticosteroid administration-to-birth interval and outcomes of preterm neonates. Obstet Gynecol. 2015;125(6):1377-1384. [DOI] [PubMed] [Google Scholar]
- 7.Kuk JY, An JJ, Cha HH, et al. . Optimal time interval between a single course of antenatal corticosteroids and delivery for reduction of respiratory distress syndrome in preterm twins. Am J Obstet Gynecol. 2013;209(3):256.e1-256.e7. [DOI] [PubMed] [Google Scholar]
- 8.Wilms FF, Vis JY, Pattinaja DA, et al. . Relationship between the time interval from antenatal corticosteroid administration until preterm birth and the occurrence of respiratory morbidity. Am J Obstet Gynecol. 2011;205(1):49.e1-49.e7. [DOI] [PubMed] [Google Scholar]
- 9.Waters TP, Mercer B. Impact of timing of antenatal corticosteroid exposure on neonatal outcomes. J Matern Fetal Neonatal Med. 2009;22(4):311-314. [DOI] [PubMed] [Google Scholar]
- 10.Ring AM, Garland JS, Stafeil BR, Carr MH, Peckman GS, Pircon RA. The effect of a prolonged time interval between antenatal corticosteroid administration and delivery on outcomes in preterm neonates: a cohort study. Am J Obstet Gynecol. 2007;196(5):457.e1-457.e6. [DOI] [PubMed] [Google Scholar]
- 11.Peaceman AM, Bajaj K, Kumar P, Grobman WA. The interval between a single course of antenatal steroids and delivery and its association with neonatal outcomes. Am J Obstet Gynecol. 2005;193(3, pt 2):1165-1169. [DOI] [PubMed] [Google Scholar]
- 12.Sehdev HM, Abbasi S, Robertson P, et al. . The effects of the time interval from antenatal corticosteroid exposure to delivery on neonatal outcome of very low birth weight infants. Am J Obstet Gynecol. 2004;191(4):1409-1413. [DOI] [PubMed] [Google Scholar]
- 13.Vermillion ST, Soper DE, Newman RB. Is betamethasone effective longer than 7 days after treatment? Obstet Gynecol. 2001;97(4):491-493. [DOI] [PubMed] [Google Scholar]
- 14.Ballard PL, Granberg P, Ballard RA. Glucocorticoid levels in maternal and cord serum after prenatal betamethasone therapy to prevent respiratory distress syndrome. J Clin Invest. 1975;56(6):1548-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Subtil D, Tiberghien P, Devos P, et al. . Immediate and delayed effects of antenatal corticosteroids on fetal heart rate: a randomized trial that compares betamethasone acetate and phosphate, betamethasone phosphate, and dexamethasone. Am J Obstet Gynecol. 2003;188(2):524-531. [DOI] [PubMed] [Google Scholar]
- 16.Ikegami M, Polk D, Jobe A. Minimum interval from fetal betamethasone treatment to postnatal lung responses in preterm lambs. Am J Obstet Gynecol. 1996;174(5):1408-1413. [DOI] [PubMed] [Google Scholar]
- 17.Hafezi-Moghadam A, Simoncini T, Yang Z, et al. . Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat Med. 2002;8(5):473-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lösel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol. 2003;4(1):46-56. [DOI] [PubMed] [Google Scholar]
- 19.Jobe AH, Soll RF. Choice and dose of corticosteroid for antenatal treatments. Am J Obstet Gynecol. 2004;190(4):878-881. [DOI] [PubMed] [Google Scholar]
- 20.Whitworth MK. Recent guidance on antenatal corticosteroids in prematurity. BMJ. 2016;352:i1655. [DOI] [PubMed] [Google Scholar]
- 21.Zeitlin J, Manktelow BN, Piedvache A, et al. ; EPICE Research Group . Use of evidence based practices to improve survival without severe morbidity for very preterm infants: results from the EPICE population based cohort. BMJ. 2016;354:i2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlo WA, McDonald SA, Fanaroff AA, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22 to 25 weeks’ gestation. JAMA. 2011;306(21):2348-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? some practical clarifications of multiple imputation theory. Prev Sci. 2007;8(3):206-213. [DOI] [PubMed] [Google Scholar]
- 24.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529-534. [DOI] [PubMed] [Google Scholar]
- 25.de Vries LS, Eken P, Dubowitz LM. The spectrum of leukomalacia using cranial ultrasound. Behav Brain Res. 1992;49(1):1-6. [DOI] [PubMed] [Google Scholar]
- 26.International Committee for the Classification of Retinopathy of Prematurity The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123(7):991-999. [DOI] [PubMed] [Google Scholar]
- 27.Bell MJ, Ternberg JL, Feigin RD, et al. . Neonatal necrotizing enterocolitis: therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gortner L, Misselwitz B, Milligan D, et al. ; members of the MOSAIC Research Group . Rates of bronchopulmonary dysplasia in very preterm neonates in Europe: results from the MOSAIC cohort. Neonatology. 2011;99(2):112-117. [DOI] [PubMed] [Google Scholar]
- 29.Källén K, Serenius F, Westgren M, Maršál K; EXPRESS Group . Impact of obstetric factors on outcome of extremely preterm births in Sweden: prospective population-based observational study (EXPRESS). Acta Obstet Gynecol Scand. 2015;94(11):1203-1214. [DOI] [PubMed] [Google Scholar]
- 30.Fellman V, Hellström-Westas L, Norman M, et al. ; EXPRESS Group . One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA. 2009;301(21):2225-2233. [DOI] [PubMed] [Google Scholar]
- 31.Johansson S, Montgomery SM, Ekbom A, et al. . Preterm delivery, level of care, and infant death in Sweden: a population-based study. Pediatrics. 2004;113(5):1230-1235. [DOI] [PubMed] [Google Scholar]
- 32.Patterson RM. Corticosteroids for fetal maturation. JAMA. 1995;274(12):943. [PubMed] [Google Scholar]
- 33.Mikolajczyk RT, Zhang J, Betran AP, et al. . A global reference for fetal-weight and birthweight percentiles. Lancet. 2011;377(9780):1855-1861. [DOI] [PubMed] [Google Scholar]
- 34.Harrell FE., Jr Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer; 2001. [Google Scholar]
- 35.Zou GY, Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res. 2013;22(6):661-670. [DOI] [PubMed] [Google Scholar]
- 36.Razaz N, Skoll A, Fahey J, Allen VM, Joseph KS. Trends in optimal, suboptimal, and questionably appropriate receipt of antenatal corticosteroid prophylaxis. Obstet Gynecol. 2015;125(2):288-296. [DOI] [PubMed] [Google Scholar]
- 37.Chawla S, Natarajan G, Shankaran S, et al. ; National Institute of Child Health and Human Development Neonatal Research Network . Association of neurodevelopmental outcomes and neonatal morbidities of extremely premature infants with differential exposure to antenatal steroids. JAMA Pediatr. 2016;170(12):1164-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKinlay CJ, Crowther CA, Middleton P, Harding JE. Repeat antenatal glucocorticoids for women at risk of preterm birth: a Cochrane Systematic Review. Am J Obstet Gynecol. 2012;206(3):187-194. [DOI] [PubMed] [Google Scholar]
- 39.Crowther CA, Anderson PJ, McKinlay CJ, et al. ; ACTORDS Follow-up Group . Mid-childhood outcomes of repeat antenatal corticosteroids: a randomized controlled trial. Pediatrics. 2016;138(4):e20160947. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Maternity unit protocols for the first course of antenatal corticosteroids.
eTable 2. Characteristics of included infants and those excluded because of missing information on timing of antenatal corticosteroids (ANS).
eTable 3. Simulation of in-hospital mortality if providing ANS hours before delivery to infants who did not receive ANS (n = 661).
eFigure 1. Cumulative distribution of administration-to-birth intervals of antenatal corticosteroids (ANS) for very preterm birth (delivered livebirths only).
eFigure 2. Administration of antenatal corticosteroids and administration-to-birth intervals (colored) by European region.
eFigure 3. Association between timing of antenatal corticosteroids (ANS) and a composite of in-hospital mortality or severe neonatal morbidity.