Abstract
Importance
Patients with germline mutations in BAP1 may develop several flesh-colored melanocytic BAP1–mutated atypical intradermal tumors (MBAITs). These tumors generally develop earlier than other BAP1–associated tumors, highlighting an important role for dermatologists in identifying and screening patients with a history suggestive of a germline mutation.
Objective
To describe 8 new families with germline mutations in BAP1 and provide a comprehensive review of reported cases.
Design, Settings and Participants
Patients were identified in an outpatient dermatology clinical setting over a 6-month period (10 mutation carriers from 8 families) and through a literature review using PubMed (205 patients).
Exposures
Mutations were identified through next-generation sequencing of saliva or blood samples, and RNA was extracted from fibroblasts cultured from a patient with an intronic variant to determine the impact of the mutation on the coding sequence.
Main Outcomes and Measures
All 215 patients were assessed for personal and/or family history and genotype. These findings were compiled and assessed for any association between genotype and phenotype.
Results
Overall, this study included 215 patients (108 women, 91 men, and 16 gender unspecified; median [range] age, 46.5 [10.0-79.0] years). Nine of the 10 patients who were identified in the outpatient dermatology setting were found to have MBAITs on clinical examination. Forty of 53 patients (75%) identified in the literature review who underwent total-body skin examinations (TBSE) were found to have MBAITs, suggesting a high penetrance in patients who have undergone TBSE. The most prevalent malignancies among BAP1 mutation carriers were uveal melanoma (n = 60 [28%]), mesothelioma (n = 48 [22%]), cutaneous melanoma (n = 38 [18%]), and renal cell carcinoma (n = 20 [9%]). A total of 71 unique mutations in BAP1 have been reported.
Conclusions and Relevance
Our results indicate that germline mutations in both coding and noncoding regions throughout the BAP1 gene can impair protein function, leading to an increased risk for several associated malignancies. Four of the 8 probands we present had no history of BAP1-associated malignancies and were assessed for germline mutations when found to have MBAITs on dermatologic examination. Dermatologists can identify patients with a high likelihood of the BAP1 cancer syndrome through personal and family history and TBSE for the presence of possible MBAITs.
This study of 10 patients identified through clinical screening and 205 patients identified through literature review describes 8 new families with germline mutations in BAP1 and provides a comprehensive review of reported cases.
Key Points
Question
How are patients with germline mutations in BAP1 most likely to present, and how are dermatologists involved in their care?
Findings
Among the 10 patients presented in this series, as well as the additional 205 patients identified in a review of the literature, melanocytic BAP1–mutated atypical intradermal tumors (MBAITs) had a significantly earlier median age of onset (32 years) compared with other BAP1-associated tumors and were present in 40 of 53 patients (75%) with documented skin examinations.
Meaning
Dermatologists play a crucial role in identifying patients with the BAP1 syndrome by screening patients diagnosed with MBAITs.
Introduction
The significance of the BAP1 gene was originally established when inactivating BAP1 mutations were found in aggressive uveal melanoma tumor specimens. Subsequent genomic evaluation of families with familial uveal melanoma and mesothelioma led to the identification of a germline tumor predisposition syndrome associated with BAP1 inactivation. The syndrome associated with BAP1 deficiency was later expanded to include a risk for renal cell carcinoma and cutaneous melanoma and continues to be associated with new malignancies.
In addition to malignant tumors, some patients with BAP1 germline mutations were found to develop a range (5-50) of skin-colored, dome-shaped, well-circumscribed papules with characteristic histomorphology, referred to as melanocytic BAP1–mutated atypical intradermal tumors (MBAITs) when first presented in the literature. Dermatologists can play a central role in the diagnosis and management of this syndrome because MBAITs are estimated to have a high penetrance and generally appear earlier than other BAP1-associated tumors. In this study, we describe 8 families with germline BAP1 mutations and discuss the typical age of onset and estimated penetrance of the various associated tumors as well as potential genotypic-phenotypic correlations found in a comprehensive literature review.
Methods
DNA Extraction
Saliva samples were collected after informed consent was acquired, and next-generation sequencing was performed with the Ion PGM platform (ThermoFisher Scientific) as standard of care. For Families 1 and 2, the BAP1 gene was targeted with a customized primer pool designed with an AmpliSeq designer (ThermoFisher Scientific). The GRCh37/hg19 reference genome and transcript NM_004656.3 were used. Results were analyzed using IonTorrent Suite 4.4 (ThermoFisher Scientific). Family 3 had blood samples sent to Prevention Genetics while Families 4 through 6 had blood samples sent to Ambry Genetics. Families 7 and 8 had saliva samples sent to Ambry Genetics for diagnosis.
Institutional review board approval was obtained from both the Northwestern and Partners institutional review boards for this study.
Sample, Messenger RNA, and Complementary DNA Generation
A skin biopsy was performed on the proband from Family 4 after consenting to a human protocol approved by the Partners institutional review board. Dermal fibroblasts were isolated and cultured using methods from Rittié et al. RNA was extracted from the fibroblasts with the RNeasy mini kit (Qiagen). Complementary DNA (cDNA) was generated with the High-Capacity RNA-to-cDNA kit (Applied Biosystems) according to the manufacturer’s instructions.
Polymerase Chain Reaction
Two primers, 5′-TAG CGA ATT CGA GTT GGC ATG AGC AAA GGA TAT GCG ATT GG-3′ and 5′-CCG CGG ATC CGA TCA TCC TCC TCG TCA TCC TC-3′, were used to amplify BAP1 exons 6 to 12 from the cDNA. Each polymerase chain reaction (PCR) contained 1 μL of cDNA, 2 μL of 10X Ex taq buffer, 1.6 μL of 2.5 mM dNTP, 0.4 μL of 100 μM of the above 2 primers, 0.1 μL of Ex taq (TAKARA), and water to a final volume of 20 μL. Cycling conditions included denaturation at 94° C for 4 minutes, followed by 35 cycles at 94° C for 30 seconds, 60° C for 30 seconds, and 72° C for 90 seconds. Polymerase chain reaction products were gel extracted with the QIAEX II Gel Extraction kit (Qiagen), and 1 μL of extracted products were used for PCR and gel purified again to get sufficient DNA for cloning.
Cloning
The PCR products were restriction digested with EcoRI and BamH1, and were cloned into vector CD516B-2. Plasmids were transformed into bacteria by routine molecular methods. Individual bacterial colonies were isolated and DNA was extracted using the QIAprep Spin Miniprep kit (Qiagen) and sequenced at the MGH sequencing core facility.
Case Identification and Statistical Analysis
Cases were identified through a literature review using PubMed, and all reported patients who tested positive for BAP1 mutations, as well as those indicated as obligate carriers, were included for review. Age distributions were compared using the Kruskal-Wallis test, and P < .05 was considered statistically significant.
Results
Case Presentation
The proband of Family 1 is a teenage female who presented with a dome-shaped papule on her upper arm that demonstrated a central pink structureless area and pigment globules at the lateral edges of the lesion on dermoscopy (Figure 1). Although not specific, these features can be seen in MBAITs. The proband has a family history of mesothelioma in her father in his 50s, renal cancer in her paternal grandfather, cutaneous melanoma in her paternal grandmother, and ovarian cancer in her maternal aunt. The presenting patient and her sister, who was also found to have several pedunculated nevi suspected to be MBAITs on clinical examination, were found to have a c.1321C>T, p. Q441X mutation in the BAP1 gene.
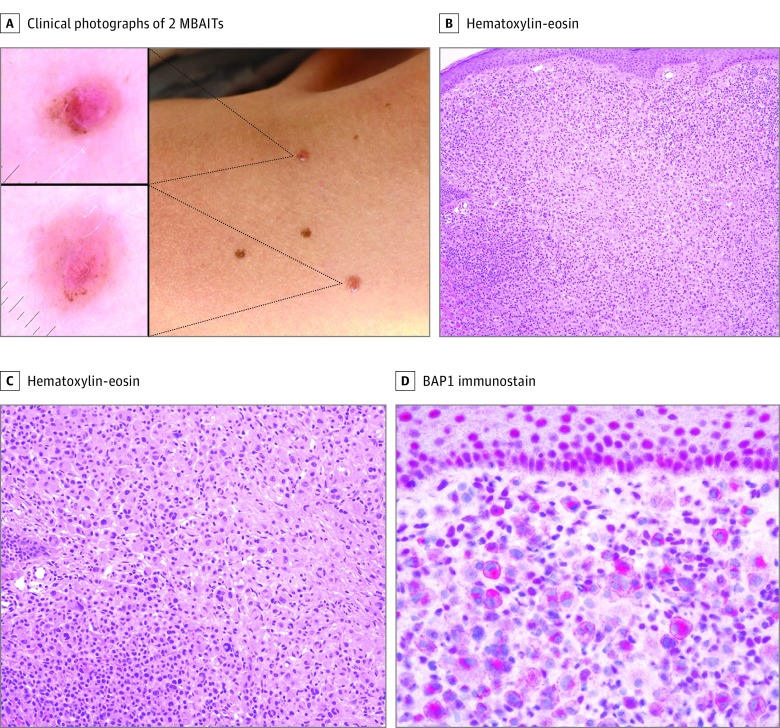
Figure 1. Clinical and Histologic Presentation of MBAIT.
A, Clinical photograph of 2 melanocytic BAP1–mutated atypical intradermal tumors (MBAITs) on the shoulder of a young female patient with a germline mutation in BAP1. Clinically, these lesions often appear as pink or flesh-colored pedunculated papules, as seen in this patient. Dermoscopy of the lesions shows pink papules with a central structureless area and peripheral pigmentation. B, Histology of an MBAIT. At low power (original magnification ×100), the sheetlike proliferation of epithelioid melanocytes can be seen. C, Medium power (original magnification ×200) shows epithelioid cells with spitzoid cytomorphology at the center of the lesion with nests of smaller conventional nevomelanocytes laterally. D, A BAP1 immunostain (original magnification ×400) shows loss of staining in the nuclei of the epithelioid spitzoid melanocytes. Keratinocytes in the epidermis show strong nuclear positivity for BAP1.
The proband of Family 2 is a female in her 30s who presented with a 3 × 2-mm pink papule with scattered foci of brown pigment on her right arm and found to be an MBAIT on histology. The patient’s paternal grandfather had mesothelioma, and her father had Parkinson disease and cutaneous melanoma. The patient was found to have a 1–base pair deletion in the 3′ untranslated region of the BAP1 gene (chr3, g.52435660delC).
The proband of Family 3 is an adolescent female diagnosed with multiple MBAITs starting at a young age. The patient was found to have a deletion in BAP1 (c.1717delC, p.L573fs*3) that has been previously published. None of the patient’s first-degree family members have any history of malignancies, and her mother and sister were found to be negative for mutations in BAP1.
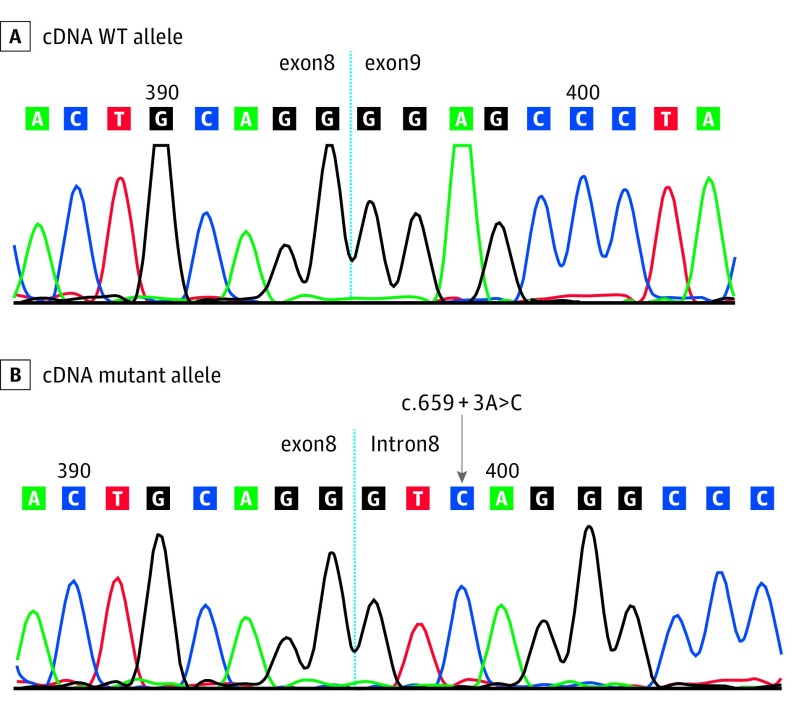
The proband of Family 4 is a female in her 50s with a history of ocular melanoma, temporal lobe meningioma, mammary ductal carcinoma in situ (DCIS), squamous cell carcinoma (SCC), and basal cell carcinoma (BCC) who was found to carry a c.659 + 3A>C intronic variant in BAP1. The patient did not demonstrate any lesions resembling MBAITs on clinical examination. She has a family history of mesothelioma, meningioma, melanoma, BCC, SCC, and other malignancies. The patient’s maternal first cousin once removed also carries the same intronic variant and has a history of 2 Spitz nevi, 1 of which was histologically confirmed to be an MBAIT. Although the mutation lies in a noncoding region, we surmised that the variant could have an impact on splicing. To prove this, we obtained skin fibroblasts from the patient and analyzed the BAP1 RNA transcript. As shown in Figure 2, the patient’s wild-type allele exhibits normal joining of exons 8 and 9 while the mutated transcript shows allele-specific retention of intron 8. This variant causes transcriptional read-through and introduces a putative novel termination codon approximately 120 codons downstream of the exon 8 junction.
Figure 2. Chromatogram of cDNA Isolated From Family 4.
The mutant allele shows retention of intron 8 due to aberrant splicing while the wild-type (WT) allele shows normal joining of exons 8 and 9. cDNA indicates complementary DNA.
The proband of Family 5 is a male in his 50s with history of meningioma in his 30s, lentigo maligna and BCC in his 40s, as well as renal cell cancer, prostate cancer, peritoneal mesothelioma, and 2 MBAITs in his 50s found to carry a deletion of exon 3 in the BAP1 gene. During initial consultation, he was found to have skin phototype II, some solar lentigines in photo-exposed areas and a moderate density of clinically atypical nevi. He has a family history of liver, bladder, and breast cancer, as well as glioma and several cancers of unknown type.
The proband of Family 6 is a female with a history of multiple MBAITs starting in her 20s who was found to have a c.1416delG, p.S473Vfs*9 mutation in BAP1. On dermatologic examination, the patient had a moderate density of benign appearing nevi as well as an 8-mm mutilobulated pink plaque histologically diagnosed as an MBAIT. The patient has a family history of 2 melanomas in her mother, a benign brain tumor in her maternal aunt, breast cancer in her maternal grandmother, and mesothelioma in her paternal grandfather. All other family members, including over 30 first-degree relatives and a brother in his 30s were reported to be unaffected, although their surveillance status was unknown to the patient.
The proband of Family 7 is a female in her 40s diagnosed with a T2b BAP1–associated nevoid melanoma of the right parietal scalp and several MBAITs. She demonstrated several flesh-colored pedunculated lesions with varying degrees of peripheral pigmentation thought to be additional MBAITs on clinical examination. She was found to carry a c.771_772insTACTA, p.A258Yfs*2 mutation in BAP1. She has a family history of uveal and cutaneous melanoma and breast cancer in her paternal grandmother, BCC, SCC, thyroid, prostate, and colon cancer in her father, glioblastoma mutliforme in her paternal uncle, and several MBAITs in her daughter.
The proband of Family 8 is a male with a c.79dupG, p.V27fs BAP1 mutation who was diagnosed with grade II peritoneal mesothelioma in his 30s. The patient’s 2 brothers, who are also in their 30s, are unaffected. He has a family history of leukemia, breast, and brain cancer, as well other unknown malignancies on his maternal side. The patient was found to have a nevus of the iris on opthamlologic examination, as well as several uniform hyperpigmented papules, some of which were exophytic, on dermatologic examination.
Penetrance
The prevalence and median age of onset of each associated neoplasm in our series and in the literature are shown in the Table. There was a significant difference in age of onset between different types of BAP1-associated tumors (P < .001) with MBAITs demonstrating the earliest age of onset (Figure 3). Of the 10 patients that we present with BAP1 mutations confirmed with genetic testing, 90% (9 of 10) were found to have suspected MBAITs on clinical examination. The median (range) age of presentation of these lesions in our series was 31 (10-56) years. In their review, Rai et al reported that only 43 of 174 individuals reported with the BAP1 syndrome were assessed via TBSE, with 31 (72%) of these patients presenting with MBAITs. With the addition of the patients in our series with both clinically suspected and confirmed lesions, 75% (40 of 53) of patients with the BAP1 syndrome assessed via TBSE were found to have MBAITs.
Table. Median Age of Onset and Prevalence of Characteristic Tumors in 215 Patients With BAP1 Syndrome.
| Tumor | Cases, No. | Estimated Penetrance, %a | Median Age of Diagnosis in the Literature, y | Median Age of Diagnosis in Our Series, y | Median Age of Diagnosis in General Population, y |
|---|---|---|---|---|---|
| Uveal melanoma | 60 | 28.0 | 53 | 59 | 61 |
| Mesothelioma | 48 | 22.0 | 56 | 46 | 74 |
| Cutaneous melanoma | 38 | 18.0 | 41 | 43 | 61 |
| MBAITs | 36 | 17.0 | 32 | 31 | 24 |
| Renal cell carcinoma | 20 | 9.0 | 47 | 51 | 64 |
| Basal cell carcinoma | 14 | 6.5 | 52 | 41 | 75 |
Abbreviation: MBAITs, melanocytic BAP1–associated intradermal tumors.
The estimated penetrance is based on the prevalence of each tumor reported in BAP1 patients identified in the literature and in our series.
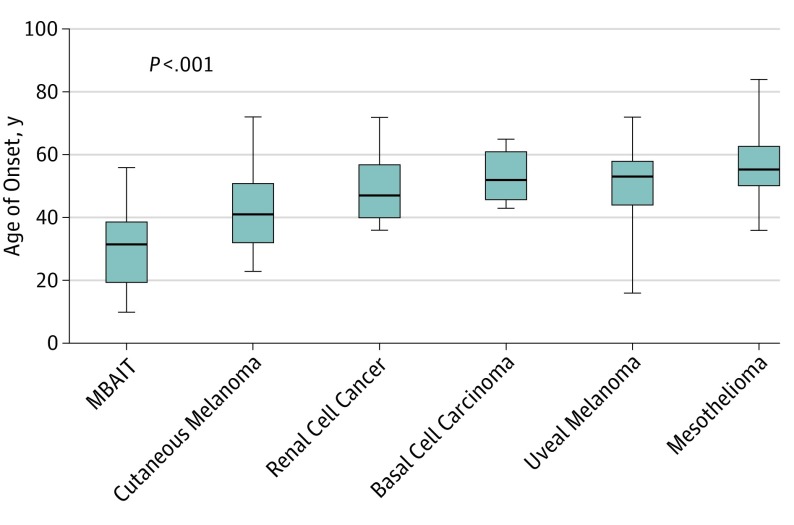
Figure 3. Median Ages of Onset of Tumors Associated With the BAP1 Syndrome.
The ages of onset were found to be significantly different from one another by the Kruskal-Wallis test. As shown in the graph, melanocytic BAP1–mutated atypical intradermal tumors (MBAITs) have the earliest age of onset in patients with the BAP1 syndrome, followed by cutaneous melanoma.
In a review of the relevant literature, a total of 215 patients from 87 families have been reported with the addition of our 8 families. Sixty of these patients developed uveal melanoma (28%), 48 patients developed mesothelioma (22%), 38 developed cutaneous melanoma (18%), and 20 developed renal cell carcinoma (9%). Other reported malignancies seen in patients found to have germline mutations in BAP1 included basal cell carcinoma, meningioma, breast cancer, lung adenocarcinoma, pancreatic cancer, and thyroid cancer.
Genotypic-Phenotypic Associations
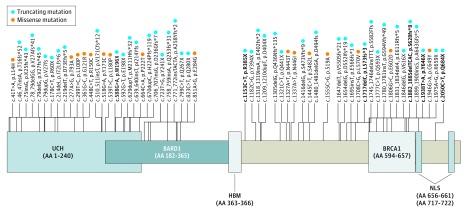
With the addition of our families, a total of 71 unique mutations in BAP1 were identified (Figure 4). Six mutations were found to occur in multiple families (eTable 1 in the Supplement). A majority of these variants (5 of 6) have not been described among the general population while 1 variant (c.2050C>T, p.Q684X) was identified in 0.0008% of the general population. A majority of the mutations reported (46 of 71) were predicted to result in protein truncation owing to frameshift or nonsense mutations. Ten mutations were reported to affect splicing, and 15 mutations were missense point mutations.
Figure 4. Diagram of the BAP1 Protein With Reported Mutations.
Reported mutations that affect coding regions of the BAP1 gene are shown with an approximation of their relative location of impact on the resulting protein. Mutations reported in multiple families are bolded. BARD1 indicates BARD1 binding domain; BRCA1, BRCA1 binding domain; HBM, HCF binding motif; NLS, nuclear localization signal; UCH, ubiquitin carboxy-terminal hydrolase.
Of all patients included, 37% (79 of 215) were found to have mutations expected to impact the first 240 amino acids of the protein, which represent the catalytic UCH (ubiquitin carboxy-terminal hydrolase) domain. Fifty percent of all patients diagnosed with melanoma (19 of 38) and 75% of patients diagnosed with more than 1 melanoma (6 of 8) were found to have mutations that affected this domain (eTable 2 in the Supplement). Three patients from 2 different families were found to have multiple basal cell carcinomas, and all carried mutations affecting the UCH domain.
Forty-eight patients were diagnosed with mesothelioma (eTable 3 in the Supplement). Because the median age of onset of mesothelioma is 56 years in patients with the BAP1 syndrome, we compared the 48 patients with mesothelioma to patients with the BAP1 syndrome 56 years or older who did not develop mesothelioma (n = 29). Eight of these 29 patients (28%) had missense mutations, 19 (66%) had mutations expected to be truncating, and 2 (7%) had mutations that affected splicing with unknown effect on protein structure (eTable 4 in the Supplement). Of the 48 patients diagnosed with mesothelioma, 46 (96%) carried truncating mutations. The 2 patients with nontruncating missense mutations were diagnosed with mesothelioma at ages much older than the median (71 and 72 years).
Discussion
The histopathology of melanocytic BAP1–mutated atypical intradermal tumors is highly characteristic and shows a central population of epithelioid melanocytes, often with spitzoid cytomorphology, in the dermis flanked by more conventional nevomelanocytes laterally. Commonly, MBAITs are pink or flesh-colored dome-shaped papules with a structureless central area and some lateral focal pigment globules on dermoscopy; MBAITs arise from conventional nevi, most commonly with initiating oncogenic mutations in BRAF, which are represented by the lateral pigment globules that may be seen on dermoscopy. Biallelic inactivation of BAP1 leads to the proliferation of a second subclone of larger epithelioid melanocytes which typically lack pigment and may result in a central pink or flesh-colored structureless area that can be seen on both dermoscopy and clinical examination. Polymorphous vessels may be seen in this central structureless area.
Immunohistochemistry can be a very helpful adjunctive study for the assessment of MBAITs. Because BAP1 is a nuclear protein, cells with 2 wild-type copies of the gene should demonstrate strong nuclear positivity. Cells with monoallelic inactivation, whether somatic or germline, will show nuclear staining but may additionally have cytoplasmic staining if the non–wild-type allele involves a mutation, such as a truncating mutation, that affects the nuclear localization sequence (NLS) at the C terminus of the protein. Cells with biallelic inactivation of BAP1 will demonstrate complete loss of nuclear staining with or without some cytoplasmic staining, again depending on whether mutations in either of the 2 alleles impact the NLS.
In their review, Rai et al found that 11 of the 31 patients (35%) who underwent full TBSEs had multiple MBAITs on clinical examination. Additionally, a majority of patients with the BAP1 syndrome reported to undergo TBSEs were found to have MBAITs (40 of 53 [75%]). Hence, we suspect that the penetrance for MBAITs is likely higher than that reported in the literature and in fact may be the most penetrant of the various related tumors in these patients. This apparent penetrance, however, is limited by clinically directed screening of patients with the BAP1 syndrome. The true prevalence could only be determined with certainty through prospective research driven assessment.
In addition to the relatively high estimated penetrance of MBAITs, the other significant feature of these lesions is the relatively young age of onset compared with other associated tumors (Figure 3). Wiesner et al noted that BAP1-deficient melanocytic tumors in patients with germline mutations in BAP1 often begin to develop during the second decade of life and progressively increase in number as the patient ages. The median age of onset of MBAITs in patients with germline BAP1 mutations is 32. However, these tumors may have been present earlier in some patients and not found until they were diagnosed with a BAP1-associated malignancy and underwent TBSEs later in life. Additionally, 4 of our 8 probands presented only with MBAITs in their 30s or earlier, which then led to the diagnosis of a germline syndrome. The diagnosis of an MBAIT is not possible on clinical examination alone without histologic confirmation, yet the presence of multiple lesions with characteristic features, as well as a positive pertinent clinical or family history, may be an indication for a dermatologist to discuss genetic testing. Alternatively, a dermatologist familiar with the syndrome may be alerted to the possibility of a germline mutation based on the histologic features of a biopsied lesion, even if the lesion was not suspected to be an MBAIT on clinical examination. Dermatologists therefore play an important role in recognizing patients who may carry germline mutations in BAP1 and referring them to undergo genetic testing as well as screening for the other associated tumors.
Although no clear associations between genotype and phenotype have been identified in the BAP1 syndrome, we found that almost all patients who develop mesothelioma carry germline truncating mutations of the BAP1 gene. All truncating mutations that have been identified in patients with the BAP1 syndrome occur before the nuclear localization sequence, which can result in cytoplasmic retention of the BAP1 protein. Aberrant BAP1 protein has been shown to form amyloid aggregates that can accumulate in the cytoplasm of cells. We hypothesize that these aggregates may contribute to the development of mesothelioma by contributing to chronic inflammation and cytotoxic effects. The chronic inflammation induced by asbestos fibers in asbestos-related sporadic mesothelioma has been shown to play a central role in carcinogenesis and to lead the generation of reactive oxygen and nitrogen species that damage DNA. We postulate that a similar mechanism may be responsible, at least in part, for carcinogenesis in cells with cytoplasmic aggregates of BAP1 protein. Accordingly, a majority of BAP1 patients who were found to develop mesothelioma were not exposed to asbestos. Also, interestingly, overall survival for BAP1-associated mesothelioma is better than for non–BAP1-associated mesothelioma, while patients with BAP1-associated uveal melanoma and renal cell carcinoma have a worse prognosis than non–BAP1-associated cases.
Additionally, patients who develop multiple cutaneous melanomas or BCCs have been found to be more likely to carry mutations that occur in the UCH catalytic domain of the protein, the area responsible for the deubiquinating function of BAP1. Mutations in the UCH domain have been shown to lead to an inability to recruit BAP1 to sites of DNA damage, where it plays a role in repair through homologous recombination. Both the nuclear localization sequence at the C terminus of the protein and the deubiquinating catalytic domain are necessary for BAP1 to act as a tumor suppressor. This observation, however, is of unknown significance and based on a limited sample size. Also, importantly, our findings in Family 4 in this study demonstrate that mutations in noncoding regions of the gene can also result in the BAP1 cancer syndrome. Hence, intronic variants, which may not be reported by all commercial assays, cannot be overlooked.
Limitations
This study was based on a relatively small sample size (n = 215) and includes retrospective data about personal and family history of malignancies. A majority of the data was collected from the text and tables of published manuscripts, as well as from published supplemental material. Patients identified may have developed subsequent tumors after the studies included for review were published. Some studies did not consistently report age at presentation and/or malignancy diagnosis and did not indicate whether skin examinations were performed to assess for the presence of MBAITs. Some patients were unable to recall which specific malignancies they had a family history of and there may have been a slight recall bias related to tumors associated with the BAP1 syndrome. While the findings identified in this analysis are suggestive, prospective studies of the growing cohort of patients identified with the BAP1 syndrome are needed to further assess penetrance and genotypic-phenotypic correlation.
Conclusions
The BAP1 syndrome demonstrates autosomal dominant inheritance, so all at-risk family members should be tested. We suggest that affected patients undergo TBSEs every 6 months and are referred to ophthalmology for uveal melanoma screening. All patients with the BAP1 syndrome identified by dermatologists should be referred to genetics and may be followed by nephrology or pulmonology at specialized centers with knowledge of the BAP1 syndrome. Screening recommendations such as those put forth by Pilarski et al and those recommended by a consensus panel of mesothelioma experts in 2015 are of value for physicians treating patients with the BAP1 syndrome, yet prospective studies of these families are necessary to generate evidence-based screening protocols.
eTable 1. BAP1 mutations seen in multiple families and associated phenotype in affected family members.
eTable 2. Patients with multiple melanomas. As seen in the table, most patients with multiple melanomas had truncating or missense mutations in the UCH domain of the BAP1 protein.
eTable 3. Patients with mesothelioma and BAP1 mutations (ages provided where available).
eTable 4. Patients with germline BAP1 mutations at or above the age of 56 without a diagnosis of mesothelioma.
References
- 1.Testa JR, Cheung M, Pei J, et al. . Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43(10):1022-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiesner T, Obenauf AC, Murali R, et al. . Germline mutations in BAP1 predispose to melanocytic tumors. Nat Genet. 2011;43(10):1018-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdel-Rahman MH, Pilarski R, Cebulla CM, et al. . Germline BAP1 mutation predisposes to uveal melanoma, lung adenocarcinoma, meningioma, and other cancers. J Med Genet. 2011;48(12):856-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Popova T, Hebert L, Jacquemin V, et al. . Germline BAP1 mutations predispose to renal cell carcinomas. Am J Hum Genet. 2013;92(6):974-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farley MN, Schmidt LS, Mester JL, et al. . A novel germline mutation in BAP1 predisposes to familial clear-cell renal cell carcinoma. Mol Cancer Res. 2013;11(9):1061-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung M, Talarchek J, Schindeler K, et al. . Further evidence for germline BAP1 mutations predisposing to melanoma and malignant mesothelioma. Cancer Genet. 2013;206(5):206-210. [DOI] [PubMed] [Google Scholar]
- 7.Wadt KA, Aoude LG, Johansson P, et al. . A recurrent germline BAP1 mutation and extension of the BAP1 tumor predisposition spectrum to include basal cell carcinoma. Clin Genet. 2015;88(3):267-272. [DOI] [PubMed] [Google Scholar]
- 8.de la Fouchardière A, Cabaret O, Pètre J, et al. . Primary leptomeningeal melanoma is part of the BAP1-related cancer syndrome. Acta Neuropathol. 2015;129(6):921-923. [DOI] [PubMed] [Google Scholar]
- 9.McDonnell KJ, Gallanis GT, Heller KA, et al. . A novel BAP1 mutation is associated with melanocytic neoplasms and thyroid cancer. Cancer Genet. 2016;209(3):75-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carbone M, Ferris LK, Baumann F, et al. . BAP1 cancer syndrome: malignant mesothelioma, uveal and cutaneous melanoma, and MBAITs. J Transl Med. 2012;10:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rittié L, Fisher GJ. Isolation and culture of skin fibroblasts. Methods Mol Med. 2005;117:83-98. [DOI] [PubMed] [Google Scholar]
- 12.Carbone M, Flores EG, Emi M, et al. . Combined genetic and genealogic studies uncover a large BAP1 cancer syndrome kindred tracing back nine generations to a common ancestor from the 1700s. PLoS Genet. 2015;11(12):e1005633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohar JA, Cheung M, Talarchek J, et al. . Germline BAP1 mutational landscape of asbestos-exposed malignant mesothelioma patients with family history of cancer. Cancer Res. 2016;76(2):206-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cebulla CM, Binkley EM, Pilarski R, et al. . Analysis of BAP1 germline gene mutation in young uveal melanoma patients. Ophthalmic Genet. 2015;36(2):126-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howlader N, Noone AM, Krapcho M, et al. National Cancer Institute. Bethesda, MD. SEER Cancer Statistics Review, 1975-2009 (Vintage 2009. Populations). https://seer.cancer.gov/archive/csr/1975_2009_pops09/. Accessed June 19, 2017.
- 16.Ludgate MW, Fullen DR, Lee J, et al. . The atypical Spitz tumor of uncertain biologic potential: a series of 67 patients from a single institution. Cancer. 2009;115(3):631-641. [DOI] [PubMed] [Google Scholar]
- 17.Verkouteren JA, Smedinga H, Steyerberg EW, Hofman A, Nijsten T. Predicting the risk of a second basal cell carcinoma. J Invest Dermatol. 2015;135(11):2649-2656. [DOI] [PubMed] [Google Scholar]
- 18.Njauw CN, Kim I, Piris A, et al. . Germline BAP1 inactivation is preferentially associated with metastatic ocular melanoma and cutaneous-ocular melanoma families. PLoS One. 2012;7(4):e35295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiesner T, Fried I, Ulz P, et al. . Toward an improved definition of the tumor spectrum associated with BAP1 germline mutations. J Clin Oncol. 2012;30(32):e337-e340. [DOI] [PubMed] [Google Scholar]
- 20.Rai K, Pilarski R, Cebulla CM, Abdel-Rahman MH. Comprehensive review of BAP1 tumor predisposition syndrome with report of two new cases. Clin Genet. 2016;89(3):285-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aoude LG, Gartside M, Johansson P, et al. . Prevalence of germline BAP1, CDKN2A, and CDK4 mutations in an Australian population-based sample of cutaneous melanoma cases. Twin Res Hum Genet. 2015;18(2):126-133. [DOI] [PubMed] [Google Scholar]
- 22.Gerami P, Yélamos O, Lee CY, et al. . Multiple cutaneous melanomas and clinically atypical moles in a patient with a novel germline BAP1 mutation. JAMA Dermatol. 2015;151(11):1235-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klebe S, Driml J, Nasu M, et al. . BAP1 hereditary cancer predisposition syndrome: a case report and review of literature. Biomark Res. 2015;3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330(600):1410-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheung M, Kadariya Y, Talarchek J, et al. . Germline BAP1 mutation in a family with high incidence of multiple primary cancers and a potential gene-environment interaction. Cancer Lett. 2015;369(2):261-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wadt K, Choi J, Chung JY, et al. . A cryptic BAP1 splice mutation in a family with uveal and cutaneous melanoma, and paraganglioma. Pigment Cell Melanoma Res. 2012;25(6):815-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Höiom V, Edsgärd D, Helgadottir H, et al. . Hereditary uveal melanoma: a report of a germline mutation in BAP1. Genes Chromosomes Cancer. 2013;52(4):378-384. [DOI] [PubMed] [Google Scholar]
- 28.Busam KJ, Wanna M, Wiesner T. Multiple epithelioid Spitz nevi or tumors with loss of BAP1 expression: a clue to a hereditary tumor syndrome. JAMA Dermatol. 2013;149(3):335-339. [DOI] [PubMed] [Google Scholar]
- 29.Ribeiro C, Campelos S, Moura CS, Machado JC, Justino A, Parente B. Well-differentiated papillary mesothelioma: clustering in a Portuguese family with a germline BAP1 mutation. Ann Oncol. 2013;24(8):2147-2150. [DOI] [PubMed] [Google Scholar]
- 30.Aoude LG, Wadt K, Bojesen A, et al. . A BAP1 mutation in a Danish family predisposes to uveal melanoma and other cancers. PLoS One. 2013;8(8):e72144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gossage L, Murtaza M, Slatter AF, et al. . Clinical and pathological impact of VHL, PBRM1, BAP1, SETD2, KDM6A, and JARID1c in clear cell renal cell carcinoma. Genes Chromosomes Cancer. 2014;53(1):38-51. [DOI] [PubMed] [Google Scholar]
- 32.Maerker DA, Zeschnigk M, Nelles J, et al. . BAP1 germline mutation in two first grade family members with uveal melanoma. Br J Ophthalmol. 2014;98(2):224-227. [DOI] [PubMed] [Google Scholar]
- 33.Pilarski R, Cebulla CM, Massengill JB, et al. . Expanding the clinical phenotype of hereditary BAP1 cancer predisposition syndrome, reporting three new cases. Genes Chromosomes Cancer. 2014;53(2):177-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Betti M, Casalone E, Ferrante D, et al. . Inference on germline BAP1 mutations and asbestos exposure from the analysis of familial and sporadic mesothelioma in a high-risk area. Genes Chromosomes Cancer. 2015;54(1):51-62. [DOI] [PubMed] [Google Scholar]
- 35.Betti M, Aspesi A, Biasi A, et al. . CDKN2A and BAP1 germline mutations predispose to melanoma and mesothelioma. Cancer Lett. 2016;378(2):120-130. [DOI] [PubMed] [Google Scholar]
- 36.Gupta MP, Lane AM, DeAngelis MM, et al. . Clinical characteristics of uveal melanoma in patients with germline BAP1 mutations. JAMA Ophthalmol. 2015;133(8):881-887. [DOI] [PubMed] [Google Scholar]
- 37.Turunen JA, Markkinen S, Wilska R, et al. . BAP1 germline mutations in Finnish patients with uveal melanoma. Ophthalmology. 2016;123(5):1112-1117. [DOI] [PubMed] [Google Scholar]
- 38.Aoude LG, Vajdic CM, Kricker A, Armstrong B, Hayward NK. Prevalence of germline BAP1 mutation in a population-based sample of uveal melanoma cases. Pigment Cell Melanoma Res. 2013;26(2):278-279. [DOI] [PubMed] [Google Scholar]
- 39.Guénard F, Durocher F. BAP1 (BRCA1 associated protein-1 [ubiquitin carboxy-terminal hydrolase]). Atlas Genet Cytogenet Oncol Haematol. 2010;14(7):670-672. [Google Scholar]
- 40.Lek M, Karczewski KJ, Minikel EV, et al. ; Exome Aggregation Consortium . Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536(7616):285-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhattacharya S, Hanpude P, Maiti TK. Cancer associated missense mutations in BAP1 catalytic domain induce amyloidogenic aggregation: a new insight in enzymatic inactivation. Sci Rep. 2015;5:18462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bononi A, Napolitano A, Pass HI, Yang H, Carbone M. Latest developments in our understanding of the pathogenesis of mesothelioma and the design of targeted therapies. Expert Rev Respir Med. 2015;9(5):633-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choe N, Tanaka S, Kagan E. Asbestos fibers and interleukin-1 upregulate the formation of reactive nitrogen species in rat pleural mesothelial cells. Am J Respir Cell Mol Biol. 1998;19(2):226-236. [DOI] [PubMed] [Google Scholar]
- 44.Huang SX, Jaurand MC, Kamp DW, Whysner J, Hei TK. Role of mutagenicity in asbestos fiber-induced carcinogenicity and other diseases. J Toxicol Environ Health B Crit Rev. 2011;14(1-4):179-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baumann F, Flores E, Napolitano A, et al. . Mesothelioma patients with germline BAP1 mutations have 7-fold improved long-term survival. Carcinogenesis. 2015;36(1):76-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eletr ZM, Yin L, Wilkinson KD. BAP1 is phosphorylated at serine 592 in S-phase following DNA damage. FEBS Lett. 2013;587(24):3906-3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scheuermann JC, de Ayala Alonso AG, Oktaba K, et al. . Histone H2A deubiquitinase activity of the Polycomb repressive complex PR-DUB. Nature. 2010;465(7295):243-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu H, Pak H, Hammond-Martel I, et al. . Tumor suppressor and deubiquitinase BAP1 promotes DNA double-strand break repair. Proc Natl Acad Sci U S A. 2014;111(1):285-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pilarski R, Rai K, Cebulla C, Abdel-Rahman M. BAP1 Tumor Predisposition Syndrome In: Pagon RA, Adam MP, Ardinger HH, et al. , eds. GeneReviews. [Internet] Seattle, WA: University of Washington, Seattle; 2016. Oct 13:1993-2017. http://www.ncbi.nlm.nih.gov/books/NBK390611/. Accessed June 19, 2017. [PubMed] [Google Scholar]
- 50.Carbone M, Kanodia S, Chao A, et al. . Consensus Report of the 2015 Weinman International Conference on Mesothelioma. J Thorac Oncol. 2016;11(8):1246-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. BAP1 mutations seen in multiple families and associated phenotype in affected family members.
eTable 2. Patients with multiple melanomas. As seen in the table, most patients with multiple melanomas had truncating or missense mutations in the UCH domain of the BAP1 protein.
eTable 3. Patients with mesothelioma and BAP1 mutations (ages provided where available).
eTable 4. Patients with germline BAP1 mutations at or above the age of 56 without a diagnosis of mesothelioma.