Abstract
Importance
Early-life epilepsies are often a consequence of numerous neurodevelopmental disorders, most of which are proving to have genetic origins. The role of genetic testing in the initial evaluation of these epilepsies is not established.
Objective
To provide a contemporary account of the patterns of use and diagnostic yield of genetic testing for early-life epilepsies.
Design, Setting, and Participants
In this prospective cohort, children with newly diagnosed epilepsy with an onset at less than 3 years of age were recruited from March 1, 2012, to April 30, 2015, from 17 US pediatric hospitals and followed up for 1 year. Of 795 families approached, 775 agreed to participate. Clinical diagnosis of the etiology of epilepsy were characterized based on information available before genetic testing was performed. Added contributions of cytogenetic and gene sequencing investigations were determined.
Exposures
Genetic diagnostic testing.
Main Outcomes and Measures
Laboratory-confirmed pathogenic variant.
Results
Of the 775 patients in the study (367 girls and 408 boys; median age of onset, 7.5 months [interquartile range, 4.2-16.5 months]), 95 (12.3%) had acquired brain injuries. Of the remaining 680 patients, 327 (48.1%) underwent various forms of genetic testing, which identified pathogenic variants in 132 of 327 children (40.4%; 95% CI, 37%-44%): 26 of 59 (44.1%) with karyotyping, 32 of 188 (17.0%) with microarrays, 31 of 114 (27.2%) with epilepsy panels, 11 of 33 (33.3%) with whole exomes, 4 of 20 (20.0%) with mitochondrial panels, and 28 of 94 (29.8%) with other tests. Forty-four variants were identified before initial epilepsy presentation. Apart from dysmorphic syndromes, pathogenic yields were highest for children with tuberous sclerosis complex (9 of 11 [81.8%]), metabolic diseases (11 of 14 [78.6%]), and brain malformations (20 of 61 [32.8%]). A total of 180 of 446 children (40.4%), whose etiology would have remained unknown without genetic testing, underwent some testing. Pathogenic variants were identified in 48 of 180 children (26.7%; 95% CI, 18%-34%). Diagnostic yields were greater than 15% regardless of delay, spasms, and young age. Yields were greater for epilepsy panels (28 of 96 [29.2%]; P < .001) and whole exomes (5 of 18 [27.8%]; P = .02) than for chromosomal microarray (8 of 101 [7.9%]).
Conclusions and Relevance
Genetic investigations, particularly broad sequencing methods, have high diagnostic yields in newly diagnosed early-life epilepsies regardless of key clinical features. Thorough genetic investigation emphasizing sequencing tests should be incorporated into the initial evaluation of newly presenting early-life epilepsies and not just reserved for those with severe presentations and poor outcomes.
This cohort study of children with newly diagnosed epilepsy assesses patterns of use and diagnostic yield of genetic testing for early-life epilepsies.
Key Points
Question
What is the diagnostic yield of genetic testing when used for children with newly presenting early-life epilepsy?
Finding
In this cohort study of 775 children, diagnostic yields overall were 40%, with epilepsy gene-sequencing panels and whole-exome sequencing having substantially greater diagnostic yields than chromosomal microarray. In the absence of a clinically identified cause, testing yields were greater than 15% and as high as 47% depending on patient subgroups.
Meaning
Genetic testing, especially with sequencing-based methods, should be incorporated into the routine initial evaluation of early-life epilepsy.
Introduction
Approximately 2 in 1000 children develop epilepsy in the first 3 years of life. Early-life epilepsies (ELEs) represent many diverse diseases, often with devastating and lasting consequences. Previously, most ELEs were relegated to the undifferentiated category of symptomatic (sometimes “secondary” or “catastrophic”) generalized epilepsy, with a few rare electroclinical syndromes (eg, West syndrome or infantile spasms) specifically recognized. Causes in half or more of the patients remain unknown.
Neuroimaging and, particularly, genetic diagnostic technologies have advanced rapidly in the last 20 years and may provide a basis for disease-targeted therapies. Although neuroimaging is the standard of care in the initial evaluation of ELEs, genetic testing has never been recommended for evaluation of ELEs, especially in the initial diagnostic workup. This practice continues despite a growing literature directed at gene discovery for ELE, which has used individual techniques such as whole-exome sequencing (WES), chromosome microarray (CMA), epilepsy panels, and single-gene testing for highly selected patients. None, however, addresses the value of genetic testing as a primary diagnostic technology at the outset of the disorder.
We conducted a prospective, observational cohort study to provide a contemporary account of the clinical epidemiology of newly diagnosed ELE as evaluated in US pediatric epilepsy centers. We examined the use and yield of genetic testing performed at and during the year after initial diagnosis and its contributions to elucidating the etiology of ELE.
Methods
Children with newly presenting ELE were prospectively recruited through 17 US pediatric epilepsy centers from March 1, 2012, to April 30, 2015. Eligibility criteria were seizure onset before the third birthday and initial diagnosis of epilepsy established at a participating hospital. Each hospital systematically identified all eligible patients. Study data are from review of medical records only. Infants who began having seizures during the neonatal period were also included provided the seizures were unprovoked and not due to an immediate response to an acute insult. Study approval was obtained from the institutional review boards of Ann & Robert H. Lurie Children’s Hospital of Chicago, Oregon Health Services University, Seattle Children’s Hospital, New York Presbyterian Hospital, University of California, San Francisco Beniof Children’s Hospital, Mayo Clinic, CS Mott’s Children’s Hospital, Nationwide Children’s Hospital, National Children’s Medical Center, Johns Hopkins All Children’s Hospital, St. Christopher’s Hospital for Children, Colorado Children’s Hospital, Lucile Packard Children’s Hospital, Cook Children’s Health Care System, Children’s Hospital of Philadelphia, Boston Children’s Hospital, and Massachusetts General Hospital. Written informed consent was obtained from parents of eligible patients.
Data collection targeted the diagnostic evaluation (neuroimaging, metabolic, and genetic), diagnoses of etiology, epilepsy syndrome, and history and results of examination. Data from before epilepsy diagnosis and through 1 year after initial diagnosis were used to determine underlying etiology.
Data were reviewed by site investigators and entered into a Research Electronic Data Capture database. Based on history and results of clinical examinations, neuroimaging, and metabolic testing, etiology was categorized as acquired brain injuries (ie, hypoxic-ischemic encephalopathy, intraventricular hemorrhage, stroke, trauma, and infections), focal cortical dysplasia, other brain malformations and abnormalities (eg, lissencephaly, polymicrogyria, and septo-optic dysplasia), tuberous sclerosis complex, other neurocutaneous diseases, metabolic diseases, recognizable dysmorphic syndromes (eg, Down syndrome and Wolf-Hirschhorn syndrome), and other disorders (eg, tumors). Microcephaly without specific structural abnormality was considered a brain malformation. When etiology could not be assigned to one of these categories, it was considered “unexplained.” The unexplained group was further divided into those with normal developmental status (including mild or equivocal delays) and those with developmental delay based on the clinician’s report from the examination and history initially and during the year after diagnosis.
The use and yield of genetic testing in each category for etiology were determined based on results of all testing performed before, at, and during the year immediately following initial epilepsy diagnosis. Genetic testing included tests generating cytogenetic, copy number, or DNA sequencing data. Metabolic testing and clinical examinations, while used in genetic diagnosis, were not considered to be genetic testing for these purposes. All testing was for clinical purposes and based on the discretion of the treating clinician. Variants were reviewed in light of current information by 2 of us (a board-certified clinical cytogeneticist [M.K.] and a board-certified clinical molecular geneticist [J.J.A.]). When warranted, interpretations were updated. Patterns of use and yield of genetic testing were specifically examined in children with initially unexplained etiology with respect to age at onset, presence of infantile spasms, and developmental delay.
Analyses were performed in SAS, version 9.4 (SAS Institute). Bivariate analyses were performed in PROC SURVEYFREQ and multivariable logistic regression analyses in PROC GLIMMIX to adjust for clustering of observations by center treated as a random variable. Confidence intervals were constructed with standard errors that incorporate clustering of patients by site. P < .05 was the minimal level accepted as reflecting statistical significance.
Results
Twenty families declined participation, and 775 eligible children were recruited (367 girls and 408 boys). The number of children per site varied from 4 to 131 (median, 31). The median age at onset of epilepsy was 7.5 months (interquartile range [IQR], 4.2-16.5 months), and the median age at diagnosis was 8.7 months (IQR, 5.0-19.2 months). Onset occurred at less than 12 months of age in 509 children (65.7%), 12 to 23 months of age in 151 children (19.5%), and 24 to 35 months of age in 115 children (14.8%).
Within 1 year of initial presentation with epilepsy, 272 children (35.1%) were diagnosed with infantile spasms (spasms), 63 (8.1%) had other specific electroclinical diagnoses, and 440 (56.8%) had nonsyndromic epilepsy presentations. The electroclinical diagnoses were Dravet syndrome (n = 11); myoclonic epilepsy of infancy (n = 9); myoclonic-atonic (astatic) epilepsy (n = 6); febrile seizures-plus (n = 5); Ohtahara syndrome, benign epilepsy of infancy, benign familial infantile epilepsy, and absence epilepsy (n = 4 each); benign familial neonatal epilepsy, early myoclonic encephalopathy, and epilepsy with myoclonic absence (n = 3 each); malignant migrating focal seizures in infancy and Lennox-Gastaut syndrome (n = 2 each); and myoclonic epilepsy with nonprogressive disorders, benign epilepsy with centrotemporal spikes, and gelastic seizures with hypothalamic hamartoma (n = 1 each). Thirty-four children with initially nonsyndromic presentations evolved to spasms, and 2 with spasms evolved to Lennox-Gastaut syndrome. All 36 are counted only once in the spasms group.
Evaluations and Findings
Neuroimaging
Overall, 620 children (80.0%) underwent epilepsy protocol magnetic resonance imaging (MRI) at initial evaluation (n = 534) or within the year following (n = 86). Ninety-four children (12.1%) underwent nonepilepsy protocol MRI, 11 children (1.4%) underwent computed tomographic scans or ultrasonography, and 50 children (6.5%) had no imaging performed during the study. For all 725 neuroimaging studies combined, 273 (37.7%) yielded findings that either provided a specific diagnosis (eg, lissencephaly or tumor) or indicated a developmental or progressive brain disorder.
Metabolic Testing
Various metabolic tests were performed for 384 children (49.5%), including tests of blood (359 [46.3%]), urine (244 [31.5%]), and cerebral spinal fluid (133 [17.2%]). Sixteen children received a diagnosis of the following metabolic diseases: Leigh syndrome (n = 4), nonketotic hyperglycinemia (n = 3), Zellweger syndrome (n = 2), mitochondrial diseases (n = 2), and 1 child each of methylmalonic acidemia, vitamin B12 dependency, pyruvate dehydrogenase deficiency, GM1 gangliosidosis, and Alper-Huttenlocher disease.
Initial Etiology Assignment Before Genetic Testing
Based on history and results of clinical examinations, neuroimaging, and metabolic testing, the initial etiology assignments were acquired brain injuries (n = 95), focal cortical dysplasia (n = 21), other brain malformations and abnormalities (n = 91), tuberous sclerosis complex (n = 20), other neurocutaneous disorders (n = 12), metabolic diseases (n = 16), dysmorphic syndromes (n = 45), other disorders (n = 29), unexplained with developmental delay (n = 122), and unexplained with normal development (n = 324).
Genetic Testing
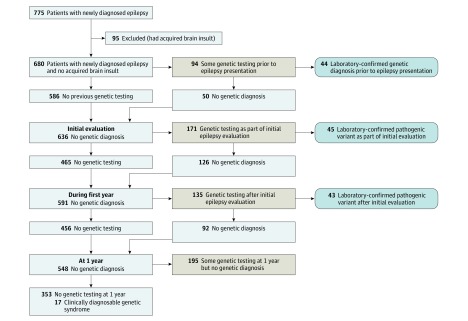
To target children in whom neurogenetic factors might play an explanatory role, analyses focused on children without documented acquired brain injury (n = 680). Of these children, 327 (48.1%) underwent some genetic testing: 94 before initial epilepsy evaluations, 171 during initial evaluations, and 135 during the following year. Sixty children underwent testing at multiple time points.
At the time of initial evaluations, 44 children already had the following laboratory-confirmed genetic diagnoses: trisomy 21 (n = 18), other trisomies and tetrasomies (n = 4), chromosomal deletions or duplications of varying sizes detected by karyotyping or CMA (n = 14), and single- or multiple-nucleotide variants identified through sequencing (n = 8). Testing as part of the epilepsy evaluation (through 1 year) identified explanatory pathogenic variants in 88 more children for a total of 132 (40.4%), including 4 with variants of uncertain significance reclassified as pathogenic (Figure 1; eTable in the Supplement).
Figure 1. Use and Yield of Genetic Testing From Before to 1 Year After Initial Epilepsy Evaluation.
Flow diagram of genetic testing in the cohort.
Ten children were carriers for autosomal recessive disorders, which corresponded to their clinical diagnoses in 2 children. The first child had frontally predominant lissencephaly with cerebellar hypoplasia associated with a heterozygous pathogenic RELN (OMIM 600514) mutation (eFigure in the Supplement). The second child was clinically diagnosed with Leigh syndrome and had a heterozygous pathogenic variant of NDUFAF5 (OMIM 612360). Sixty-six other children had variants of uncertain significance in potentially relevant genes. Twenty children had clinical genetic diagnoses that were not confirmed through genetic testing (3 also had a variant of uncertain significance): 7 with Down syndrome, 11 with tuberous sclerosis complex, 1 with neurofibromatosis, and 1 with Kabuki syndrome.
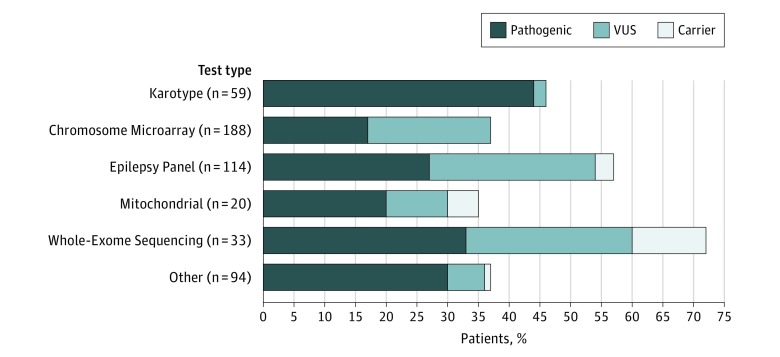
Chromosomal microarray was the most commonly used test (n = 188), followed by a sequencing-based epilepsy gene panel (n = 114), karyotypes (n = 59), WES (n = 33), mitochondrial gene tests (nuclear and mitochondrial; n = 20), and various disease-targeted tests (n = 94; eg, for tuberous sclerosis complex or lissencephaly). The proportion of children tested varied among the 17 sites (median, 49% [IQR, 40%-62%]). When genetic testing was performed, the median pathogenic yield across centers was 40% (IQR, 36%-45%).
Diagnostic (pathogenic) yields from each type of test were meaningfully high. For the 3 broad tests, the yields were as follows: 32 of 188 children for CMA (17.0%; 95% CI, 11%-23%), 31 of 114 children for epilepsy panels (27.2%; 95% CI, 17%-38%), and 11 of 33 children for WES (33.3%; 95% CI, 16%-51%). Variants of unknown significance and heterozygous mutation in autosomal recessive genes were found for all test types (Figure 2). Of 22 patients with both CMA and WES, 8 of 21 (38.1%) with negative CMA results had diagnostic results of WES, whereas the results of WES were negative and the results of CMA were diagnostic for 1 patient. Similarly, of 44 children with both CMAs and epilepsy panels, 10 of 43 (23.3%) with negative CMA results had diagnostic epilepsy panels. The converse occurred for 1 patient.
Figure 2. Findings for Different Kinds of Genetic Tests Used in the Cohort.
Yield of different genetic tests. VUS indicates variants of uncertain significance.
Genetic testing was frequently used regardless of initial etiology (Table 1). For most categories of etiology, the overall yield exceeded 20%. One notable exception was focal cortical dysplasia, for which all test results (on blood-derived DNA) were negative.
Table 1. Use and Yield of Genetic Testing by Initial Etiology Designation in Children Without Acquired Brain Injuriesa.
| Initial Etiologic Group | Children, No. (%) | ||
|---|---|---|---|
| Nonacquired (n = 680) |
Any Genetic Testingb (n = 347) |
Pathogenic Variant Foundb (n = 132) |
|
| Brain malformations | 112 (16.5) | 67 (60) | 20 (30) |
| Focal cortical dysplasia | 21 (3.1) | 6 (29) | 0 |
| Other malformation and structural anomalies | 91 (13.4) | 61 (67) | 20 (33) |
| Neurocutaneous diseases | 32 (4.7) | 16 (50) | 13 (81) |
| Tuberous sclerosis | 20 (2.9) | 11 (55) | 9 (82) |
| Other neurocutaneous diseasesc | 12 (1.8) | 5 (42) | 4 (80) |
| Metabolic diseases | 16 (2.4) | 14 (88) | 11 (79) |
| Clinical dysmorphic syndromes | 45 (6.6) | 38 (84) | 37 (97) |
| Down syndrome | 25 (3.7) | 18 (72) | 18 (100) |
| Other syndromesd | 20 (2.9) | 20 (100) | 19 (95) |
| Other etiologye | 29 (4.3) | 12 (41) | 3 (25) |
| Unexplained etiology | 446 (65.6) | 180 (40) | 48 (27) |
| Developmental delay | 122 (17.9) | 93 (76) | 28 (30) |
| Normal development | 324 (47.6) | 87 (27) | 20 (23) |
Children could undergo more than 1 type of test.
Denominator for each percentage is in the preceding column in each row.
Such as neurofibromatosis or Sturge-Weber syndrome.
Such as Wolf-Hirschhorn syndrome.
Such as tumors, hippocampal atrophy, postnatal strokes, or lesions of unclear nature (eg, tumor vs malformation).
Genetic Testing Yield With Initially Unexplained Etiology
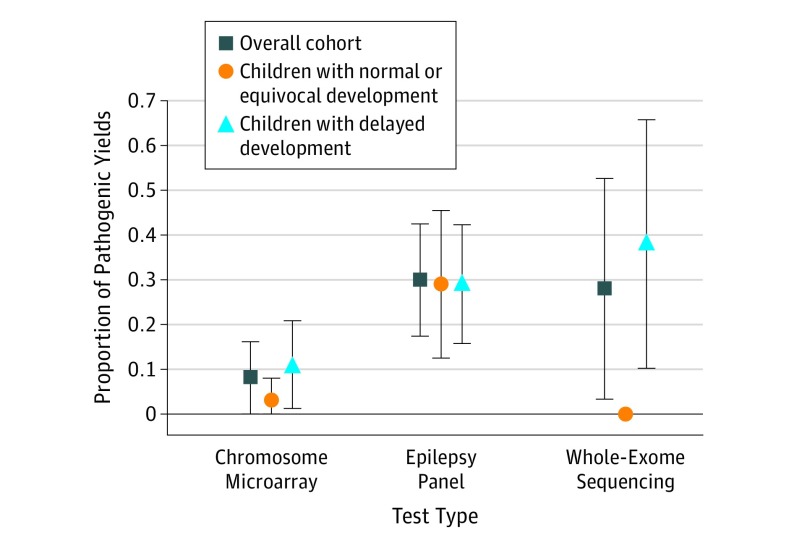
In the group of children with unexplained etiology, 180 of 446 children (40.4%) received some form of genetic testing before (n = 19), as part of (n = 103), and in the year immediately following (n = 93) their initial epilepsy evaluation (35 had testing at multiple times). Pathogenic variants were reported in 48 of 180 patients tested (26.7%; 95% CI, 18%-34%). Chromosome microarray yields (8 of 101 [7.9%]; 95% CI, 0%-16%) were markedly lower than yields for panels (28 of 96 [29.2%]; 95% CI, 17%-42%; P < .001) and for WES (5 of 18 [27.8%]; 95% CI, 3%-52%; P = .02). Yields for these tests did not vary markedly by developmental delay (Figure 3). Pathogenic variants were identified before (n = 4 [all developmentally delayed]), during (n = 18), and in the year immediately following (n = 26) initial epilepsy evaluations.
Figure 3. Genetic Test Yields in Children With Otherwise Unexplained Etiology.
Genetic test results in children with unexplained etiology. Vertical bars indicate 95% CIs.
Genetic testing was ordered 2 to 3 times as often for infants (<1 year) than for older patients, for those with vs without definite developmental delay, and for those with spasms initially and during the year after initial epilepsy diagnosis (Table 2). In a multivariable logistic model, each factor independently contributed to the likelihood of being tested. On bivariate analysis, diagnostic yields did not significantly differ by age at onset or developmental delay but were substantially lower overall for children with spasms and especially when limited to infants. Because spasms are strongly associated with delays and early age at onset, all 3 factors were strongly associated with pathogenic test results in a multivariable model.
Table 2. Use and Pathogenic Yield of Genetic Testing for Children With Unexplained Etiology.
| Characteristic | Children, No. | % of Children Tested (95% CI) | No. of Children With Pathogenic Yield (n = 42) |
% of Children With Pathogenic Yield (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Total (n = 442a) |
Tested (n = 167) |
Bivariateb | Multivariablec | Bivariateb | Multivariablec | ||
| Age at onset, mo | |||||||
| <12 | 250 | 124 | 50 (39-60) | 62 (51-72) | 36 | 29 (21-37) | 27 (18-38) |
| ≥12 | 192 | 43 | 22 (17-28) | 44 (31-58) | 8 | 19 (5-32) | 9 (4-21) |
| P value | <1 × 10−4 | .006 | .17 | .01 | |||
| Developmental delayd | |||||||
| None or equivocal | 316 | 83 | 26 (19-33) | 36 (26-47) | 18 | 22 (12-32) | 10 (5-21) |
| Definite | 126 | 84 | 67 (58-76) | 70 (58-80) | 26 | 31 (21-41) | 24 (14-38) |
| P value | <1 × 10−4 | <1 × 10−4 | .18 | .02 | |||
| Type of epilepsy | |||||||
| Nonsyndromic epilepsy or other syndromes | 340 | 91 | 27 (20-34) | 36 (27-46) | 32 | 35 (24-46) | 32 (21-46) |
| Infantile spasmse | 102 | 76 | 75 (61-88) | 70 (56-81) | 12 | 16 (8-23) | 7 (3-16) |
| P value | <1 × 10−4 | <1 × 10−4 | .009 | <.001 | |||
| Developmental delayd,f | (n = 250) | (n = 124) | (n = 36) | ||||
| None or equivocal | 168 | 59 | 35 (24-46)) | 41 (33-50) | 14 | 24 (14-34) | 17 (9-29) |
| Definite | 82 | 65 | 79 (71-87) | 78 (67-86) | 22 | 34 (23-45) | 42 (28-56) |
| P value | <1 × 10−4 | <1 × 10−4 | .20 | .01 | |||
| Type of epilepsy | |||||||
| Nonsyndromic epilepsy or other syndromes | 153 | 53 | 35 (25-44) | 46 (36-56) | 25 | 47 (33-62) | 52 (37-66) |
| Infantile spasmse | 97 | 71 | 73 (59-88) | 75 (64-83) | 11 | 15 (7-24) | 12 (6-22) |
| P value | <1 × 10−4 | <1 × 10−4 | <.001 | <1 × 10−4 | |||
A total of 4 children who were evaluated prior to the onset of epilepsy and who received genetic diagnoses are excluded. All had seizure onset at 1 year of age or younger and were developmentally delayed. Two had infantile spasms.
Overall percentage adjusted for site clustering as a random effect.
Overall percentage after adjustment for other factors. Site is treated as a random effect.
Noted at initial diagnosis or within the year following initial diagnosis.
At initial diagnosis or within 1 year of initial diagnosis.
Multivariable model not adjusted for site due to failure to converge.
Discussion
Our results provide a contemporary assessment of the use of genetic testing in US pediatric epilepsy centers and its diagnostic value for children with newly presenting ELE. Whereas most studies focus on genetic testing in convenience samples of patients selected for severe presentations and outcomes, we targeted all eligible patients at participating centers with ELE at initial diagnostic presentation. After excluding children with acquired brain injuries, the precipitating etiology could be linked to a specific genetic factor in 40% of children, including those with brain malformations, metabolic diseases, or dysmorphic syndromes. Genetic testing provided a diagnosis in one-fourth of children whose cause would have otherwise remained unresolved.
Magnetic resonance imaging is recommended in the evaluation of ELE, and there was a high use of neuroimaging (725 of 775 [93.5%]), mostly with MRI (714 of 775 [92.1%]). Consequently, most brain malformations in this cohort have likely been identified, although some subtle focal cortical dysplasias may not be discernible until myelination is more complete. Recent international recommendations emphasize metabolic testing for infants and toddlers with refractory epilepsy. Where neonatal metabolic screening for inborn errors of metabolism is not commonly available, this practice is good policy. In our cohort, however, only 1 child had a metabolic condition that typically would be identified at birth (methylmalonic acidemia) but was only found later. Most children with neurometabolic diseases had other signs of metabolic disorders, which were then confirmed by genetic testing. This same report recommended genetic testing only for selected children “as the screening to identify those in need of specific genetic analysis is based on tertiary settings.”(p1188) Although knowing how to use genetic investigations, interpret findings, and report results and the availability of appropriate genetic counseling are critical, we found that, like neuroimaging, current clinical genetic testing methods have substantial diagnostic yields regardless of clinical features certainly higher than the many metabolic tests that are frequently ordered. These results suggest that genetic testing should be incorporated into the routine initial evaluation of ELEs to reach an accurate diagnosis as soon as possible.
To our knowledge, CMA is currently the only test with a tangentially relevant indication, unexplained developmental delays. For children with initially unexplained etiology, however, the yield of CMA was substantially less than for sequencing methods (panels and WES). Thus, prioritizing sequencing-based tests over CMA may be a more efficient diagnostic strategy for children with normal results of imaging and noninformative histories regardless of developmental status. Whether WES or epilepsy gene panels are comparably efficient is unclear. Currently, WES is more expensive because it requires trios and extensive genetic counseling. Its sensitivity is also variable due to inadequate coverage of some genes. As genetic testing technologies rapidly evolve, the relative costs and benefits of current and future technologies will likely change as well. Whole-genome sequencing, which can detect variants ranging in size from a single-nucleotide change through large chromosomal variants, may replace many of the current testing technologies in the future.
Our findings highlight the difficulties in characterizing the etiology of developmental brain disorders. Traditional divisions of structural, metabolic, and genetic seem increasingly inadequate because many brain malformations and inborn metabolic diseases are fundamentally genetic. The genetic-structural distinction is further challenged by observations that ion channelopathies, the quintessential “genetic” epilepsy, can be associated with striking abnormalities in brain structure. Although results of genetic testing were negative for children with focal cortical dysplasias, these lesions are often due to somatic mutations that may only be detectable through very deep sequencing of testing of brain tissue–derived DNA. In the future, therapies may be directed toward molecular pathways and may result in a fundamental shift in how etiologies are grouped and classified for clinical purposes.
Limitations
Important limitations of this study include that it is not population-based; there may be a selection of patients sent to hospital centers according to severity at initial presentation. Patients were not further selected, however, and they represent newly presenting patients initially diagnosed at these centers. Given that participating hospitals are located across the United States and serve diverse communities, we suspect that our findings are relevant to other centers in this country or elsewhere. They do not, however, reflect non–hospital-based practice.
Data come from review of medical records, and pediatric epileptologists at each site ensured the quality of the recorded data elements. Genetic testing reports, however, were not always available for review, especially when testing occurred before epilepsy presentation. In those instances, the variants in the medical record, as reported and used by the treating clinicians, were accepted.
Children did not undergo systematic genetic testing, although all tests were performed at Clinical Laboratory Improvement Amendments–certified facilities. Tests were ordered by physicians at their discretion. Consequently, there were expected trends in the use of genetic testing by key clinical features. Diagnostic yields, however, were meaningfully high regardless of these features, although they were low for spasms. Ideally, all children should receive the same thorough, comprehensive test battery.
Expanding comprehensive genetic testing to all children with ELE could yield many benefits, including stratification of patients in trials of disease-targeted therapies. Currently, there is little evidence to guide selection of therapies based on genetic diagnosis; most recommendations reflect expert opinion (eg, for Dravet syndrome, largely due to SCN1A [OMIM 182389] variants) and anecdotes (eg, for KCNQ2 [OMIM 602235]-associated and SCN2A [OMIM 182390]-associated epilepsies); in our study, there were 12 children with SCN1A variants, 2 children with KCNQ2-associated epilepsies, and no children with SCN2A-associated epilepsies. The lack of evidence to guide treatment based on genetic diagnosis occurs because the individual genetic disorders are rare and currently not routinely identified due to an uneven and sometimes unenthusiastic uptake of genetic testing. If testing is not performed and genetic diagnoses are not made, there is no basis for identifying optimal, targeted treatments. Prioritizing thorough, comprehensive genetic testing as part of the initial epilepsy evaluation could make precision medicine part of standard clinical practice. Ideally, the inclusion of genetic testing would result in better health outcomes and reduce costs due to overuse of unnecessary but currently reimbursed diagnostic modalities, including metabolic testing.
Aside from treatment selection, multiple benefits accrue to the health system and the family by obtaining a genetic diagnosis. It ends the diagnostic odyssey during which parents and physicians spend untold amounts of time searching for an explanation for a child’s epilepsy and reduces associated costs. A genetic diagnosis allows for genetic counseling of parents who may wish to have more children and the consideration of risk for other siblings once they are ready to plan families. A genetic diagnosis creates the opportunity to participate in research studies of new therapies and to find optimal therapy and management approaches for the child or for others in the future. The most successful rare disease network, Children’s Oncology Group, has been effective in turning clinical care into research and feeding results back into care, continuously improving survivorship and other outcomes of young patients with cancer. Finally, a genetic diagnosis allows families to seek out others and advocate for research into these rare conditions through a variety of channels, such as the National Organization for Rare Disorders.
Conclusions
Growing evidence suggests that early, effective intervention for seizures may modify the severity of developmental, behavioral, and other outcomes for children with ELE. This evidence provides added impetus to move the diagnosis of the specific cause to the point of initial presentation. Our study provides an initial assessment of the potential diagnostic value of such a strategy and suggests that it is time to provide greater emphasis on and support for thorough genetic evaluations, particularly sequencing-based evaluations, for children with newly presenting epilepsies in the first few years of life.
eTable. Pathogenic Variants Reported in the Cohort Including 132 for Which the Findings Were Explanatory of the Child’s Condition and 10 for Which the Gene Was Associated With Autosomal Recessive in Heritance and Could Not Alone Explain the Child’s Condition
eFigure. Sagittal and Axial Images in a Patient With Heterozygous Frameshift Mutation in RELN (c.329 dup; p. Gly111Argfs*7)
References
- 1.Camfield CS, Camfield PR, Gordon K, Wirrell E, Dooley JM. Incidence of epilepsy in childhood and adolescence: a population-based study in Nova Scotia from 1977 to 1985. Epilepsia. 1996;37(1):19-23. [DOI] [PubMed] [Google Scholar]
- 2.Commission on Classification and Terminology of the International League Against Epilepsy Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989;30(4):389-399. [DOI] [PubMed] [Google Scholar]
- 3.Camfield C, Camfield P. Twenty years after childhood-onset symptomatic generalized epilepsy the social outcome is usually dependency or death: a population-based study. Dev Med Child Neurol. 2008;50(11):859-863. [DOI] [PubMed] [Google Scholar]
- 4.Glauser TA. Topiramate in the catastrophic epilepsies of childhood. J Child Neurol. 2000;15(suppl 1):S14-S21. [DOI] [PubMed] [Google Scholar]
- 5.Glauser TA. Following catastrophic epilepsy patients from childhood to adulthood. Epilepsia. 2004;45(suppl 5):23-26. [DOI] [PubMed] [Google Scholar]
- 6.Cho JH, Hong SB, Jung YJ, et al. Evaluation of algorithms for intracranial EEG (iEEG) source imaging of extended sources: feasibility of using iEEG source imaging for localizing epileptogenic zones in secondary generalized epilepsy. Brain Topogr. 2011;24(2):91-104. [DOI] [PubMed] [Google Scholar]
- 7.Cukiert A, Cukiert CM, Burattini JA, et al. A prospective long-term study on the outcome after vagus nerve stimulation at maximally tolerated current intensity in a cohort of children with refractory secondary generalized epilepsy. Neuromodulation. 2013;16(6):551-556. [DOI] [PubMed] [Google Scholar]
- 8.De Negri M, Cremonte M, Veneselli E, et al. Secondary generalized epilepsy in childhood: EEG patterns and correlation with responsiveness to benzodiazepines or ACTH (preliminary note). Brain Dev. 1988;10(6):375-381. [DOI] [PubMed] [Google Scholar]
- 9.Egli M, Mothersill I, O’Kane M, O’Kane F. The axial spasm—the predominant type of drop seizure in patients with secondary generalized epilepsy. Epilepsia. 1985;26(5):401-415. [DOI] [PubMed] [Google Scholar]
- 10.Kugoh T, Hosokawa K. A trial of discontinuation of barbiturates in patients with secondary generalized epilepsy. Jpn J Psychiatry Neurol. 1986;40(4):655-662. [DOI] [PubMed] [Google Scholar]
- 11.Gaillard WD, Chiron C, Cross JH, et al. ; ILAE, Committee for Neuroimaging, Subcommittee for Pediatrics . Guidelines for imaging infants and children with recent-onset epilepsy. Epilepsia. 2009;50(9):2147-2153. [DOI] [PubMed] [Google Scholar]
- 12.Hirtz D, Ashwal S, Berg A, et al. Practice parameter: evaluating a first nonfebrile seizure in children: report of the Quality Standards Subcommittee of the American Academy of Neurology, the Child Neurology Society, and the American Epilepsy Society. Neurology. 2000;55(5):616-623. [DOI] [PubMed] [Google Scholar]
- 13.Nunes VD, Sawyer L, Neilson J, Sarri G, Cross JH. Diagnosis and management of the epilepsies in adults and children: summary of updated NICE guidance. BMJ. 2012;344:e281. [DOI] [PubMed] [Google Scholar]
- 14.Allen AS, Berkovic SF, Cossette P, et al. ; Epi4K Consortium; Epilepsy Phenome/Genome Project . De novo mutations in epileptic encephalopathies. Nature. 2013;501(7466):217-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin HC, Kim GE, Pagnamenta AT, et al. ; WGS500 Consortium . Clinical whole-genome sequencing in severe early-onset epilepsy reveals new genes and improves molecular diagnosis. Hum Mol Genet. 2014;23(12):3200-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veeramah KR, Johnstone L, Karafet TM, et al. Exome sequencing reveals new causal mutations in children with epileptic encephalopathies. Epilepsia. 2013;54(7):1270-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helbig KL, Farwell Hagman KD, Shinde DN, et al. Diagnostic exome sequencing provides a molecular diagnosis for a significant proportion of patients with epilepsy. Genet Med. 2016;18(9):898-905. [DOI] [PubMed] [Google Scholar]
- 18.Olson H, Shen Y, Avallone J, et al. Copy number variation plays an important role in clinical epilepsy. Ann Neurol. 2014;75(6):943-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemke JR, Riesch E, Scheurenbrand T, et al. Targeted next generation sequencing as a diagnostic tool in epileptic disorders. Epilepsia. 2012;53(8):1387-1398. [DOI] [PubMed] [Google Scholar]
- 20.Mercimek-Mahmutoglu S, Patel J, Cordeiro D, et al. Diagnostic yield of genetic testing in epileptic encephalopathy in childhood. Epilepsia. 2015;56(5):707-716. [DOI] [PubMed] [Google Scholar]
- 21.Otsuka M, Oguni H, Liang JS, et al. STXBP1 mutations cause not only Ohtahara syndrome but also West syndrome—result of Japanese cohort study. Epilepsia. 2010;51(12):2449-2452. [DOI] [PubMed] [Google Scholar]
- 22.Schwarz N, Hahn A, Bast T, et al. Mutations in the sodium channel gene SCN2A cause neonatal epilepsy with late-onset episodic ataxia. J Neurol. 2016;263(2):334-343. [DOI] [PubMed] [Google Scholar]
- 23.Weckhuysen S, Ivanovic V, Hendrickx R, et al. ; KCNQ2 Study Group . Extending the KCNQ2 encephalopathy spectrum: clinical and neuroimaging findings in 17 patients. Neurology. 2013;81(19):1697-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Claes L, Del-Favero J, Ceulemans B, Lagae L, Van Broeckhoven C, De Jonghe P. De novo mutations in the sodium-channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am J Hum Genet. 2001;68(6):1327-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torkamani A, Bersell K, Jorge BS, et al. De novo KCNB1 mutations in epileptic encephalopathy. Ann Neurol. 2014;76(4):529-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.SAS Institute Inc The SURVEYFREQ Procedure: SAS/STAT 9.3 User’s Guide. Cary, NC: SAS Institute Inc; 2011. [Google Scholar]
- 28.SAS Institute Inc The GLIMMIX Procedure: SAS/STAT 9.3 User’s Guide. Cary, NC: SAS Institute, Inc; 2011. [Google Scholar]
- 29.Wilmshurst JM, Gaillard WD, Vinayan KP, et al. Summary of recommendations for the management of infantile seizures: Task Force Report for the ILAE Commission of Pediatrics. Epilepsia. 2015;56(8):1185-1197. [DOI] [PubMed] [Google Scholar]
- 30.Michelson DJ, Shevell MI, Sherr EH, Moeschler JB, Gropman AL, Ashwal S. Evidence report: genetic and metabolic testing on children with global developmental delay: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2011;77(17):1629-1635. [DOI] [PubMed] [Google Scholar]
- 31.Miller DT, Adam MP, Aradhya S, et al. Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet. 2010;86(5):749-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ankala A, da Silva C, Gualandi F, et al. A comprehensive genomic approach for neuromuscular diseases gives a high diagnostic yield. Ann Neurol. 2015;77(2):206-214. [DOI] [PubMed] [Google Scholar]
- 33.Consugar MB, Navarro-Gomez D, Place EM, et al. Panel-based genetic diagnostic testing for inherited eye diseases is highly accurate and reproducible, and more sensitive for variant detection, than exome sequencing. Genet Med. 2015;17(4):253-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorokhova S, Cerino M, Mathieu Y, et al. Comparing targeted exome and whole exome approaches for genetic diagnosis of neuromuscular disorders. Appl Transl Genom. 2015;7:26-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51(4):676-685. [DOI] [PubMed] [Google Scholar]
- 36.Barkovich AJ, Dobyns WB, Guerrini R. Malformations of cortical development and epilepsy. Cold Spring Harb Perspect Med. 2015;5(5):a022392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Almannai M, Marom R, Sutton VR. Newborn screening: a review of history, recent advancements, and future perspectives in the era of next generation sequencing. Curr Opin Pediatr. 2016;28(6):694-699. [DOI] [PubMed] [Google Scholar]
- 38.Platzer K, Yuan H, Schütz H, et al. GRIN2B encephalopathy: novel findings on phenotype, variant clustering, functional consequences and treatment aspects [published online April 4, 2017]. J Med Genet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poduri A, Evrony GD, Cai X, Walsh CA. Somatic mutation, genomic variation, and neurological disease. Science. 2013;341(6141):1237758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jamuar SS, Lam AT, Kircher M, et al. Somatic mutations in cerebral cortical malformations. N Engl J Med. 2014;371(8):733-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matthews PM, Edison P, Geraghty OC, Johnson MR. The emerging agenda of stratified medicine in neurology. Nat Rev Neurol. 2014;10(1):15-26. [DOI] [PubMed] [Google Scholar]
- 42.Wirrell EC, Laux L, Donner E, et al. Optimizing the diagnosis and management of Dravet syndrome: recommendations from a North American consensus panel. Pediatr Neurol. 2017;68:18-34.e3. [DOI] [PubMed] [Google Scholar]
- 43.Millichap JJ, Park KL, Tsuchida T, et al. KCNQ2 encephalopathy: features, mutational hot spots, and ezogabine treatment of 11 patients. Neurol Genet. 2016;2(5):e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dilena R, Striano P, Gennaro E, et al. Efficacy of sodium channel blockers in SCN2A early infantile epileptic encephalopathy. Brain Dev. 2017;39(4):345-348. [DOI] [PubMed] [Google Scholar]
- 45.Kutscher EJ, Joshi SM, Patel AD, Hafeez B, Grinspan ZM. Barriers to genetic testing for pediatric Medicaid beneficiaries with epilepsy [published online April 20, 2017]. Pediatr Neurol. 2017;S0887-8994(16)31047-5. [DOI] [PubMed] [Google Scholar]
- 46.Trevathan E. So what? does the test lead to improved health outcomes? Neurology. 2011;77(17):1586-1587. [DOI] [PubMed] [Google Scholar]
- 47.Joshi C, Kolbe DL, Mansilla MA, Mason SO, Smith RJ, Campbell CA. Reducing the cost of the diagnostic odyssey in early onset epileptic encephalopathies. Biomed Res Int. 2016;2016:6421039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ashwal S, Michelson D, Plawner L, Dobyns WB; Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society . Practice parameter: evaluation of the child with microcephaly (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2009;73(11):887-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grosse SD, Wordsworth S, Payne K. Economic methods for valuing the outcomes of genetic testing: beyond cost-effectiveness analysis. Genet Med. 2008;10(9):648-654. [DOI] [PubMed] [Google Scholar]
- 50.Brunklaus A, Dorris L, Ellis R, et al. The clinical utility of an SCN1A genetic diagnosis in infantile-onset epilepsy. Dev Med Child Neurol. 2013;55(2):154-161. [DOI] [PubMed] [Google Scholar]
- 51.López-Pisón J, García-Jiménez MC, Monge-Galindo L, et al. Our experience with the aetiological diagnosis of global developmental delay and intellectual disability: 2006-2010. Neurologia. 2014;29(7):402-407. [DOI] [PubMed] [Google Scholar]
- 52.National Academy of Sciences Comprehensive Cancer Care for Children and Their Families: Summary of a Joint Workshop by the Institute of Medicine and the American Cancer Society. Washington, DC: The National Academies Press; 2015. [PubMed] [Google Scholar]
- 53.NORD National Organization for Rare Disorders. https://rarediseases.org/. Accessed September 4, 2016.
- 54.Berg AT. Epilepsy, cognition, and behavior: the clinical picture. Epilepsia. 2011;52(suppl 1):7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Callaghan FJ, Lux AL, Darke K, et al. The effect of lead time to treatment and of age of onset on developmental outcome at 4 years in infantile spasms: evidence from the United Kingdom Infantile Spasms Study. Epilepsia. 2011;52(7):1359-1364. [DOI] [PubMed] [Google Scholar]
- 56.Eisermann MM, DeLaRaillère A, Dellatolas G, et al. Infantile spasms in Down syndrome—effects of delayed anticonvulsive treatment. Epilepsy Res. 2003;55(1-2):21-27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Pathogenic Variants Reported in the Cohort Including 132 for Which the Findings Were Explanatory of the Child’s Condition and 10 for Which the Gene Was Associated With Autosomal Recessive in Heritance and Could Not Alone Explain the Child’s Condition
eFigure. Sagittal and Axial Images in a Patient With Heterozygous Frameshift Mutation in RELN (c.329 dup; p. Gly111Argfs*7)