Abstract
Importance
Moderate/severe and even mild paravalvular regurgitation (PVR) are associated with increased mortality following transcatheter aortic valve replacement (TAVR) with first and second generations of transcatheter valves.
Objective
To examine the incidence, evolution, and effect on 1-year outcomes of PVR following TAVR with a third-generation balloon-expandable transcatheter heart valve.
Design, Setting, and Participants
Prespecified analysis of PVR in the Placement of Aortic Transcatheter Valves (PARTNER) II SAPIEN 3 trial, conducted between October 1, 2013, and September 3, 2014. Multicenter, nonrandomized registry of 1661 patients at intermediate or high surgical risk undergoing TAVR with the SAPIEN 3. Patients with severe, symptomatic aortic stenosis and high/intermediate surgical risk were enrolled in the registry at 51 sites in the United States and Canada.
Interventions
Transcatheter aortic valve replacement with the SAPIEN 3 valve.
Main Outcomes and Measures
Paravalvular regurgitation was assessed in a core laboratory at 30 days and 1 year according to a 5-class scheme: 0, none or trace; 1, mild; 2, mild to moderate; 3, moderate; 4, moderate to severe; and 5, severe. We assessed the effect of PVR on 1-year mortality and heart failure rehospitalization.
Results
Among the 1661 included in the registry, 1592 received a SAPIEN 3 valve and had assessment of PVR. Of these patients, 55.7% had none-trace PVR, 32.6% had mild, 8.2% had mild to moderate, and 3.5% had at least moderate PVR at 30 days. At 1 year, 9.3% of patients had died and 14.2% had been rehospitalized. Only patients with at least moderate PVR had higher 1-year mortality (hazard ratio [HR], 2.40; 95% CI, 1.30-4.43; P = .005) and composite of mortality/rehospitalization (HR, 2.35; 95% CI, 1.52-3.62; P < .001). In a paired comparison including 1213 patients, 73% of the patients with at least moderate PVR at 30 days showed a reduction in PVR severity of at least 1 PVR class at 1 year.
Conclusions and Relevance
In this series of patients undergoing TAVR with the SAPIEN 3 valve, at least moderate PVR was rare but associated with increased risk of death and heart failure rehospitalization at 1 year. Even the upper range of the mild class in the 3-class grading scheme (ie, mild to moderate in the 5-class scheme) had no significant effect on short-term mortality or rehospitalization. Most patients with at least moderate PVR at 30 days showed a decrease of PVR severity grade at 1 year.
Trial Registration
clinicaltrials.gov Identifier: NCT01314313.
Key Points
Question
What is the incidence, evolution, and effect on 1-year outcomes of paravalvular regurgitation assessed using a 5-class grading scheme following transcatheter aortic valve replacement with the new generation of balloon-expandable SAPIEN 3 valve?
Findings
In this secondary analysis of a randomized clinical trial, moderate paravalvular regurgitation (PVR) was observed in 3.5% of patients at 30 days and was associated with a 2.4- to 2.7-fold increase in the risk of mortality and rehospitalization at 1 year. Seventy-three percent of the patients with at least moderate PVR at 30 days showed a reduction in PVR severity at 1 year, and mild to moderate PVR (8.2% of patients) was associated with increased risk of aortic valve reintervention but had no significant effect on 1-year mortality or rehospitalization.
Meaning
In this series of patients with severe AS undergoing transcatheter aortic valve replacement with the SAPIEN 3, at least moderate PVR was rare but associated with increased risk of death and heart failure rehospitalization at 1 year; most patients with at least moderate PVR at 30 days showed a reduction in PVR grade at 1 year.
This analysis of the PARTNER II trial examines the incidence, evolution, and effect on 1-year outcomes of paravalvular regurgitation following transcatheter aortic valve replacement with a third-generation balloon-expandable transcatheter heart valve.
Introduction
Transcatheter aortic valve replacement (TAVR) is now well established in the treatment of high-risk and inoperable patients with severe aortic stenosis (AS). Studies have shown that TAVR is equivalent to surgical aortic valve replacement in patients at intermediate surgical risk. However, paravalvular regurgitation (PVR) is an important complication of TAVR that has been shown to be associated with increased mortality for both the balloon-expandable and the self-expanding transcatheter heart valves (THV). Moderate or severe PVR occurs in 5% to 25% of patients undergoing TAVR and is associated with a 2- to 3-fold increase in mortality. The studies on the effect of mild PVR on outcomes have yielded conflicting results. New generations of THV, such as the SAPIEN 3 (Edwards Lifesciences), have been developed to reduce PVR.
Standardized and accurate measurement of PVR becomes an important means for establishing prognosis after TAVR and for determining the effectiveness of various THV iterations or designs. Several studies have used the 3-class grading scheme (mild, moderate, and severe) proposed in the most recent guidelines to report the severity of PVR. To better understand the possible contribution to differences in grading attributable to the broad categories of mild, moderate, and severe and to align the regurgitation severity classifications with the commonly used clinical nomenclature that often uses more granular categories, we proposed using an expanded, 5-class grading scheme (eTable in Supplement 1).
The objectives of this study were to (1) examine the effect of PVR graded using this 5-class scheme on 1-year mortality, rehospitalization for heart failure, and aortic valve reintervention and (2) assess the change in PVR severity between 30 days and 1 year in patients undergoing TAVR with the new-generation SAPIEN 3 THV.
Methods
Study Design and Patient Population
The Placement of Aortic Transcatheter Valves (PARTNER) II SAPIEN 3 trial was a prospective, multicenter registry that enrolled patients with symptomatic severe AS. The Edwards SAPIEN 3 balloon-expandable THV consists of bovine pericardial leaflets sutured to a cobalt chromium frame. The lower portion of the frame is covered with a polyethylene terephthalate skirt to reduce PVR. The design, inclusion and exclusion criteria, and primary results of this trial have been reported. The trial protocol is available in Supplement 2.The trial was approved by the institutional review boards of each participating site, and written informed consent was provided by all patients.
In total, 1661 patients were enrolled in the SAPIEN 3 registry at 51 hospitals in the United States, of whom 1651 received a SAPIEN 3 valve implantation (eFigure 1 in Supplement 1). Among these 1651 patients, 1592 had a transthoracic echocardiogram (TTE) available at 30-day follow-up or discharge and were included in this analysis (eFigure 1 in Supplement 1); 558 (35.1%) were considered inoperable or at high risk for surgical aortic valve replacement, whereas 1034 (64.9%) were considered at intermediate risk. Procedures were performed via transfemoral (n = 1384; 86.9%), transapical (n = 131; 8.2%), or transaortic (n = 77; 4.8%) access depending on preprocedural vascular assessment. Patients were required to have an aortic annular area between 273 mm2 and 680 mm2, appropriate for treatment with a 20-mm, 23-mm, 26-mm, or 29-mm SAPIEN 3 THV (eMethods in Supplement 1).
Doppler-Echocardiographic Assessment of PVR
All baseline and follow-up TTEs were interpreted by a consortium of 3 echocardiography core laboratories based at Québec Heart and Lung Institute, Columbia University Medical Center, and MedStar Health Research Institute. The PVR was assessed at 30 days and 1 year after TAVR using a multiparameter integrative approach as previously described (eMethods and eTable in Supplement 1). Patients with missing 30-day TTE data were imputed using discharge echocardiographic results. Patients were assigned to a given core laboratory so that all echocardiograms from a given patient were assessed by the same laboratory.
The PVR was graded according to a 5-class scheme as follows (eTable in Supplement 1): 0, none or trace; 1, mild; 2, mild to moderate; 3, moderate; 4, moderate to severe; and 5, severe. These 5 classes of grading can easily be collapsed into the 3-class scheme recommended by the American Society of Echocardiography guidelines (eTable 1 in Supplement 1).
Prior to the start of core laboratory analyses in the SAPIEN 3 study, the 3 core laboratories read together a sample of 160 TTE examinations selected from the PARTNER II trial to standardize the methods for grading PVR among the 3 laboratories. During the course of the study, the intercore laboratory agreement in the grading of PVR severity was assessed in a subset of 64 echocardiographic examinations that were randomly selected and analyzed by the 3 laboratories.
Study Outcomes
The outcomes for this study were 1-year all-cause mortality, cardiovascular mortality, rehospitalization for heart failure, composite of death or rehospitalization, and need for valve reintervention after TAVR procedure. All events were adjudicated. We also assessed the change in the prevalence and severity of PVR between 30 days and 1 year in the subset of 1213 patients who had a TTE available at both 30 days and 1 year (eFigure 1 in Supplement 1). Patients with aortic valve reintervention between 30 days and 1 year were excluded from this analysis.
Statistical Analysis
Continuous variables are presented as mean (SD) and compared with the use of the 1-way analysis of variance; pairwise comparisons are reported if the overall test was significant. Categorical variables were compared with the use of the χ2 test or Fisher exact tests.
Survival curves for time-to-event variables were constructed on the basis of all available follow-up data with the use of Kaplan-Meier estimates and were compared with the use of the log-rank test. Multivariable analysis was performed with the Cox proportional hazards model. The PVR was entered into the models in binary (≥moderate vs <moderate PVR). Other variables entered into the multivariable models for adjustment were the Society of Thoracic Surgeons score and the variables that showed a statistically significant difference between the PVR groups. A paired comparison of the rate of PVR at 1 year vs at 30 days was performed using the McNemar test. Statistical significance was defined at 2-sided P value of less than .05. All statistical analyses were performed with the use of SAS software, version 9.4 (SAS Institute Inc).
The database for the study is maintained at the Cardiovascular Research Foundation, where independent analyses can be requested by investigators with statistical assistance provided. All of the analyses were performed with data from the as-implanted population. Data are based on an extract date of September 2, 2016, and December 10, 2015, for the inoperable/high-risk and intermediate-risk cohorts, respectively.
Results
Incidence of PVR at 30 Days
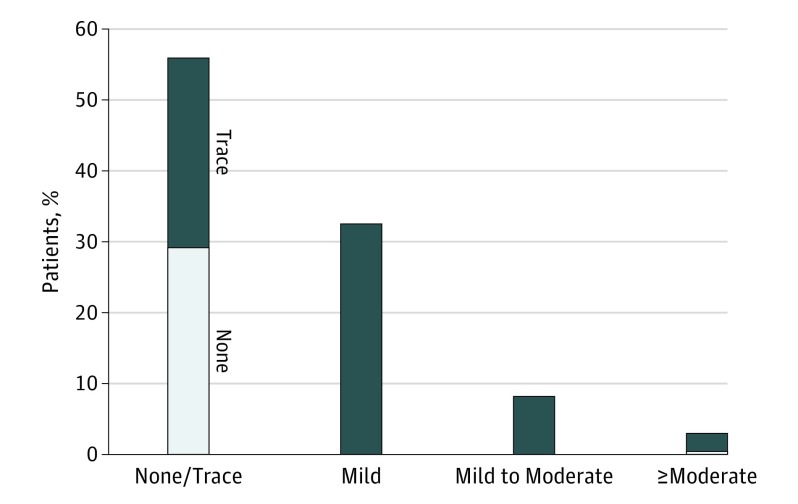
Of the 1592 patients included in this analysis, 887 patients (55.7%) had none (n = 462) or trace (n = 425) PVR, 519 (32.6%) had mild PVR, 131 (8.2%) had mild to moderate PVR, 7 (3.0%) had moderate PVR, and 8 (0.5%) had moderate to severe PVR at 30 days post-TAVR (Figure 1). There were no patients with severe PVR. We thus grouped together patients with moderate and those with moderate-to-severe PVR into 1 single group: ie, at least moderate PVR group (n = 55; 3.5%) (Figure 1 and Table 1). In the 3-class scheme, this would translate into: 55.7% with none or trace PVR (n = 887), 40.8% with mild PVR (n = 650), and 3.5% with moderate PVR (n = 55). The results of the intercore laboratory variability analysis are shown in the eResults in Supplement 1.
Figure 1. Incidence of Paravalvular Regurgitation (PVR) at 30 Days.
In the ≥moderate column, the dark blue indicates moderate PVR (n = 47), and the light blue indicates moderate to severe PVR (n = 8).
Table 1. Baseline and Procedural Characteristics of Patients According to PVR.
| Baseline Characteristics | No. (%) | ||||
|---|---|---|---|---|---|
| None/Trace PVR (n = 887, 55.7%) |
Mild PVR (n = 519, 32.6%) |
Mild to Moderate PVR (n = 131, 8.2%) |
At Least Moderate PVR (n = 55, 3.5%) |
P Value | |
| Cohort assignmenta | |||||
| Inoperable/high risk | 351 (39.6) | 146 (28.1)a | 45 (34.4) | 16 (29.1) | <.001 |
| Intermediate risk | 536 (60.4) | 373 (71.9)a | 86 (65.6) | 39 (70.9) | |
| Age, mean (SD), y | 81.6 (7.5) | 82.9 (6.8)a | 82.4 (6.6) | 84.1(5.5)a | .001 |
| Male sex | 550 (62.0) | 308 (59.3) | 71 (54.2) | 30 (54.5) | .25 |
| BMI, mean (SD) | 29.4 (6.7) | 27.8 (5.8)a | 27.7 (6.4)a | 26.1 (4.3)a | <.001 |
| STS score,b mean (SD) | 6.6 (3.0) | 6.3 (2.6) | 6.5 (3.5) | 6.4 (2.6) | .12 |
| Logistic EuroSCORE II,c mean (SD) | 6.8 (6.1) | 6.3 (5.3) | 6.1 (5.6) | 6.3 (5.5) | .25 |
| NYHA class III or IV | 718 (80.9) | 392 (75.5) | 98 (74.8) | 42 (76.4) | .07 |
| Coronary artery disease | 656 (74.0) | 366 (70.5%) | 94 (71.8) | 32 (58.2) | .06 |
| Previous myocardial infarction | 164 (18.5%) | 82 (15.8%) | 25 (19.1%) | 9 (16.4%) | .59 |
| Previous CABG | 280 (31.6) | 150 (28.9) | 34 (26.0) | 17 (30.9) | .51 |
| Previous PCI | 295 (33.3) | 162 (31.2) | 50 (38.2) | 16 (29.1%) | .44 |
| Previous stroke | 80 (9.0) | 55 (10.6) | 33 (25.2) | 4 (7.3) | .52 |
| Peripheral vascular disease | 289 (32.6) | 148 (28.5) | 33 (25.2) | 14 (25.5) | .16 |
| COPD | |||||
| Any | 342 (38.7) | 165 (31.9)a | 35 (26.7)a | 18 (32.7) | .009 |
| Oxygen dependent | 70 (7.9) | 32 (6.2) | 11 (8.4) | 3 (5.5) | .45 |
| Creatinine >2 mg/dL | 97 (10.9) | 39 (7.5) | 12 (9.2) | 3 (5.5) | .13 |
| Atrial fibrillation | 334 (37.7) | 206 (39.7) | 51 (38.9) | 26 (47.3) | .51 |
| Permanent pacemaker | 121 (13.6) | 71 (13.7) | 25 (19.1) | 9 (16.4) | .37 |
| Severe pulmonary hypertension | 31 (3.5) | 16 (3.1) | 4 (3.1) | 2 (3.6) | .97 |
| Diabetes | 350 (39.5) | 155 (29.9)a | 32 (24.4)a | 10 (18.2)a | <.001 |
| Hypertension | 842 (94.9) | 479 (92.3) | 113 (86.3)a,d | 50 (90.9) | .002 |
| Endocarditis | 3 (0.3) | 2 (0.4) | 1 (0.8) | 1 (1.8) | .40 |
| Frailty | 143 (16.1) | 72 (13.9) | 32 (24.4)a,d | 12 (21.8) | .02 |
| Porcelain aorta | 16 (1.8) | 5 (1.0) | 3 (2.3) | 2 (3.6) | .34 |
| LV ejection fraction, mean (SD), % | 57.3 (13.9) | 57.7 (14.1) | 59.8 (13.5) | 61.2 (12.9) | .09 |
| Stroke volume index, mean (SD), mL/m2 | 37.0 (8.8) | 37.4 (9.6) | 37.8 (8.9) | 38.9 (9.3) | .34 |
| Mean transaortic gradient, mean (SD), mm Hg | 45.8 (13.1) | 45.7 (13.1) | 47.3 (12.8) | 46.7 (13.5) | .63 |
| Mitral regurgitation moderate or severe | 76/849 (9.0) | 50/500 (10.0) | 17/123 (13.8) | 11/53 (20.8)a,d | .02 |
| Aortic regurgitation moderate or severe | 51/856 (6.0) | 25/504 (5.0) | 19/126 (15.1)a,d | 7/54 (13.0)a,d | <.001 |
| Procedural characteristics | |||||
| THV size | |||||
| 20 mm | 10 (1.1) | 28 (5.4)a | 9 (6.9)a | 5 (9.1)a | <.001 |
| 23 mm | 257 (29.0) | 185 (35.6)a | 56 (42.7)a | 25 (45.5)a | <.001 |
| 26 mm | 398 (44.9) | 205 (39.5) | 50 (38.2) | 20 (36.4) | .12 |
| 29 mm | 222 (25.0) | 101 (19.5)a | 16 (12.2)a | 5 (9.1)a | <.001 |
| Oversizing by area, No. (mean [SD], %) | 474 (11.1 [10.4]) | 331 (6.2 [9.1])a | 75 (2.7 [8.4])a,d | 31 (−1.1 [6.9])a,d | <.001 |
| Access | |||||
| Transfemoral | 748 (84.3) | 468 (90.2)a | 119 (90.8) | 49 (89.1) | .007 |
| Transapical | 90 (10.1) | 29 (5.6)a | 7 (5.3) | 5 (9.1) | .01 |
| Transaortic | 49 (5.5) | 22 (4.2 | 5 (3.8) | 1 (1.8) | .44 |
| Conscious sedation | 138 (15.5) | 85 (16.4) | 20 (15.3) | 8 (14.5) | .97 |
| General anesthesia | 749 (84.4) | 434 (83.6) | 111 (84.7) | 47 (85.5) | .97 |
| Anesthesia duration, mean (SD), min | 191 (65) | 184 (58) | 188 (47) | 193 (55) | .22 |
| Total procedure time, mean (SD), min | 191 (59) | 185 (49) | 185 (42) | 183 (52) | .17 |
| Postdilatation | 78 (8.8) | 84 (16.2)a | 24 (18.3)a | 16 (29.1)a,d | <.001 |
| Multiple valves implanted | 4 (0.5) | 2 (0.4%) | 0 | 3 (5.5)a,d,e | <.001 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CABG, denotes coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; EuroSCORE, European System for Cardiac Operative Risk Evaluation; LV, left ventricular; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; STS, Society of Thoracic Surgeons; TAVR, transcatheter aortic valve replacement; THV, transcatheter heart valve.
SI conversion factor: To convert creatinine to micromoles per liter, multiply by 88.4.
Significant difference vs none/trace PVR.
The STS score estimates the risk of 30-day mortality following cardiac surgery and ranges from 0% to 100%, with higher scores indicating higher predicted risk of mortality. An STS score of at least 8% indicates high risk, 4% to 8% indicates intermediate risk, and less than 4% indicates low risk of 30-day mortality.
The logistic EuroSCORE II estimates the risk of operative mortality following cardiac surgery and ranges from 0% to 100%, with higher scores indicating higher predicted risk of mortality.
Significant difference vs mild PVR.
Significant difference vs mild to moderate PVR.
Comparison of Baseline and Procedural Characteristics According to PVR
Patients with at least moderate PVR were older and had smaller body mass index, lower prevalence of diabetes, and higher prevalence of at least moderate aortic regurgitation and at least moderate mitral regurgitation at baseline compared with other PVR groups (Table 1). The proportion of patients in the inoperable/high-risk cohort was higher in the none/trace PVR group than in the mild PVR group. With regards to procedural characteristics (Table 1), patients with at least moderate PVR generally received smaller THVs and had smaller percentage of valve oversizing by area. There was a graded inverse relationship between the percentage of valve oversizing and the severity of PVR. Patients with at least moderate PVR also more frequently had balloon postdilation and more than 1 THV deployed during the TAVR procedure. Postdilation or implanting a second THV at the time of TAVR was not associated with increased risk of 1-year mortality or heart failure rehospitalization. Alternative access (transapical or transaortic) was more frequently used in patients with none/trace PVR compared with those with mild PVR. Factors associated with at least moderate PVR on multivariable logistic regression analysis were smaller THV size (odds ratio for THV size, 0.61; 95% CI, 0.37-0.99; P = .04) and smaller THV oversizing (odds ratio for percentage of oversizing, 0.89; 95% CI, 0.85-0.94; P < .001).
Effect of PVR on 1-Year Outcomes
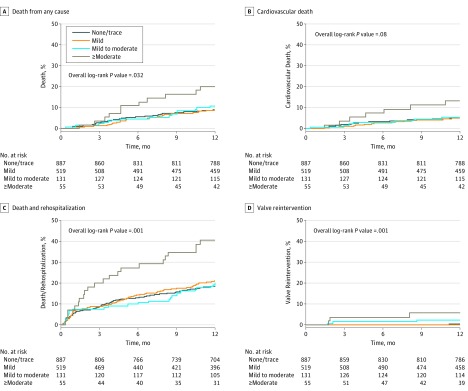
At 1 year, 147 patients (9.3%) had died (84 of cardiovascular cause [5.4%]), 220 (14.2%) had been rehospitalized, 319 (20.2%) had a composite of death or rehospitalization, and 10 (0.7%) had aortic valve reintervention after the TAVR procedure (4 valve-in-valve procedures, 4 surgical valve replacements, 1 balloon postdilation plus surgical valve replacement, and 1 closure of paravalvular leak with a vascular plug) (eFigure 1 in Supplement 1). Patients with at least moderate PVR had significantly higher 1-year rates of all-cause mortality (hazard ratio [HR], 2.40; 95% CI, 1.30-4.43; P = .005), cardiovascular mortality (HR, 2.68; 95% CI, 1.24-5.81; P = .01), rehospitalization (HR, 2.27; 95% CI, 1.34-3.83; P = .002), composite of death or rehospitalization (HR, 2.35; 95% CI, 1.52-3.62; P < .001), and valve reintervention (HR, 13.14; 95% CI, 3.39-50.85; P < .001) compared with patients in the 3 other PVR groups (Figure 2 and Table 2). Patients with mild PVR or mild to moderate PVR had similar incidence of all-cause mortality, cardiovascular mortality, and rehospitalization compared with patients with none/trace PVR. Patients with mild to moderate PVR (HR, 6.80; 95% CI, 1.37-33.7) but not those with mild PVR had higher rate of valve reintervention compared with patients with none or trace PVR (Figure 2).
Figure 2. One-Year Outcomes According to Presence and Severity of Paravalvular Regurgitation (PVR) at 30 Days.
Time-to-event curves for PVR stratified in 4 groups of PVR severity at 30-day echocardiography (none/trace; mild; mild to moderate; and ≥moderate) for death from any cause (A), cardiovascular death (B), composite of death and rehospitalization (C), and valve reintervention (D).
Table 2. Univariable and Multivariable Analyses of the Association Between at Least Moderate PVR and 1-Year Outcomes.
| End Point | Univariable Analysis |
Multivariable Analysisa |
||
|---|---|---|---|---|
| HR (95% CI)b | P Value | HR (95% CI) | P Value | |
| All-cause mortality | 2.40 (1.30-4.43) | .005 | 2.59 (1.39-4.85) | .003 |
| Cardiovascular mortality | 2.68 (1.24-5.81) | .01 | 2.87 (1.30-6.30) | .009 |
| Rehospitalization | 2.27 (1.34-3.83) | .002 | 2.27 (1.31-3.94) | .003 |
| Composite of mortality and rehospitalization | 2.35 (1.52-3.62) | .001 | 2.36 (1.50-3.69) | <.001 |
| Aortic valve reintervention | 13.14 (3.39-50.85) | <.001 | NA | NA |
Abbreviations: HR, hazard ratio; NA, not applicable; PVR, paravalvular regurgitation; STS, Society of Thoracic Surgeons.
Adjusted for age, sex, body mass index, STS score, diabetes, at least moderate baseline aortic regurgitation, and at least moderate baseline mitral regurgitation.
Hazard ratio is for at least moderate PVR vs less than moderate PVR. Multivariable analysis was not performed for aortic valve reintervention because there were only 10 events.
In multivariable models adjusted for age, sex, Society of Thoracic Surgeons score, body mass index, diabetes, at least moderate aortic regurgitation, and at least moderate mitral regurgitation at baseline, the presence of at least moderate PVR at 30 days was independently associated with increased risk of all-cause mortality (HR, 2.59; 95% CI, 1.39-4.85; P = .003), cardiovascular mortality (HR, 2.87; 95% CI, 1.30-6.30; P = .009), rehospitalization (HR, 2.27; 95% CI, 1.31-3.94; P = .003), and composite of death and rehospitalization (HR, 2.36; 95% CI, 1.50-3.69; P < .001) (Table 2). The analysis of total, (ie, paravalvular and central) aortic regurgitation provided similar results in terms of prevalence (3.8% ≥moderate regurgitation at 30 days) and effect on 1-year outcomes (data not shown).
In multiple subgroups based on cohort assignment, clinical factors, and echocardiographic parameters, there was a consistent association between at least moderate PVR at 30 days and higher 1-year all-cause mortality and rehospitalization (eFigure 2 in Supplement 1). The effect of mild to moderate or mild PVR was also similar in the different subgroups (eFigure 3 in Supplement 1).
Change in PVR Severity Between 30 Days and 1 Year
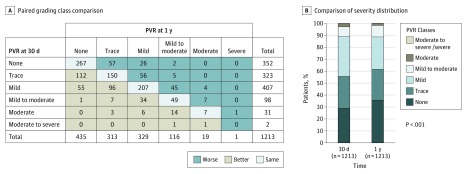
Among the 1213 patients who had an echocardiographic assessment of PVR available at 30 days and 1 year (eFigure 1 in Supplement 1), 33 (2.7%) had at least moderate PVR (31 moderate and 2 moderate to severe) at 30 days (Figure 3). Of these 33 patients, 9 (27%) remained at least moderate and 24 (73%) decreased to less than moderate PVR at 1 year, with improvement of 1 class in 14 patients, 2 classes in 7 patients, and 3 classes in 3 patients (Figure 3). On the other hand, among the 1180 patients with less than moderate PVR at 30 days, 11 (0.9%) increased to moderate PVR at 1 year. Overall, in the subset of 1213 patients with paired comparison, there were 33 patients (2.7%) with at least moderate PVR at 30 days vs 20 patients (1.6%) at 1 year (P < .001) (Figure 3). Additional results are provided in the eResults in Supplement 1.
Figure 3. Paired Comparison of Paravalvular Regurgitation (PVR) at 30 Days vs 1 Year.
A, Paired comparison of PVR grading class at 30 days and 1 year in the subset of 1213 patients who had echocardiographic assessment of PVR available at both times and who did not undergo aortic valve reintervention between 30 days and 1 year. B, Comparison of the distribution of PVR severity at 30 days vs 1 year in this subset of patients.
Discussion
The main findings of this study are (1) in this large, adjudicated prospective series of patients with severe AS undergoing TAVR with the new generation of balloon-expandable THV SAPIEN 3, the rate of at least moderate PVR at 30 days was low (3.5%); (2) the presence of at least moderate PVR at 30 days was associated with a 2.4- to 2.7-fold increase in the risk of mortality and rehospitalization, respectively; and (3) mild PVR and mild to moderate PVR were present in 32.6% and 8.2% of patients, respectively, and were not associated with increased risk of 1-year mortality or rehospitalization. As expected, at least moderate PVR was associated with markedly increased risk of aortic valve reintervention after TAVR procedure. Mild to moderate PVR but not mild PVR was associated with increased risk of reintervention. Given that mild PVR is frequent, even with the new generations of THVs, and that previous studies yielded to conflicting results with regards to its effect on outcomes, these findings provide important new insights and have major clinical implications. Seventy-three percent of the patients with at least moderate PVR at 30 days showed a reduction in PVR severity at 1 year, whereas only 0.9% of those with less than moderate PVR at 30 days had a worsening of PVR at 1 year.
Application of the 5-Class Grading Scheme to Grade PVR
The advantages and limitations of the various imaging methods, parameters, and schemes to grade PVR severity have been discussed in details in previous articles (eTable in Supplement 1). In this study, we used a 5-class grading scheme for the evaluation of PVR (eTable in Supplement 1). Although more grades would initially produce greater variability, using the 5-class scheme, which assigns in-between grades into a predetermined category, has been shown to reduce variability between echocardiography core laboratories in a substudy of the PARTNER IA trial. In this study, agreement between core laboratories was improved when the 5-class scheme was collapsed into the 3-class scheme. A 78% to 91% agreement between 3 different core laboratories for grading of PVR is exceptional when considering the inherent difficulties in assessing PVR following TAVR and the complexities of the multiparametric approach.
Incidence and Effect of PVR Following TAVR With SAPIEN 3
In this large series of patients with high or intermediate surgical risk, the rate of at least moderate PVR was close to 3%, which is about 3-fold lower compared with the rates with the previous generations of SAPIEN THVs. Among the procedural factors, valve undersizing appeared to be the main determinant of the occurrence of significant PVR in this series. The 1-year mortality HR (2.4) for at least moderate PVR in this study was actually similar to those reported in the PARTNER I trial (HR, 2.2) and in previous meta-analysis (HR, 2.3). This study thus confirms the poor outcome associated with at least moderate PVR.
However, the effect of mild PVR on outcomes is more controversial and debated. Several studies and meta-analyses indeed reported that mild PVR has a significant effect on clinical outcomes, whereas others found no effect of mild PVR. Several factors have been proposed to explain these discrepancies: (1) differences between sites or core laboratories in the grading of mild vs moderate PVR; (2) the mild PVR (in the 3-class scheme) represents a broad class, and the upper range of this mild class (ie, mild to moderate in the 5-class scheme) may have a significant effect on outcomes, whereas the lower range may not; and (3) some subsets of patients may tolerate a mild or moderate PVR, whereas others may be highly vulnerable to even a mild PVR. Using a more granular scale to grade PVR in this study, we were able to dissect out the effect of mild (ie, lower range of mild in 3-class) vs mild to moderate (ie, upper range of mild in 3-class). We found that even the upper range of mild (3 class) PVR, ie, mild to moderate (5-class), was not significantly associated with mortality or heart failure rehospitalization. Furthermore, in subgroup analyses, mild or mild to moderate PVR did not appear to have a detrimental effect on outcomes in some specific potentially more vulnerable subsets (eg, patients with no preexistent aortic regurgitation).
Regression of PVR After TAVR With the SAPIEN 3
Some studies previously reported regression of PVR after TAVR with the self-expanding CoreValve. In the CoreValve US pivotal trial, PVR was moderate or severe in 9.9% of patients at 30 days, and of the 36 patients with moderate PVR at discharge and paired data, 30 (83%) improved by at least 1 grade of regurgitation at 1 year. The regression in PVR after SAPIEN 3 TAVR reported in our study may be related to the continuing expansion of the valve skirt potentially owing to further radial deployment and/or thickening of the skirt as well as connective tissue overgrowth that may reduce the gaps between the THV and native annulus.
Clinical Implications
Given that patients with at least moderate PVR at 30 days harbor a 2.4-fold increase in 1-year mortality and that it is difficult to predict who among the survivors will exhibit a regression of PVR, it is essential to make every effort to avoid at least moderate PVR at the time of TAVR. This effort includes comprehensive periprocedural imaging to assess the presence and severity of PVR as well as the use of corrective procedures (ie, balloon postdilation) if at least moderate PVR is present. On the other hand, our results do not support the use of corrective procedure at the time of or after TAVR in the presence of mild PVR. Nonetheless, patients with mild to moderate PVR had increased rate of delayed aortic valve reintervention, and this factor may have contributed to the similar 1-year survival observed in patients with mild to moderate vs none/trace PVR at 30 days. Doppler echocardiography may underestimate the severity of PVR in some patients. Additional imaging, including cardiac magnetic resonance, should thus be considered in patients with mild or mild to moderate PVR presenting with persistent or recurrent symptoms or signs of heart failure.
Limitations
Inconsistent imaging may account for some differences in the grading of PVR between the 30-day and 1-year studies. The change in PVR severity during follow-up may also, at least in part, be related to a change in heart rate, blood pressure, or medications. Moreover, the data on PVR regression were based on a small subset of patients and may reflect a survivorship bias.
Conclusions
In this large, prospective series of patients undergoing TAVR with the SAPIEN 3 valve, at least moderate PVR was rare but associated with increased risk of death and rehospitalization at 1 year. Even the upper range of the mild class in the 3-class grading scheme, ie, the mild to moderate class in the 5-class scheme, had no significant effect on 1-year mortality or rehospitalization. However, mild to moderate PVR but not mild PVR was associated with a higher 1-year rate of aortic valve reinterventions. Longer follow-up is needed to determine the effect of mild to moderate PVR on outcomes beyond 1 year. Most patients with at least moderate PVR at 30 days, and surviving to 1 year showed a reduction in PVR grade at 1 year, which may be multifactorial.
eMethods. Preprocedural THV Sizing
eTable. Parameters and Criteria for Grading the Severity of Paravalvular Regurgitation
eFigure 1. Study Flow Chart
eFigure 2. Subgroup Analyses of the Effect of ≥ Moderate PVR at 30 Days on 1-Year Outcomes
eFigure 3. Subgroup Analyses of the Effect of Mild and Mild-to-Moderate PVR at 30 Days on 1-Year Outcomes
eResults.
Trial Protocol.
References
- 1.Leon MB, Smith CR, Mack M, et al. ; PARTNER Trial Investigators . Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597-1607. [DOI] [PubMed] [Google Scholar]
- 2.Kodali SK, Williams MR, Smith CR, et al. ; PARTNER Trial Investigators . Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366(18):1686-1695. [DOI] [PubMed] [Google Scholar]
- 3.Adams DH, Popma JJ, Reardon MJ, et al. ; U.S. CoreValve Clinical Investigators . Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370(19):1790-1798. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura RA, Otto CM, Bonow RO, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(22):e57-e185. [DOI] [PubMed] [Google Scholar]
- 5.Leon MB, Smith CR, Mack MJ, et al. ; PARTNER 2 Investigators . Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374(17):1609-1620. [DOI] [PubMed] [Google Scholar]
- 6.Thourani VH, Kodali S, Makkar RR, et al. . Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet. 2016;387(10034):2218-2225. [DOI] [PubMed] [Google Scholar]
- 7.Kodali S, Pibarot P, Douglas PS, et al. . Paravalvular regurgitation after transcatheter aortic valve replacement with the Edwards sapien valve in the PARTNER trial: characterizing patients and impact on outcomes. Eur Heart J. 2015;36(7):449-456. [DOI] [PubMed] [Google Scholar]
- 8.Popma JJ, Adams DH, Reardon MJ, et al. ; CoreValve United States Clinical Investigators . Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63(19):1972-1981. [DOI] [PubMed] [Google Scholar]
- 9.Athappan G, Patvardhan E, Tuzcu EM, et al. . Incidence, predictors, and outcomes of aortic regurgitation after transcatheter aortic valve replacement: meta-analysis and systematic review of literature. J Am Coll Cardiol. 2013;61(15):1585-1595. [DOI] [PubMed] [Google Scholar]
- 10.Pibarot P, Hahn RT, Weissman NJ, Monaghan MJ. Assessment of paravalvular regurgitation following TAVR: a proposal of unifying grading scheme. JACC Cardiovasc Imaging. 2015;8(3):340-360. [DOI] [PubMed] [Google Scholar]
- 11.Van Belle E, Juthier F, Susen S, et al. ; FRANCE 2 Investigators . Postprocedural aortic regurgitation in balloon-expandable and self-expandable transcatheter aortic valve replacement procedures: analysis of predictors and impact on long-term mortality: insights from the FRANCE2 Registry. Circulation. 2014;129(13):1415-1427. [DOI] [PubMed] [Google Scholar]
- 12.Dworakowski R, Wendler O, Halliday B, et al. . Device-dependent association between paravalvar aortic regurgitation and outcome after TAVI. Heart. 2014;100(24):1939-1945. [DOI] [PubMed] [Google Scholar]
- 13.Kodali S, Thourani VH, White J, et al. . Early clinical and echocardiographic outcomes after SAPIEN 3 transcatheter aortic valve replacement in inoperable, high-risk and intermediate-risk patients with aortic stenosis. Eur Heart J. 2016;37(28):2252-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zoghbi WA, Chambers JB, Dumesnil JG, et al. ; American Society of Echocardiography’s Guidelines and Standards Committee; Task Force on Prosthetic Valves; American College of Cardiology Cardiovascular Imaging Committee; Cardiac Imaging Committee of the American Heart Association; European Association of Echocardiography; European Society of Cardiology; Japanese Society of Echocardiography; Canadian Society of Echocardiography; American College of Cardiology Foundation; American Heart Association; European Association of Echocardiography; European Society of Cardiology; Japanese Society of Echocardiography; Canadian Society of Echocardiography . Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: a report From the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2009;22(9):975-1014. [DOI] [PubMed] [Google Scholar]
- 15.Lancellotti P, Tribouilloy C, Hagendorff A, et al. ; Scientific Document Committee of the European Association of Cardiovascular Imaging . Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2013;14(7):611-644. [DOI] [PubMed] [Google Scholar]
- 16.Lancellotti P, Pibarot P, Chambers J, et al. . Recommendations for the imaging assessment of prosthetic heart valves: a report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17(6):589-590. [DOI] [PubMed] [Google Scholar]
- 17.Hahn RT, Pibarot P, Weissman NJ, Rodriguez L, Jaber WA. Assessment of paravalvular aortic regurgitation after transcatheter aortic valve replacement: intra-core laboratory variability. J Am Soc Echocardiogr. 2015;28(4):415-422. [DOI] [PubMed] [Google Scholar]
- 18.Abdelghani M, Soliman OI, Schultz C, Vahanian A, Serruys PW. Adjudicating paravalvular leaks of transcatheter aortic valves: a critical appraisal. Eur Heart J. 2016;37(34):2627-2644. [DOI] [PubMed] [Google Scholar]
- 19.Oh JK, Little SH, Abdelmoneim SS, et al. ; CoreValve U.S. Pivotal Trial Clinical Investigators . Regression of paravalvular aortic regurgitation and remodeling of self-expanding transcatheter aortic valve: an observation from the CoreValve U.S. Pivotal Trial. JACC Cardiovasc Imaging. 2015;8(12):1364-1375. [DOI] [PubMed] [Google Scholar]
- 20.Fishbein GA, Schoen FJ, Fishbein MC. Transcatheter aortic valve implantation: status and challenges. Cardiovasc Pathol. 2014;23(2):65-70. [DOI] [PubMed] [Google Scholar]
- 21.Ribeiro HB, Le Ven F, Larose E, et al. . Cardiac magnetic resonance versus transthoracic echocardiography for the assessment and quantification of aortic regurgitation in patients undergoing transcatheter aortic valve implantation. Heart. 2014;100(24):1924-1932. [DOI] [PubMed] [Google Scholar]
- 22.Ribeiro HB, Orwat S, Hayek SS, et al. . Cardiovascular magnetic resonance to evaluate aortic regurgitation after transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;68(6):577-585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Preprocedural THV Sizing
eTable. Parameters and Criteria for Grading the Severity of Paravalvular Regurgitation
eFigure 1. Study Flow Chart
eFigure 2. Subgroup Analyses of the Effect of ≥ Moderate PVR at 30 Days on 1-Year Outcomes
eFigure 3. Subgroup Analyses of the Effect of Mild and Mild-to-Moderate PVR at 30 Days on 1-Year Outcomes
eResults.
Trial Protocol.