Key Points
Question
Can multiple en face image averaging improve the image quality of optical coherence tomography angiography in the choriocapillaris layer?
Findings
In this cross-sectional case series among 17 eyes of 17 healthy individuals, the averaged optical coherence tomography angiography images of the choriocapillaris showed a meshwork structure that appeared to resemble the histology of the human choriocapillaris. The mean (SD) diameter of the vessels was 22.8 (5.8) µm.
Meaning
The results of this study suggest that multiple en face averaging may provide better visualization of the choriocapillaris on optical coherence tomography angiography.
Abstract
Importance
Imaging of the choriocapillaris in vivo is challenging with existing technology. Optical coherence tomography angiography (OCTA), if optimized, could make the imaging less challenging.
Objective
To investigate multiple en face image averaging on OCTA images of the choriocapillaris.
Design, Setting, and Participants
Observational, cross-sectional case series at a referral institutional practice in Los Angeles, California. From the original cohort of 21 healthy individuals, 17 normal eyes of 17 participants were included in the study. The study dates were August to September 2016.
Exposures
All participants underwent OCTA imaging of the macula covering a 3 × 3-mm area using OCTA software (Cirrus 5000 with AngioPlex; Carl Zeiss Meditec). One eye per participant was repeatedly imaged to obtain 9 OCTA cube scan sets. Registration was first performed using superficial capillary plexus images, and this transformation was then applied to the choriocapillaris images. The 9 registered choriocapillaris images were then averaged. Quantitative parameters were measured on binarized OCTA images and compared with the unaveraged OCTA images.
Main Outcome and Measure
Vessel caliber measurement.
Results
Seventeen eyes of 17 participants (mean [SD] age, 35.1 [6.0] years; 9 [53%] female; and 9 [53%] of white race/ethnicity) with sufficient image quality were included in this analysis. The single unaveraged images demonstrated a granular appearance, and the vascular pattern was difficult to discern. After averaging, en face choriocapillaris images showed a meshwork appearance. The mean (SD) diameter of the vessels was 22.8 (5.8) µm (range, 9.6-40.2 µm). Compared with the single unaveraged images, the averaged images showed more flow voids (1423 flow voids [95% CI, 967-1909] vs 1254 flow voids [95% CI, 825-1683], P < .001), smaller average size of the flow voids (911 [95% CI, 301-1521] µm2 vs 1364 [95% CI, 645-2083] µm2, P < .001), and greater vessel density (70.7% [95% CI, 61.9%-79.5%] vs 61.9% [95% CI, 56.0%-67.8%], P < .001). The distribution of the number vs sizes of the flow voids was skewed in both unaveraged and averaged images. A linear log-log plot of the distribution showed a more homogeneous distribution in the averaged images compared with the unaveraged images.
Conclusions and Relevance
Multiple en face averaging can improve visualization of the choriocapillaris on OCTA images, transforming the images from a granular appearance to a level where the intervascular spaces can be resolved in healthy volunteers.
This cross-sectional case series investigates multiple en face image averaging for optical coherence tomography angiography images of the choriocapillaris.
Introduction
The choriocapillaris is the capillary plexus of the choroid located between the Sattler layer and Bruch membrane. It forms a dense freely anastomosing monolayer network of large capillaries and serves as the major source of nutrition for the retinal pigment epithelium (RPE) and outer retinal layers. Considering that the results of both clinical and histopathologic studies have suggested a relationship between the choroidal circulation and retinal disorders, including age-related macular degeneration and diabetic retinopathy, in vivo imaging of the choriocapillaris is thought to be of value.
However, imaging of the choriocapillaris in vivo is challenging with existing technology. Although dye-based angiography, in particular indocyanine green angiography, has long been considered the criterion standard for evaluation of the choroidal circulation, the limited depth resolution has made it difficult to resolve the choriocapillaris from the deeper vascular layers. In addition, the low lateral resolution of conventional fundus imaging makes it difficult to resolve the intervascular spaces and visualize the choriocapillary network. Instead, with fluorescein angiography, for example, early diffuse leakage through the fenestrated choriocapillaris only allows the choriocapillaris to be visualized as a diffuse grayish haze, the so-called choroidal blush.
Optical coherence tomography angiography (OCTA) offers a precise visualization of the retinal microvasculature of the fundus through motion contrast derived by detecting the reflectivity changes between multiple OCT B-scans. Unlike dye-based angiography, OCTA is free from limitations due to dye leakage and has a sufficiently high axial resolution such that en face images from the choriocapillaris layer can be selectively extracted. On the other hand, current OCTA technology still is limited by the low lateral resolution.
Recently, averaging of multiple en face OCTA images has been reported to improve the image quality of the retinal microvasculature and to have a significant consequence on the quantitative measurements in commercial OCTA systems. However, the association of en face image averaging with image quality of the choriocapillaris layer has not been reported to date. In this study, we evaluate the qualitative and quantitative results of image averaging on OCTA images of the choriocapillaris.
Methods
This observational, cross-sectional case series was approved by the Institutional Review Board of UCLA (University of California, Los Angeles), and was conducted in accord with the ethical standards stated in the Declaration of Helsinki. The study was carried out in conformity with the Health Insurance Portability and Accountability Act of 1996 regulations. The study dates were August to September 2016. All individuals signed written informed consent before participating in the study.
Participants
Twenty-one healthy individuals with no history of ophthalmologic or systemic diseases were recruited for this study. A normal retina was confirmed by biomicroscopy and structural OCT examination for all participants. Although mild refractive errors were allowed, individuals with high myopia (>6 diopters) were excluded.
OCTA Imaging
All participants underwent OCTA imaging of the macula covering a 3 × 3-mm area centered on the fovea using OCTA software (Cirrus 5000 with AngioPlex; Carl Zeiss Meditec). The software is approved by the US Food and Drug Administration for 510(k) clearance. One eye per individual was randomly selected and repeatedly imaged without pupil dilation to obtain 9 OCTA cube scan sets with sufficient image quality that met the acceptance criteria of the Doheny Image Reading Center as previously reported.
Generation of En Face OCTA Images
En face OCTA images of the choriocapillaris layer were generated by extracting slabs as defined previously. Because we were attempting to optimize our visualization of the choriocapillaris, 3 different choriocapillaris en face images were generated by extracting the following 3 different slabs: (1) an 8-μm-thick slab starting 29 μm posterior to the automated RPE segmentation, (2) a 6-μm-thick slab starting 31 μm posterior to the automated RPE segmentation, and (3) a 6-μm-thick slab starting 35 μm posterior to the RPE segmentation. From these 3 candidate en face images, the scan with the least projection artifact (ie, evidence of superficial retinal vessels) was selected for averaging. If the quality of the slabs was similar, the thinner and deeper slab was selected for the study. The same slab for all 9 images was used for each individual. The overall choroidal thickness was not considered in the selection of the position and thickness of the slab. To generate en face images of the superficial capillary plexus (SCP), we used the automated instrument-generated slabs.
Image Registration
Nine choriocapillaris en face images generated from 9 different OCTA cube scan sets were stacked to create a 9-frame video and were registered before multiple image averaging. Because the unaveraged choriocapillaris is mostly featureless, it is not a good candidate for primary use in registration. However, we have shown previously that the SCP yields excellent performance for use in registration operations.
A central rectangular area of 950 × 900 pixels was cropped for registration and averaging. Registration was first performed on the 9-frame video based on the SCP en face images. This same transformation information was then applied to the choriocapillaris layer (eFigure 1 in the Supplement). This registration method is described in detail in our previous publication. However, to further improve registration performance, we made one additional optimization in the present study. Specifically, the 9-frame videos were divided into 9 smaller sectors, and these smaller sectors were individually registered. After registration, these 9 sectors were then stitched back together to reconstruct the original size of the registered video.
Multiple Image Averaging
After registration, the 9 frames of the choriocapillaris were compounded into a single image by projecting the average intensity. After averaging, contrast-limited adaptive histogram equalization (https://tinyurl.com/y7x9aq3o) was performed, and the minimum intensity operation was applied between the averaged image with and without contrast-limited adaptive histogram equalization. Ten capillaries per eye were randomly selected on the averaged choriocapillaris images, and the vessel caliber was measured.
Quantitative Image Analysis of the Choriocapillaris OCTA Images
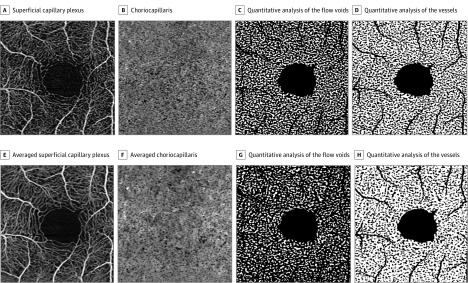
The choriocapillaris OCTA images were binarized for quantitative image analysis of the flow voids (the area without flow information) and the vessels (Figure 1). The Phansalkar method (radius, 15 pixels) was used for binarization as previously reported. Then, the thresholded images were analyzed using open source software for image analysis (“Analyze Particles” command; ImageJ) to count and measure the flow voids. Subsequently, the images were inverted, and the morphology of the vessels was analyzed using vessel density (VD) and vessel diameter index (VDI). The VD was defined as the ratio of the area occupied by vessels divided by the total area. The VDI, which represents the average vessel caliber, was calculated by dividing the total vessel area in the binarized image by the total vessel length in the skeletonized image. For both flow voids and vessels, to avoid the potential influence of the shadow of the major retinal vessels and the registration inaccuracy in the foveal avascular zone (FAZ), which has fewer landmarks for SCP registration compared with other areas, these regions were masked and eliminated from the quantitative analyses.
Figure 1. Quantitative Image Analysis of the Choriocapillaris Optical Coherence Tomography Angiography (OCTA) Images.
A, Single unaveraged OCTA image of the superficial capillary plexus. B, Single unaveraged OCTA image of the choriocapillaris. C, Binarized choriocapillaris image (B) for quantitative analysis of the flow voids. White area is the flow voids. D, Binarized choriocapillaris image (B) for quantitative analysis of the vessels. White area is the vessels. In both C and D, the shadow of the major retinal vessels and the foveal avascular zone were masked and eliminated from the analyses. E, Averaged OCTA image of the superficial capillary plexus. The image shows more continuous vessels and less background noise compared with the single unaveraged image (A). F, Averaged OCTA image of the choriocapillaris. After averaging, the poorly defined granular appearance observed in B was transformed to a meshwork appearance. G, Binarized choriocapillaris image (F) for quantitative analysis of the flow voids. White area is the flow voids. The average size of the flow voids is smaller than those in the single unaveraged image (C). H, Binarized choriocapillaris image (F) for quantitative analysis of the vessels. White area is the vessels. Vessel area is greater than those in the single unaveraged image (G).
The distribution of the number vs sizes of the flow voids was analyzed by transforming the data to a linear log-log plot to follow the power law model based on the previous report. After fitting with a regression line that followed the formula:
| log(Number) = mlog(Size) + b, |
the slope (m) and the intercept (b) were compared between the unaveraged image and the averaged image. All digital image processing was automatically executed without need for manual intervention using ImageJ (developed by Wayne Rasband, National Institutes of Health; available at http://rsb.info.nih.gov/ij/index.html).
Statistical Analysis
All values are expressed as the mean (SD), except for the quantitative measurement of the flow voids. The measurements of the flow voids are expressed using 95% CIs. Paired t tests were used to compare the flow void measurements between the single unaveraged OCTA images and the averaged images. Two-sided P < .05 was considered statistically significant. All analyses, except for the assessment of the flow void distribution, were performed using StatView (version 5.0; SAS Institute Inc). The assessment of the flow void distribution was performed using R (version 3.3.2; R Foundation for Statistical Computing).
Results
From the original cohort of 21 healthy individuals, 17 normal eyes of 17 participants met the image-quality criteria for all 9 OCTA acquisitions and were included in the study. The mean (SD) age of included participants was 35.1 (6.0) years (range, 24-49 years); 9 participants (53%) were female. Nine participants (53%) were of white race/ethnicity, 7 (41%) were Asian, and 1 (6%) was African American. Excluded from the analysis were 1 eye with at least one nonuniformly illuminated scan, 2 eyes with off-center scans, and 1 eye with significant motion.
Image registration was successfully completed in all 17 eyes, and the registered videos showed minimum evidence of misalignments in the SCP on visual inspection (Video). After registration, the averaged images showed more continuous vessels and less background noise compared with the single unaveraged images of the SCP, whereas averaging the unregistered frames resulted in blurred images (eFigure 2 in the Supplement).
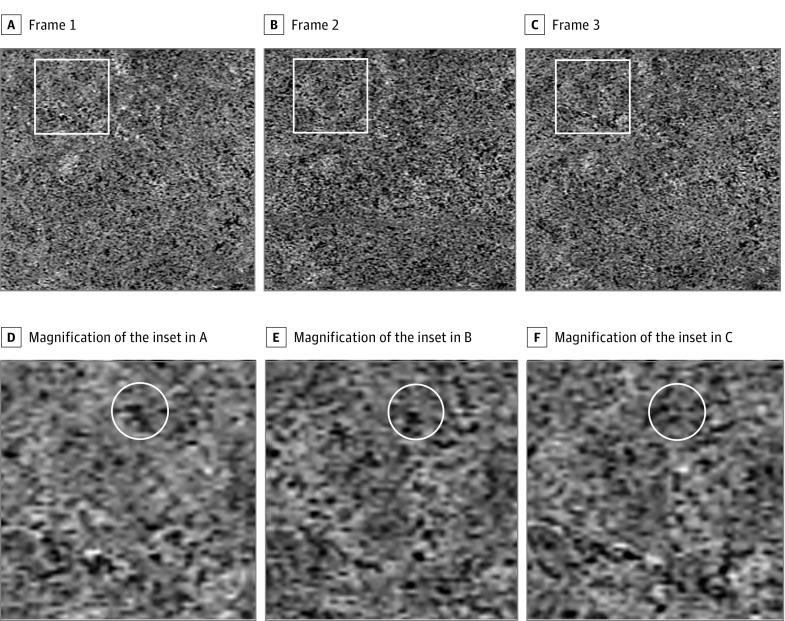
The single unaveraged choriocapillaris en face images showed a granular appearance. The bright areas are thought to represent blood flow, and the intervening dark areas are believed to indicate flow voids or areas of relative flow impairment. In the 9-frame video, this granular pattern appeared to change from frame to frame (Figure 2). The location, shape, and intensity of the flow voids also showed a difference between frames.
Figure 2. Difference in Granular Pattern of the Choriocapillaris Between the Single Unaveraged Optical Coherence Tomography Angiography Images.
A-C, Three images of the choriocapillaris slab from different optical coherence tomography angiography cube scan sets of the left eye of a woman in her early 40s. The single unaveraged choriocapillaris en face images each showed a granular appearance. The bright areas are thought to represent blood flow, and the dark areas are believed to indicate areas of impaired or absent flow (flow voids [the area without flow information]). D-F, Magnified views of the insets in A-C. The pattern of this granular appearance can be seen to change from image to image, although there is some overlap (white circle).
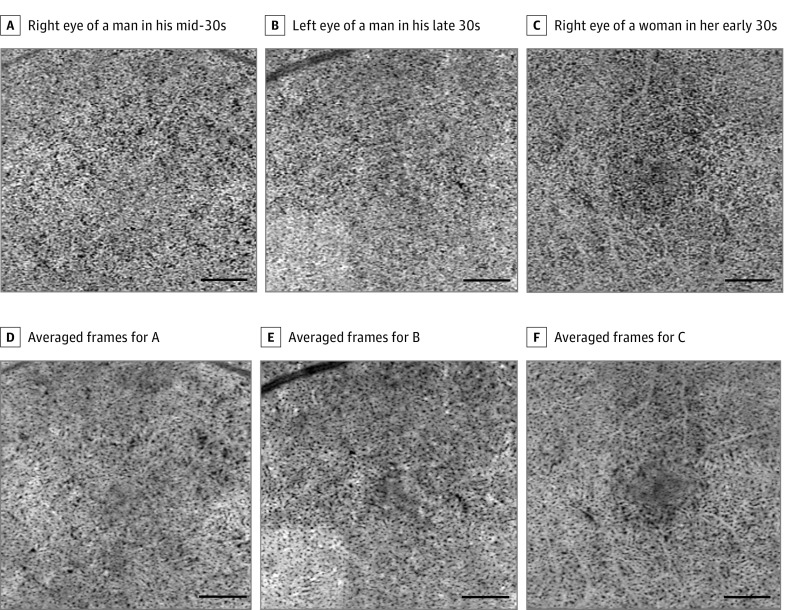
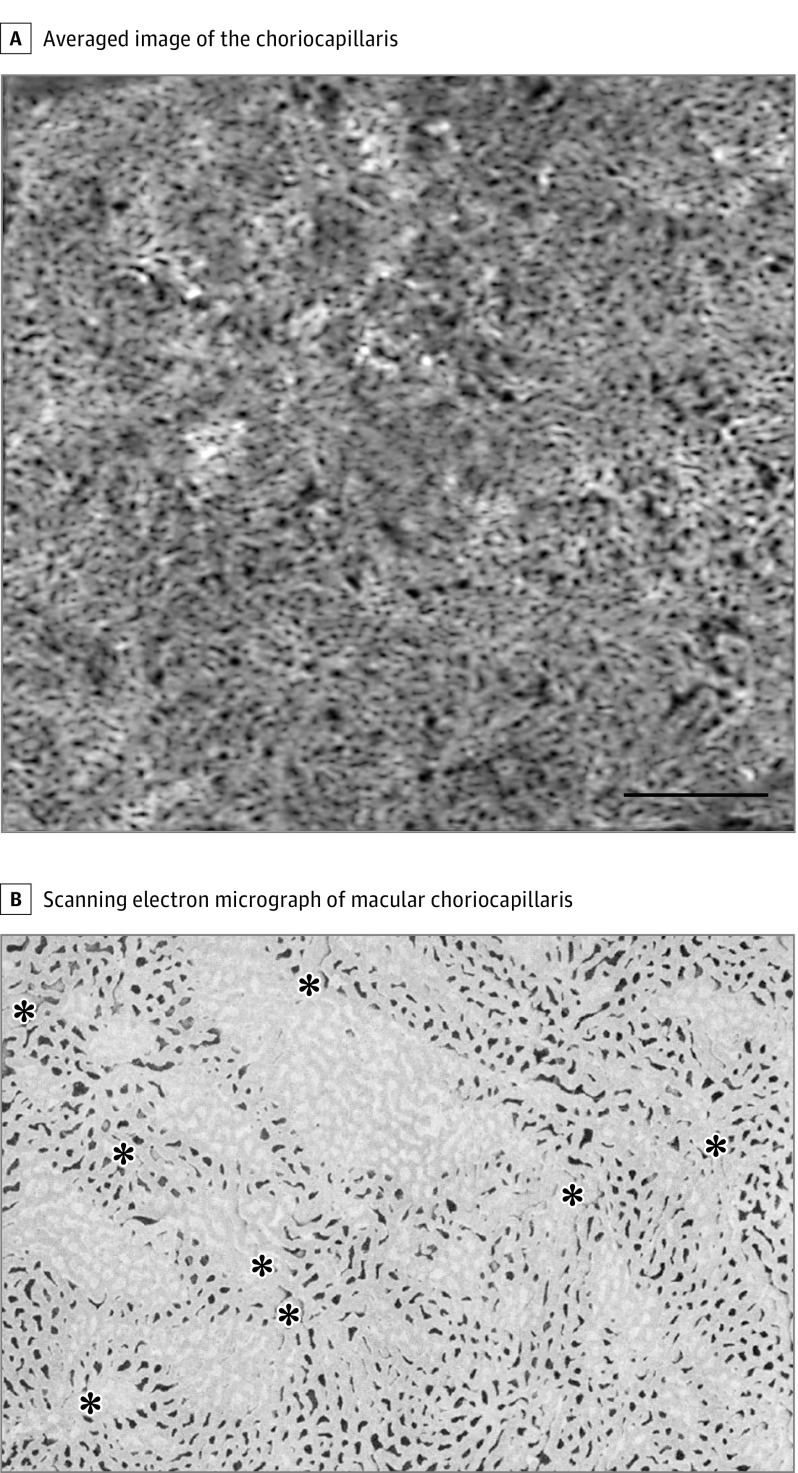
After averaging, the poorly defined granular appearance observed in the single unaveraged images was transformed to a clearly visible meshwork appearance (Figure 3). The bright area, which represents the flow, showed a somewhat continuous densely packed capillary network, and the mean (SD) diameter of the vessels was 22.8 (5.8) µm (range, 9.6-40.2 µm) outside the FAZ area. The architectural pattern of the capillary network was similar to the lobular pattern, which has been reported as the typical pattern in the posterior pole (Figure 4).
Figure 3. Comparison Between the Unaveraged and Averaged Optical Coherence Tomography Angiography (OCTA) Images of the Choriocapillaris.
A-C, Single unaveraged OCTA images of the choriocapillaris. A, The right eye of a man in his mid-30s. B, The left eye of a man in his late 30s. C, The right eye of a woman in her early 30s. D-F, The OCTA images after averaging multiple frames (A-C). The granular appearance observed in the single unaveraged images was transformed to a more apparent meshwork appearance in the averaged images. The vessels were densely packed, and the averaged images showed a smaller proportion of the dark area (flow voids [the area without flow information]). The flow voids were homogeneously distributed, and most of them appeared to be similar in size, although several larger flow voids may be seen.
Figure 4. Averaged Optical Coherence Tomography Angiography Image of the Choriocapillaris.
A, Averaged optical coherence tomography angiography image of the choriocapillaris. After averaging, en face choriocapillaris images revealed a meshwork structure reminiscent of the histology of the human choriocapillaris (B). The flow voids in the foveal avascular zone area are less detectable than those outside the foveal avascular zone area. Scale bar = 500 μm. B, Scanning electron micrograph of the corrosion vascular cast from macular choriocapillaris (original magnification ×135). Asterisks indicate postcapillary venules. The image is reproduced and adapted with permission from the study by Yoneya and Tso.
Video. Registration of the Multiple Optical Coherence Tomography Angiography Images of Superficial Capillary Plexus
Significant differences in quantitative measurements were found between the single image and the averaged image (Table and Figure 1). The number of dark spots (flow voids) was greater in the averaged image than in the single image (1423 [95% CI, 967-1909] vs 1254 [95% CI, 825-1683], P < .001). Meanwhile, the total flow void area was smaller (1.25 [95% CI, 0.90-1.60] vs 1.61 [95% CI, 1.39-1.83] mm2, P < .001), and the average size of the flow voids was smaller (911 [95% CI, 301-1521] vs 1364 [95% CI, 645-2083] µm2, P < .001) in the averaged images than those in the single unaveraged images. For vessel morphology parameters, VD (70.7% [95% CI, 61.9%-79.5%] vs 61.9% [95% CI, 56.0%-67.8%], P < .001) and VDI (6.85 [95% CI, 6.34-7.36] vs 6.06 [95% CI, 5.82-6.30], P < .001) were significantly greater in the averaged images than in the single images.
Table. Differences in Morphologic Parameters of the Choriocapillaris Between the Single Image and the Averaged Image in Optical Coherence Tomography Angiography Imaginga.
| Variable | Value (95% CI) | |
|---|---|---|
| Single Image | Averaged Image | |
| No. of flow voidsb | 1254 (825-1683) | 1423 (967-1909) |
| Total flow void area, mm2 | 1.61 (1.39-1.83) | 1.25 (0.90-1.60) |
| Total flow void area, % | 38.1 (32.2-44.0) | 29.6 (20.8-38.4) |
| Average size of the flow voids, µm2 | 1364 (645-2083) | 911 (301-1521) |
| Vessel density, % | 61.9 (56.0-67.8) | 70.7 (61.9-79.5) |
| Vessel diameter index | 6.06 (5.82-6.30) | 6.85 (6.34-7.36) |
P < .001 for all comparisons.
Flow voids: the area without flow information.
The distribution of the number vs sizes of the flow voids was skewed in both unaveraged and averaged images (eFigure 3 in the Supplement). The flow voids were homogeneously distributed, and most of them were similar in size and small, although there were several flow voids that appeared larger. After log-log transformation, significant differences in slope (m) and intercept (b) were found between the unaveraged image and the averaged image. The averaged images showed lower m (−1.61 [95% CI, −2.02 to −1.19] vs −1.36 [95% CI, −1.75 to −0.97], P < .001) and higher b (6.23 [95% CI, 4.89-7.58] vs 5.47 [95% CI, 4.09-6.85], P < .001) than the unaveraged images. The flow voids in the central macula (area of the FAZ) were more difficult to discern.
Discussion
In the present study, multiple image averaging was performed on en face choriocapillaris OCTA images of healthy volunteers. The averaging enhanced the image quality, and the poorly defined granular appearance observed in the single unaveraged images was transformed to a meshwork appearance. Moreover, averaging was associated with various quantitative parameters that could be derived from the choriocapillaris OCTA images, including the number of flow voids, the total flow void area, the average size of the flow voids, the distribution of the flow voids, VD, and VDI. The study demonstrated that multiple en face averaging was associated with better visualization of the choriocapillaris on OCTA, much like what is the case for the SCP or deep capillary plexus as shown in our previous article.
Detailed observation of the angioarchitecture of the choriocapillaris has been difficult using single unaveraged OCTA images. The unique granular appearance has been reported to be useful in distinguishing the choriocapillaris layer from other layers, such as the larger choroidal vessel layers, or the projection artifacts of the retinal vessels on the RPE layer, as well as for qualitative assessment of choriocapillaris alterations. However, a granular appearance without resolution of the capillaries does not allow for more detailed morphologic evaluations of this circulation. Despite this limitation, we and other groups have performed quantitative analyses on this granular pattern under the assumption that the bright pixels represent flow and the dark pixels represent flow voids. However, the averaged choriocapillaris images in this study show a morphologic pattern that more closely mimics the meshwork pattern observed on histology and potentially allows more precise quantitative metrics to be generated (Figure 4). For example, we were able to evaluate the caliber of the choriocapillaris in this healthy cohort and observed a mean (SD) vessel caliber of 22.8 (5.8) µm (range, 9.6-40.2 µm). This measurement seems to be in agreement with previous detailed histologic morphometric assays in the literature that have reported choriocapillaris vessel calibers of 16 to 20 µm2 or 40 to 60 µm. In addition to the vessel caliber size, the capillary network in the averaged images showed a lobular pattern arrangement that was consistent with the known angioarchitecture of the posterior pole choriocapillaris but was different from the spindle pattern at the equator or the ladder pattern of the more peripheral choriocapillaris. The application of en face image averaging enabled us to directly analyze the morphologic characteristics of the choriocapillaris. Given that the caliber of the choriocapillaris may be associated with diseases (eg, age-related macular degeneration), OCTA image averaging may allow such alterations to be studied more precisely.
Another notable observation from our study was that the granular pattern and the flow voids in the choriocapillaris appeared to change from frame to frame even after registration. There are several potential explanations for this finding. First, the pattern of the flow voids might be influenced by the decorrelation signal (differences in the OCT signal intensity or amplitude) loss, which could be detected even in the SCP (eFigure 2A in the Supplement) and also varies from scan to scan. In other words, this could simply be a type of noise that was removed by our averaging approach. Second, although 9 en face slabs from the same individual were generated based on the same segmentation protocol, minor differences in segmentation that we could not detect might have been associated with the appearance of the choriocapillaris en face images. A third possibility is that there are true dynamic changes in the choriocapillaris that are occurring from frame to frame. In that case, our averaging approach may be removing real and important information. On the other hand, the averaged images much more closely resembled the histology than the unaveraged images (Figure 4). Regardless, the fact that the flow voids change from frame to frame has some implications for previous studies that have evaluated these patterns in health and disease. Indeed, significant differences in quantitative measurements were found between the original image and the averaged images in this study. Smaller area, decreased size of the flow voids, larger VD, and greater VDI were observed in the averaged images and suggested that the averaged images had more continuous vessel segments. Furthermore, averaging significantly decreased the slope value and increased the offset value of the distribution of the number vs sizes of the flow voids, suggesting that the flow voids were distributed more homogeneously in the averaged images than in the unaveraged images. Performing quantitative assessments on the averaged images as opposed to the single images may yield more reliable and stable measurements, but this hypothesis needs to be confirmed in future studies.
Limitations
Our multiple en face image-averaging approach has some limitations. First, although the vessel caliber measured in this study showed good agreement with previously reported histologic measurements, subtle misalignment between the registered frames (which may be difficult for a human operator to detect) may artifactitiously enlarge the vessel caliber after averaging. Moreover, differences in slab thickness and depth among individuals could potentially also have influenced the quantitative measurements. In this analysis, the same slab was applied to most participants (n = 12). Second, the precision of registration in mostly featureless areas, such as the FAZ, may not be as good as in areas of the SCP, where there are many good vascular features and vessel branches to facilitate automatic image alignment. Therefore, although the apparent reduced number of flow voids in the central fovea may be due to real reduction in the intercapillary spaces, it may also simply be an artifact of less precise registration and averaging in this region. Third, we have only performed this investigation on healthy eyes thus far. In eyes with severe retinal vascular diseases with extensive nonperfusion eliminating retinal vessel features, our registration approach will presumably not work as precisely and could blur visualization of the choriocapillaris and undermine the quantitative analyses. Future studies on diseased eyes should be able to better define the best application of this technique. Fourth, the averaged OCTA choriocapillaris images in this study were not directly compared with indocyanine green angiography or histology from the same eyes. Fifth, another limitation of our approach is that it requires multiple OCTA images. It is unclear whether 9 images are required. Based on our experience with the SCP, we would suspect that fewer images (range, 3-5) may be sufficient to yield high-quality images. Even with a reduced number, obtaining multiple images has implications with respect to clinical practicality. On the other hand, if multiple acquisitions are required, OCTA is considerably less time-consuming compared with conventional dye-based angiography. Sixth, a final limitation is that our approach at present is not fully automated and requires postprocessing, albeit with publicly available free software. However, the approach should be easy to implement in a fully automated fashion in commercial devices.
Conclusions
Multiple en face averaging was associated with better visualization of the choriocapillaris on OCTA imaging. This technique holds promise for detailed morphologic assessment of the choriocapillaris in the living human eye.
eFigure 1. Image Registration Strategy for Enhanced Choriocapillaris Imaging
eFigure 2. Averaging Optical Coherence Tomography Angiography En Face Images of the Superficial Capillary Plexus
eFigure 3. Assessment of the Distribution of the Number vs Sizes of the Flow Voids
References
- 1.Guyer DR, Schachat AP, Green WR The choroid: structural considerations. In: Ryan SJ, Schachat AP, Wilkinson CP, Hinton DR, eds. Retina. Vol 1. 4th ed Edinburgh, Scotland: Mosby; 2006:33-42. [Google Scholar]
- 2.Olver JM. Functional anatomy of the choroidal circulation: methyl methacrylate casting of human choroid. Eye (Lond). 1990;4(pt 2):262-272. [DOI] [PubMed] [Google Scholar]
- 3.Yoneya S, Tso MO. Angioarchitecture of the human choroid. Arch Ophthalmol. 1987;105(5):681-687. [DOI] [PubMed] [Google Scholar]
- 4.Mullins RF, Johnson MN, Faidley EA, Skeie JM, Huang J. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52(3):1606-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLeod DS, Grebe R, Bhutto I, Merges C, Baba T, Lutty GA. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50(10):4982-4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullins RF, Schoo DP, Sohn EH, et al. The membrane attack complex in aging human choriocapillaris: relationship to macular degeneration and choroidal thinning. Am J Pathol. 2014;184(11):3142-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almeida DR, Zhang L, Chin EK, et al. Comparison of retinal and choriocapillaris thicknesses following sitting to supine transition in healthy individuals and patients with age-related macular degeneration. JAMA Ophthalmol. 2015;133(3):297-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staurenghi G, Flower RW. Clinical observations supporting a theoretical model of choriocapillaris blood flow in treatment of choroidal neovascularization associated with age-related macular degeneration. Am J Ophthalmol. 2002;133(6):801-808. [DOI] [PubMed] [Google Scholar]
- 9.Yanoff M, Duker JS, Augsburger JJ. Ophthalmology. 3rd ed St Louis, MO: Mosby Elsevier; 2009. [Google Scholar]
- 10.Nagiel A, Sadda SR, Sarraf D. A promising future for optical coherence tomography angiography. JAMA Ophthalmol. 2015;133(6):629-630. [DOI] [PubMed] [Google Scholar]
- 11.Jia Y, Tan O, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012;20(4):4710-4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spaide RF, Klancnik JM Jr, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015;133(1):45-50. [DOI] [PubMed] [Google Scholar]
- 13.Uji A, Balasubramanian S, Lei J, Baghdasaryan E, Al-Sheikh M, Sadda SR. Impact of multiple en face image averaging on quantitative assessment from optical coherence tomography angiography images. Ophthalmology. 2017;124(7):944-952. [DOI] [PubMed] [Google Scholar]
- 14.Mo S, Phillips E, Krawitz BD, et al. Visualization of radial peripapillary capillaries using optical coherence tomography angiography: the effect of image averaging. PLoS One. 2017;12(1):e0169385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 16.Spaide RF. Choriocapillaris flow features follow a power law distribution: implications for characterization and mechanisms of disease progression. Am J Ophthalmol. 2016;170:58-67. [DOI] [PubMed] [Google Scholar]
- 17.Park IK, Chun YS, Kim KG, Yang HK, Hwang JM. New clinical grading scales and objective measurement for conjunctival injection. Invest Ophthalmol Vis Sci. 2013;54(8):5249-5257. [DOI] [PubMed] [Google Scholar]
- 18.Chun YS, Yoon WB, Kim KG, Park IK. Objective assessment of corneal staining using digital image analysis. Invest Ophthalmol Vis Sci. 2014;55(12):7896-7903. [DOI] [PubMed] [Google Scholar]
- 19.Spaide RF. Visualization of the posterior vitreous with dynamic focusing and windowed averaging swept source optical coherence tomography. Am J Ophthalmol. 2014;158(6):1267-1274. [DOI] [PubMed] [Google Scholar]
- 20.Jain N, Jia Y, Gao SS, et al. Optical coherence tomography angiography in choroideremia: correlating choriocapillaris loss with overlying degeneration. JAMA Ophthalmol. 2016;134(6):697-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole ED, Novais EA, Louzada RN, et al. Visualization of changes in the choriocapillaris, choroidal vessels, and retinal morphology after focal laser photocoagulation using OCT angiography. Invest Ophthalmol Vis Sci. 2016;57(9):OCT356-OCT361. [DOI] [PubMed] [Google Scholar]
- 22.Lane M, Moult EM, Novais EA, et al. Visualizing the choriocapillaris under drusen: comparing 1050-nm swept-source versus 840-nm spectral-domain optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57(9):OCT585-OCT590. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Image Registration Strategy for Enhanced Choriocapillaris Imaging
eFigure 2. Averaging Optical Coherence Tomography Angiography En Face Images of the Superficial Capillary Plexus
eFigure 3. Assessment of the Distribution of the Number vs Sizes of the Flow Voids