Key Points
Question
Is reflectance confocal microscopy reliable enough to discern benign from malignant facial lesions when clear-cut dermoscopic criteria of lentigo maligna are absent?
Findings
This study of 61 patients with 75 facial locations (63 pigmented macules and 12 control photodamaged skin) demonstrated that reflectance confocal microscopy enhances the diagnosis of ambiguous pigmented facial macules with high sensitivity (91.7%) and specificity (86.8%). Most diagnostic confocal features had a dermoscopic, histopathologic, and immunohistochemical correlation.
Meaning
Reflectance confocal microscopy improves lentigo maligna diagnosis. False-positive findings obtained with reflectance confocal microscopy in photodamaged skin are due to the presence of basal melanocyte hyperplasia and intraepidermal Langerhans cells.
Abstract
Importance
Pigmented facial macules on photodamaged skin are a clinical, dermoscopic, and histopathologic challenge.
Objectives
To clinically and dermoscopically characterize, by means of reflectance confocal microscopy (RCM), ambiguous pigmented facial macules and establish a correlation between RCM, histopathologic, and immunohistochemical findings.
Design, Setting, and Participants
A prospective study of ambiguous pigmented facial macules on photodamaged skin was conducted in a tertiary referral center for dermatology between January 1, 2009, and December 31, 2015. Sixty-one patients with 63 ambiguous pigmented facial macules and 12 control photodamaged facial areas were included in the study. Melanocyte density in 1-mm basal layers was determined in skin biopsy specimens from all lesions stained with hematoxylin-eosin and immunohistochemical markers (melan-A, microphthalmia-associated transcription factor, and SRY-related HMG-box gene 10). Dermoscopic, RCM images, and histopathologic preparations were systematically evaluated for the presence of lentigo maligna (LM) criteria. Confocal evaluation was blinded to clinical and dermoscopic diagnosis. Sensitivity and specificity of RCM for LM diagnosis and κ value to establish correlations between dermoscopy, RCM, and histopathology were performed.
Main Outcomes and Measures
Sensitivity and specificity of RCM for LM diagnosis.
Results
Of the 61 patients included in the study, 31 (51%) were women; mean (SD) age was 71.8 (13.1) years. Twenty-four of the 63 (38%) lesions were diagnosed as LM or LM melanoma (LMM) and 39 (62%) as benign pigmented lesions. Reflectance confocal microscopy enhanced the diagnosis of pigmented facial macules with 91.7% sensitivity and 86.8% specificity. Multivariate analysis showed 2 dermoscopic and 2 confocal features associated with LM or LMM: (1) asymmetric follicular pigmentation and targetlike structures, and (2) round, large pagetoid cells and follicular localization of atypical cells, respectively. Continuous proliferation of atypical melanocytes was found in 21 (88%) LM or LMM and in 3 (77%) benign lesions. Asymmetric pigmented follicular openings by dermoscopy correlated with follicular localization of pagetoid cells by RCM (κ = 0.499, P < .001). The presence of 3 or more atypical cells at the dermal-epidermal junction (DEJ) by RCM correlated with hyperplasia of melanocytes in hematoxylin-eosin sections (κ = 0.422, P < .001).
Conclusions and Relevance
Reflectance confocal microscopy improves LM diagnosis in photodamaged skin with good histopathologic correlation although false-positive and false-negative cases exist. False-positives obtained with RCM in photodamaged skin are due to the presence of basal melanocyte hyperplasia and intraepidermal Langerhans cells. Histopathologic features of these lesions sometimes are not enough for a definite diagnosis and immunohistochemical studies may be required.
This study compares the use of reflectance confocal microscopy with other measures used in diagnosis of pigmented facial macules.
Introduction
Pigmented facial macules (PFMs) are commonly evaluated in clinical practice, especially in elderly patients with sun-damaged skin. In this clinical setting, one must distinguish benign PFMs from lentigo maligna (LM) or lentigo maligna melanoma (LMM). Dermoscopy has provided some help in this scenario. However, some of these lesions exhibit overlapping features, and classical dermoscopic LM criteria may not be useful to detect early facial melanomas.
Reflectance confocal microscopy (RCM) has further improved diagnosis of these lesions. Multiple studies have demonstrated its accuracy in skin cancer diagnosis with algorithms applicable in clinical practice. Reproducibility and correlation with histopathology have also been established. Reflectance confocal microscopy is also useful in the distinction between LM and benign PFMs, LM surveillance, presurgical mapping of LM, and posttreatment follow-up.
Histopathologic diagnosis of PFMs on sun-damaged skin is also difficult owing to the presence of atypical melanocytic proliferations both in normal-looking sun-exposed skin and in benign PFMs. Immunohistochemical stains are often useful to render a correct diagnosis. The most frequently used markers are melan-A and human melanoma black-45 (HMB-45). Both markers are cytoplasmic and may overestimate the number of melanocytes, which are often hyperplastic in sun-damaged skin. New markers, such as microphthalmia-associated transcription factor (MITF) and sex-determining region Y (SRY)-related high mobility group (HMG)-box gene 10 (SOX10) (nuclear markers), are more useful in these cases.
Methods
Clinical and dermoscopically ambiguous PFMs from patients attending a referral pigmented lesion unit of dermatology at a tertiary university hospital were included prospectively between January 1, 2009, and December 31, 2015. Ambiguous PFMs exhibited at least 1 criterion for LM (asymmetric pigmented follicular openings, annular-granular pattern, pigmented rhomboidal structures, homogeneous areas [obliterated hair follicles], increased density of vascular network, targetlike patterns, darkening at dermatoscopic examination, and red rhomboidal structures), but not enough features to ensure the diagnosis, or, in the absence of LM criteria, they did not present clear-cut criteria for benignity. All patients gave written informed consent to be included in the study, which was approved by the ethics committee of the Hospital del Mar-Parc de Salut Mar, Barcelona, Spain. There was no financial compensation.
Lesions included in the study were primary lesions or possible recurrent, previously treated lesions after cryotherapy, radiotherapy, or surgery. We selected 12 patients from our series to study nonlesional, photodamaged skin areas on the contralateral site to the lesion included as controls.
Clinical and dermoscopic images of all lesions were obtained using a camera (PowerShot G10; Canon) attached to a contact polarized light dermoscopy device (DermLite FOTO; 3Gen). All lesions were evaluated for the presence of LM dermoscopic features by 3 investigators (I.G.-M., S.M., and S.S.) according to Stolz criteria, as well as criteria more recently described.
Reflectance confocal microscopy examination was performed in all lesions (VivaScope 1500; Caliber I.D.). Sequential images were recorded in horizontal 4 × 4- to 8 × 8-mm mosaics (VivaBlock; Caliber I.D.) at 3 levels of the skin (epidermis, dermal-epidermal junction [DEJ], and dermis) and vertical sequential images (VivaStack; Caliber I.D.) in the more representative areas of the lesion. The RCM images were later evaluated by 2 investigators (I.G.-M. and S.S.) blinded to dermoscopic images and histopathologic diagnosis, using the Guitera algorithm.
The Guitera algorithm calculates an LM score based on 6 criteria. Two major criteria (nonedged dermal papilla and round large pagetoid cells) are scored +2 points each and 3 minor criteria (nucleated cells in dermal papilla, ≥3 atypical cells at the dermal-epidermal junction [DEJ], and follicular localization of atypical cells) are scored +1 point each. There is also 1 negative (benign) criterion (broadened honeycomb pattern of the epidermis) that receives a score of −1 point. An LM score of 2 or higher is suspicious of at least melanoma in situ. One or more 4-mm punch biopsies were obtained from all lesions at the most suspicious areas after clinical, dermoscopic, and RCM evaluation. Histopathologic features were systematically evaluated by 4 investigators: a pathologist (C.B.) and 3 dermatopathologists (I.G.-M., R.M.P., and S.S.). The diagnosis of LM or LMM was considered in the presence of uniform atypia and continuous proliferation of melanocytes along the DEJ and down adnexal structures. All histopathologic evaluators were blinded to the clinical information and, when present, previous histopathologic diagnosis.
The immunohistochemical study was performed with SRY-related HMG-box gene 10 (SOX10) (1:50 dilution, polyclonal, Cell Marque; Sigma-Aldrich Co), MITF-1 (clone C5/D5, prediluted; Ventana Medical Systems Inc), and melan-A (1:50 dilution, clone A103; Dako); the latter was combined with Ki-67 stain. CD1a (1:20 dilution, clone MTB1; Leica Biosystems) was used to reveal Langerhans cells in select cases for better characterization of RCM parameters. The detection system used was Liquid DAB+Substrate (K3468 Chromogen system; Dako; Denmark), except for melan-A, which was revealed with permanent red (Dako). Positive controls were included in each session.
The density of melanocytes in the basal layer and the number of positive intraepidermal cells (pagetoid spreading) were assessed in hematoxylin-eosin (H&E) sections and with melan-A, MITF, and SOX10. They were assessed by counting the number of labeled cells over 1 mm of basal layer.
Statistical Analysis
In univariate analysis, dichotomous variables were evaluated by the χ2 test. In multivariate analysis of dermoscopic and RCM features, a binary logistic regression was performed to differentiate between LM/LMM and non-LM/LMM. For the analysis of numeric variables, a 2-tailed, unpaired t test was calculated. Pearson correlation coefficient was used to compare H&E, melan-A, MITF, and SOX10 stains, and a 2-tailed, paired t test was used to determine correlations between different stainings. The Cohen κ coefficient was applied to measure agreement between dermoscopic, RCM, and histopathologic findings regarding qualitative parameters. Statistical evaluation was performed using SPSS statistical software for Windows, version 15.0 (SPSS Inc).
Results
Clinical Findings
A total of 63 clinically and dermoscopically atypical PFMs from 61 white patients (31 women [51%]), with ages ranging from 26 to 93 years (mean [SD], 71.8 [13.1] years), were included in the study. The lesions corresponded to 24 (38%) facial LM/LMM (17 LM and 7 LMM) and 39 (62%) non-LM/LMM (3 melanocytic nevi, 1 blue nevus, 9 dermal pigmentations, 9 pigmented actinic keratoses, 7 solar lentigines, 5 seborrheic keratoses, 4 pigmented basal cell carcinomas, and 1 lichen planuslike keratosis). All lesions were located on sun-exposed areas, including the cheek (27), nose (12), forehead (11), eyelid (5), scalp (4), neck (3), or lip (1).
In our series, 27 lesions were completely excised (18 LM/LMM, 4 pigmented basal cell carcinomas, 4 nevi, and 1 seborrheic keratosis); 4 were treated with radiotherapy (4 LM), 4 with topical immunotherapy (2 pigmented actinic keratoses and 2 LM), 5 with cryotherapy (5 pigmented actinic keratoses), and 23 as “wait and see” (4 seborrheic keratoses, 9 dermal pigmentations, 2 pigmented actinic keratoses, 7 solar lentigines, and 1 lichen planuslike keratosis). The time of follow-up for “wait-and-see” patients and patients treated with radiotherapy, topical immunotherapy, or cryotherapy varied from 18 months to 7 years.
Dermoscopic Findings
The pattern most often observed in LM/LMM was pigmented rhomboidal structures, followed by asymmetric pigmented follicular openings and targetlike structures (Table). Fifty-six of 63 (89%) ambiguous PFMs had at least 1 criterion for LM diagnosis. Thirty-two (82%) of non-LM/LMM lesions had at least 1 criterion suggestive of LM; the most frequent was the increased density of the vascular network and the annular-granular pattern (Table). In multivariate analysis, only asymmetric pigmented follicular openings (non-LM/LMM, 7 [18%] vs LM/LMM, 16 [67%]; P = .03) and targetlike patterns (3 [8%] vs 10 [42%]; P = .002) were statistically significant (eFigure 1 in the Supplement). Control cases did not present relevant dermoscopic findings (Table).
Table. Dermoscopic, RCM, Histopathologic, and Immunohistochemical Findings in 63 Pigmented Macules and 12 Sun-Damaged Controls.
| Technique | Variable Evaluated | Non-LM/LMM (n = 39) |
LM/LMM (n = 24) |
P Value for Non-LM/LMM vs LM/LMMa | Control Cases (n = 12) | P Value for Non-LM/LMM vs Controla |
|---|---|---|---|---|---|---|
| Dermoscopy, No. (%) | Asymmetric pigmented follicular openings | 7 (18) | 16 (67) | <.001b | 0 | .18 |
| Annular-granular pattern | 16 (41) | 10 (42) | >.99 | 0 | .01 | |
| Pigmented rhomboidal structures | 12 (31) | 18 (75) | .001 | 0 | .047 | |
| Homogeneous areas (obliterated hair follicles) | 4 (10) | 5 (21) | .28 | 0 | .56 | |
| Increased density of vascular network | 16 (41) | 10 (42) | >.99 | 1 (8) | .17 | |
| Targetlike patterns | 3 (8) | 10 (42) | .003b | 0 | >.99 | |
| Darkening at dermatoscopic examination | 6 (15) | 2 (8) | .70 | 0 | .32 | |
| Red rhomboidal structures | 5 (13) | 4 (17) | .72 | 1 (8) | >.99 | |
| Dustlike dots | 4 (10) | 7 (29) | .06 | 0 | .18 | |
| Fingerprint | 5 (13) | 2 (8) | .70 | 1 (8) | >.99 | |
| Pseudonetwork | 22 (56) | 18 (75) | .18 | 1 (8) | .003 | |
| RCM, No. (%) | Nonedged dermal papillac | 0 | 4 (17) | .02 | 0 | NI |
| Round, large pagetoid cellsc | 5 (13) | 16 (67) | <.001b | 0 | .32 | |
| ≥3 Atypical cells at DEJc | 9 (23) | 19 (79) | <.001 | 0 | .09 | |
| Follicular localization of pagetoid cells and/or atypical junctional cellsc | 5 (13) | 13 (54) | .001b | 0 | .32 | |
| Nucleated cells in dermal papillac | 1 (3) | 6 (25) | .01 | 0 | >.99 | |
| Broadened honeycombed patternc | 4 (10) | 0 | .29 | 1 (8) | >.99 | |
| Epidermal disarray | 10 (26) | 16 (67) | .002 | 3 (25) | >.99 | |
| Epidermal cords and/or bulbous projections | 15 (38) | 2 (8) | .01 | 1 (8) | .07 | |
| Dendritic pagetoid cells | 20 (51) | 20 (83) | .02 | 0 | .001 | |
| Edged papilla | 15 (38) | 2 (8) | .01 | 3 (25) | .50 | |
| Nonvisible papilla | 23 (59) | 18 (75) | .28 | 9 (75) | .003 | |
| Melanophages | 25 (64) | 14 (58) | .79 | 1 (8) | .002 | |
| Enlarged vessels | 11 (28) | 2 (9)d | .11 | 0 | .09 | |
| Score LM, mean (SD) | 0.54 (0.91) | 3.25 (1.29) | <.001 | −0.08 (0.29) | .001 | |
| Histopathology, No. (%) | Epidermal atrophy | 14 (36) | 11 (46) | .60 | 4 (33) | >.99 |
| Flattening of dermal papilla | 10 (26) | 8 (33) | .57 | 3 (25) | >.99 | |
| Epidermal hyperplasia | 17 (44) | 6 (25) | .18 | 1 (8) | .04 | |
| Basal melanocytic hyperplasia H&E | 5 (13) | 21 (88) | <.001 | 0 | .56 | |
| Basal pigmentation | 19 (49) | 18 (75) | .06 | 2 (17) | .09 | |
| Atypia in keratinocytes | 19 (49) | 6 (25) | .07 | 4 (33) | .35 | |
| Atypia in melanocytes | 3 (8) | 21 (88) | <.001 | 0 | >.99 | |
| Perifollicular localization of melanocytes | 1 (3) | 13 (54) | <.001 | 0 | >.99 | |
| Melanocytes in rete ridges | 4 (10) | 10 (42) | .01 | 0 | .56 | |
| Junctional melanocytic nests | 2 (5) | 16 (67) | <.001 | 0 | >.99 | |
| Pagetoid spreading in 1 mm | 0 | 13 (54) | <.001 | 0 | NI | |
| Melanophages | 28 (72) | 20 (83) | .37 | 0 | <.001 | |
| Inflammatory infiltrate | 31 (82)e | 19 (79) | .82 | 6 (50) | .06 | |
| Immunohistochemistry | Hyperplasia of basal melanocytes, No. (%) | |||||
| H&E | 5 (13) | 21 (88) | <.001 | 0 | .32 | |
| Melan-A | 14 (36) | 23 (96) | <.001 | 0 | .02 | |
| MITF | 14 (36) | 23 (96) | <.001 | 0 | .02 | |
| SOX10 | 6 (15) | 21 (88) | <.001 | 0 | .32 | |
| Density of basal melanocytes, mean (SD), melanocytes/mm | ||||||
| H&E | 9.1 (6.7) | 29.8 (17.0) | <.001 | 10.3 (5.5) | .60 | |
| Melan-A | 26.5 (15.3) | 67.4 (27.1) | <.001 | 19.3 (7.0) | .03 | |
| MITF | 27 (16.1) | 54 (19.9) | <.001 | 21.3 (7.4) | .24 | |
| SOX10 | 27 (14.5) | 57.4 (25.5) | <.001 | 21.9 (9.1) | .26 | |
| Pagetoid spreading, No. (%) | ||||||
| H&E | 0 | 13 (54) | <.001 | 0 | NI | |
| Melan-A | 0 | 13 (54) | <.001 | 0 | NI | |
| MITF | 0 | 13 (54) | <.001 | 0 | NI | |
| SOX10 | 0 | 13 (54) | <.001 | 0 | NI | |
| No. of pagetoid cells, mean (SD), melanocytes/mm | ||||||
| H&E | 0.2 (0.7) | 3.6 (4.0) | <.001 | 0 | .40 | |
| Melan-A | 0.2 (1.0) | 5.2 (5.9) | .001 | 0 | .06 | |
| MITF | 0.1 (0.5) | 4.5 (6.0) | .002 | 0 | .07 | |
| SOX10 | 0.7 (3.0) | 4.8 (5.4) | .003 | 0 | .03 | |
Abbreviations: DEJ, dermal-epidermal junction; H&E, hematoxylin-eosin; LM, lentigo maligna; LMM, lentigo maligna melanoma; MITF, microphthalmia-associated transcription factor; NI, not indicated; RCM, reflectance confocal microscopy; SOX10, SRY-related HMG (high mobility box group)-box gene 10.
P value from univariate analysis.
Statistically significant also in multivariate analysis.
Criteria of LM algorithm according to Guitera et al.
Data available on 22 lesions.
Data available on 38 lesions.
RCM Findings
The more frequent criteria observed in LM/LMM were the presence of atypical cells at the DEJ; round, large pagetoid cells (intraepidermal cells); and follicular localization of atypical cells. Nonedged papilla, nucleated cells in papilla, and epidermal disarray were also associated with LM/LMM (Table). The presence of intraepidermal dendritic cells was a common finding in both non-LM/LMM and LM/LMM. Epidermal cords or bulbous projections and edged papilla were RCM features of benignity (protective criteria). In multivariate analysis, only round, large pagetoid cells (non-LM/LMM, 5 [13%] vs LM/LMM, 16 [67%]; P < .001) and follicular localization of atypical cells (non-LM/LMM, 5 [13%] vs LM/LMM, 13 [54%]; P = .002) were statistically significant for cancer (eFigure 1 in the Supplement).
The Guitera diagnostic algorithm was applied in all cases and, in quantitative analysis, it demonstrated that the mean (SD) RCM score was significantly higher in LM/LMM (3.25 [1.29]) than in non-LM/LMM lesions (0.54 [0.91]); P < .001. According to that procedure, lesions with a score 2 or higher are suspicious for cancer. Examples of a malignant and a benign lesion are seen in Figure 1 and eFigure 2 in the Supplement, respectively.
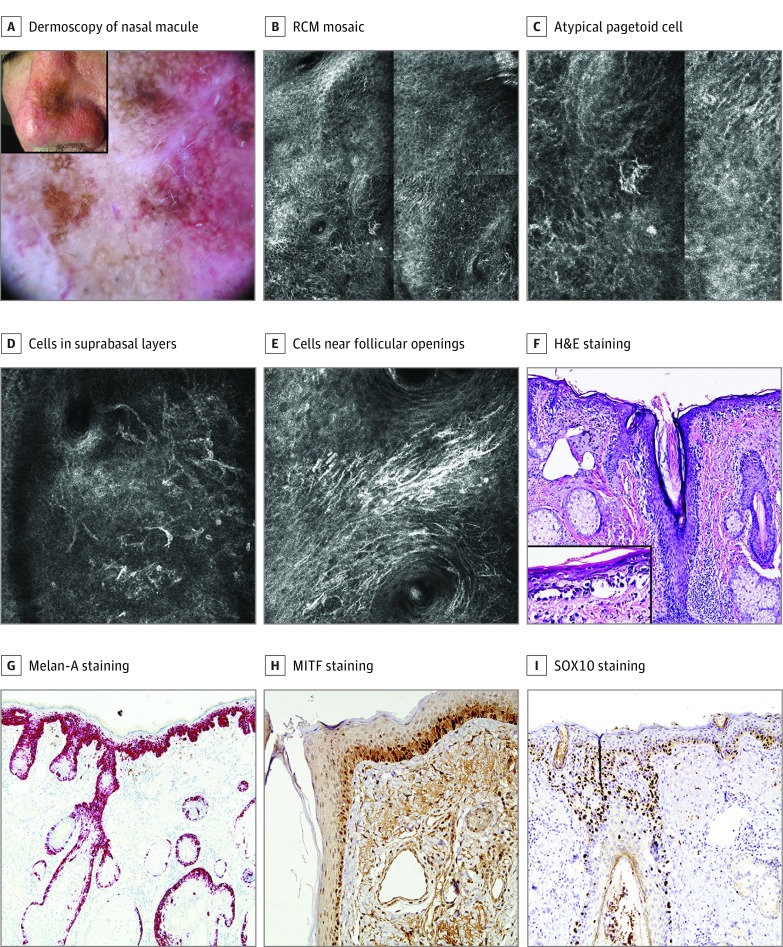
Figure 1. Lentigo Maligna in a Man in His 60s.
A, A 25-mm brown macule on the nose (inset). Dermoscopy of the central area showed a pseudonetwork, brown rhomboidal structures, focal annular-granular pattern, focal asymmetric pigmented follicular openings, increased density of vascular network with red rhomboidal structures and targetlike patterns. A scar from a previous, nonconfirmatory biopsy is visible. B, Reflectance confocal microscopy (RCM) mosaic (1 × 1 mm) at the epidermal layer showing epidermal disarray with atypical pagetoid cells. C, Detail of an atypical pagetoid cell. D, RCM image (0.5 × 0.5 mm). Dendritic pagetoid cells and round, large pagetoid cells are observed in suprabasal layers. E, RCM image (0.5 × 0.5 mm). Abundant dendritic cells and dendritic processes near the follicular openings. F, Biopsy specimen revealed an atypical proliferation of melanocytes in an atrophic epidermis with extension along follicular structures. Elastosis in the upper dermis. Hematoxylin-eosin (H&E), original magnification ×200. Inset: atypical intraepidermal melanocytes in more detail (H&E, original magnification ×400). G, Melan-A staining, original magnification ×100, labeling the atypical proliferation of melanocytes (cytoplasm) in the epidermis and adnexa. Isolated pagetoid melanocytes are observed. H, Microphthalmia-associated transcription factor (MITF) staining, original magnification ×100, labeling the nucleus of melanocytes. I, SRY-related HMG (high mobility group)-box gene 10 (SOX10) staining, original magnification ×100, with the same features as MITF.
According to the Guitera algorithm, we obtained 5 false-positive (FP) and 2 false-negative (FN) results. All FP results presented abundant dendritic pagetoid cells (Figure 2), 4 lesions had round, large pagetoid cells, and 3 exhibited atypical cells at the DEJ. Two FP results corresponded to postradiotherapy pigmentation. Lesions with FP results have not recurred after 5 to 7 years of follow-up. Regarding FN cases, 1 case was an incipient lesion that had been previously biopsied and the other was a PFM on the cheek previously treated with multiple cycles of cryotherapy. The latter lesion exhibited the so-called medusahead-like structures at the DEJ (Figure 3), described by Gonzalez et al, that were not considered in the algorithm but could have been a clue for a correct diagnosis. This finding was not observed in any other case of the study.
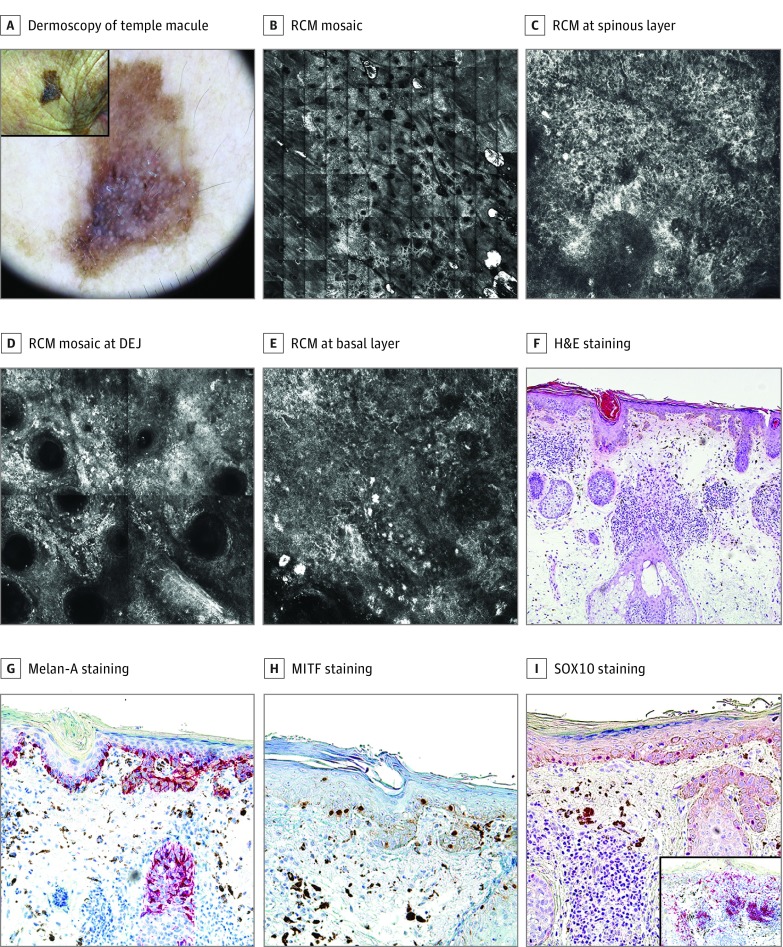
Figure 2. Benign Facial Macule in a Woman in Her 90s: False-Positive According to the Reflectance Confocal Microscopy (RCM) Algorithm.
A, A 14-mm pigmented macule on the right temple (inset). Dermoscopically, it showed multiple colors, pseudonetwork, asymmetric, pigmented follicular openings, pigmented rhomboidal structures, and blue-white veil. B, RCM mosaic (6 × 6 mm), revealing refractile structures on the periphery and a central area of hyporefractility. C, RCM image (0.5 × 0.5 mm) at the spinous layer showing epidermal disarray and atypical refractile cells. D, RCM mosaic (1 × 1 mm) at the dermal-epidermal junction (DEJ) showing atypical cells and bright refractile particles suggesting inflammation. E, RCM image (0.5 × 0.5 mm) at basal layer with dendritic cells as well as atypical cells and melanophages in the papillary dermis. F, Hematoxylin-eosin (H&E) stain, original magnification ×100, showing atrophic epidermis with basal pigmentation and without melanocytic proliferation. At the dermal level, melanophages and an intense inflammatory infiltrate are observed. G, Melan-A staining, original magnification ×200, showing an apparent hyperplasia of dendritic melanocytes without atypia or pagetoid spreading at the basal layer. Melanophages in the upper dermis. H, Microphthalmia-associated transcription factor (MITF) staining, original magnification ×200, revealing a normal number of melanocytes in basal layers. Melanophages in the upper dermis. I, SRY-related HMG (high mobility group)-box gene 10 (SOX10) staining, original magnification ×100, showing an absence of melanocytic hyperplasia and an intense inflammatory infiltrate in the dermis with melanophages. Inset: CD1a labeling multiple Langerhans cells either in the whole epidermis, adnexal structures, or in the dermal inflammatory infiltrate.
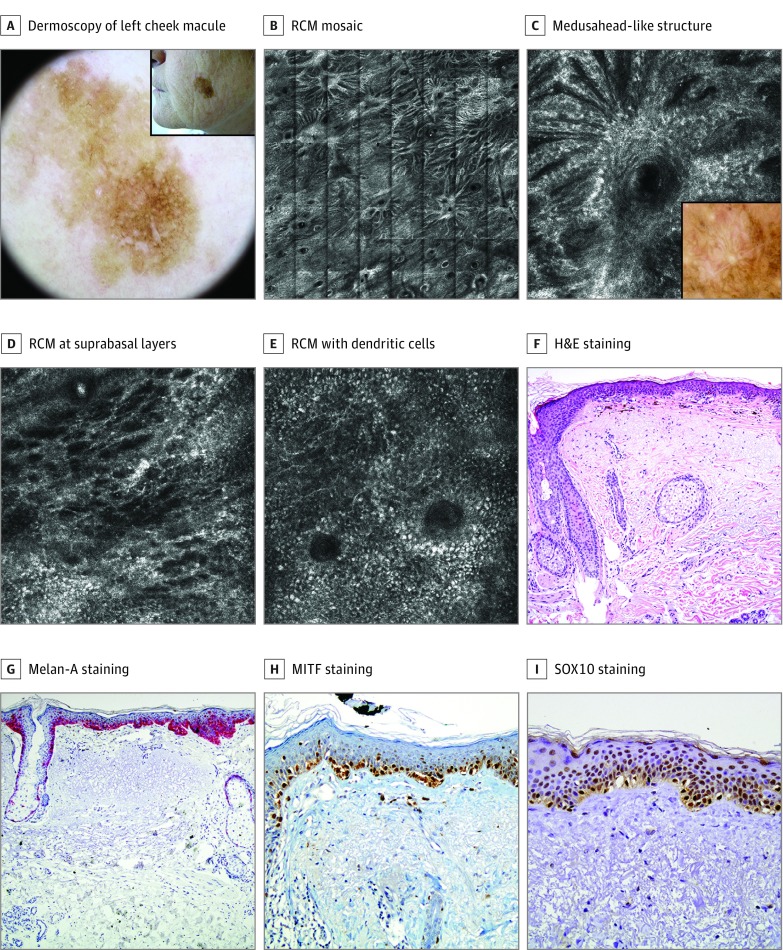
Figure 3. Lentigo Maligna in a Woman in Her 60s: False-Negative According to the Reflectance Confocal Microscopy (RCM) Algorithm.
A, An 11-mm, brown macule on the left cheek treated 6 times with cryotherapy (inset). Dermoscopically, it showed a brown pseudonetwork, pigmented rhomboidal structures, and targetlike patterns. B, An RCM mosaic (4 x 4 mm) showed an abundant number of follicular openings with radiating refractile strands composed by confluent, elongated clusters of atypical cells all over the circumference. C, An RCM image (0.5 × 0.5 mm). Detail of medusahead-like structure. Inset: correlation with dermoscopy. D, An RCM image (0.5 × 0.5 mm). Epidermal disarray with abundant dendritic pagetoid cells at suprabasal layers. E, An RCM image (0.5 × 0.5 mm). Dendritic cells with perifollicular distribution. F, Biopsy specimen revealed an atypical proliferation of melanocytes with extension along the follicular structures. Slight melanophagia and severe elastosis in the dermis. Hematoxylin-eosin (H&E), original magnification ×100. G, Melan-A staining, original magnification ×100, labeling the atypical hyperplasia of melanocytes in epidermis and adnexa. Isolated pagetoid melanocytes are visualized. H, Microphthalmia-associated transcription factor (MITF) staining, original magnification ×100, labeling the nucleus of atypical melanocytes. I, SRY-related HMG (high mobility group)-box gene 10 (SOX10) staining, original magnification ×100, expressing the same features as MITF.
No control case showed RCM criteria suggestive of LM. However, in some cases, mild to moderate degrees of epidermal disarray were present.
Histopathologic and Immunohistochemical Findings
Histopathologic criteria that allowed us to differentiate benign PFMs from LM included basal melanocytic hyperplasia, atypia of melanocytes, perifollicular location of melanocytes, and pagetoid melanocytes (Table). Continuous proliferation of atypical melanocytes was found in 21 (88%) LM/LMM and 3 (77%) non-LM/LMM lesions. The presence of junctional melanocytic nests was observed in 16 of 24 (67%) LM/LMM vs 2 of 39 (0.05%) non-LM/LMM lesions (P < .001). The 2 non-LM/LMM lesions with melanocytic nests corresponded to melanocytic nevi without atypical melanocytes. Quantitative analysis of immunohistochemical markers showed statistically significant differences in the count of basal melanocytes per 1 mm of basal layer (melanocyte density) and in the count of intraepithelial melanocytes (pagetoid spreading) between non-LM/LMM and LM/LMM with the 3 immunostains (melan-A, MITF, and SOX10) (Table). Pearson correlation coefficients between the different stainings were higher than 0.8, with the better correlation being between melan-A and MITF, with values of 0.884 (P = .02) in basal melanocytes and 0.921 (P < .001) in pagetoid cells. In malignant lesions, melan-A labeled higher numbers of basal melanocytes compared with MITF (t = 4.149; P < .001) and SOX10 (t = 2.295; P = .03), whereas MITF and SOX10 had similar findings (t = 0.788; P = .44). No significant difference was found among benign lesions in the positivity of basal melanocytes with the different immunostains. The 12 control photodamaged areas were diagnosed as compatible with sun-damaged skin.
Correlation Between Techniques
Dermoscopy-RCM
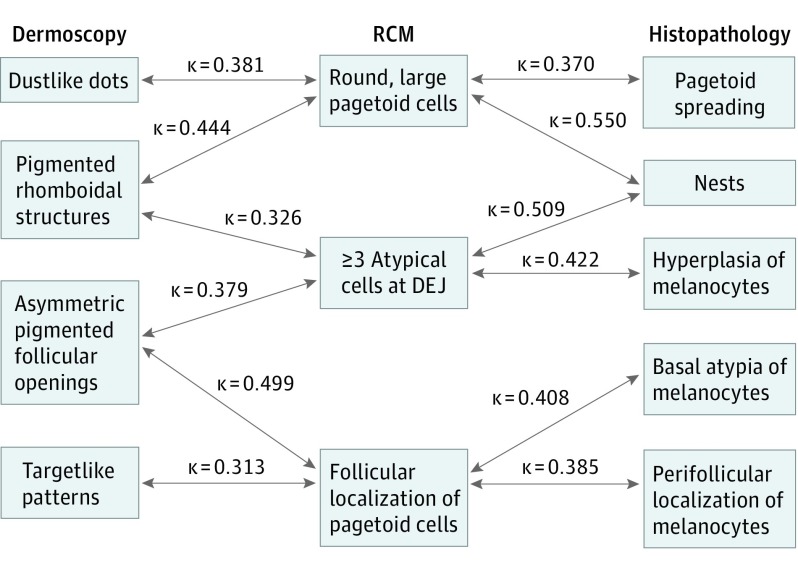
Pigmented rhomboidal structures in dermoscopy correlated well with round, large pagetoid cells (κ = 0.444, P < .001) and with 3 or more atypical cells at the DEJ by RCM (κ = 0.326, P = .005). In addition, asymmetric pigmented follicular openings correlated with follicular localization of pagetoid cells (κ = 0.499, P < .001) and 3 or more atypical cells at the DEJ by RCM (κ = 0.379, P = .001). Dustlike dots in dermoscopy correlated with round, large pagetoid cells by RCM (κ = 0.381, P < .001) and dermoscopic targetlike patterns with follicular localization of pagetoid cells by RCM (κ = 0.313, P = .006) (Figure 4).
Figure 4. Correlation Between Techniques .
Correlation between dermoscopy, reflectance confocal microscopy (RCM), and histopathology. DEJ indicates dermal-epidermal junction.
RCM-Histopathology and Immunohistochemistry
Among the RCM criteria, round, large pagetoid cells correlated with pagetoid spread (κ = 0.370, P < .001) and junction nests (κ = 0.550, P < .001) in the histopathologic study (Figure 4). With t test analysis, the presence of round, large intraepidermal cells by RCM was also associated with a higher average of immunostained pagetoid cells, but not with the H&E pagetoid cell count.
The presence of 3 or more atypical cells at the DEJ by RCM correlated with nests of melanocytes (κ = 0.509, P < .001), hyperplasia of melanocytes in H&E sections (κ = 0.422, P < .001), melan-A (κ = 0.438, P < .001), MITF (κ = 0.438, P < .001) and SOX10 (κ = 0.455, P < .001) stains. Follicular localization of pagetoid cells by RCM correlated with basal atypia of melanocytes (κ = 0.408, P < .001) and perifollicular localization of melanocytes (κ = 0.385, P = .001).
The observation of dendritic pagetoid cells by RCM did not correlate with pagetoid cells on histopathologic examination. Indeed, from 20 LM/LMM lesions in which intraepithelial dendritic cells were present on RCM, only 11 had pagetoid cells with melanocytic stainings. In non-LM/LMM cases with intraepithelial dendritic cells on RCM (20 cases; 5 were FP by RCM), CD1a showed the presence of abundant intraepidermal Langerhans cells in 15 of 18 cases.
Discussion
In this study, we found that the criteria of Stolz et al are insufficient for the diagnosis of LM, because at least 1 Stolz et al criterion is always present in ambiguous PFMs. The criteria of LM more recently described have shown little help in distinguishing LM in our study except for the targetlike structures; this limitation is probably due to the small number of cases and the presence of incipient lesions or lesions that were previously treated. Therefore, RCM can help to establish the diagnosis without a biopsy in a sensitive area, such as the face.
In applying the Guitera et al RCM algorithm, we obtained an RCM sensitivity of 91.7% with a RCM specificity of 86.8% owing to 2 FN and 5 FP results. These percentages are similar to those described by the original authors. In a recent study from Menge et al, which analyzed 63 LM sites from 17 participants, histopathologic interpretations were concordant in 56 cases, with 7 FP results and no FN results, achieving 100% sensitivity and 71% specificity. The authors attributed the observed FP results to the presence of actinically damaged skin. In the present study, the 5 FP results were due to the presence of round, large pagetoid cells and/or atypical cells in the basal layer. Histopathologic and immunohistochemical analysis confirmed that intraepidermal cells present in some benign lesions corresponded to CD1a-positive Langerhans cells. In 2 FP cases, the confounding confocal findings were due to postradiotherapy changes inducing both basal melanocyte hyperplasia and intraepidermal Langerhans cells.
The most frequently observed confocal feature in LM/LMM lesions was the presence of atypical cells in the basal layer (19 of 24 [79%] cases), which is also a histologic criterion for LM. However, this confocal feature was also present in nearly a quarter (9 of 39 [23%]) of benign PFMs. These atypical basal cells were almost always of dendritic morphology on RCM and corresponded with basal melanocytic hyperplasia on histopathologic examination. These cells tended to coalesce and had histologic atypia in LM. Some benign lesions in photodamaged skin may also exhibit melanocytic hyperplasia that, in some cases, can be marked and appear as dendritic cells at the DEJ (>3 per field/500 µm) in RCM. Moreover, melanocytic hyperplasia can sometimes present atypia, especially after sun exposure or treatment with radiotherapy. In this situation, we would have an FP result on RCM. In our study, nonlesional control sites did not show confocal findings that could lead to misdiagnosing LM, and the density of basal melanocytes in control sites was lower than in non-LM/LMM lesions, but the difference was significant only with melan-A.
The confluence of atypical basal melanocytes corresponded on dermoscopy with pigmented rhomboidal structures and asymmetric pigmented follicular openings. The correspondence between dermoscopic and confocal findings in PFM has recently been explored by de Carvalho et al, with similar findings.
Another important confocal feature in LM is the presence of round, large pagetoid cells observed in 16 LM/LMM lesions. The visualization of intraepithelial cells is also common in benign lesions in photodamaged skin, but they usually have a dendritic morphology. As we have demonstrated in our study, these cells correspond to Langerhans cells that are especially common in inflamed lesions or after treatments, such as cryotherapy, topical immunotherapy, or radiotherapy, and exhibit a similar morphology and reflectance to melanocytes; these results raise reasonable doubts and confusion with LM. As described in the literature, Langerhans cells may also appear in an inflamed nevus or eczematous nevus of Meyerson. We did not observe dendritic intraepidermal cells in nonlesional sun-damaged skin.
In the histopathologic analysis, we identified 6 statistically significant criteria that may help to differentiate LM/LMM from benign lesions. However, in clinical practice, immunohistochemical evaluation of melanocytic lesions is a useful additional tool in the diagnosis of PFMs. Melan-A staining is the most used in the diagnosis of melanocytic lesions. Notwithstanding, some authors have questioned its usefulness in photodamaged skin in the differential diagnosis of melanoma, since healthy skin, solar lentigines, and actinic keratoses may present a significant number of cells stained with melan-A. In this sense, some studies have investigated the role of newer nuclear markers, such as MITF and SOX10, in the diagnosis of melanocytic intraepithelial lesions. Two studies showed MITF to be more specific than melan-A for diagnosing LM, but SOX10 had a lower sensitivity. In our work, we have demonstrated that malignant lesions have a significantly higher number of basal and intraepidermal melanocytes than benign lesions. In addition, round, large intraepidermal cells observed on RCM examination correlated with pagetoid proliferation demonstrated by immunostaining but not by H&E staining. Thus, the performance of immunostains could be recommended at least when confocal examination shows round, large intraepidermal cells.
The correlation between the 3 stainings was very good, but labeling with melan-A was slightly higher than with MITF and SOX10 in malignant lesions. In benign lesions, melan-A, MITF, and SOX10 markers stained very similarly. Melan-A, MITF, and SOX10 were more sensitive than H&E in the detection of melanocytes and can help in some cases to demonstrate the melanocytic hyperplasia of LM/LMM. In this regard, and in accordance with previous studies, melan-A could overestimate the number of melanocytes compared with MITF and SOX10. However, in our study, these differences were statistically significant only in the group of malignant lesions, probably owing to a higher density of melanocytes in these sort of lesions.
Limitations
A limitation of our study is that definite diagnosis was established according to a 4-mm punch biopsy evaluation chosen by RCM findings in 36 cases (only 27 lesions completely excised). This small sample size may have caused confocal FN results according to histopathology if the sample was not perfectly representative of the lesion, especially in cases with borderline findings on histopathologic examination (presence of atypical basal melanocytes or melanocyte nests without clear-cut criteria for LM). A longer follow-up of the cases will give us a definite diagnosis. Another limitation of the study is that only 12 control sites were examined.
Conclusions
Reflectance confocal microscopy is a noninvasive technique that complements dermoscopy in the differential diagnosis of PFMs and improves the diagnostic accuracy of LM/LMM. However, in photodamaged skin, FP results are possible due to the presence of basal melanocytic hyperplasia and intraepidermal Langerhans cells. Although histopathologic examination remains the standard technique, immunohistochemistry may be useful in the detection of incipient LM, with similar staining profiles among the different markers, except for a higher sensitivity for melan-A in the detection of melanocytes in case of malignant lesions.
eFigure 1. Dermoscopic and Confocal Features Statistically Significant in Multivariate Analysis for LM Diagnosis
eFigure 2. Benign Macule in a Woman in Her 70s
References
- 1.Stolz W, Schiffner R, Burgdorf WH. Dermatoscopy for facial pigmented skin lesions. Clin Dermatol. 2002;20(3):276-278. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka M, Sawada M, Kobayashi K. Key points in dermoscopic differentiation between lentigo maligna and solar lentigo. J Dermatol. 2011;38(1):53-58. [DOI] [PubMed] [Google Scholar]
- 3.Pralong P, Bathelier E, Dalle S, Poulalhon N, Debarbieux S, Thomas L. Dermoscopy of lentigo maligna melanoma: report of 125 cases. Br J Dermatol. 2012;167(2):280-287. [DOI] [PubMed] [Google Scholar]
- 4.Lallas A, Argenziano G, Moscarella E, Longo C, Simonetti V, Zalaudek I. Diagnosis and management of facial pigmented macules. Clin Dermatol. 2014;32(1):94-100. [DOI] [PubMed] [Google Scholar]
- 5.Guitera P, Haydu LE, Menzies SW, et al. Surveillance for treatment failure of lentigo maligna with dermoscopy and in vivo confocal microscopy: new descriptors. Br J Dermatol. 2014;170(6):1305-1312. [DOI] [PubMed] [Google Scholar]
- 6.Pellacani G, Guitera P, Longo C, Avramidis M, Seidenari S, Menzies S. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127(12):2759-2765. [DOI] [PubMed] [Google Scholar]
- 7.Segura S, Puig S, Carrera C, Palou J, Malvehy J. Development of a two-step method for the diagnosis of melanoma by reflectance confocal microscopy. J Am Acad Dermatol. 2009;61(2):216-229. [DOI] [PubMed] [Google Scholar]
- 8.Guitera P, Menzies SW, Longo C, Cesinaro AM, Scolyer RA, Pellacani G. In vivo confocal microscopy for diagnosis of melanoma and basal cell carcinoma using a two-step method: analysis of 710 consecutive clinically equivocal cases. J Invest Dermatol. 2012;132(10):2386-2394. [DOI] [PubMed] [Google Scholar]
- 9.Pellacani G, Vinceti M, Bassoli S, et al. Reflectance confocal microscopy and features of melanocytic lesions: an internet-based study of the reproducibility of terminology. Arch Dermatol. 2009;145(10):1137-1143. [DOI] [PubMed] [Google Scholar]
- 10.Segura S, Pellacani G, Puig S, et al. In vivo microscopic features of nodular melanomas: dermoscopy, confocal microscopy, and histopathologic correlates. Arch Dermatol. 2008;144(10):1311-1320. [DOI] [PubMed] [Google Scholar]
- 11.Pellacani G, Longo C, Malvehy J, et al. In vivo confocal microscopic and histopathologic correlations of dermoscopic features in 202 melanocytic lesions. Arch Dermatol. 2008;144(12):1597-1608. [DOI] [PubMed] [Google Scholar]
- 12.Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J Invest Dermatol. 2010;130(8):2080-2091. [DOI] [PubMed] [Google Scholar]
- 13.de Carvalho N, Farnetani F, Ciardo S, et al. Reflectance confocal microscopy correlates of dermoscopic patterns of facial lesions help to discriminate lentigo maligna from pigmented nonmelanocytic macules. Br J Dermatol. 2015;173(1):128-133. [DOI] [PubMed] [Google Scholar]
- 14.Menge TD, Hibler BP, Cordova MA, Nehal KS, Rossi AM. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): a prospective study. J Am Acad Dermatol. 2016;74(6):1114-1120. [DOI] [PubMed] [Google Scholar]
- 15.Guitera P, Moloney FJ, Menzies SW, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149(6):692-698. [DOI] [PubMed] [Google Scholar]
- 16.El Shabrawi-Caelen L, Kerl H, Cerroni L. Melan-A: not a helpful marker in distinction between melanoma in situ on sun-damaged skin and pigmented actinic keratosis. Am J Dermatopathol. 2004;26(5):364-366. [DOI] [PubMed] [Google Scholar]
- 17.Helm K, Findeis-Hosey J. Immunohistochemistry of pigmented actinic keratoses, actinic keratoses, melanomas in situ and solar lentigines with melan-A. J Cutan Pathol. 2008;35(10):931-934. [DOI] [PubMed] [Google Scholar]
- 18.Black WH, Thareja SK, Blake BP, Chen R, Cherpelis BS, Glass LF. Distinction of melanoma in situ from solar lentigo on sun-damaged skin using morphometrics and MITF immunohistochemistry. Am J Dermatopathol. 2011;33(6):573-578. [DOI] [PubMed] [Google Scholar]
- 19.Nybakken GE, Sargen M, Abraham R, Zhang PJ, Ming M, Xu X. MITF accurately highlights epidermal melanocytes in atypical intraepidermal melanocytic proliferations. Am J Dermatopathol. 2013;35(1):25-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buonaccorsi JN, Prieto VG, Torres-Cabala C, Suster S, Plaza JA. Diagnostic utility and comparative immunohistochemical analysis of MITF-1 and SOX10 to distinguish melanoma in situ and actinic keratosis: a clinicopathological and immunohistochemical study of 70 cases. Am J Dermatopathol. 2014;36(2):124-130. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez S, Gill M, Halpern AC, eds. Reflectance Confocal Microscopy of Cutaneous Tumors. London: Informa UK; 2008. [Google Scholar]
- 22.Alarcon I, Carrera C, Alos L, Palou J, Malvehy J, Puig S. In vivo reflectance confocal microscopy to monitor the response of lentigo maligna to imiquimod. J Am Acad Dermatol. 2014;71(1):49-55. [DOI] [PubMed] [Google Scholar]
- 23.Kai AC, Richards T, Coleman A, Mallipeddi R, Barlow R, Craythorne EE. Five-year recurrence rate of lentigo maligna after treatment with imiquimod. Br J Dermatol. 2016;174(1):165-168. [DOI] [PubMed] [Google Scholar]
- 24.Richtig E, Arzberger E, Hofmann-Wellenhof R, Fink-Puches R. Assessment of changes in lentigo maligna during radiotherapy by in-vivo reflectance confocal microscopy: a pilot study. Br J Dermatol. 2015;172(1):81-87. [DOI] [PubMed] [Google Scholar]
- 25.Longo C, Segura S, Cesinaro AM, Bassoli S, Seidenari S, Pellacani G. An atypical Meyerson’s naevus: a dermoscopic, confocal microscopic and immunohistochemical description of one case. J Eur Acad Dermatol Venereol. 2007;21(3):414-416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Dermoscopic and Confocal Features Statistically Significant in Multivariate Analysis for LM Diagnosis
eFigure 2. Benign Macule in a Woman in Her 70s