This systematic review and meta-analysis evaluates the comparative effectiveness and adverse events of cognitive behavioral therapy and pharmacotherapy for childhood anxiety disorders.
Key Points
Question
What is the comparative effectiveness of cognitive behavioral therapy and pharmacotherapy for childhood anxiety disorders?
Findings
In this systematic review and meta-analysis, selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and cognitive behavioral therapy were all effective in reducing anxiety symptoms. Selective serotonin reuptake inhibitor and serotonin-norepinephrine reuptake inhibitor use were associated with various adverse events that were mostly not serious.
Meaning
The choice of treatments should be based on values, preferences, availability of services, and adverse effect profile.
Abstract
Importance
Childhood anxiety is common. Multiple treatment options are available, but existing guidelines provide inconsistent advice on which treatment to use.
Objectives
To evaluate the comparative effectiveness and adverse events of cognitive behavioral therapy (CBT) and pharmacotherapy for childhood anxiety disorders.
Data Sources
We searched MEDLINE, EMBASE, PsycINFO, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and SciVerse Scopus from database inception through February 1, 2017.
Study Selection
Randomized and nonrandomized comparative studies that enrolled children and adolescents with confirmed diagnoses of panic disorder, social anxiety disorder, specific phobias, generalized anxiety disorder, or separation anxiety and who received CBT, pharmacotherapy, or the combination.
Data Extraction and Synthesis
Independent reviewers selected studies and extracted data. Random-effects meta-analysis was used to pool data.
Main Outcomes and Measures
Primary anxiety symptoms (measured by child, parent, or clinician), remission, response, and adverse events.
Results
A total of 7719 patients were included from 115 studies. Of these, 4290 (55.6%) were female, and the mean (range) age was 9.2 (5.4-16.1) years. Compared with pill placebo, selective serotonin reuptake inhibitors (SSRIs) significantly reduced primary anxiety symptoms and increased remission (relative risk, 2.04; 95% CI, 1.37-3.04) and response (relative risk, 1.96; 95% CI, 1.60-2.40). Serotonin-norepinephrine reuptake inhibitors (SNRIs) significantly reduced clinician-reported primary anxiety symptoms. Benzodiazepines and tricyclics were not found to significantly reduce anxiety symptoms. When CBT was compared with wait-listing/no treatment, CBT significantly improved primary anxiety symptoms, remission, and response. Cognitive behavioral therapy reduced primary anxiety symptoms more than fluoxetine. The combination of sertraline and CBT significantly reduced clinician-reported primary anxiety symptoms and response more than either treatment alone. Head-to-head comparisons were sparse, and network meta-analysis estimates were imprecise. Adverse events were common with medications but not with CBT and were not severe. Studies were too small or too short to assess suicidality with SSRIs or SNRIs. One trial showed a statistically nonsignificant increase in suicidal ideation with venlafaxine. Cognitive behavioral therapy was associated with fewer dropouts than pill placebo or medications.
Conclusions and Relevance
Evidence supports the effectiveness of CBT and SSRIs for reducing childhood anxiety symptoms. Serotonin-norepinephrine reuptake inhibitors also appear to be effective based on less consistent evidence. Head-to-head comparisons between various medications and comparisons with CBT represent a need for research in the field.
Introduction
Childhood anxiety disorders are the most common mental health diagnoses, with prevalence rates of 15% to 20%, and are associated with significant impairment.1,2 Multiple treatment options are available for childhood anxiety disorders, including psychotherapy, pharmacotherapy, and combined treatment approaches. Treatment guidelines recommend cognitive behavioral therapy (CBT) and selective serotonin reuptake inhibitors (SSRIs) as first-line interventions and also discuss the potential benefits of other interventions, such as serotonin-norepinephrine reuptake inhibitors (SNRIs), benzodiazepine, and tricyclic antidepressant.3 However, to our knowledge, comparative effectiveness or, in some cases, the absolute effectiveness of these treatments has not been established. The objectives of this systematic review and meta-analysis are to evaluate the comparative effectiveness of CBT and pharmacotherapy for childhood anxiety disorders and to evaluate adverse events (AEs) associated with these treatments.
Methods
The reporting of this systematic review complies with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statements.4 We developed the study protocol with input from patient representatives, clinical and research experts, and professional organizations. The study protocol is registered in the International Prospective Register of Systematic Reviews (CRD42016046542).
Data Sources and Searches
We searched MEDLINE, EMBASE, PsycINFO, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and SciVerse Scopus from databases inception to February 1, 2017. Relevant systematic reviews, conference proceedings, and reference mining of relevant publications were used to identify additional literature. A reference librarian executed the search strategy (eAppendix in the Supplement). An independent librarian peer-reviewed the strategy.
Study Selection
Consistent with the current conceptualization of anxiety disorders (ie, Diagnostic and Statistical Manual of Mental Disorders [Fifth Edition]), we included the following disorders: panic disorder, social anxiety disorder (and the precursor, avoidant disorder), specific phobias, generalized anxiety disorder (and the precursor, overanxious disorder), and separation anxiety. The decision to examine the anxiety disorders as a class reflects the outcome literature in which most studies typically combine at least 3 (ie, social anxiety disorder, generalized anxiety disorder, and separation anxiety) and sometimes more disorders together. An inclusive definition of anxiety disorder also maximizes the size of the available literature and provides the potential to examine differences in outcome between disorders. The high level of comorbidity between diagnoses makes separating them difficult and of questionable clinical relevance. Obsessive-compulsive disorder and posttraumatic stress disorder were excluded because their treatment approaches are generally different from other types of anxiety.
Cognitive behavioral therapy was defined as attempts to change cognition and behavior consisting of some combination of cognitive restructuring, relaxation training, and exposure therapy. This intervention was studied and delivered clinically through individual therapy sessions with children and parent participation. Accordingly, only studies examining face-to-face child CBT with varying degrees of parent involvement were included. Alternative delivery formats, such as parent-only interventions, bibliotherapy, or computer-based treatment, and non-CBT therapies were excluded.
Eligible studies (1) examined children and adolescents between ages 3 and 18 years with confirmed diagnoses of panic disorder, social anxiety disorder, specific phobias, generalized anxiety disorder, or separation anxiety and who received CBT or any medication, alone or in combination; (2) included at least 1 of the controls (CBT, medication, pill placebo, wait-listing/no treatment, or attention control/treatment as usual); and (3) reported outcomes of interest (primary anxiety symptoms, remission, relapse, or any AEs). We included randomized clinical trials (RCTs) and nonrandomized comparative studies. Singe-cohort observational studies, case reports, and case series were included if they reported any AEs. We did not restrict publication time or study location. We excluded in vitro studies, narrative reviews, editorials, letters, and erratum.
Independent reviewers, working in duplicates, screened the titles and abstracts of all citations and then the full text of eligible references. Discrepancies between the reviewers were resolved through discussions and consensus. If consensus was not reached, a third reviewer was added to resolve the difference.
Data Extraction and Quality Assessment
We developed a pilot-tested standardized data extraction form. We used the Cochrane Collaboration Risk of Bias tool to assess risk of bias.5 Overall risk of bias across the various domains was made by focusing on random allocation, allocation concealment, and blinding (high risk of bias in any of these domains led to a high overall rating). For observational studies, we selected items from the Newcastle-Ottawa Scale, with focus on the representativeness of the population, selection of the cohorts, ascertainment of exposure and outcomes, adequacy of follow-up, and possible conflicts of interest.6 Data extraction and quality assessment were completed by pairs of independent reviewers.
Main Outcome Measures
The outcomes of interest were primary anxiety symptoms, treatment response, and clinical remission. We also evaluated dropout, dropout owing to AEs, and AEs. Primary anxiety symptoms were defined as standardized measures of child anxiety symptoms completed by the child, parent, or a clinician. Clinician report refers to independent evaluators providing ratings as part of a research protocol. Treatment response was defined as (1) loss of principle anxiety diagnosis or (2) Clinical Global Impression–Severity scale score of 1 or 2; remission was defined as (1) loss of all anxiety diagnoses or (2) Clinical Global Impression–Improvement scale score of 1 or 2. We categorized AEs into symptoms related to abdominal/gastrointestinal tract/appetite, behavior change, cold/infection/allergies, headache/dizzy/vision problems, fatigue/somnolence, difficulty sleeping, unintentional injury, and suicide/suicidal ideation/self-harm. Adverse events were deemed to be serious if they were described as serious by the included studies or led to discontinuation of treatment, significant morbidity, or mortality.
Data Synthesis and Analysis
We used the intention-to-treat principle. The main analyses were based on the postintervention findings, while treatment effects during follow-up were evaluated based on less than 6-month follow-up and more than 6-month follow-up. For continuous outcomes, we calculated standardized mean differences (SMDs) based on Cohen d and related 95% CIs. For binary outcomes, we calculated relative risks (RRs) and corresponding 95% CIs. We calculated rate ratios for count data (AEs). We used the DerSimonian and Laird random-effects method with the Knapp and Hartung adjustment of the variance to pool direct (head-to-head) comparisons when the number of the included studies was larger than 2.7 The fixed-effects model based on the Mantel and Haenszel method was used when there were only 2 studies. For primary outcomes, we conducted network meta-analyses based on random-effects meta-regression.8 Network consistency was evaluated using the node-splitting method.9
We used the I2 indicator to evaluate heterogeneity, in which an arbitrary cutoff of greater than 60% suggests substantial heterogeneity. Based on direct comparisons, we conducted the following preplanned subgroup analyses: age, comorbidity (attention-deficit/hyperactivity disorder vs no attention-deficit/hyperactivity disorder; autism vs no autism; any comorbidity vs no comorbidity), school refusal, type of anxiety, treatment settings (school, mental health clinic, or outpatient primary care), length of follow-up (postintervention, less than 6-month follow-up vs more than 6-month follow-up). One-way analysis of variance tests were used to quantitatively compare subgroups. We were unable to conduct other subgroup analyses (race/ethnicity, parent education level, family income, disease severity, treatment sequence, and provider) owing to studies not providing sufficient stratified data per subgroup variable. Publication bias was evaluated by inspecting asymmetry of funnel plots and the Egger regression test when the number of studies in an analysis exceeded 20. A 2-tailed P value <.05 was considered statistically significant. All statistical analyses were conducted using Stata version 14.1 (StataCorp).
Grading the Quality of Evidence
We used the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) approach. Randomized clinical trials start with an initial rating of high and nonrandomized comparative studies with low. For each comparison and outcomes, we assessed method limitations of the included studies, precision, directness, consistency, and the likelihood of publication bias.
Results
Description of the Evidence
Our search identified 32 156 relevant citations. A total of 115 unique studies identified from 127 publications were eventually included in the analyses (eFigure 1 in the Supplement). From these studies, 7719 patients were included, with a mean (range) age of 9.2 (5.4-16.1) years, and 4290 (55.6%) were female. Seventy studies (60.7%) included patients with separation anxiety disorder, 72 (62.6%) with generalized anxiety disorder, 82 (71.3%) with social anxiety disorder, 52 (45.2%) with specific phobia, and 36 (31.3%) with panic disorder; 67 studies (58.3%) included patients without any comorbidity and 46 studies (40.0%) included children with anxiety and comorbidity (attention-deficit/hyperactivity disorder, autism, oppositional defiant disorder, obsessive-compulsive disorder, or other internalizing disorders). The medications were SSRIs (ie, sertraline, fluoxetine, fluvoxamine, or paroxetine), SNRIs (ie, atomoxetine, duloxetine, or venlafaxine), tricyclic antidepressant (ie, clomipramine or imipramine), and benzodiazepine (ie, clonazepam). The detailed characteristics of the included studies are listed in eTables 1-8 in the Supplement.
The overall risk of bias was moderate to high because of lack of blinding for patients, care professional who provided interventions to patients, and outcome assessors for RCTs and unclear risk of conflicts of interest for nonrandomized comparative studies (eFigures 2 and 3 in the Supplement). When SSRIs were compared with pill placebo, trials with adequate blinding of outcome assessors produced larger reductions in clinician-reported primary anxiety symptoms than those with no or unclear blinding. The effect of blinding outcome assessor was not significant for the comparison of CBT vs wait-listing/no treatment and not feasible for the comparison of SNRIs vs pill placebo, as these trials reported adequate blinding of outcome assessors. We found potential publication bias when CBT was compared with wait-listing/no treatment on all 3 reports of primary anxiety symptoms (child, parent, and clinician reports). We were unable to evaluate publication bias for the other comparisons owing to small numbers of the included studies (n < 20).
Medications vs Pill Placebo
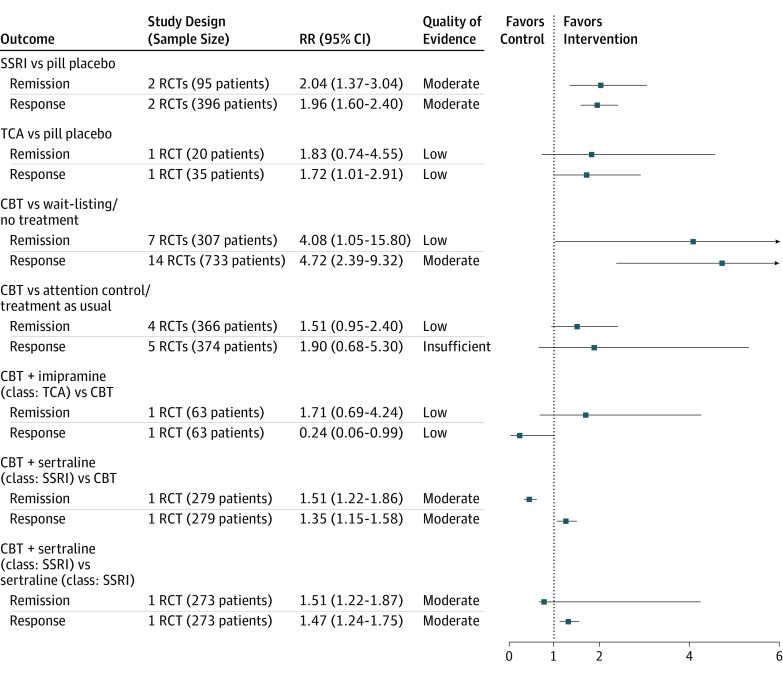
Figure 1 and Figure 2 summarize pooled effect size and quality of evidence (QOE) by medication class. Compared with pill placebo, as a class, SSRIs were significantly more effective at reducing primary anxiety symptoms (parent report: SMD, −0.61; 95% CI, −1.03 to −0.20; I2 = 55.1%; moderate QOE; clinician report: SMD, −0.65; 95% CI, −1.10 to −0.21; I2 = 73.4%; moderate QOE) and significantly more likely to increase remission (RR, 2.04; 95% CI, 1.37-3.04; I2 = not applicable [NA]; moderate QOE) and response (RR, 1.96; 95% CI, 1.60-2.40; I2 = 0%; moderate QOE). Serotonin-norepinephrine reuptake inhibitors significantly reduced clinician-reported primary anxiety symptoms (SMD, −0.45; 95% CI, −0.81 to −0.10; I2 = 0%; high QOE), while tricyclic antidepressants marginally increased the likelihood of treatment response (RR, 1.72; 95% CI, 1.01-2.91; I2 = NA; low QOE). We did not find significant improvement associated with using benzodiazepines.
Figure 1. Pooled Effect Size and Quality of Evidence for Primary Anxiety Symptoms (Measured by Child, Parent, and Clinician Report).
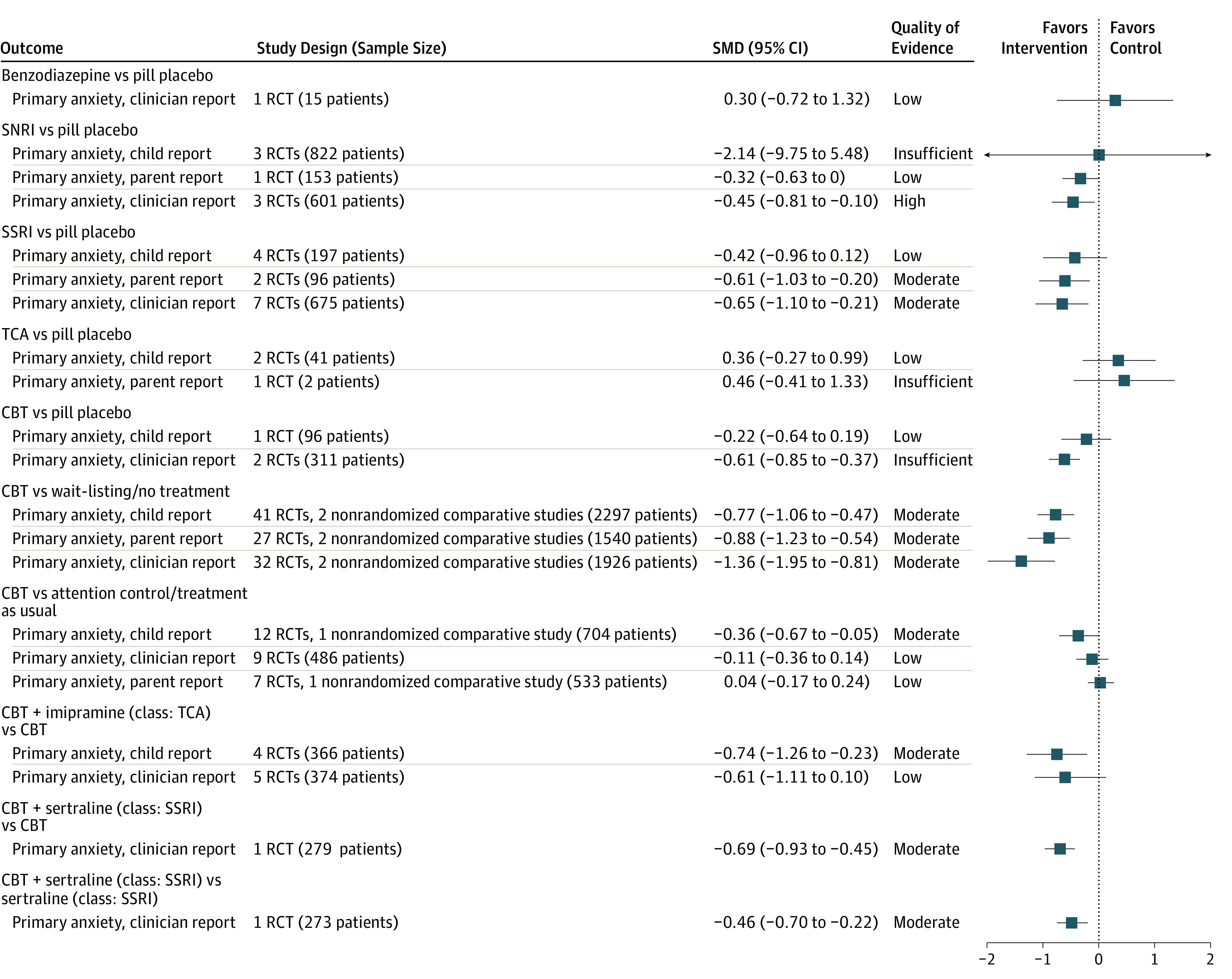
CBT indicates cognitive behavioral therapy; RCT, randomized clinical trial; SMD, standardized mean difference; SNRI, serotonin-norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressants.
Figure 2. Pooled Effect Size and Quality of Evidence for Treatment Response and Remission.
CBT indicates cognitive behavioral therapy; RCT, randomized clinical trial; RR, relative risk; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressants.
In terms of specific drugs, compared with pill placebo, atomoxetine, duloxetine, venlafaxine, fluoxetine, fluvoxamine, paroxetine, and sertraline significantly improved clinician-reported anxiety symptoms. In addition, atomoxetine improved anxiety symptoms by child report, while sertraline improved anxiety symptoms by parent report. Fluoxetine significantly increased response and remission while paroxetine improved response (eTable 9 in the Supplement).
CBT vs Wait-listing/No Treatment, Attention Control/Treatment as Usual, or Pill Placebo
When CBT was compared with wait-listing/no treatment, CBT significantly improved 3 reports of primary anxiety symptoms (child report: SMD, −0.77; 95% CI, −1.06 to −0.47; I2 = 86.5%; moderate QOE; parent report: SMD, −0.88; 95% CI, −1.23 to −0.54; I2 = 81.2%; moderate QOE; clinician report: SMD, −1.38; 95% CI, −1.95 to −0.81; I2 = 88.3%; moderate QOE), treatment response (RR, 4.72; 95% CI, 2.39-9.32; I2 = 80.4%; moderate QOE), and remission (RR, 4.08; 95% CI, 1.05-15.80; I2 = 80.8%; low QOE). Compared with attention control/treatment as usual, CBT only reduced primary anxiety symptoms per child report (SMD, −0.36; 95% CI, −0.67 to −0.05; I2 = 60.5%; moderate QOE). Figure 1 and Figure 2 show the comparison between CBT and wait-listing/no treatment, attention control/treatment as usual, and pill placebo.
Combination of CBT and Medications
The combination of imipramine and CBT reduced primary anxiety symptoms by child report compared with CBT alone (SMD, −0.74; 95% CI, −1.26 to −0.23; I2 = NA; moderate QOE) (Figure 1). The combination of sertraline and CBT significantly reduced primary anxiety symptoms by clinician report and improved treatment response and remission more than either treatment alone (moderate QOE) (Figure 1 and Figure 2).
CBT vs Medications
Two RCTs compared CBT with medications.10,11,12,13,14,15,16,17,18 Compared with fluoxetine, CBT was more effective in improving primary anxiety symptoms (clinician report: SMD, −0.78; 95% CI, −1.18 to −0.37; I2 = NA; moderate QOE). No significant difference was found between CBT and sertraline (class: SSRI) (eTable 9 in the Supplement).
Medications vs Medications
Two RCTs provided data.19,20 Compared with clomipramine, fluoxetine was more effective in improving primary anxiety symptoms (child report: low QOE), and no significant difference was found between venlafaxine and atomoxetine (eTable 9 in the Supplement).
Network Meta-analysis
We did not find significant difference between CBT and any medication or across different medications. Pairwise comparisons by class and medication are listed in eTables 10-19 in the Supplement. Network meta-analysis was mostly consistent (only a single comparison was not) (eTables 20-29 in the Supplement).
Adverse Events
Sixty-four studies (62 RCTs and 2 nonrandomized comparative studies) and 18 single-cohort observational studies reported AEs. Adverse events were reported with most drugs, but none were serious (eTable 30 in the Supplement). Cognitive behavioral therapy was associated with fewer dropouts than pill placebo.
No deaths were reported in any studies. Three studies reported on suicide/suicidal ideation/self-harm.12,13,14,15,16,17,18,21,22 The Child/Adolescent Anxiety Multimodal Study13 found no suicide attempts in patients receiving CBT, sertraline, combination of CBT and sertraline, and pill placebo and no statistical difference between groups on suicidal ideation.12,13,14,15,16,17,18 In 1 RCT of 293 children with generalized social anxiety disorder, March et al21 compared venlafaxine extended release with pill placebo and found 3 patients with suicidal ideation (3 of 140) in the venlafaxine group and no incidence in the pill placebo group (P = .18). In an observational study, Renaud et al22 found no suicide attempts or ideation among 12 children treated with SSRIs and benzodiazepines.
Eighteen single-cohort observational studies22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39 reported AEs related to different drugs. Those AEs included gastrointestinal symptoms, behavior change, difficulty sleeping, headache, fatigue, and somnolence. No serious AEs were reported.
Subgroup Analysis
eTables 31-39 in the Supplement list the findings based on subgroup analyses. When CBT was compared with wait-listing/no treatment, postintervention treatment response was significantly higher than that reported at less than 6 months follow-up (postintervention: RR, 4.72; 95% CI, 2.39-9.32; less than 6-month follow-up: RR, 1.50; 95% CI, 0.71-3.17). Postintervention reduction of primary anxiety symptoms by child report was also significantly larger than those reported after more than 6-month follow-up (postintervention: SMD, −0.77; 95% CI, −1.06 to −0.47; more than 6-month follow-up: SMD, −0.08; 95% CI, −0.56 to 0.39) as well as remission (postintervention: RR, 4.08; 95% CI, 1.05-15.80; more than 6-month follow-up: RR, 0.71; 95% CI, 0.57-1.03). We did not find significant differences based on age, comorbidity, school refusal, treatment settings, and diagnosis.
Discussion
In this systematic review, we evaluated the comparative effectiveness and safety of CBT and medications for childhood anxiety disorders. We included 115 studies in the analyses. Compared with pill placebo, SSRIs and SNRIs improved anxiety symptoms, although symptom reduction was only significant based 2 or 1 of 3 sources, respectively. The effect of benzodiazepines and tricyclic antidepressants on anxiety symptoms, response, and remission was only supported by insufficient or low strength of evidence. Data on head-to-head comparisons across drugs were sparse. Compared with wait-listing/no treatment, CBT significantly reduced primary anxiety symptoms and improved response and remission. The combination of SSRIs and CBT reduced primary anxiety symptoms and improved treatment response and remission compared with either approach alone. Adverse events (mostly not serious) were common with medications but not with CBT. Cognitive behavioral therapy was also associated with fewer dropouts than pill placebo or medications.
The current analysis is consistent with previous ones, such as a Cochrane systematic review40concluding that CBT is an effective treatment for childhood and adolescent anxiety disorders. However, the current review extends the empirical support for CBT by finding moderate support for the superiority of CBT over attention control/treatment as usual. Moreover, the current analyses found no differences or fewer dropout rates between CBT and pill placebo, wait-listing/no treatment, or attention control/treatment as usual. This finding refutes the belief that patients find CBT aversive and unacceptable.
In regards to medication, the current analyses are consistent with previous systematic reviews of psychopharmacologic interventions suggesting that SSRIs and SNRIs have demonstrated effectiveness in the reduction of anxiety symptoms. However, the support for SSRIs is somewhat lessened by the fact that superiority over pill placebo was not found with child report. This issue of inconsistent support is more concerning for SNRIs, where support for effectiveness was only found through clinician report and not through parent or child report of symptoms. In addition, the inconsistent support for SNRIs and the lack of support for the effectiveness of benzodiazepines should be interpreted in the context of previous concerns regarding an increased risk for suicidality in the treatment of adolescent depression.41
In terms of AEs, to our knowledge, the current review provides the most comprehensive evaluation to date and suggests that AEs tended to not be serious and generally did not lead to discontinuation. Likewise, we found a low risk for serious AEs. There was no evidence of suicidal behavior or ideation associated with the use of SSRIs in children with anxiety, although one trial42 showed a statistically nonsignificant increase in suicidal ideation with venlafaxine. This contrasted with the well-reported 2-fold increase in suicidal behavior and/or suicidal thoughts associated with SSRIs in children and adolescents treated for any diagnosis, a finding which led to the black box warning.43,44 This discrepancy could be because of the lack of a standardized mechanism for coding and assessing akathisia, aggression, hostility, and suicidal events in pediatric trials and the resulting underreporting of harm events.45
Limitations
Our study had limitations. Despite anxiety being a common disorder in children, the body of evidence was relatively small. Few studies evaluated long-term effects of the available treatments. Components of interventions and description of participant comorbidities, demographic information, and social support was either lacking or was provided without stratification per intervention. The lack of stratification based on severity prevented us from evaluating the effect of severity on outcome, which underscores the fact that the literature has not provided the data to inform treatment guidelines recommending different approaches based on level of severity. The synthesis of data on AEs in particular is limited by the fact that most CBT studies do not evaluate AEs and by the lack of a structured consistent approach to measurement in medication studies. The findings from subgroup analyses should be considered as hypothesis generating and, thus, need to be interpreted with caution. Although adequate blinding of patients, care professional, and outcome assessors is crucial to reduce potential bias, this is a challenge for the included treatment studies. Most of the examinations of CBT used a no treatment comparison, which does not allow for blinding of child and parent reporters. As such, parents and children may have stronger expectations for the benefits in CBT than in other treatments and may exaggerate treatment effects. However, the strength of the pill placebo blind in psychopharmacological trials has been questioned, suggesting that medication trials may also not be free of bias.46 Moreover, the superiority of combined SSRIs and CBT over either intervention is also susceptible to bias, as children and parents were aware that they were receiving both active treatments and those in the other groups knew they were not. We also found indications of potential publication bias when CBT was compared with wait-listing on primary anxiety symptoms. We were unable to statistically evaluate publication bias for most other comparisons because of small numbers of studies (n <20). Finally, because most of the studies were conducted in rigorous conditions with stringent quality control and study protocols, the findings may be difficult to reproduce in routine care and may provide efficacy rather than effectiveness inferences. Future studies are also needed to examine alternatives to traditional CBT, such as technology-assisted therapy, parent-based interventions, and non-CBT approaches.
Conclusions
Evidence most consistently supports the effectiveness of CBT and SSRIs for reducing childhood anxiety symptoms. Serotonin-norepinephrine reuptake inhibitors also appear to be effective based on less consistent evidence. Selective serotonin reuptake inhibitor and SNRI use are associated with various AEs that are mostly not serious, but studies were too small or too short to assess suicidality with SSRIs or SNRIs. Head-to-head comparisons between various medications and comparing with CBT represent a need for research in the field.
eAppendix. Search strategy.
eFigure 1. Flow chart.
eFigure 2. Risk of bias assessment for randomized control trials.
eFigure 3. Risk of bias assessment for nonrandomized comparative studies.
eTable 1. Characteristics of the included studies comparing medications vs pill placebo.
eTable 2. Characteristics of the included studies comparing medications vs medications.
eTable 3. Characteristics of the included studies comparing CBT vs medications.
eTable 4. Characteristics of the included studies comparing CBT vs wait-listing/no treatment.
eTable 5. Characteristics of the included studies comparing CBT vs pill placebo.
eTable 6. Characteristics of the included studies comparing CBT vs attention control/treatment as usual.
eTable 7. Characteristics of studies evaluating combination of CBT with medications vs CBT.
eTable 8. Characteristics of single-cohort observational studies with adverse events.
eTable 9. Pooled effect size and quality of evidence for primary anxiety symptoms (measured by clinician, child, and parent).
eTable 10. Pairwise comparison for primary anxiety symptoms (clinician report) by medication class.
eTable 11. Pairwise comparison for primary anxiety symptoms (clinician report) by medication.
eTable 12. Pairwise comparison for primary anxiety symptoms (child report) by medication class.
eTable 13. Pairwise comparison for primary anxiety symptoms (child report) by medication.
eTable 14. Pairwise comparison for primary anxiety symptoms (parent report) by medication class.
eTable 15. Pairwise comparison for primary anxiety symptoms (parent report) by medication.
eTable 16. Pairwise comparison for remission by medication class.
eTable 17. Pairwise comparison for remission by medication.
eTable 18. Pairwise comparison for response by medication class.
eTable 19. Pairwise comparison for response by medication.
eTable 20. Network consistency test for network of primary anxiety symptoms (clinician report) by medication class.
eTable 21. Network consistency test for network of primary anxiety symptoms (clinician report) by medication.
eTable 22. Network consistency test for network of primary anxiety symptoms (child report) by medication class.
eTable 23. Network consistency test for network of primary anxiety symptoms (child report) by medication.
eTable 24. Network consistency test for network of primary anxiety symptoms (parent report) by medication class.
eTable 25. Network consistency test for network of primary anxiety symptoms (parent report) by medication.
eTable 26. Network consistency test for network of remission by medication class.
eTable 27. Network consistency test for network of remission by medication.
eTable 28. Network consistency test for network of response by medication class.
eTable 29. Network consistency test for network of response by medication.
eTable 30. Pooled effect size and quality of evidence for adverse events (including dropouts, dropouts due to any adverse events, and adverse events).
eTable 31. Subgroup analysis: age.
eTable 32. Subgroup analysis: comorbidity.
eTable 33. Subgroup analysis: ADHD.
eTable 34. Subgroup analysis: autism.
eTable 35. Subgroup analysis: school refusal.
eTable 36. Subgroup analysis: diagnosis.
eTable 37. Subgroup analysis: treatment settings.
eTable 38. Subgroup analysis: follow-up less than 6 months.
eTable 39. Subgroup analysis: follow-up longer than 6 months.
eReferences.
References
- 1.Beesdo K, Knappe S, Pine DS. Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM-V. Psychiatr Clin North Am. 2009;32(3):483-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ezpeleta L, Keeler G, Erkanli A, Costello EJ, Angold A. Epidemiology of psychiatric disability in childhood and adolescence. J Child Psychol Psychiatry. 2001;42(7):901-914. [DOI] [PubMed] [Google Scholar]
- 3.Connolly SD, Bernstein GA; Work Group on Quality Issues . Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(2):267-283. [DOI] [PubMed] [Google Scholar]
- 4.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Hoboken, NJ: The Cochrane Collaboration; 2011. [Google Scholar]
- 6.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed August 2, 2017.
- 7.Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22(17):2693-2710. [DOI] [PubMed] [Google Scholar]
- 8.White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods. 2012;3(2):111-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29(7-8):932-944. [DOI] [PubMed] [Google Scholar]
- 10.Beidel DC, Turner SM, Sallee FR, Ammerman RT, Crosby LA, Pathak S. SET-C versus fluoxetine in the treatment of childhood social phobia. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1622-1632. [DOI] [PubMed] [Google Scholar]
- 11.Scharfstein LA, Beidel DC, Finnell LR, Distler A, Carter NT. Do pharmacological and behavioral interventions differentially affect treatment outcome for children with social phobia? Behav Modif. 2011;35(5):451-467. [DOI] [PubMed] [Google Scholar]
- 12.Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753-2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rynn MA, Walkup JT, Compton SN, et al. Child/Adolescent Anxiety Multimodal Study: evaluating safety. J Am Acad Child Adolesc Psychiatry. 2015;54(3):180-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piacentini J, Bennett S, Compton SN, et al. 24- and 36-week outcomes for the Child/Adolescent Anxiety Multimodal Study (CAMS). J Am Acad Child Adolesc Psychiatry. 2014;53(3):297-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ginsburg GS, Kendall PC, Sakolsky D, et al. Remission after acute treatment in children and adolescents with anxiety disorders: findings from the CAMS. J Consult Clin Psychol. 2011;79(6):806-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keeton CP, Ginsburg GS, Drake KL, et al. Benefits of child-focused anxiety treatments for parents and family functioning. Depress Anxiety. 2013;30(9):865-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez A, Peris TS, Vreeland A, et al. Parental anxiety as a predictor of medication and CBT response for anxious youth. Child Psychiatry Hum Dev. 2015;46(1):84-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nail JE, Christofferson J, Ginsburg GS, et al. Academic impairment and impact of treatments among youth with anxiety disorders. Child Youth Care Forum. 2015;44(3):327-342. doi: 10.1007/s10566-014-9290-x [DOI] [Google Scholar]
- 19.da Costa CZ, de Morais RM, Zanetta DM, et al. Comparison among clomipramine, fluoxetine, and placebo for the treatment of anxiety disorders in children and adolescents. J Child Adolesc Psychopharmacol. 2013;23(10):687-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rynn MA, Riddle MA, Yeung PP, Kunz NR. Efficacy and safety of extended-release venlafaxine in the treatment of generalized anxiety disorder in children and adolescents: two placebo-controlled trials. Am J Psychiatry. 2007;164(2):290-300. [DOI] [PubMed] [Google Scholar]
- 21.March JS, Entusah AR, Rynn M, Albano AM, Tourian KA. A randomized controlled trial of venlafaxine ER versus placebo in pediatric social anxiety disorder. Biol Psychiatry. 2007;62(10):1149-1154. [DOI] [PubMed] [Google Scholar]
- 22.Renaud J, Birmaher B, Wassick SC, Bridge J. Use of selective serotonin reuptake inhibitors for the treatment of childhood panic disorder: a pilot study. J Child Adolesc Psychopharmacol. 1999;9(2):73-83. [DOI] [PubMed] [Google Scholar]
- 23.Biederman J. Clonazepam in the treatment of prepubertal children with panic-like symptoms. J Clin Psychiatry. 1987;48(suppl):38-42. [PubMed] [Google Scholar]
- 24.Birmaher B, Waterman GS, Ryan N, et al. Fluoxetine for childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry. 1994;33(7):993-999. [DOI] [PubMed] [Google Scholar]
- 25.Chavira DA, Stein MB. Combined psychoeducation and treatment with selective serotonin reuptake inhibitors for youth with generalized social anxiety disorder. J Child Adolesc Psychopharmacol. 2002;12(1):47-54. [DOI] [PubMed] [Google Scholar]
- 26.Chutko LS, Surushkina SY, Nikishena IS, et al. Treatment of anxiety disorders in school maladaptation with adaptol. Neurosci Behav Physiol. 2011;41(5):520-524. doi: 10.1007/s11055-011-9448-z [DOI] [Google Scholar]
- 27.Compton SN, Grant PJ, Chrisman AK, Gammon PJ, Brown VL, March JS. Sertraline in children and adolescents with social anxiety disorder: an open trial. J Am Acad Child Adolesc Psychiatry. 2001;40(5):564-571. [DOI] [PubMed] [Google Scholar]
- 28.D’Amato G. Chlordiazepoxide in management of school phobia. Dis Nerv Syst. 1962;23:292-295. [PubMed] [Google Scholar]
- 29.Dummit ES III, Klein RG, Tancer NK, Asche B, Martin J. Fluoxetine treatment of children with selective mutism: an open trial. J Am Acad Child Adolesc Psychiatry. 1996;35(5):615-621. [DOI] [PubMed] [Google Scholar]
- 30.Fairbanks JM, Pine DS, Tancer NK, et al. Open fluoxetine treatment of mixed anxiety disorders in children and adolescents. J Child Adolesc Psychopharmacol. 1997;7(1):17-29. [DOI] [PubMed] [Google Scholar]
- 31.Isolan L, Pheula G, Salum GA Jr, Oswald S, Rohde LA, Manfro GG. An open-label trial of escitalopram in children and adolescents with social anxiety disorder. J Child Adolesc Psychopharmacol. 2007;17(6):751-760. [DOI] [PubMed] [Google Scholar]
- 32.Karabekiroglu K, Karakurt MN, Yuce M, Say GNT. Fluoxetine for the treatment of childhood and adolescence social phobia: factors playing a role in efficacy. Klinik Psikofarmakoloji Bulteni. 2011;21(4):317-324. [Google Scholar]
- 33.Lepola U, Leinonen E, Koponen H. Citalopram in the treatment of early-onset panic disorder and school phobia. Pharmacopsychiatry. 1996;29(1):30-32. [DOI] [PubMed] [Google Scholar]
- 34.Mancini C, Van Ameringen M, Oakman JM, Farvolden P. Serotonergic agents in the treatment of social phobia in children and adolescents: a case series. Depress Anxiety. 1999;10(1):33-39. [DOI] [PubMed] [Google Scholar]
- 35.Masi G, Toni C, Mucci M, Millepiedi S, Mata B, Perugi G. Paroxetine in child and adolescent outpatients with panic disorder. J Child Adolesc Psychopharmacol. 2001;11(2):151-157. [DOI] [PubMed] [Google Scholar]
- 36.Mrakotsky C, Masek B, Biederman J, et al. Prospective open-label pilot trial of mirtazapine in children and adolescents with social phobia. J Anxiety Disord. 2008;22(1):88-97. [DOI] [PubMed] [Google Scholar]
- 37.Simeon JG, Ferguson HB. Alprazolam effects in children with anxiety disorders. Can J Psychiatry. 1987;32(7):570-574. [DOI] [PubMed] [Google Scholar]
- 38.Simeon JG, Knott VJ, Dubois C, et al. Buspirone therapy of mixed anxiety disorders in childhood and adolescence: a pilot-study. J Child Adol Psychop. 1994;4(3):159-170. doi: 10.1089/cap.1994.4.159 [DOI] [Google Scholar]
- 39.Zwier KJ, Rao U. Buspirone use in an adolescent with social phobia and mixed personality disorder (cluster A type). J Am Acad Child Adolesc Psychiatry. 1994;33(7):1007-1011. [DOI] [PubMed] [Google Scholar]
- 40.Manassis K, Russell K, Newton AS. The Cochrane Library and the treatment of childhood and adolescent anxiety disorders: an overview of reviews. Evid Based Child Health. 2010;5:541-554. doi: 10.1002/ebch.508 [DOI] [Google Scholar]
- 41.Brent DA, Emslie GJ, Clarke GN, et al. Predictors of spontaneous and systematically assessed suicidal adverse events in the treatment of SSRI-resistant depression in adolescents (TORDIA) study. Am J Psychiatry. 2009;166(4):418-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.March JS, Entusah AR, Rynn M, Albano AM, Tourian KA. A randomized controlled trial of venlafaxine ER versus placebo in pediatric social anxiety disorder. Biol Psychiatry. 2007;62(10):1149-1154. [DOI] [PubMed] [Google Scholar]
- 43.Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63(3):332-339. [DOI] [PubMed] [Google Scholar]
- 44.US Food and Drug Administration . Relationship between psychotropic drugs and pediatric suicidality: review and evaluation of clinical data. http://www.fda.gov/ohrms/dockets/ac/04/briefing/2004-4065b1-10-TAB08-Hammads-Review.pdf. Accessed August 02, 2017.
- 45.Sharma T, Guski LS, Freund N, Gøtzsche PC. Suicidality and aggression during antidepressant treatment: systematic review and meta-analyses based on clinical study reports. BMJ. 2016;352:i65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perlis RH, Ostacher M, Fava M, Nierenberg AA, Sachs GS, Rosenbaum JF. Assuring that double-blind is blind. Am J Psychiatry. 2010;167(3):250-252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Search strategy.
eFigure 1. Flow chart.
eFigure 2. Risk of bias assessment for randomized control trials.
eFigure 3. Risk of bias assessment for nonrandomized comparative studies.
eTable 1. Characteristics of the included studies comparing medications vs pill placebo.
eTable 2. Characteristics of the included studies comparing medications vs medications.
eTable 3. Characteristics of the included studies comparing CBT vs medications.
eTable 4. Characteristics of the included studies comparing CBT vs wait-listing/no treatment.
eTable 5. Characteristics of the included studies comparing CBT vs pill placebo.
eTable 6. Characteristics of the included studies comparing CBT vs attention control/treatment as usual.
eTable 7. Characteristics of studies evaluating combination of CBT with medications vs CBT.
eTable 8. Characteristics of single-cohort observational studies with adverse events.
eTable 9. Pooled effect size and quality of evidence for primary anxiety symptoms (measured by clinician, child, and parent).
eTable 10. Pairwise comparison for primary anxiety symptoms (clinician report) by medication class.
eTable 11. Pairwise comparison for primary anxiety symptoms (clinician report) by medication.
eTable 12. Pairwise comparison for primary anxiety symptoms (child report) by medication class.
eTable 13. Pairwise comparison for primary anxiety symptoms (child report) by medication.
eTable 14. Pairwise comparison for primary anxiety symptoms (parent report) by medication class.
eTable 15. Pairwise comparison for primary anxiety symptoms (parent report) by medication.
eTable 16. Pairwise comparison for remission by medication class.
eTable 17. Pairwise comparison for remission by medication.
eTable 18. Pairwise comparison for response by medication class.
eTable 19. Pairwise comparison for response by medication.
eTable 20. Network consistency test for network of primary anxiety symptoms (clinician report) by medication class.
eTable 21. Network consistency test for network of primary anxiety symptoms (clinician report) by medication.
eTable 22. Network consistency test for network of primary anxiety symptoms (child report) by medication class.
eTable 23. Network consistency test for network of primary anxiety symptoms (child report) by medication.
eTable 24. Network consistency test for network of primary anxiety symptoms (parent report) by medication class.
eTable 25. Network consistency test for network of primary anxiety symptoms (parent report) by medication.
eTable 26. Network consistency test for network of remission by medication class.
eTable 27. Network consistency test for network of remission by medication.
eTable 28. Network consistency test for network of response by medication class.
eTable 29. Network consistency test for network of response by medication.
eTable 30. Pooled effect size and quality of evidence for adverse events (including dropouts, dropouts due to any adverse events, and adverse events).
eTable 31. Subgroup analysis: age.
eTable 32. Subgroup analysis: comorbidity.
eTable 33. Subgroup analysis: ADHD.
eTable 34. Subgroup analysis: autism.
eTable 35. Subgroup analysis: school refusal.
eTable 36. Subgroup analysis: diagnosis.
eTable 37. Subgroup analysis: treatment settings.
eTable 38. Subgroup analysis: follow-up less than 6 months.
eTable 39. Subgroup analysis: follow-up longer than 6 months.
eReferences.