Key Points
Question
Is macular pigment density increased with dietary supplementation containing lutein, zeaxanthin, ω-3 polyunsaturated fatty acids, and vitamins?
Findings
Among 120 participants (239 eyes) in the Lutein Influence on Macula of Persons Issued From AMD Parents (LIMPIA) randomized clinical trial with a 6-month treatment period followed by a 6-month follow-up period, no increase in macular pigment optical density was observed when measured with the modified MPD-Visucam 200 (Carl Zeiss Meditec) or the modified Heidelberg Retina Angiograph (Heidelberg Engineering) in treated patients (n = 60) compared with patients receiving placebo (n = 60).
Meanings
Macular pigment density may increase after dietary supplementation, but it is not measurable.
Abstract
Importance
Nutritional uptake of lutein, zeaxanthin, and ω-3 polyunsaturated fatty acids may increase macular pigment optical density (MPOD) and thereby protect against the development of age-related macular degeneration (AMD).
Objectives
To estimate the efficiency of dietary supplementation containing lutein, zeaxanthin, ω-3 polyunsaturated fatty acids, and vitamins to increase the density of macular pigment in first-generation offspring of parents with neovascular AMD.
Design, Setting, and Participants
This study was a randomized clinical trial (Lutein Influence on Macula of Persons Issued From AMD Parents [LIMPIA]) with a 6-month treatment period, followed by a 6-month follow-up period. Analyses were based on the intent-to-treat principle. The setting was 2 university hospitals in France (at Bordeaux and Dijon) from January 2011 (first participant first visit) to February 2013 (last participant last visit). The analysis was conducted from January to November 2016. Participants were 120 individuals free of any retinal ocular disease. They were first-generation offspring of parents with neovascular AMD.
Interventions
Participants were randomized in a 1:1 ratio to receive either 2 daily dietary supplementation capsules or placebo for 6 months.
Main Outcomes and Measures
The primary assessment criterion was the evolution of MPOD after 6 months of supplementation (value of both eligible eyes) measured using the modified MPD-Visucam 200 (Carl Zeiss Meditec) and the modified Heidelberg Retina Angiograph (Heidelberg Engineering) (HRA) at 0.98° eccentricity. The statistical analysis was adjusted for hospital and for risk factors.
Results
Overall, 120 participants (60 in each group) were included, and 239 eyes were analyzed (119 in the lutein plus zeaxanthin [L + Z] group and 120 in the placebo group). Their mean (SD) age was 56.7 (6.6) years, and 71.7% (n = 86) were female. A statistically significant increase in plasma lutein and zeaxanthin was shown in the L + Z group after 3 months and 6 months of treatment compared with the placebo group. However, the difference between groups in the evolution of MPOD measured by HRA 0.98° eccentricity between 6 months and baseline was 0.036 (95% CI, −0.037 to 0.110) (P = .33).
Conclusions and Relevance
Among first-generation offspring of parents with neovascular AMD in the LIMPIA trial, MPOD as measured with the modified HRA and the MPD-Visucam was not modified after 6 months of lutein and zeaxanthin dietary supplementation despite plasma levels showing continuous exposure to lutein and zeaxanthin. Further research is necessary to understand the mechanism of absorption and metabolism of these nutrients in the macula, the best way to measure MPOD, and the clinical benefit for the patients.
Trial Registration
clinicaltrials.gov Identifier: NCT01269697
This randomized clinical trial estimates the efficiency of dietary supplementation containing lutein, zeaxanthin, ω-3 polyunsaturated fatty acids, and vitamins to increase the density of macular pigment in first-generation offspring of parents with neovascular age-related macular degeneration.
Introduction
Age-related macular degeneration (AMD) leads to photoreceptor degeneration of the macula and to loss of central vision. The role of antioxidants in preventing the onset of macular damage was demonstrated in the interventional Age-Related Eye Disease Study (AREDS), which showed that dietary supplementation with antioxidants (vitamin C, vitamin E, and beta carotene) and oligo-elements (zinc) may slow the evolution of some forms of AMD. Macular pigment protects the macula from photooxidation through its light-screening capacity and its antioxidant activity. Lutein and zeaxanthin are the main components of macular pigment. These 2 xanthophylls have a protective role against retinal oxidation through the absorption of damaging blue light, neutralization of photosensitizers and reactive oxygen species, and scavenging of free radicals.
There is growing interest in the potential role of dietary lutein and zeaxanthin to prevent the development of AMD. A first step toward the demonstration of a preventive effect of macular pigment for AMD consists in the evaluation of the effect of lutein and zeaxanthin supplementation on plasma and retinal concentrations in individuals with AMD. Previous randomized clinical trials showed increased plasma and retinal concentrations of lutein and zeaxanthin after dietary supplementation in patients with early AMD and in a population of women aged 60 to 80 years.
Regarding risk factors, multiple environmental and genetic risk factors may predispose to the development of AMD. The genetic component appears to explain a large proportion of risk variation, and individuals with a family history of AMD have a higher risk of developing AMD. Moreover, individuals with a confirmed family history of AMD have significantly lower levels of macular pigment optical density (MPOD) than individuals with no known family history of the disease. Whether dietary supplements containing lutein and zeaxanthin may increase MPOD in those with high genetic risk of AMD remains unknown. Therefore, we performed a randomized clinical trial based on the hypothesis that dietary supplementation containing lutein, zeaxanthin, ω-3 polyunsaturated fatty acids, and antioxidants could increase the density of macular pigment in first-generation offspring of parents with neovascular AMD.
Methods
Study Design and Treatments
The Lutein Influence on Macula of Persons Issued From AMD Parents (LIMPIA) is a phase 3, double-blind, randomized clinical trial (clinicaltrials.gov Identifier NCT01269697) performed at 2 university hospitals in France (at Bordeaux and Dijon) from January 2011 (first participant first visit) to February 2013 (last participant last visit). It aimed to evaluate the efficacy of daily dietary supplementation with lutein, zeaxanthin, ω-3 polyunsaturated fatty acids, and antioxidants to increase MPOD through elevated concentrations of plasma lutein and zeaxanthin (trial protocol in Supplement 1). The analysis was conducted from January to November 2016.
Adult participants aged 40 to 70 years were required to have at least one parent with a history of neovascular AMD and have an Early Treatment Diabetic Retinopathy Study (ETDRS) visual acuity of 20/25 or better. In addition, they had to remain free of any sign of late AMD for both eyes or other eye disease that would have made AMD evaluation and visual acuity measurements unlikely or difficult (ie, severe glaucoma, myopia of at least −6 diopters, or other severe retinopathy). Individuals with a history of cataract surgery or opacities preventing the evaluation of retinal photographs (cataract or corneal dystrophy) and individuals with food supplementation in the previous year or predicted to be noncompliant were excluded. The trial was conducted in compliance with the Good Clinical Practice guideline and the Declaration of Helsinki. The study protocol and questionnaires were reviewed and approved by the ethics committee of Centre Hospitalier Universitaire Bordeaux. All participants were apprised about the study and gave written informed consent.
Participants were randomized in a 1:1 ratio to receive either 2 daily dietary nutritional supplementation capsules or placebo for 6 months (M6). Each participant was treated for 6 months with capsules of a dietary supplement (2 times daily, before breakfast and lunch) containing lutein (5 mg), zeaxanthin (1 mg), vitamin C (90 mg), vitamin E (15 mg), zinc (7.5 mg), copper (<0.5 mg), and resveratrol (0.5 mg), as well as 33 mg of fish oil (Epax; Ålesund, Norway) that included 50% ω-3 (Nutrof Total; Laboratoires Théa) or a matched placebo containing paraffin provided by Laboratoires Théa. All other dietary supplements containing lutein or zeaxanthin were not allowed throughout the study. Treatments were allocated online by a clinical research associate using a centralized randomization list stratified by hospital (random block sizes of 4 and 6).
Study Evaluations
Follow-up visits were scheduled after 3 months (M3) and M6 of treatment and then during a 6-month extended follow-up period at 9 months (M9) and 12 months (M12). Standardized case report forms were used to record demographics and medical and family history at baseline (D0), including AMD phenotype. A standardized food questionnaire was administered to assess previous consumption of lutein, zeaxanthin, docosahexaenoic acid, and fish oil. All participants received a complete ophthalmic examination with refraction, slitlamp biomicroscopy, and ophthalmoscopy. Lens opacity was assessed at slitlamp biomicroscopy using the Lens Opacities Classification System, version II. Visual function was recorded by measuring best-corrected visual acuity and contrast sensitivity with the Pelli-Robson contrast sensitivity test. The AMD status was assessed by ophthalmoscopy, color retinal photographs, and spectral-domain optical computed tomography (SD-OCT SPECTRALIS; Heidelberg Engineering). All participants underwent MPOD measurements of both eyes at D0 and then at M3, M6, M9, and M12, first with the modified MPD-Visucam 200 (Carl Zeiss Meditec) and then the modified Heidelberg Retina Angiograph (Heidelberg Engineering) (HRA) on the same day through dilated pupils (one drop of tropicamide [0.5%] and phenylephrine hydrochloride [2.5%]) as described previously. The MPOD measurements were performed at each hospital by a single examiner (M.-B.R. in Bordeaux and C.C.-G. in Dijon). Compliance was evaluated by a questionnaire and by collecting empty blister packs.
MPOD Measurements
The macular pigment density module for the MPD-Visucam 200 used the reflectance of a single 460-nm wavelength based on a single blue-reflection ophthalmoscopy image to determine MPOD and its spatial distribution. Shading was used that approximates the reflectance of the fundus in the absence of macular pigment. The MPD-Visucam 200 is based on a 3-dimensional parabolic function automatically fitted to fundus reflectance at peripheral locations. The individual was positioned in front of the ophthalmoscope and was instructed to look at a target inside. The fundus was illuminated by a monochromatic blue light. The following 4 MPOD parameters were automatically calculated: maximum optical density (MPOD measured at the peak), mean right eye (mean MPOD within the measurement area), area (area where macular pigment could be detected), and volume (sum of all optical densities) as recommended by the manufacturer (Carl Zeiss Meditec).
For HRA measurements, each participant was positioned in front of the camera and was instructed to look straight ahead. After focusing the scanning laser ophthalmoscope on the macular region, sequences of 208 images were captured at 488 nm (well absorbed) and 514 nm (minimally absorbed) at least 30 seconds after retinal bleaching as described by Trieschmann et al. The MPOD maps were generated by digital subtraction of the log autofluorescence images. We recorded MPOD at 0° eccentricity, and we located circles centered on the fovea at eccentricities of 0.50°, 1.00°, 2.00°, and 6.00°; the mean MPOD values were calculated for each using the software provided by the manufacturer of the device (Heidelberg Engineering). The MPOD was expressed in optical density units. Typical macular pigment distribution can follow 3 different patterns (with a central peak, a ringlike pattern, or a steady decline), and MPOD was in most cases optically undetectable at 6.00° eccentricity.
Analysis of Blood Samples
Plasma lipids were analyzed at the biochemistry department of Centre Hospitalier Universitaire Bordeaux from fasting blood samples collected at baseline and M12. Plasma lutein and zeaxanthin measurements were performed at DSM Nutritional Products (Kaiseraugst, Switzerland). Plasma samples were analyzed for lutein (sum of all E isomers and Z isomers) and zeaxanthin (sum of all E isomers and Z isomers).
Statistical Analysis
The sample size was calculated based on an expected mean MPOD of 0.36 at M6 with the HRA at 0.98° eccentricity in the placebo group, an expected increase of at least 20% (ie, difference of 0.07 U) in the group receiving lutein and zeaxanthin supplementation, and a common SD of 0.13 U. With a power of 80% and a 2-sided type I error of 5%, a total of 53 participants were to be recruited in each group using a 2-sample t test, assuming equal variances (proc power in SAS, version 9.1; SAS Institute Inc). Taking into account potential withdrawals, the total sample size was increased to 120 participants. Moreover, this sample size allowed sufficient power for comparison of the mean plasma lutein and zeaxanthin concentrations, assuming an expected increase of at least 30% in the experimental group compared with a hypothesized mean (SD) of approximately 192 (88) µg/L. All statistical analyses were performed using SAS version 9.1 (SAS Institute Inc).
Data were described using usual statistics, including means (SDs) and first quartile, third quartile, minimum, and maximum values for quantitative variables. Frequencies and percentages were used for qualitative or ordinal variables. Differences between treatment groups were analyzed using a linear mixed model (fixed effect) with random intercept (allowing to take into account the correlation between data of the 2 eyes for each individual) adjusted for age, sex, body mass index (BMI), smoking, and hospital. Analyses were based on the intent-to-treat principle, with missing data equaling failure (for each visit, missing values were replaced by the lowest observed value for both treatment groups combined). One-sided P < .05 was the threshold of statistical significance.
Results
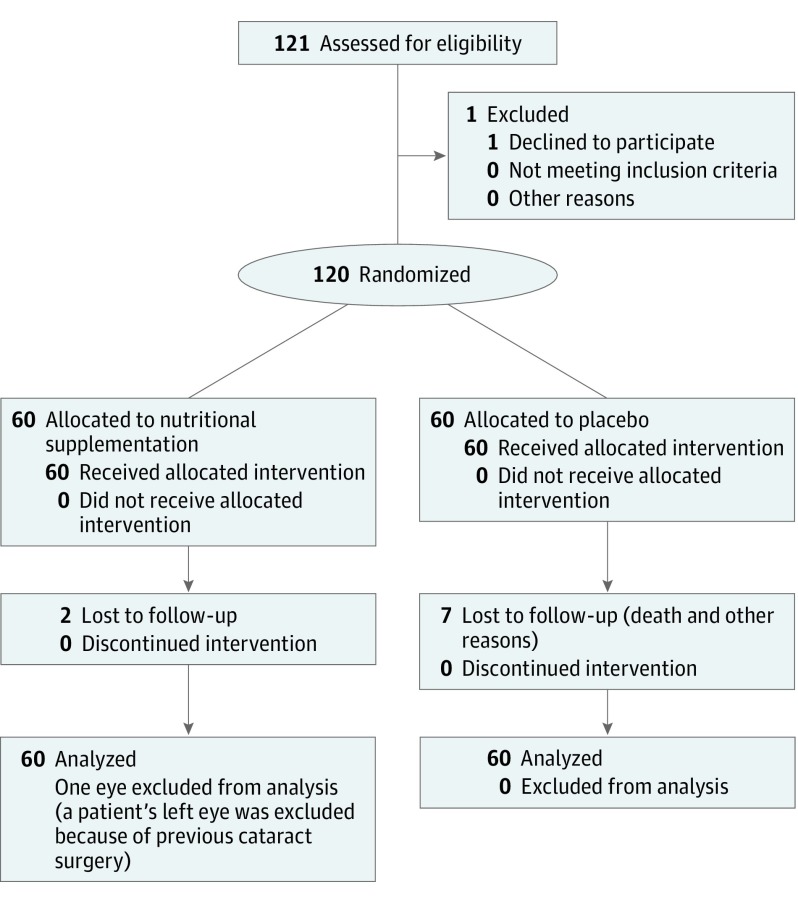
A total of 120 participants (60 in each group) were included at 2 university hospitals in France (at Bordeaux and Dijon). Their mean (SD) age was 56.7 (6.6) years, and 71.7% (n = 86) were female. A total of 239 eyes were analyzed (119 in the lutein plus zeaxanthin [L + Z] group and 120 in the placebo group); one eye was excluded from analysis because of previous cataract surgery (Figure 1).
Figure 1. CONSORT Diagram.
CONSORT indicates Consolidated Standards of Reporting Trials.
Main Baseline Characteristics
The 2 groups (L + Z and placebo) did not differ by age, sex, BMI, smoking, medical history, or plasma lipid concentrations at baseline (Table 1). Mothers (97 of 118 participants) had AMD onset at a mean (SD) age of 78 (8) years, and fathers (25 of 100 participants) had AMD onset at a mean (SD) age of 76 (8) years. Four participants (6.7%) in the L + Z group had a previous ocular history (treated glaucoma in 2 individuals). The food questionnaire showed that 15.0% (n = 9) of participants in the L + Z group and 11.7% (n = 7) of participants in the placebo group were users of vitamins or other dietary supplements. The mean (SD) lutein and zeaxanthin consumption during the previous year was 1139 (597) × 103 U in the L + Z group vs 949 (578) × 103 U in the placebo group.
Table 1. Baseline Sociodemographic, Behavioral, and Plasma Characteristics Among 120 Participants.
| Variable | L + Z (n = 60) | Placebo (n = 60) |
|---|---|---|
| Age, mean (SD) [range], y | 57.6 (6.5) [42-71] |
55.8 (6.6) [44-68] |
| Female, No. (%) | 47 (78.3) | 39 (65.0) |
| Parent history of AMD, No./total No. (%) | ||
| Mother | 52/59 (88.1) | 45/59 (76.3) |
| Father | 9/50 (18.0) | 16/50 (32.0) |
| BMI, mean (SD) | 24.8 (4.8) | 24.8 (4.1) |
| Smoking, No. (%) | ||
| Never | 25 (41.7) | 28 (46.7) |
| Former | 20 (33.3) | 18 (30.0) |
| Current | 15 (25.0) | 14 (23.3) |
| Ocular history, No. (%) | 4 (6.7) | 0 |
| Iris color, No./total No. (%) | ||
| Blue | 13/58 (22.4) | 11 (18.3) |
| Green | 9/58 (15.5) | 11 (18.3) |
| Brown | 36/58 (62.1) | 38 (63.3) |
| Medical history, No. (%) | ||
| Hypertension | 13 (21.7) | 11 (18.3) |
| Diabetes | 2 (3.3) | 1 (1.7) |
| Hypercholesterolemia | 11 (18.3) | 12 (20.0) |
| Previous vitamin or food supplementation, No. (%) | ||
| Yes | 9 (15.0) | 7 (11.7) |
| No | 51 (85.0) | 53 (88.3) |
| Lutein and zeaxanthin consumption during the previous year, mean (SD) [range], ×103 U | 1139 (597) [181-3928] |
949 (578) [74-2261] |
| Total cholesterol, mean (SD) [range], mg/dL | 223 (1) [166-294] |
223 (1) [155-290] |
| High-density lipoprotein cholesterol, mean (SD) [range], mg/dL | 69 (0) [44-114] |
69 (1) [38-133] |
| Triglycerides, mean (SD) [range], mg/dL | 105 (1) [43-251] |
105 (1) [31-347] |
| ETDRS visual acuity | (n = 116) | (n = 120) |
| Letters, mean (SD) [range] | 86.7 (4.1) [63-95] |
86.7 (4.5) [74-98] |
| Contrast sensitivity | (n = 119) | (n = 120) |
| Mean (SD) [range] | 1.7 (0.2) [0.0-2.0] |
1.7 (0.1) [1.4-2.0] |
| Lens extraction, No./total No. (%) | 0/117 | 2/118 (1.7) |
| Lens opacity LOCS II≥2, No./total No. (%) | ||
| Nuclear | 34/119 (28.6) | 32/120 (26.7) |
| Cortical | 6/119 (5.0) | 13/120 (10.8) |
| Subcapsular | 5/119 (4.2) | 6/120 (5.0) |
| SD-OCT, No./total No. (%) | ||
| Classic drusen | 12/73 (16.4) | 16/74 (21.6) |
| Other macular abnormality | 1/73 (1.4) | 0/74 |
| Retinophotography, No./total No. (%) | ||
| Normal | 93/118 (78.8) | 90/120 (75.0) |
| Abnormala | 25/118 (21.2) | 30/120 (25.0) |
Abbreviations: AMD, age-related macular degeneration; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); ETDRS, Early Treatment Diabetic Retinopathy Study; L + Z, lutein plus zeaxanthin; LOCS II, Lens Opacities Classification System, version II; SD-OCT, spectral-domain optical computed tomography.
SI conversion factors: To convert cholesterol level to millimoles per liter, multiply by 0.0259; to convert triglycerides level to millimoles per liter, multiply by 0.0113.
Mainly small drusen.
There were no relevant differences between the L + Z and placebo groups in ocular baseline characteristics (Table 1). Retinophotographs were normal in approximately 75% of eyes for both groups. No reticular drusen or features of late AMD were identified in either group. Large drusen were found in 2 eyes (1.7%) in the L + Z group and in 3 eyes (2.5%) in the placebo group, and small drusen were observed in 23 eyes (19.3%) in the L + Z group and in 25 eyes (20.8%) in the placebo group. On SD-OCT, 12 of 73 eyes (16.4%) in the L + Z group and 16 of 74 eyes (21.6%) in the placebo group had classic drusen.
Evolution of Plasma Lutein and Zeaxanthin Concentrations
As summarized in Table 2, plasma lutein and zeaxanthin concentrations were increased from 0.38 to 0.58 µmol/L for lutein and from 0.08 to 0.14 µmol/L for zeaxanthin after M3 of supplementation in the L + Z group, while no significant changes were shown in the placebo group. The plasma levels of lutein and zeaxanthin remained elevated during M6 after randomization and then decreased to baseline values at M3 after treatment discontinuation (at M9). Differences between treatment groups were statistically significant at M3 and M6. Small but statistically significant differences were shown between groups after M3 of treatment discontinuation for lutein and after M3 and M6 of treatment discontinuation for zeaxanthin.
Table 2. Plasma Lutein and Zeaxanthin Concentrations Among 120 Participants.
| Variable | L + Z (n = 60) | Placebo (n = 60) | Between-Group Differencea | |
|---|---|---|---|---|
| Estimate (95% CI) | P Value | |||
| Total Lutein, µmol/L | ||||
| Baseline concentration | (n = 59) | (n = 59) | ||
| Mean (SD) | 0.38 (0.15) | 0.37 (0.16) | NA | NA |
| Mean change from baseline | ||||
| M3 minus D0 | (n = 59) | (n = 57) | ||
| Mean (SD) | 0.58 (0.41) | −0.02 (0.09) | 0.609 (0.501 to 0.716) | <.001 |
| M6 minus D0 | (n = 59) | (n = 55) | ||
| Mean (SD) | 0.59 (0.39) | −0.04 (0.12) | 0.634 (0.528 to 0.740) | <.001 |
| M9 minus D0 | (n = 57) | (n = 56) | ||
| Mean (SD) | 0.02 (0.10) | −0.02 (0.12) | 0.048 (0.008 to 0.088) | .02 |
| M12 minus D0 | (n = 58) | (n = 55) | ||
| Mean (SD) | 0.01 (0.10) | −0.02 (0.11) | 0.033 (−0.005 to 0.072) | .09 |
| Total Zeaxanthin, µmol/L | ||||
| Baseline concentration | (n = 59) | (n = 59) | ||
| Mean (SD) | 0.08 (0.04) | 0.09 (0.04) | NA | NA |
| Mean change from baseline | ||||
| M3 minus D0 | (n = 59) | (n = 57) | ||
| Mean (SD) | 0.14 (0.09) | −0.01 (0.03) | 0.142 (0.118 to 0.166) | <.001 |
| M6 minus D0 | (n = 59) | (n = 55) | ||
| Mean (SD) | 0.14 (0.09) | −0.01 (0.03) | 0.145 (0.119 to 0.170) | <.001 |
| M9 minus D0 | (n = 57) | (n = 56) | ||
| Mean (SD) | 0.01 (0.04) | −0.01 (0.03) | 0.021 (0.008 to 0.033) | .001 |
| M12 minus D0 | (n = 58) | (n = 55) | ||
| Mean (SD) | 0.01 (0.03) | −0.01 (0.03) | 0.018 (0.006 to 0.029) | .003 |
Abbreviations: D0, baseline; L + Z, lutein plus zeaxanthin; M3, 3 months; M6, 6 months; M9, 9 months; M12, 12 months; NA, not applicable.
Linear mixed model with random intercept on available data adjusted for age, sex, body mass index, smoking, and hospital.
Macular Pigment Measurements
The MPOD measured with the modified HRA and changes in MPOD from baseline are listed for each degree of eccentricity in eTable 1 in Supplement 2. Results did not show any significant change from baseline in either treatment group for the mean MPOD within each degree of eccentricity at each visit. There was no statistically significant difference between groups.
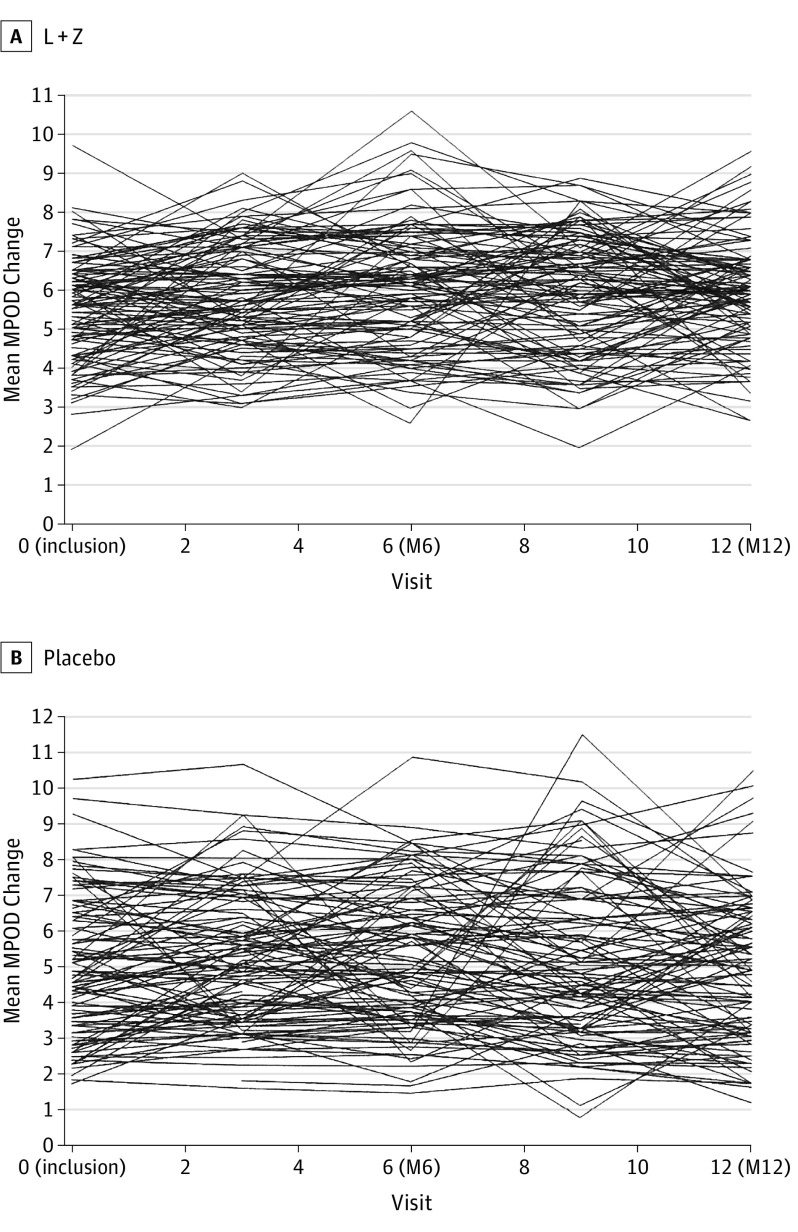
As summarized in Figure 2, the mean (SD) MPOD change between baseline and M6 within 0.98° eccentricity was 0.05 (0.15) in the L + Z group (observed in 108 participants) and 0.03 (0.16) in the placebo group (observed in 107 participants). With missing data equaling failure, the mean (SD) evolution was 0.01 (0.22) in the L + Z group and −0.03 (0.22) in the placebo group (Table 3). There was no statistically significant difference between groups in MPOD variation using the unadjusted linear model or the adjusted model.
Figure 2. Primary End Point of Macular Pigment Optical Density (MPOD) Measured by the Modified Heidelberg Retina Angiograph (Heidelberg Engineering) at 0.98° Eccentricity for the Treated and Placebo Participants.
Shown are results in the 2 study groups. L + Z indicates lutein plus zeaxanthin; M6, 6 months; and M12, 12 months.
Table 3. Macular Pigment Optical Density (MPOD) as Measured With the Modified Heidelberg Retina Angiograph (Heidelberg Engineering) at 0.98° Eccentricity (Primary Efficacy Criterion) Among 239 Eyesa.
| Variable | L + Z (n = 119) | Placebo (n = 120) |
|---|---|---|
| Baseline MPOD | (n = 116) | (n = 117) |
| Mean (SD) | 0.55 (0.13) | 0.56 (0.18) |
| Median | 0.56 | 0.54 |
| Q1 to Q3 | 0.44 to 0.64 | 0.41 to 0.69 |
| Range | 0.19 to 0.97 | 0.26 to 1.04 |
| M6 MPOD | (n = 110) | (n = 110) |
| Mean (SD) | 0.61 (0.15) | 0.58 (0.18) |
| Median | 0.61 | 0.56 |
| Q1 to Q3 | 0.50 to 0.71 | 0.44 to 0.71 |
| Range | 0.26 to 1.06 | 0.24 to 1.10 |
| Mean change from baseline (M6 minus M0)b | (n = 119) | (n = 120) |
| Mean (SD) | 0.01 (0.22) | −0.03 (0.22) |
| Median | 0.02 | 0.01 |
| Q1 to Q3 | −0.06 to 0.13 | −0.08 to 0.07 |
| Range | −0.50 to 0.61 | −0.50 to 0.50 |
Abbreviations: L + Z, lutein plus zeaxanthin; M0, 0 months; M6, 6 months; Q1, first quartile; Q3, third quartile.
The between-group differences were not significant. In the unadjusted model, the estimate (95% CI) was 0.034 (−0.039 to 0.108), P = .36. In the adjusted model (linear mixed model with random intercept adjusted for age, sex, body mass index, smoking, and hospital), the estimate (95% CI) was 0.036 (−0.037 to 0.110), P = .33.
With missing data equaling failure.
Using the modified MPD-Visucam 200, no relevant differences were shown in MPOD between the L + Z and placebo groups. These results are summarized in eTable 2 in Supplement 2.
Discussion
In this randomized clinical trial of a 6-month dietary supplementation with lutein and zeaxanthin, ω-3 polyunsaturated fatty acids, and antioxidants that included first-generation offspring of parents with neovascular AMD, intake of dietary supplementation led to a rapid and sustained increase in plasma lutein and zeaxanthin concentrations but did not translate into elevation of macular pigment density. The MPOD after lutein and zeaxanthin supplementation was measured by the modified HRA at each eccentricity (0.51°, 0.98°, 1.99°, and 6.00°) and by the reflectance methods using the modified MPD-Visucam 200.
In contrast, previous randomized controlled interventional studies using Visucam measurement or heterochromatic flicker photometry have shown that nutrient formulations containing lutein and zeaxanthin produced a statistically significant increase in macular pigment density among individuals with AMD and in a population of older women. Although the supplement formulations differed in those studies, the dosages of lutein and zeaxanthin and the duration of intervention are consistent with the LIMPIA randomized clinical trial, and the elevations of plasma lutein and zeaxanthin concentrations are similar. Therefore, the absence of MPOD elevation after lutein and zeaxanthin supplementation in the LIMPIA trial cannot be due to nutrient malabsorption. It is known that MPOD responses to lutein and zeaxanthin supplementation are variable across individuals. In previous investigations, 20% to 50% of relevant study participants had low plasma response, low retinal response, or both. In the Lutein Nutrition Effects Measured by Autofluorescence (LUNA) study, among patients with AMD receiving lutein and zeaxanthin supplementation, there was an increase in plasma lutein from 0.16 mg/mL at baseline to 0.593 mg/mL concomitant with a mean MPOD increase by 16% after 6 months.
Despite increased plasma lutein and zeaxanthin after dietary supplementation, a substantial proportion of participants herein did not show a detectable increase in MPOD over the 6-month study period. We believe that the absence of increased MPOD could be due to the macular saturation in a young population with virtually no risk factors for AMD besides the family history. Trieschmann et al reported that the greatest increase in MPOD at 0.50° eccentricity in individuals receiving supplemental lutein and zeaxanthin was found in those with the lowest baseline MPOD. It was suggested that the mechanisms underlying retinal capture or stabilization of macular lutein and zeaxanthin may be saturable. Similarly, in a 12-month interventional study among patients with nonexudative AMD, the time course of MPOD using a formulation containing lutein, zeaxanthin, and ω-3 polyunsaturated fatty acids showed a rapid increase within M3, reached a plateau after M6, and demonstrated sustained values until 1M2, consistent with a saturable mechanism.
In a young, healthy population, dietary supplementation with lutein and zeaxanthin resulted in a statistically significant (although modest) increase of 4% to 5% in MPOD after M6 (corresponding to MPOD variation from 0.44 to 0.47 U using heterochromatic flicker photometry), while no effect was shown after M3 despite statistically significant increases in plasma lutein and zeaxanthin concentrations (2.3 times and 6.3 times from baseline, respectively). The rise in MPOD at M6 was only weakly related to the plasma lutein level measured at M3.
Nevertheless, the baseline mean MPOD among participants in the LIMPIA trial (0.6 U at 0.51° eccentricity) was much higher compared with studies in other populations that included patients with AMD. Nolan et al previously showed that individuals with a family history of AMD had lower MPOD compared with individuals without a family history of AMD. However, besides a family history of AMD, most of the participants in the LIMPIA trial had no risk factors that may influence the concentration of carotenoids in the retina, such as high BMI, smoking, diabetes, or dyslipidemia.
Our study enrolled mostly women (78.3% [47 of 60] in the L + Z group), who tend to have a poorer response to lutein and zeaxanthin supplementation than men. Results from the Carotenoids in Age-Related Eye Disease Study (CAREDS) indicated that women with high lutein and zeaxanthin levels in their diet and plasma also had high MPOD. However, only 3% of MPOD variation was explained by lutein and zeaxanthin supplementation, and 8% of MPOD variation could be justified by dietary, lifestyle, medical, and physical variables.
Taken together, results of the LIMPIA trial suggest poor capture of plasma lutein and zeaxanthin in the macula, consistent with a young, female population without major risk factors except for a family history of neovascular AMD. Further research is necessary to identify the best method to measure macular pigment.
Limitations
Limitations of our study are related to participants who were phakic. Therefore, we cannot extrapolate MPOD measurement results in pseudophakic eyes.
Conclusions
The LIMPIA trial showed no significant difference in MPOD with lutein and zeaxanthin dietary supplementation after M6 of treatment despite increased plasma levels of lutein and zeaxanthin in first-generation offspring of parents with neovascular AMD. Additional research is needed to better evaluate macular pigment density and to understand cellular uptake and metabolism of these nutrients within the macula.
Trial Protocol
eTable 1. Macular Pigment Optical Density (Modified HRA) Within Each Eccentricity
eTable 2. Macular Pigment Optical Density (MPD Visucam)
References
- 1.Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS Report No. 8 [published correction appears in Arch Ophthalmol. 2008;126(9):1251]. Arch Ophthalmol. 2001;119(10):1417-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Widomska J, Subczynski WK. Why has nature chosen lutein and zeaxanthin to protect the retina? J Clin Exp Ophthalmol. 2014;5(1):326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.García-Layana A, Recalde S, Alamán AS, Robredo PF. Effects of lutein and docosahexaenoic acid supplementation on macular pigment optical density in a randomized controlled trial. Nutrients. 2013;5(2):543-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson EJ, Chung HY, Caldarella SM, Snodderly DM. The influence of supplemental lutein and docosahexaenoic acid on serum, lipoproteins, and macular pigmentation. Am J Clin Nutr. 2008;87(5):1521-1529. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Bedell M, Zhang K. Age-related macular degeneration: genetic and environmental factors of disease. Mol Interv. 2010;10(5):271-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seddon JM, Ajani UA, Mitchell BD. Familial aggregation of age-related maculopathy. Am J Ophthalmol. 1997;123(2):199-206. [DOI] [PubMed] [Google Scholar]
- 7.Klaver CC, Wolfs RC, Assink JJ, van Duijn CM, Hofman A, de Jong PT. Genetic risk of age-related maculopathy: population-based familial aggregation study. Arch Ophthalmol. 1998;116(12):1646-1651. [DOI] [PubMed] [Google Scholar]
- 8.Nolan JM, Stack J, O’Connell E, Beatty S. The relationships between macular pigment optical density and its constituent carotenoids in diet and serum. Invest Ophthalmol Vis Sci. 2007;48(2):571-582. [DOI] [PubMed] [Google Scholar]
- 9.Chylack LT Jr, Leske MC, McCarthy D, Khu P, Kashiwagi T, Sperduto R. Lens Opacities Classification System II (LOCS II). Arch Ophthalmol. 1989;107(7):991-997. [DOI] [PubMed] [Google Scholar]
- 10.Creuzot-Garcher C, Koehrer P, Picot C, Aho S, Bron AM. Comparison of two methods to measure macular pigment optical density in healthy subjects. Invest Ophthalmol Vis Sci. 2014;55(5):2941-2946. [DOI] [PubMed] [Google Scholar]
- 11.Trieschmann M, Beatty S, Nolan JM, et al. . Changes in macular pigment optical density and serum concentrations of its constituent carotenoids following supplemental lutein and zeaxanthin: the LUNA study. Exp Eye Res. 2007;84(4):718-728. [DOI] [PubMed] [Google Scholar]
- 12.Merle BM, Maubaret C, Korobelnik JF, et al. . Association of HDL-related loci with age-related macular degeneration and plasma lutein and zeaxanthin: the Alienor study. PLoS One. 2013;8(11):e79848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolf-Schnurrbusch UE, Röösli N, Weyermann E, Heldner MR, Höhne K, Wolf S. Ethnic differences in macular pigment density and distribution. Invest Ophthalmol Vis Sci. 2007;48(8):3783-3787. [DOI] [PubMed] [Google Scholar]
- 14.Arnold C, Winter L, Fröhlich K, et al. . Macular xanthophylls and ω-3 long-chain polyunsaturated fatty acids in age-related macular degeneration: a randomized trial. JAMA Ophthalmol. 2013;131(5):564-572. [DOI] [PubMed] [Google Scholar]
- 15.Dawczynski J, Jentsch S, Schweitzer D, Hammer M, Lang GE, Strobel J. Long term effects of lutein, zeaxanthin and omega-3-LCPUFAs supplementation on optical density of macular pigment in AMD patients: the LUTEGA study. Graefes Arch Clin Exp Ophthalmol. 2013;251(12):2711-2723. [DOI] [PubMed] [Google Scholar]
- 16.Mares JA, LaRowe TL, Snodderly DM, et al. ; CAREDS Macular Pigment Study Group and Investigators . Predictors of optical density of lutein and zeaxanthin in retinas of older women in the Carotenoids in Age-Related Eye Disease Study, an ancillary study of the Women’s Health Initiative. Am J Clin Nutr. 2006;84(5):1107-1122. [DOI] [PubMed] [Google Scholar]
- 17.Hammond CJ, Liew SH, Van Kuijk FJ, et al. . The heritability of macular response to supplemental lutein and zeaxanthin: a classic twin study. Invest Ophthalmol Vis Sci. 2012;53(8):4963-4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyers KJ, Johnson EJ, Bernstein PS, et al. . Genetic determinants of macular pigments in women of the Carotenoids in Age-Related Eye Disease Study. Invest Ophthalmol Vis Sci. 2013;54(3):2333-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammond BR Jr, Curran-Celentano J, Judd S, et al. . Sex differences in macular pigment optical density: relation to plasma carotenoid concentrations and dietary patterns. Vision Res. 1996;36(13):2001-2012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Macular Pigment Optical Density (Modified HRA) Within Each Eccentricity
eTable 2. Macular Pigment Optical Density (MPD Visucam)