Abstract
Importance
Benign melanotic macules (MAC) are the most frequent cause of lip pigmentation and sometimes difficult to differentiate from lip melanoma (MEL).
Objectives
To report in vivo reflectance confocal microscopy (RCM) features of normal lips of different phototypes and to identify features that assist in distinguishing MEL from MAC using dermoscopy and RCM.
Design, Setting, and Participants
For this retrospective observational study, 2 groups of patients from 2 tertiary referral centers for melanoma (Sydney Melanoma Diagnostic Centre and Melanoma Institute Australia) were recruited between June 2007 and January 2015. Group 1 included patients with normal lips and different phototypes, and Group 2 consisted of patients with MAC and MEL; RCM and dermoscopy were used for lips analysis.
Main Outcomes and Measures
Overall, 92 RCM features were correlated with clinical history, dermoscopic images, and histopathology in all patients with MEL and 5 patients with MAC.
Results
Images from the vermillion and/or mucosal part of the lip were recorded from 10 patients with clinically normal lips (mean [SD] age, 34.5 [6.1] years), 16 patients with MAC (mean [SD] age, 49.6 [17.9] years), and 5 patients with 6 cases of MEL (1 patient had a recurrent lesion; mean [SD] age, 56.2 [15.5] years). In normal lips, the draped pattern—a previously described MAC RCM feature—was identified in all cases. In MEL, the following findings were frequent and significantly different from MAC: epidermal disarray; pagetoid infiltration of dendritic and/or round cells; a nonspecific architectural pattern at the dermoepidermal junction (DEJ); nonhomogenously distributed papillae; continuous (lentiginous) proliferation of cells with marked atypia at the DEJ, especially in interpapillary spaces; a higher number of dendritic cells (especially roundish); and atypical round cells at the DEJ. The cellular body area of dendritic cells was about the double in MEL compared with MAC. An RCM lip algorithm was developed that provided 100% sensitivity and 88% specificity for the diagnosis of MEL of the vermillion and mucosal part of the lip. With dermoscopy, MAC were correctly classified as benign in 13 of 16 cases (81%) and MEL were classified as equivocal or malignant in 5 of 6 cases (83%).
Conclusions and Relevance
Reflectance confocal microscopy can assist in the differential diagnosis of lip MEL and MAC. An RCM Lip Score that we developed based on study results is proposed and needs to be validated on an independent data set.
This case series study reports in vivo reflectance confocal microscopy (RCM) features of normal lips of different phototypes and attempts to identify features that assist in distinguishing melanoma from benign melanotic macules using dermoscopy and RCM.
Key Points
Question
Does reflectance confocal microscopy (RCM) add useful information to clinical and dermoscopic findings for diagnosing melanoma (MEL) and melanotic macules (MAC) of the vermillion and mucosal lip?
Finding
In this case series study, 92 RCM features were analyzed with dermoscopic images in 10 normal lips, 16 cases of MAC, and 6 cases MEL. Some RCM features can help in differentiating melanomas from melanotic macules, and the presence of epidermal disarray, pagetoid cells, nonhomogeneously distributed papillae and large, and round and numerous dendritic cells around and between the papillae are especially helpful.
Meaning
There are useful RCM features that can assist in the differential diagnosis of MEL and MAC.
Introduction
Pigmented lesions on the lip are a frequent cause for consultation or referral to dermatologists. Benign melanotic macules (MAC) are the most frequent cause of lip pigmentation and can usually be distinguished from melanoma (MEL) using clinical and dermoscopic criteria. However, the diagnosis of MAC can be challenging, and close follow-up and/or a lip biopsy may be needed to establish a diagnosis with confidence. Biopsying lip lesions is troublesome for patients because it is often associated with discomfort, pain and sometimes scarring.
Reflectance confocal microscopy (RCM) is a noninvasive imaging technique that allows in vivo microscopic visualization of the skin, providing horizontal views of the skin layers up to 200 µm deep and even deeper in mucosa. The lip is a complex structure with 3 different anatomical areas: external area (keratinized epithelium with hair follicles, sebaceous, and sweat glands), vermillion (thin nonkeratinising squamous mucosa devoid of adnexal structures with tall connective tissue papillae and rich underlying vasculature), and inner zone (nonkeratinising squamous mucosa). Reflectance confocal microscopy features in normal oral mucosa, including the lip, have been described, but information about findings in patients with different skin phototypes and in different anatomical regions of the lip is, to the best of our knowledge, lacking in the current literature. This information could assist in the diagnostic evaluation of pigmented lip lesions.
Several diagnostic RCM features and algorithms for cutaneous MEL have been proposed and evaluated; however, reports about the diagnostic use of RCM for lesions on the lips are very limited. Furthermore, basal atypical dendritic cells, an RCM feature of cutaneous MEL, can be found in MAC and may limit the diagnostic accuracy of RCM.
The objectives of this study were to report RCM features of normal lips not described previously and to identify features that may assist in distinguishing between benign and malignant pigmented macules of the lip with dermoscopy and RCM.
Methods
Patients
This retrospective observational study analyzed patients older than 18 years who presented to the Sydney Melanoma Diagnostic Centre (Royal Prince Alfred Hospital) or Melanoma Institute Australia between June 2007 and January 2015 and had their lip evaluated by RCM. All available clinical information, photographs, dermoscopy, and RCM images were extracted from RCM databases of both institutions, and the patients were divided into 2 groups. Group 1 was recruited to document RCM features of clinically normal lips and describe differences between phototypes in 3 different anatomical areas: vermillion border, vermillion, and inner mucosae. Group 1 comprised 10 voluntary patients recruited from April 2013 to June 2013. Group 2 consisted of patients with pigmented lesions on the lip, including 16 MAC (from 16 patients) and 6 MEL (from 5 patients, with 1 having a recurrent lesion), with images recorded from the vermillion and/or mucosal part of the lip (Table 1). Lesions suspicious for malignancy, either clinically or on dermoscopy and/or under RCM evaluation, were biopsied. The rest of the lesions were followed clinically according to standard clinical practice for at least 6 months, most of them with digital monitoring. Histopathology was available for all MEL and in 5 out of 16 MAC and was assessed by a dermatopathologist (R.A.S.). Immunostaining with MelanA was performed in 4 MEL and 1 MAC, and immunostaining against CD1a was performed in 1 MEL.
Table 1. Clinical Characteristics of Patients.
| Characteristic | No. (%) | Melanotic Macules vs Melanomas, P Value | ||
|---|---|---|---|---|
| Group 1 | Group 2 | |||
| Normal Lips (n = 10) |
Melanotic Macules (n = 16) |
Melanomasa (n = 6) |
||
| Age, mean (SD), y | 34.5 (6.1) | 49.6 (17.9) | 56.2 (15.5) | .46 |
| Sex | ||||
| Male | 3 (30) | 3 (19) | 2 (40) | .55 |
| Female | 7 (70) | 13 (81) | 3 (60) | |
| Localization | ||||
| Upper lip | 0 | 1 (6) | 5 (83) | .001 |
| Lower lip | 10 (100) | 15 (94) | 1 (17) | |
| Anatomic areab | .009 | |||
| Skin/vermillion | 0 (0) | 2 (33) | ||
| Vermillion | 7 (44) | 2 (33) | ||
| Vermillion/inner mucosae | 8 (50) | 0 | ||
| Inner mucosae | 1 (6) | 2 (33) | ||
| Personal history of melanoma | .61 | |||
| Yes | 0 | 4 (25) | 3 (50) | |
| No | 10 (100) | 10 (63) | 3 (50) | |
| NA | 0 | 2 (12) | 0 | |
| Family history of melanoma | >.99 | |||
| Yes | 0 | 1 (6) | 0 | |
| No | 10 (100) | 12 (75) | 5 (83) | |
| NA | 3 (19) | 1 (17) | ||
| Phototype | .31 | |||
| 1 | 0 | 2 (12) | 1 (17) | |
| 2 | 3 (30) | 2 (12) | 3 (50) | |
| 3 | 5 (50) | 7 (44) | 1 (17) | |
| 4 | 2 (20) | 1 (6) | 0 | |
| NA | 0 | 4 (25) | 1 (17) | |
Abbreviation: NA, not available.
Two cases were primary melanomas (2 patients) and 4 cases were recurrent melanomas (3 patients).
In normal lips, the vermillion border, vermillion, and inner mucosae were examined.
This study was approved by the human ethics review committee from Royal Prince Alfred Hospital and Melanoma Institute Australia, and informed and signed consent was obtained from all patients according to principles of the Declaration of Helsinki.
Dermoscopy
Dermoscopy images were obtained using SolarScan (Polartechnics Ltd), Vivascope Macrocamera (Caliber I.D.), or Heine-20 (Heine Dermatology) attached to a digital camera (Canon Inc). Dermoscopic images were analyzed following published criteria by one of the authors (S.W.M.) without having access to clinical data, and classified as benign, equivocal, or malignant.
RCM Instruments and Acquisition Procedure
Reflectance confocal microscopy images were acquired using the Vivascope 1500 (Caliber) in 30 cases and Vivascope 3000 (Caliber) in 1 case. The field of view is wider with Vivascope 1500 (a square of 8 × 8 mm) than Vivascope 3000 (handheld, 1 × 1 mm) and allows for a sequence of montage images (“Vivablock” images). Instrument and acquisition procedures are described elsewhere. All images corresponding to each patients’ lip were reviewed and evaluated.
RCM Analysis and Diagnosis
Reflectance confocal microscopy features were described and evaluated by 2 investigators by consensus (P.U., H.C.), blinded to clinical information apart from location and Vivascope Macrocamera image.
A series of 92 features corresponding to previous observations and new descriptors were considered at 3 different depth levels in each case. All these features were evaluated for their presence or absence (binary nonparametric data). Description and definitions are summarized in Table 2.
Table 2. RCM Features Findings of Pigmented Lesions on the Lipsa.
| RCM Feature | No. (%) | P Value | |
|---|---|---|---|
| Melanotic Macules (n = 16) |
Melanomas (n = 6) |
||
| Suprabasal Epidermis | |||
| Regular architectural patterns | 15/16 (94) | 2/6 (33) | .009 |
| Regular HC pattern | 15/16 (94) | 2/6 (33) | .009 |
| Cobblestone pattern | 2/16 (13) | 0/6 (0) | >.99 |
| Atypical architectural patterns | 7/16 (44) | 6/6 (100) | .046 |
| Epidermal disarray, disarranged pattern | 5/16 (31) | 6/6 (100) | .01 |
| Atypical HC | 3/16 (19) | 4/6 (67) | .05 |
| HC Atypical and disarray | 3/16 (19) | 4/6 (67) | .05 |
| Broadened HC | 2/16 (13) | 0/6 (0) | >.99 |
| Diamond shape HC pattern with irregular diamond-shaped large cells, >20 μm | 1/16 (6) | 0/6 (0) | >.99 |
| Atypical cobblestone pattern | 1/16 (6) | 2/6 (33) | .17 |
| Other findings | |||
| Corneal cyst | 0/16 (0) | 0/6 (0) | |
| Grainy image | 7/16 (44) | 1/6 (17) | .35 |
| Presence of Pagetoid cells | 4/16 (25) | 6/6 (100) | .003 |
| Pleomorphism, variability of pagetoid cell morphology | 1/4 (25) | 3/6 (50) | .57 |
| Density of pagetoid cells | |||
| Classification 1 | |||
| Low: <5 cells/mm2 | 2/4 (50) | 3/6 (50) | >.99 |
| Moderate: 5-10 cells/mm2 | 2/4 (50) | 1/6 (17) | .50 |
| High: >10 cells/mm2 | 0/4 (0) | 2/6 (33) | .47 |
| Classification 2 | |||
| >3 Pagetoid cells in five (0.5 × 0.5mm2) images | 1/4 (25) | 4/6 (67) | .52 |
| >3 Pagetoid cells in a cluster | 0/4 (0) | 3/6 (50) | .20 |
| Distribution of pagetoid cells | |||
| Localized | 2/4 (50) | 3/6 (50) | >.99 |
| Sparse | 2/4 (50) | 1/6 (17) | .50 |
| Widespread | 0/4 (0) | 2/6 (33) | .47 |
| Other | |||
| Dark pagetoid cells (hyporeflective) | 0/16 (0) | 0/6 (0) | |
| Widespread pagetoid infiltration | 0/16 (0) | 2/6 (33) | .06 |
| Round pagetoid cells | 0/16 (0) | 3/6 (50) | .01 |
| Dendritic pagetoid cells | 4/16 (25) | 5/6 (83) | .02 |
| Dermal Epidermal Junction | |||
| Architectural patterns | |||
| Ringed pattern | 16/16 (100) | 4/6 (67) | .06 |
| Polycyclic papillae | 12/16 (75) | 2/6 (33) | .14 |
| Meshwork pattern | 0/16 (0) | 1/6 (17) | .27 |
| Clod pattern | 0/16 (0) | 0/6 (0) | |
| Nonspecific pattern | 1/16 (6) | 3/6 (50) | .046 |
| Trabecullar pattern or draped pattern | 11/15 (73) | 3/6 (50) | .61 |
| Distribution of papillae | |||
| Homogeneously distributed papilllae | 12/16 (75) | 1/6 (17) | .02 |
| Nonhomogeneously distributed papillae | 4/16 (25) | 5/6 (83) | .02 |
| Papillae/interpapillary space | |||
| Edged papillae | 9/16 (56) | 1/6 (17) | .16 |
| Papillae rimmed by highly reflective epithelial cells | 7/16 (44) | 1/6 (17) | .35 |
| Nonedged papillae | 16/16 (100) | 6/6 (100) | |
| Absence of a hyperreflective basal layer and presence 2-3 dendritic bright cells per papillae | 15/16 (94) | 3/6 (50) | .046 |
| Pearl necklace | 0/16 (0) | 1/6 (17) | .27 |
| Continuous (lentiginous) proliferation of atypical enlarged bright cells | 2/16 (13) | 5/6 (83) | .004 |
| Nonvisible papillae | 3/16 (19) | 3/6 (50) | .28 |
| Large interpapillary space | 0/16 (0) | 0/6 (0) | |
| Cellular atypia | 16/16 (100) | 6/6 (100) | |
| General characteristics of large visible atypical cells | |||
| Nonpleomorphic monomorphous small cells typical | 0/16 (0) | 0/6 (0) | |
| Mild cellular atypia | 14/16 (88) | 0/6 (0) | <.001 |
| Marked cellular atypia | 3/16 (19) | 6/6 (100) | .001 |
| More than 3 atypical cells at the junction in 5 images, field of view 0.5 × 0.5 mm | 14/16 (88) | 6/6 (100) | >.99 |
| Numerosity 1 | |||
| Low (<5 cells/mm2) | 2/16 (13) | 0/6 (0) | >.99 |
| Moderate (5-10 cells/mm2) | 2/16 (13) | 0/6 (0) | >.99 |
| High (>10 cells/mm2) | 12/16 (75) | 6/6 (100) | .54 |
| Numerosity 2: Number of atypical cells in 1 mm2, (SD) | 18.20 (11.38) | 88.2 (115.71) | .02 |
| Numerosity, >20 dendritic cells in 1 mm2 or four 0.5 × 0.5 mm fields of view | 6/15 (40) | 5/5 (100) | .04 |
| Numerosity, >30 dendritic cells in 1 mm2 or four 0.5 × 0.5 mm fields of view | 2/15 (13) | 3/5 (60) | .07 |
| Numerosity, >40 dendritic cells in 1 mm2 or four 0.5 × 0.5 mm fields of view | 1/15 (7) | 2/5 (49) | .14 |
| Numerosity 3: maximun number dendritic cells per papillae | |||
| ≤2 cells | 5/15 (33) | 1/5 (20) | >.99 |
| 3-4 cells | 10/15 (67) | 2/5 (40) | .35 |
| ≥5 cells | 0/15 (0) | 2/5 (40) | .05 |
| Aspect of atypical cells | |||
| Atypical cells with dark halo | 0/16 (0) | 1/6 (17) | .27 |
| Atypical dendritic cells | 16/16 (100) | 5/6 (83) | .27 |
| Atypical round cells | 1/16 (6) | 5/6 (83) | .001 |
| Distribution of atypical cells | |||
| Localized | 1/16 (6) | 1/6 (17) | .48 |
| Sparse | 15/16 (94) | 3/6 (50) | .046 |
| Widespread | 0/16 (0) | 2/6 (33) | .06 |
| Distribution of atypical cells in relationship with the papillae | 15/16 (94) | 6/6 (100) | |
| Peripapillar | 14/16 (88) | 6/6 (100) | >.99 |
| Interpapillar | 6/16 (38) | 6/6 (100) | .02 |
| Morphology of dendritic cells-1 | 16/16 (100) | 5/6 (83) | |
| Elongated | 16/16 (100) | 4/5 (80) | .24 |
| Plump | 8/16 (50) | 5/5 (100) | .11 |
| Moderate-high amount of plump cells | 6/16 (38) | 5/5 (100) | .04 |
| Both | 8/16 (50) | 5/5 (100) | .11 |
| Morphology of dendritic cells-2 | 16/16 (100) | 5/6 (83) | |
| Stellate | 16/16 (100) | 4/5 (80) | .24 |
| Moderate-high amount stellate cells | 16/16 (100) | 2/5 (40) | .008 |
| Triangular | 8/16 (50) | 2/5 (40) | >.99 |
| Fusiform | 10/16 (63) | 4/5 (80) | .62 |
| Roundish | 2/16 (13) | 5/5 (100) | .001 |
| Cytomorphology of dendritic cells | |||
| Body area | |||
| Average area soma, (SD), μm2 | 123.13 (31.92) | 260.42 (60.86) | .002 |
| Soma area >150 μm2 | 4/16 (25) | 3/5 (60) | .28 |
| Soma area >160 μm2 | 2/16 (13) | 5/5 (100) | .001 |
| Soma area >200 μm2 | 0/16 (0) | 4/5 (80) | <.001 |
| Body length | |||
| Average length, (SD) | 16.56 (2.60) | 21.08 (3.06) | .01 |
| Length >19 μm | 4/16 (25) | 4/5 (80) | .048 |
| Length >20 μm | 2/16 (13) | 4/5 (80) | .01 |
| Body width | |||
| Average width, (SD) | 8.93 (1.82) | 14.14 (2.17) | .002 |
| Width >12 μm | 1/16 (6) | 4/5 (80) | .004 |
| Ratio length/width | |||
| Average ratio length/width, (SD) | 1.90 (0.35) | 1.50 (0.13) | .01 |
| Ratio length/width >1.7 | 10/16 (63) | 1/5 (20) | .15 |
| Ratio length/width >1.8 | 9/16 (56) | 0/5 (0) | .045 |
| Junctional changes | 0/16 (0) | 3/6 (50) | .01 |
| Junctional nest | 0/16 (0) | 1/6 (17) | .27 |
| Junctional clusters, 3 or 4 cells | 0/16 (0) | 3/6 (50) | .01 |
| Junctional thickenings | 0/16 (0) | 2/6 (33) | .06 |
| Sheet of cells | 1/16 (6) | 3/6 (50) | .046 |
| Papillary Dermis | |||
| Nests/other collections of cells | 0/16 (0) | 1/6 (17) | .27 |
| Regular dense nest | 0/0 (0) | 0/6 (0) | |
| Dishomogenous nest (dense and sparse nests) | 0/0 (0) | 0/6 (0) | |
| Cerebriform nest | 0/0 (0) | 0/6 (0) | |
| Sparse cell nests | 0/16 (0) | 1/6 (17) | .27 |
| Nucleated cells within tumor islands | 0/16 (0) | 1/6 (17) | .27 |
| Nucleated cells within the papilla | 1/16 (6) | 2/6 (33) | .17 |
| Plump bright cells within the papillae | 16/16 (100) | 5/6 (83) | .27 |
| Plump bright cells in large aggregation within the papillary dermis | 13/16 (81) | 2/6 (33) | .05 |
| Plump bright cells and/or small bright particles in papillae | 15/16 (94) | 5/6 (83) | .48 |
| Plump bright cells sparse | 11/16 (69) | 4/6 (67) | >.99 |
| Plump bright cells confluent | 6/16 (38) | 1/6 (17) | .62 |
| Big, round bright cells within the papillae | 7/16 (44) | 0/6 (0) | .12 |
| Big, dendritic, bright within the papillae | 7/16 (44) | 0/6 (0) | .12 |
| Vessels | |||
| Vessels visible | 16/16 (100) | 4/6 (67) | .06 |
| Horizontal vessel | 0/0 (0) | 0/6 (0) | |
| Convoluted glomerular-like vessel | 0/16 (0) | 0/6 (0) | |
| Fibers | Evaluable in 8/9 | Evaluable in 4/6 | |
| Collagen bundles | 2/9 (22) | 3/4 (65) | .22 |
| Reticulated fibers | 8/9 (89) | 1/4 (25) | .05 |
| Broadened reticulated collagen fibers | 0/9 (0) | 2/4 (50) | .08 |
| Thick cordons | 0/9 (0) | 0/4 (0) | |
| Other findings | |||
| Network of dendritic cells and dendritic procesess | 6/16 (38) | 1/6 (17) | .62 |
| Small basal pigmented cells (keratinocytes) | 11/16 (69) | 2/6 (33) | .18 |
Abbreviations: HC, honeycombed; RCM, reflectance confocal microscopy.
The following RCM features for basal cell carcinomas were scored, but because they were negative were not included in the table: polarized in the HC, epidermal shadow, dendriticlike features within tumor islands, basaloid cord or nodules, fibrillar polarized pattern around tumor, linear telangiectasialike horizontal vessel, clefting.
Dendritic cells at the DEJ were counted in representative areas of 1 mm2 or in 4 0.5 × 0.5-mm fields of view. For the morphological analysis of dendritic cells at the DEJ (body area, body length, body width), the 3 largest cells from representative areas were selected and measured using Adobe Photoshop CS5 Extended version 12.0.4 (Adobe Systems Incorporated).
Seven categorical RCM features with the lowest P values, excluding numerosity and cytomorphology of dendritic cells because of their difficulty to be assessed routinely, were used for calculating a lip RCM score (Table 3). All cases were blindly reviewed using this scoring system (P.G.), and 7 diagnostic features—5 positive (+1 or +2) and 2 negative (−1)—were scored. A score 4 or greater corresponded to the threshold for the diagnosis of MEL.
Table 3. RCM Lip Scorea for Diagnosis.
| RCM Featureb | Pointsc | Interrater Agreement κ Coefficient (95% CI) |
|---|---|---|
| Regular honeycombed pattern | -1 | 0.69 (0.31-1.08) |
| Epidermal disarray, disarranged pattern | +1 | 0.9 (0.71-1.09) |
| Presence of dendritic or roundish pagetoid cells | +2 | 0.91 (0.74-1.08) |
| Homogeneous distributed papilllae | −1 | 0.81 (0.55-1.06) |
| Dishomogeneous distributed papillae | +1 | 0.64 (0.32-0.95) |
| Marked cellular atypia at the DEJa | +1 | 1 |
| Interpapillar distribution of atypical cells in relationship with the papillae | +1 | 0.91 (0.74-1.08) |
Abbreviations: DEJ, dermoepidermal junction; RCM, reflectance confocal microscopy.
An RCM score is calculated adding every positive and negative RCM feature.
An RCM feature is defined as: numerous cells irregular in size, shape, and reflectivity, round to oval or stellate, occasionally with branching dendriticlike structures, and/or distributed throughout the lesion.
An RCM score of 4 or greater corresponded to the threshold for the diagnosis of melanoma.
Statistics
Statistical analysis were performed using JMP 9 statistical software (SAS Institute Inc) and P values <.05 were regarded as statistically significant. Absolute and relative frequencies of the observations were obtained for each RCM feature. Significant differences between MEL and benign lesions were evaluated using Fisher exact test (2-sided). For continuous variables, the median between MEL and benign lesions were analyzed using Mann-Whitney U test. Interrater reliability in the interpretation of RCM method was computed using the κ-statistic including 95% CIs and using random RCM images.
Results
Study Population
The study population comprised 31 patients analyzed by RCM, separated into 2 groups (Table 1). Benign melanotic macules were mainly located on the vermillion and/or inner mucosae of the lower lip, while MEL involved the vermillion or the vermillion and adjacent skin of the upper lip in most cases. Other clinical characteristics of the patients are presented in Table 1.
RCM in Normal Lips
The previously described draped pattern was identified in all the cases in the vermillion, being a normal finding. It consists of elongated polycyclic papillae with well-demarcated borders without dendritic cells (eFigure 1 in the Supplement). In darker phototypes the papillae were better defined than lighter phototypes, especially in the vermillion (eFigure 1 in the Supplement), and in 2 patients with phototype IV, some small dendritic cells around the papillae were identified in the inner mucosae and in the upper part of the vermillion (around 60-70 µm depth).
RCM in Pigmented Lip Lesions
Reflectance confocal microscopy of 16 MAC and 6 MEL was performed on the vermillion and/or inner mucosae of the lip. All RCM features scored, absolute and relative values, percentages, and P values are presented in Table 2.
Superficial Layer Features
Atypical architectural patterns, rather than the honeycombed pattern, were always observed in MEL and in 44% of MAC, especially epidermal disarray. A regular honeycomb pattern was strongly correlated with benign pigmented lesions (94% vs 33%, respectively; P = .009). Pagetoid infiltration was identified in all MEL and 4 cases of MAC (25%) (P = .003) (eFigure 2 in the Supplement). In MAC, the pagetoid cells were only dendritic; whereas in MEL, pagetoid cells were exclusively dendritic in 3 cases, round in 1 case, and both in 2 cases.
At the Dermoepidermal Junction Level
In RCM mosaics, the pigmented area was discernable as a hyperreflective region (eFigure 3 in the Supplement). If the lesion was in the vermillion, the draped pattern, as in normal lips, was evident (eFigure 3 in the Supplement). An atypical draped pattern was detected in 1 MEL (Figure 1B and C). A ringed architectural pattern was more common in MAC (100%) rather than MEL (67%) (P = .06) (eFigure 3 in the Supplement); however, a nonspecific pattern was commonly seen in MEL. The papillae were nonhomogeneously distributed in 83% of MEL and 25% of MAC (eFigure 2 in the Supplement). Nonedged papilla, a common feature for skin melanoma, was observed in all benign and malignant cases, and 94% of MAC and 50% of MEL (P = .046) showed an absence of a hyperreflective basal layer and presence of 2 to 3 bright dendritic cells per papilla(eFigure 3 and eFigure 4 in the Supplement). Atypical dendritic cells were detected in 100% of MAC and 83% of MEL (Figure 2) (eFigure 3 and eFigure 4 in the Supplement), and atypical round cells were more characteristic of MEL than MAC (83% vs 6%; P = .001) (eFigure 5 in the Supplement). The cellular atypia was marked in 19% of MAC and in 100% of MEL (P = .001). Continuous (lentiginous) proliferation of atypical enlarged bright cells was observed in 13% of MAC and 83% of MEL (P = .004) (eFigure 5 in the Supplement). The atypical cells involved interpapillary spaces (corresponding to rete ridges) in 38% of MAC and 100% of MEL (P = .02) (Figure 1 and Figure 2). The mean (SD) number of atypical dendritic cells per mm2 was higher in MEL (18.20 [11.38]) than in MAC (88.2 [115.71]) (P = .02), and more than 20 dendritic cells per mm2 was more frequent in MEL (100%) than MAC (40%) (P = .04).
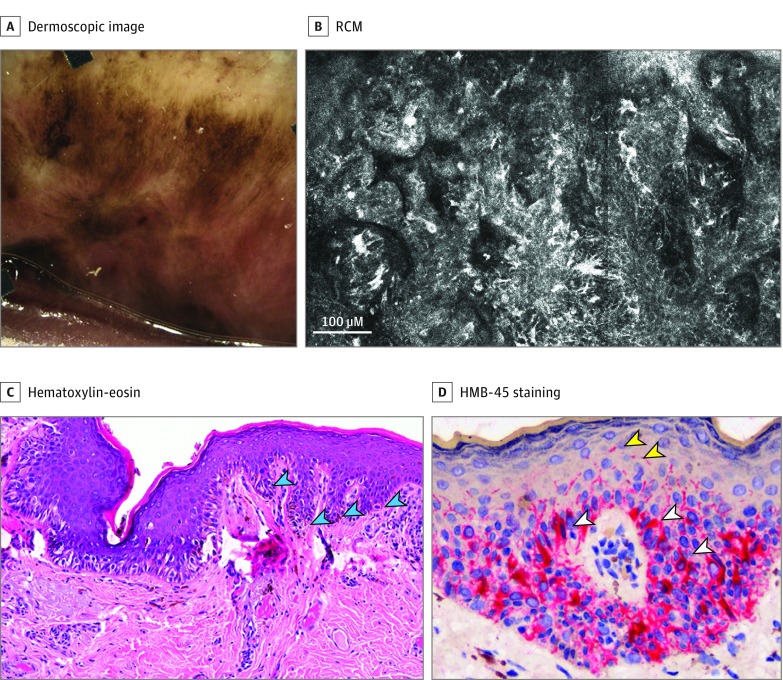
Figure 1. Patient With an Upper Lip Lentigo Maligna.
A, Dermoscopic image shows an asymmetric multicomponent pattern with multiple colors. It was classified as malignant. B, Reflectance confocal microscopy (RCM) at the dermoepidermal junction shows an atypical draped or trabecular pattern, with multiple atypical dendritic cells rimming the papillae and in-between. C, Histopathology (original magnification ×100) shows a lentiginous proliferation of atypical melanocytes with sparse pagetoid cells; elongated rete ridges (cyan arrowheads) explain the draped pattern seen by RCM. D, HMB-45 staining (original magnification ×400) reveals multiple dendritic melanocytic cells (white arrowheads) and dendritic projections to the upper epidermal levels (yellow arrowheads).
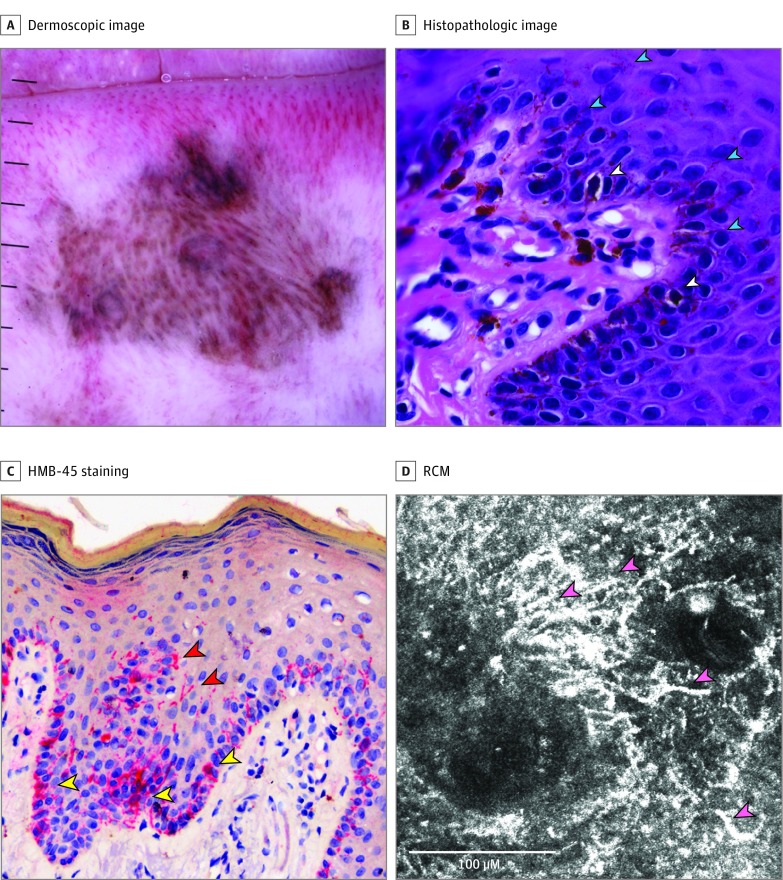
Figure 2. Patient With Melanotic Macule of the Lower Lip.
A, Dermoscopic image shows an asymmetric patterned lesion made of parallel and curved lines, globules and/or clods, dots, and multiple colors. B, Hematoxylin-eosin (original magnification ×400) shows basal pigmented keratinocytes, some of them mildly atypical; basal melanocytes, some of them clearly dendritic (white arrowheads), dendritic processes (cyan arrowheads), multiple melanophages and pigment incontinence in the dermis. C, HMB-45 staining (original magnification ×400) shows basal melanocytes (yellow arrowheads) and dendritic processes in the upper levels of the epidermis (red arrowheads). D, Reflectance confocal microscopy (RCM) reveals a dendritic network (cells and dendritic processes) in the interpapillary space (rete ridges [pink arrowheads]).
Cytomorphology of Dendritic Cells at DEJ on RCM
A moderate-to-high number of stellate dendritic cells was more frequent in MAC and roundish dendritic cells were more frequent in MEL (Table 2). Different dimensions of the atypical dendritic cells were analyzed: the mean (SD) cellular body area was higher in MEL (260.42 [60.82] μm2) compared with MAC (123.13 [31.92] µm2) (P = .002); the body length and width were longer in MEL; and MEL cells were “plumper” because the mean (SD) ratio between length and width was smaller in MEL (1.50 [0.14]) than in MAC (1.90 [0.35]) (P = .01) (Table 2).
In the Papillary Dermis on RCM
Plump bright cells within the papillae were detected in almost all benign and malignant cases. In some cases, small round and dendritic nucleated cells were present in the papillae in 44% of MAC and 0% of MEL (eFigure 3 in the Supplement). The types of vessels and fibers were not different between MAC and MEL (Table 2).
A network of dendritic cells and dendritic processes, a new RCM feature in pigmented lesions of the lips, was detected in 38% of MAC and 17% of MEL (P = .62). The dendritic processes were mainly located in the upper layers of the epidermis, while the network of dendritic cells was commonly found at the DEJ (Figure 1D and Figure 2B-D). The latter was normally associated with basal pigmented cells and plump bright cells within the papillae in the same areas (eFigure 6 in the Supplement). Three of 4 MAC (75%) with dendritic pagetoid cells were associated with dendritic processes, a network of dendritic cells, basal pigmented keratinocytes, and plump bright cells in the papillae. The same finding was detected in 1 MEL (20%) (P = .21).
Dermoscopy of Pigmented Lip Lesions
We evaluated dermoscopic patterns, colors, symmetry of pattern and the diagnosis with an expert in dermoscopy (eTable 1 in the Supplement). A structureless pattern was more frequently found in MEL compared with MAC (83% vs 25%; P = .02). A pattern characterized by lines, mostly parallel or curved rather than reticular, was more frequent in MAC. Three or more patterns were present in 31% of MAC and 67% of MEL (P = .18). Three or more colors were present in 19% of MAC and 33% of MEL (P > .58), brown and gray being the most frequent colors in both groups. The presence of asymmetry was common in MAC (69%) and MEL (83%). In MAC, if gray was present, 92% had plump bright cells in large aggregation within the papillary dermis by RCM (P = .07). Also, 85% of MAC with gray color lacked symmetry of pattern (P = .02). Considering malignant and equivocal as a possible MEL diagnosis, dermoscopy had a sensitivity of 83% and specificity of 81% for MEL diagnosis. Of note, 1 case of MEL was diagnosed by dermoscopy as benign, corresponding to a recurrent MEL case where a scar was prominent (eFigure 5A-C in the Supplement).
Diagnosis of Pigmented Lip Lesions Using RCM
A scoring system termed “Lip Score” was developed using the main differentiating RCM features. Using the Lip Score, RCM correctly identified all MEL as malignant and diagnosed 88% of the MAC as benign, having a sensitivity of 100% and specificity of 88% for MEL diagnosis if the score was 4 or greater.
Discussion
The clinical distinction between MAC and MEL of the lip can be challenging; here we demonstrate that in vivo RCM is useful for diagnosis. We extensively analyzed a retrospective series of 16 MAC and 6 lip MEL using in vivo RCM which, to the best of our knowledge, represents the largest series of lip MEL analyzed by RCM reported to date. We confirmed preliminary reports, and also highlight some novel RCM findings in MAC and lip MEL. Furthermore, we analyzed 10 lower lip controls with different phototypes to describe normal RCM features.
In cutaneous MEL, in vivo RCM has demonstrated that it can improve the accuracy of diagnosis. Important criteria are nonedged papillae and cellular atypia at the dermoepidermal junction. However, both criteria are also commonly present in MAC, and therefore are not useful for discriminating them from MEL. Recently, Debarbieux et al analyzed confocal images of 56 labial or genital pigmented lesions, including 22 lip MAC and 2 lip MEL, and reported that 86% of benign lesions have a ringed pattern and sparse bright dendritic cells in the basal layer with the basal epithelial cells being otherwise less reflective. We detected small sparse dendritic cells (by RCM and in some cases by conventional histopathology) around the papillae in all the MAC and some scattered dendritic cells around the papillae in the inner lip and in the upper part of the vermillion of 2 patients with normal lips with phototype IV. All of our cases but 1 were recorded using Vivascope 1500, which enables performing RCM mosaics and scans a larger surface area than using Vivascope 3000. This allowed us to analyze the papillae distribution, observing that nonhomogeneously distributed papillae is a common characteristic of MEL.
In MEL, basal atypical dendritic cells predominate over basal round cells. Basal dendritic cells were detected in higher number than in MAC and were located not only around the papillae but also in the interpapillary spaces (rete ridges). This feature was termed “intraepithelial bright cells” by Debarbieux et al, and it was also detected in 27% of MAC in our study. In MEL, dendritic cells had a higher body surface, were longer, wider and were “fatter” or “roundish”, with a smaller length:width ratio (eFigure 4 in the Supplement).
The draped pattern, or “trabecular pattern” as we prefer to name it because it resembles the liver trabeculae, can be explained by elongated rete ridges and papillae found in the vermillion lip. It has been described as a normal RCM feature in clinically noninvolved vulvar mucosae and as a pathological RCM feature found in genital and lip melanotic macules. We detected this pattern in normal vermillion and in all the cases where the pigmentation was in this location. Moreover, we described an atypical draped or trabecular pattern in 1 case (Figure 1).
Dermoscopic evaluation correctly classified 81% of MAC as benign and 83% of MEL as equivocal or malignant. One case of MEL was classified as benign, probably because it corresponded to a MEL recurrence over a scar. However, in this case, in vivo RCM detected an atypical honeycombed pattern, pagetoid cells, and nonhomogeneously distributed papillae, all features commonly seen in lip MEL. Reflectance confocal microscopy appears to have a higher sensitivity than dermoscopy in diagnosing lip MEL, although both technologies are probably complementary.
Limitations
Some limitations of our study are its (predominantly) retrospective nature and the small sample size. The latter did not allow us to perform logistic regression and discriminant analysis. Although we created the Lip Score, it needs to be validated in an independent test set. Variable interobserver reproducibility of pathological diagnosis of oral pigmented lesions, particularly for intermediate and/or borderline lesions, is another potential limitation of our study. While MAC and MEL can be readily distinguished by routine histopathology in most instances, occasionally cases with many features of MAC occur that are associated with a moderately dense atypical melanocytic hyperplasia. Distinguishing MAC associated with an atypical melanocytic hyperplasia from an early and/or evolving MEL in situ can be very difficult and is at least in part subjective. For pigmented lesions occurring at some other sites such as the nail matrix, a number of studies have documented differences in the density of melanocytes in benign (subungual melanocytic macules) and MEL which could also be useful in the assessment of lip pigmented lesions. Nevertheless, because the density of melanocytes is used as a diagnostic feature for pathological diagnosis of such cases, the described differences of melanocyte density in these studies may simply reflected the cutoffs used by the authors to make diagnoses rather than representing an independent objective criterion. Indeed some cases of MAC associated with an atypical melanocytic hyperplasia may indicate an unstable (and possibly genetically abnormal) melanocytic proliferation that could in some cases progress to melanoma in situ. A careful clinical follow-up and rebiopsying after a period of observation may be advisable particularly if the lesion progresses, becomes more atypical, or the clinical features do not correlate with the pathological features, especially in small biopsy specimens.
Conclusions
In vivo RCM is increasingly being incorporated into the routine dermatological practice, and soon portable devices will be readily available. As a consequence, it is important that dermatologists are aware that melanotic macules of the lips frequently show some RCM features that are typically also seen in cutaneous melanoma. On the other hand, other RCM features can help differentiating melanomas from melanotic macules, especially the presence of pagetoid cells, nonhomogeneously distributed papillae, and the presence of large round and numerous dendritic cells around and between the papillae. Using our observations, we have proposed a diagnostic RCM Lip Score for diagnosing pigmented lip lesions, but this will need to be validated in a larger independent study cohort.
eTable 1. Dermoscopic findings in melanotic macules and melanomas of the lip
eFigure 1. In vivo confocal images from normal lower lip showing the draped pattern in two patients
eFigure 2. Patient with a recurrent in situ melanoma on her lower lip
eFigure 3. Melanotic macule from the vermillion and part of the inner mucosae in a patient with an Asiatic background
eFigure 4. RCM representative examples of differences in dendritic cells morphology between melanoma and melanotic macules
eFigure 5. Patients with lip melanomas
eFigure 6. Patient with a changing lower lip pigmented macule
References
- 1.Blum A, Simionescu O, Argenziano G, et al. Dermoscopy of pigmented lesions of the mucosa and the mucocutaneous junction: results of a multicenter study by the International Dermoscopy Society (IDS). Arch Dermatol. 2011;147(10):1181-1187. [DOI] [PubMed] [Google Scholar]
- 2.Ronger-Savle S, Julien V, Duru G, Raudrant D, Dalle S, Thomas L. Features of pigmented vulval lesions on dermoscopy. Br J Dermatol. 2011;164(1):54-61. [DOI] [PubMed] [Google Scholar]
- 3.Lin J, Koga H, Takata M, Saida T. Dermoscopy of pigmented lesions on mucocutaneous junction and mucous membrane. Br J Dermatol. 2009;161(6):1255-1261. [DOI] [PubMed] [Google Scholar]
- 4.Mannone F, De Giorgi V, Cattaneo A, Massi D, De Magnis A, Carli P. Dermoscopic features of mucosal melanosis. Dermatol Surg. 2004;30(8):1118-1123. [DOI] [PubMed] [Google Scholar]
- 5.Puig S, Carrera C, Lovato L, Hanke-Martinez M. Acral volar skin, facial skin and mucous membrane In: Hofmann-Wellenhof R, Pellicani G, Malvehy J, Soyer HP, eds. Reflectance confocal microscopy for skin diseases. Heidelberg, Germany: Springer; 2012:33-38. [Google Scholar]
- 6.White WM, Rajadhyaksha M, González S, Fabian RL, Anderson RR. Noninvasive imaging of human oral mucosa in vivo by confocal reflectance microscopy. Laryngoscope. 1999;109(10):1709-1717. [DOI] [PubMed] [Google Scholar]
- 7.Contaldo M, Agozzino M, Moscarella E, Esposito S, Serpico R, Ardigò M. In vivo characterization of healthy oral mucosa by reflectance confocal microscopy: a translational research for optical biopsy. Ultrastruct Pathol. 2013;37(2):151-158. [DOI] [PubMed] [Google Scholar]
- 8.Pellacani G, Cesinaro AM, Seidenari S. Reflectance-mode confocal microscopy of pigmented skin lesions—improvement in melanoma diagnostic specificity. J Am Acad Dermatol. 2005;53(6):979-985. [DOI] [PubMed] [Google Scholar]
- 9.Pellacani G, Guitera P, Longo C, Avramidis M, Seidenari S, Menzies S. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127(12):2759-2765. [DOI] [PubMed] [Google Scholar]
- 10.Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J Invest Dermatol. 2010;130(8):2080-2091. [DOI] [PubMed] [Google Scholar]
- 11.Guitera P, Menzies SW, Longo C, Cesinaro AM, Scolyer RA, Pellacani G. In vivo confocal microscopy for diagnosis of melanoma and basal cell carcinoma using a two-step method: analysis of 710 consecutive clinically equivocal cases. J Invest Dermatol. 2012;132(10):2386-2394. [DOI] [PubMed] [Google Scholar]
- 12.Erfan N, Hofman V, Desruelles F, et al. Labial melanotic macule: a potential pitfall on reflectance confocal microscopy. Dermatology. 2012;224(3):209-211. [DOI] [PubMed] [Google Scholar]
- 13.Debarbieux S, Perrot JL, Erfan N, et al. ; Groupe d’Imagerie Cutanée Non Invasive de la Société Française de Dermatologie . Reflectance confocal microscopy of mucosal pigmented macules: a review of 56 cases including 10 macular melanomas. Br J Dermatol. 2014;170(6):1276-1284. [DOI] [PubMed] [Google Scholar]
- 14.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 15.Rajadhyaksha M, González S, Zavislan JM, Anderson RR, Webb RH. In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison with histology. J Invest Dermatol. 1999;113(3):293-303. [DOI] [PubMed] [Google Scholar]
- 16.Pellacani G, Cesinaro AM, Seidenari S. Reflectance-mode confocal microscopy for the in vivo characterization of pagetoid melanocytosis in melanomas and nevi. J Invest Dermatol. 2005;125(3):532-537. [DOI] [PubMed] [Google Scholar]
- 17.Scope A, Benvenuto-Andrade C, Agero AL, et al. In vivo reflectance confocal microscopy imaging of melanocytic skin lesions: consensus terminology glossary and illustrative images. J Am Acad Dermatol. 2007;57(4):644-658. [DOI] [PubMed] [Google Scholar]
- 18.Losi A, Longo C, Cesinaro AM, et al. Hyporeflective pagetoid cells: a new clue for amelanotic melanoma diagnosis by reflectance confocal microscopy. Br J Dermatol. 2014;171(1):48-54. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari B, Soyer HP, Casari A, Pellacani G. Semeiology and pattern analysis in melanocytic lesions In: Hofmann-Wellenhof R, Pellacani G, Malvehy J, Soyer HP, eds. Reflectance confocal microscopy for skin diseases. Heidelberg, Germany: Springer; 2012:41-58. [Google Scholar]
- 20.Langley RG, Burton E, Walsh N, Propperova I, Murray SJ. In vivo confocal scanning laser microscopy of benign lentigines: comparison to conventional histology and in vivo characteristics of lentigo maligna. J Am Acad Dermatol. 2006;55(1):88-97. [DOI] [PubMed] [Google Scholar]
- 21.Cinotti E, Perrot JL, Labeille B, Adegbidi H, Cambazard F. Reflectance confocal microscopy for the diagnosis of vulvar melanoma and melanosis: preliminary results. Dermatol Surg. 2012;38(12):1962-1967. [DOI] [PubMed] [Google Scholar]
- 22.Nori S, Rius-Díaz F, Cuevas J, et al. Sensitivity and specificity of reflectance-mode confocal microscopy for in vivo diagnosis of basal cell carcinoma: a multicenter study. J Am Acad Dermatol. 2004;51(6):923-930. [DOI] [PubMed] [Google Scholar]
- 23.Agero AL, Busam KJ, Benvenuto-Andrade C, et al. Reflectance confocal microscopy of pigmented basal cell carcinoma. J Am Acad Dermatol. 2006;54(4):638-643. [DOI] [PubMed] [Google Scholar]
- 24.Ulrich M, Roewert-Huber J, González S, Rius-Diaz F, Stockfleth E, Kanitakis J. Peritumoral clefting in basal cell carcinoma: correlation of in vivo reflectance confocal microscopy and routine histology. J Cutan Pathol. 2011;38(2):190-195. [DOI] [PubMed] [Google Scholar]
- 25.Cinotti E, Couzan C, Perrot JL, et al. In vivo confocal microscopic substrate of grey colour in melanosis. J Eur Acad Dermatol Venereol. 2015;29(12):2458-2462. [DOI] [PubMed] [Google Scholar]
- 26.André J, Sass U, Richert B, Theunis A. Nail pathology. Clin Dermatol. 2013;31(5):526-539. [DOI] [PubMed] [Google Scholar]
- 27.Amin B, Nehal KS, Jungbluth AA, et al. Histologic distinction between subungual lentigo and melanoma. Am J Surg Pathol. 2008;32(6):835-843. [DOI] [PubMed] [Google Scholar]
- 28.Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am J Surg Pathol. 2007;31(12):1902-1912. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Dermoscopic findings in melanotic macules and melanomas of the lip
eFigure 1. In vivo confocal images from normal lower lip showing the draped pattern in two patients
eFigure 2. Patient with a recurrent in situ melanoma on her lower lip
eFigure 3. Melanotic macule from the vermillion and part of the inner mucosae in a patient with an Asiatic background
eFigure 4. RCM representative examples of differences in dendritic cells morphology between melanoma and melanotic macules
eFigure 5. Patients with lip melanomas
eFigure 6. Patient with a changing lower lip pigmented macule