Key Points
Question
What is the association between secondary mitral regurgitation and morbidity and mortality of patients with left ventricular dysfunction?
Findings
In this meta-analysis of 53 studies and 45 900 patients, secondary mitral regurgitation in patients with ischemic or idiopathic cardiomyopathies was associated with a significant worsening of survival. Although secondary mitral regurgitation is known to be dynamic and load dependent, it was consistently associated with higher mortality regardless of its severity.
Meaning
Secondary mitral regurgitation may be a strong marker of adverse outcomes in patients with left ventricular dysfunction.
Abstract
Importance
The outcomes of patients with left ventricular (LV) dysfunction and secondary mitral regurgitation (SMR) are still controversial.
Objective
To clarify the role of SMR in the outcomes of patients with ischemic or idiopathic cardiomyopathies.
Data Sources
MEDLINE, ISI Web of Science, and Scopus databases were searched for studies published up to March 2017.
Study Selection
Studies reporting data on outcomes in patients with SMR were included. Duplicate publication data, studies lacking data on SMR grade and its correlation with outcomes, mixed data on SMR and primary mitral regurgitation, studies not clearly reporting the outcome of interest, and studies with fewer than 100 patients were excluded. Of the initial 3820 articles identified, 1.4% were finally included.
Data Extraction and Synthesis
The study met PRISMA requirements. Two of us independently screened articles for fulfillment of inclusion criteria.
Main Outcomes and Measures
The primary outcome, set after data collection, was the incidence of all-cause mortality in patients with and without SMR. Secondary outcomes included hospitalization for heart failure (HF), cardiac mortality, and a composite end point of death, HF hospitalization, and cardiac transplant.
Results
Fifty-three studies and 45 900 patients were included in the meta-analysis. The mean (SD) length of follow-up was 40.8 (22.2) months. In 26 of 36 studies reporting LV function by SMR grade, increasing SMR severity was associated with worse LV function. When SMR was categorized as present or absent, all-cause mortality was significantly higher in the patients with SMR (17 studies, 26 359 patients; risk ratio [RR],1.79; 95% CI, 1.47-2.18; P < .001, I2 = 85%); when SMR was qualitatively graded, the incidence of all-cause mortality was significantly increased in patients having any degree of SMR compared with patients not having SMR (21 studies, 21 081 patients; RR, 1.96; 95% CI, 1.67-2.31; P < .001, I2 = 74%). Finally, when SMR was quantitatively graded, it remained associated with an increased all-cause mortality rate (9 studies, 3649 patients; RR, 1.97; 95% CI, 1.71-2.27; P < .001, I2 = 0%). Moreover, SMR was associated with an increased risk of hospitalization for HF (16 studies, 10 171 patients; RR, 2.26; 95% CI, 1.92-2.67; P < .001, I2 = 41%), cardiac mortality (12 studies, 11 896 patients; RR, 2.62; 95% CI, 1.87-3.69; P < .001, I2 = 74%), and death, HF, and transplant (11 studies, 8256 patients; RR, 1.63; 95% CI, 1.33-1.99; P < .001, I2 = 78%).
Conclusions and Relevance
To our knowledge, this study is the first meta-analysis to date to demonstrate that SMR, even when mild, correlates with adverse outcomes in patients with ischemic or idiopathic cardiomyopathies. Because SMR is an intrinsic consequence of LV dysfunction, causality between SMR and mortality should not be implied.
This meta-analysis clarifies the role of secondary mitral regurgitation in the outcomes of patients with ischemic or idiopathic cardiomyopathies.
Introduction
Secondary mitral regurgitation (SMR) occurs when normal or almost normal mitral leaflets are prevented from adequate coaptation by underlying left ventricular (LV) dysfunction, mitral annular dilation, or both. It is the most common valve disease, with an incidence of less than 1% before age 55 years but reaching 9% after age 75 years. Secondary mitral regurgitation is generally divided into SMR due to ischemic heart disease or nonischemic cardiomyopathies, although it can also be a consequence of chronic atrial enlargement. In ischemic heart disease, even mild SMR has been shown to increase mortality. In the Survival and Ventricular Enlargement (SAVE) trial, patients with SMR were more likely to experience cardiovascular mortality and severe heart failure (HF) than those without SMR. Grigioni et al showed adjusted relative risks of total and cardiac mortality associated with the presence of ischemic mitral regurgitation (MR) of 1.88 and 1.83, respectively, compared with patients without ischemic MR. The results from an MR substudy of the Surgical Treatment for Ischemic Heart Failure (STICH) trial confirmed the previous observational studies, demonstrating an adverse prognosis of untreated ischemic MR in a cohort of patients with ischemic cardiomyopathy treated with current guideline-directed medical therapy. However, several other studies did not find that SMR predicted worse survival among patients with ischemia. Similarly, in patients with nonischemic cardiomyopathy, the presence of SMR has been linked to different outcomes, such as death, HF hospitalization, or transplant. In the Beta-blocker Evaluation of Survival Trial (BEST), an MR vena contracta width less than 0.4 cm predicted worse outcomes. Similarly, Cioffi et al and Rossi et al found that SMR was an independent predictor of mortality even in patients without ischemia. On the other hand, studies by Patel et al and Boriani et al did not confirm these results. In the setting of such conflicting findings, we performed a meta-analysis to clarify whether patients with ischemic or idiopathic cardiomyopathies and SMR have an adverse prognosis.
Methods
Literature Search and Study Selection
The study was designed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) requirements. MEDLINE, ISI Web of Science, and Scopus databases were searched for studies published up to March 2017. Studies were identified using the major Medical Subject Headings functional mitral regurgitation OR ischemic mitral regurgitation OR secondary mitral regurgitation combined with outcome OR mortality OR survival. English was set as a language restriction. Two of us (A.S. and P.A.G.) independently examined the title and abstract of citations. The full texts of potentially eligible trials were obtained, and disagreements were resolved by discussion. To identify additional relevant studies, the full text and bibliography of all potential articles were also retrieved in detail. Abstracts, meeting proceedings, and personal communications were not used for the purpose of this study.
Eligibility Criteria
Studies were included if they reported data on outcomes associated with estimation of SMR grade. Studies were excluded if any of the following criteria applied: (1) duplicate publication data, (2) lack of data on SMR grade and its correlation with outcomes, (3) mixed data on SMR and organic MR populations, (4) outcome of interest not clearly reported or impossible to extract or calculate from the published results, and (5) less than 100 patients. The length of follow-up was not set as a restriction.
Data Extraction
Two of us (A.S. and P.A.G.) independently screened articles for fulfillment of inclusion criteria. The baseline characteristics, associations of SMR grade with cardiovascular risk factors, and outcomes were abstracted. Moreover, associations of SMR grade with LV ejection fraction (LVEF) and LV end-systolic volume (LVESV) were also abstracted. Two of us (A.S. and P.A.G.) compared selected trials, and discrepancies were resolved by consensus.
End Points and Definitions
The primary end point (or outcome) of this study was the incidence of all-cause mortality in patients not having SMR compared with patients having SMR. The included studies reported SMR differently; therefore, the primary outcome analysis has been divided into the 3 following sections: (1) SMR present vs absent detected at echocardiography or ventriculography, (2) SMR detected at echocardiography and qualitatively graded, and (3) SMR detected at echocardiography and quantitatively graded. As secondary end points, we analyzed (1) hospitalization for HF, (2) cardiac mortality, and (3) a composite end point of death, HF hospitalization, and cardiac transplant.
Quality Assessment
Risk of bias for each included study was assessed using the Newcastle-Ottawa Scale for quality assessment. This scale allows the assessment of the internal validity of cohort studies included in a meta-analysis on the basis of the following 3 main items: (1) selection (adequate selection and definition of groups), (2) comparability (comparability of 2 groups for a selected variable and comparability for other variables), and (3) outcome (modality of assessment, enough length of follow-up, and adequacy of follow-up). Based on these criteria, studies with 4 stars for selection, 2 stars for comparability, and 3 stars for outcome were defined as having low risk of bias. Studies with 2 or 3 stars for selection, 1 star for comparability, and 2 stars for outcome were defined as having medium risk of bias. Any study with 1 star for selection or outcome or 0 stars for any of the 3 domains was defined as having high risk of bias.
Statistical Analysis
Two of us (A.S. and P.A.G.) independently extracted for each study the most comprehensively adjusted or unadjusted odds ratios, hazard ratios, and their 95% CIs, as well as the means (SDs). When multiple data were available for the same study, adjusted data were prioritized. Estimates of effect were calculated with a random-effects model and expressed as risk ratios (RRs). Statistical significance was set at P ≤ .05 (2 tailed). Heterogeneity was assessed by Cochran Q statistic and I2 test. Significant heterogeneity was considered present for P < .10 or I2>50%. Meta-regression analysis was performed to assess the potential influence of important covariates (included in eTable 1 in the Supplement) on between-study heterogeneity (significance at P ≤ .05). If no significant covariates were found to be heterogeneous, the leave-one-out sensitivity analysis was carried out to evaluate the key studies with substantial influence on between-study heterogeneity. Additional sensitivity analyses have been performed for the same purpose, including (1) removal of studies having low risk of bias, (2) removal of studies having moderate to high risk of bias, (3) removal of studies with proximal isovelocity surface area assessment of SMR, and (4) removal of studies reporting unadjusted estimates (eTable 2 in the Supplement). A fixed-effects model was used to confirm the results in case of significant heterogeneity.
Publication bias was assessed using funnel plots. When significant publication bias was found, it was further explored by Egger test, consisting of a linear regression of the intervention effect estimates on their standard errors, weighting by 1 divided by the variance of the intervention effect estimate.
All data analyses were performed using statistical software. ProMeta (version 2; IDoStatistics) and RevMan (version 5.2; Cochrane Community) were used.
Results
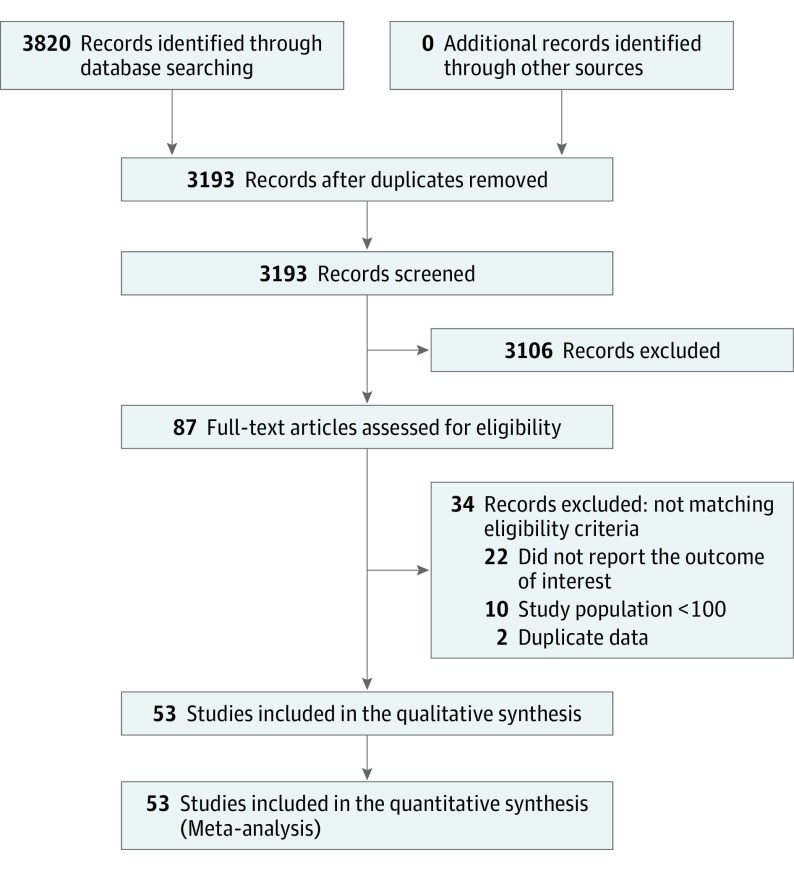
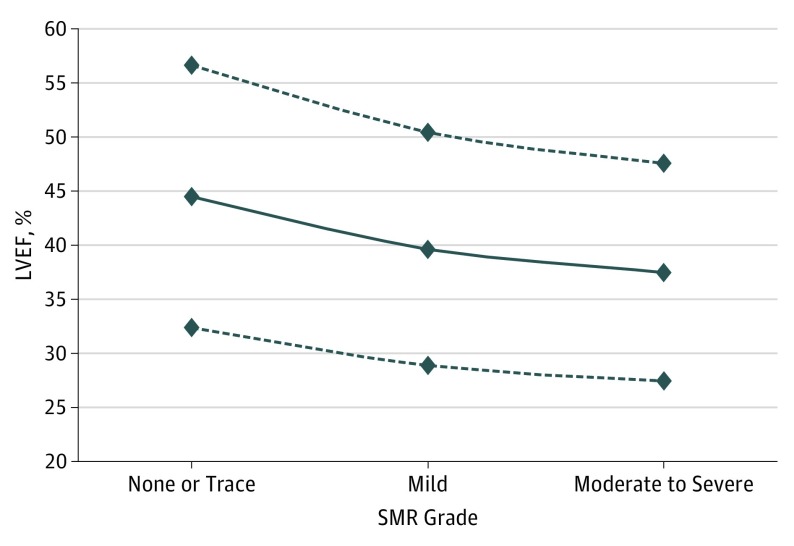
The initial literature search identified 3820 articles, of which 87 were retrieved for more detailed evaluation, and 53 trials (1.4%) were finally included in the study, enrolling 45 900 patients (Figure 1 and eTable 1 in the Supplement). The mean (SD) length of follow-up in our study population was 40.8 (22.2) months. The LVEF and LVESV values according to SMR grade in the included studies are listed in eTable 3 in the Supplement. As shown, in 26 of 36 studies that reported these data, there was a significant association between LV dysfunction and SMR severity, with higher grades of SMR associated with worse LV function. Figure 2 shows a plot of averaged LVEF values for each grade of SMR. Because severe SMR is uncommon, these studies combined moderate to severe SMR into a single group.
Figure 1. Meta-analysis Flowchart.
Figure 2. Relationship Between Left Ventricular Ejection Fraction (LVEF) and Secondary Mitral Regurgitation (SMR) Grade.
The variation in LVEF is shown according to different grades of SMR. The solid line represents averaged LVEF values for the 36 studies reporting the data; dotted lines represent the 95% CIs.
Primary Outcome of All-Cause Mortality
The association between SMR and all-cause mortality was systematically addressed. As summarized in eTable 4 in the Supplement, the detection and grading of SMR differed across the included studies; therefore, the all-cause mortality analysis has been divided into the 3 sections discussed below.
SMR Present vs Absent Detected at Echocardiography or Ventriculography
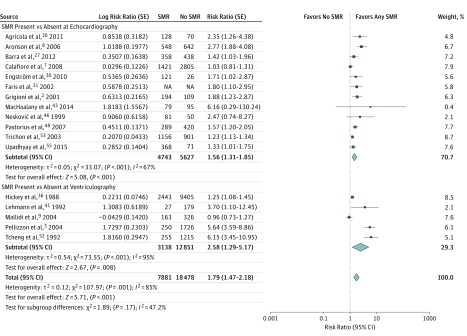
In patients having any degree of SMR, the incidence of all-cause mortality was significantly increased compared with patients not having SMR (17 studies, 26 359 patients; RR, 1.79; 95% CI, 1.47-2.18; P < .001, I2 = 85%) (Figure 3). When stratifying the analysis for all-cause mortality according to the method used to detect SMR (echocardiography or ventriculography), both subgroups exhibited a significant association between the presence of SMR and all-cause mortality, with no difference in the magnitude of the analyzed outcome between the subgroups (χ2 = 1.89, P for interaction = .17).
Figure 3. Influence of Secondary Mitral Regurgitation (SMR) on All-Cause Mortality in Studies Defining SMR as Present or Absent.
Shown are random-effects risk ratios and 95% CIs for all-cause mortality according to the SMR detection method used. NA indicates not applicable.
SMR Detected at Echocardiography and Qualitatively Graded
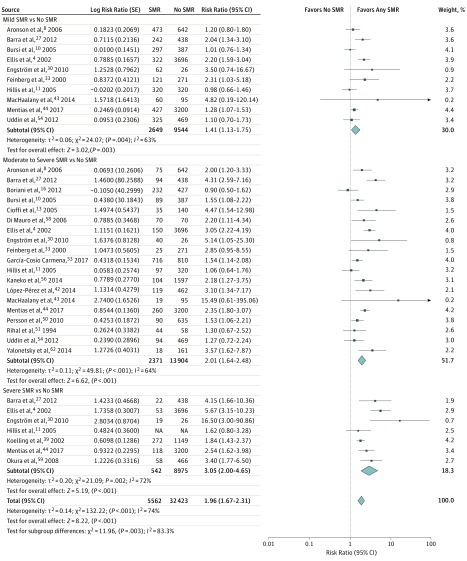
In patients having SMR, the incidence of all-cause mortality was significantly increased compared with patients not having SMR (21 studies, 21 081 patients; RR, 1.96; 95% CI, 1.67-2.31; P < .001, I2 = 74%) (Figure 4). When stratifying the analysis for all-cause mortality according to SMR grading (mild, moderate, and severe), a significant association between the presence of SMR and all-cause mortality persisted in all the subgroups, with a significant difference in the magnitude of the analyzed outcome between subgroups (χ2 = 11.96, P for interaction = .003).
Figure 4. Influence of Secondary Mitral Regurgitation (SMR) on All-Cause Mortality in Studies Grading SMR Qualitatively.
Shown are random-effects risk ratios and 95% CIs for all-cause mortality according to SMR grade. NA indicates not applicable.
SMR Detected at Echocardiography and Quantitatively Graded
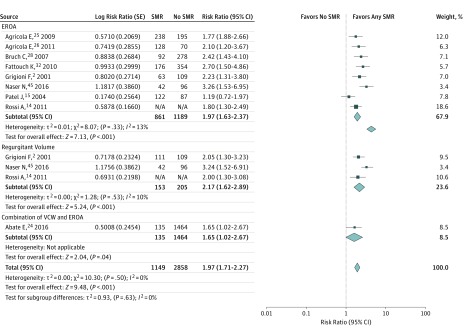
In patients having SMR, the incidence of all-cause mortality was significantly increased compared with patients not having SMR (9 studies, 3649 patients; RR, 1.97; 95% CI, 1.71-2.27; P < .001, I2 = 0%) (Figure 5). When stratifying the analysis for all-cause mortality according to the SMR quantification method, a significant association between the presence of SMR and all-cause mortality persisted in all the subgroups, with no difference in the magnitude of the analyzed outcome between subgroups (χ2 = 0.93, P for interaction = .63).
Figure 5. Influence of Secondary Mitral Regurgitation (SMR) on All-Cause Mortality in Studies Grading SMR Quantitatively.
Shown are random-effects risk ratios and 95% CIs for all-cause mortality according to SMR grading. The cutoff for EROA is 0.2 cm2, and the cutoff for regurgitant volume is 30 mL. EROA indicates effective regurgitant orifice area; NA, not applicable; VCW, vena contracta width.
Secondary Outcomes
Hospitalization for HF
In patients having SMR, the rate of hospitalization for HF was significantly higher compared with patients not having SMR (16 studies, 10 171 patients; RR, 2.26; 95% CI, 1.92-2.67; P < .001, I2 = 41%) (eFigure 1 in the Supplement). When the analysis was stratified according to the degree of SMR, all subgroups demonstrated a significant association between the presence of SMR and HF hospitalization, with no differences between subgroups (χ2 = 0.73, P = .70).
Cardiac Mortality
The incidence of cardiac mortality was significantly higher in patients having any SMR compared with patients not having SMR (12 studies, 11 896 patients; RR, 2.62; 95% CI, 1.87-3.69; P < .001, I2 = 74%). These results are shown in eFigure 2 in the Supplement.
Composite End Point of Death, HF Hospitalization, and Cardiac Transplant
In patients having SMR, the incidence of the composite end point of death, HF hospitalization, and cardiac transplant was significantly higher compared with patients not having SMR (11 studies, 8256 patients; RR, 1.63; 95% CI, 1.33-1.19; P < .001, I2 = 78%) (eFigure 3 in the Supplement). When the analysis was stratified according to the degree of SMR, all subgroups demonstrated a significant association between the presence of SMR and the composite end point, with no differences between subgroups (χ2 = 5.12, P = .08).
Study Quality Assessment and Heterogeneity
Heterogeneity refers to the variation in study outcomes between studies. To assess the influence of study quality (bias) on heterogeneity, we applied the Newcastle-Ottawa Scale for quality assessment to the primary studies included in the meta-analysis. All included studies fell into the low or medium categories of risk of bias (eTable 5 in the Supplement), allowing us to identify 2 subgroups (low or medium) and to perform a subgroup analysis of our primary outcome.
Sensitivity Analysis
The significantly higher incidence of all-cause mortality in the patients with SMR vs the patients without SMR was confirmed when meta-analyses were repeated by removing 1 study at a time (eTable 6 in the Supplement). All the additional sensitivity analyses performed were consistent with the previous results (eTable 7 in the Supplement). Moreover, the analysis of all-cause mortality was repeated using a fixed-effects model, which confirmed the significantly higher mortality rate in the patients with SMR vs the patients without SMR (eFigure 4 in the Supplement). Our results were consistent even when the analysis for the primary outcome was performed with inclusion of only the studies reporting adjusted estimates (eTable 7 and eFigure 5 in the Supplement). Finally, when the primary end point was tested in specific subgroups of patients with ischemic or idiopathic cardiomyopathies, the presence of any degree of SMR was consistently associated with a significantly higher all-cause mortality in both subgroups (eFigure 6 in the Supplement).
Meta-regression Analysis and Publication Bias
To explore the potential influence of modifiers on the association between SMR and the all-cause mortality outcome, we performed a meta-regression analysis of the baseline characteristics of the included studies. Meta-regression analysis showed no relationship between all the analyzed effect modifiers and the primary outcome of interest (P > .05 for all) except for the covariates of New York Heart Association (NYHA) class III or IV and length of follow-up (eTable 8 and eFigure 7 in the Supplement).
The funnel plots did not show any publication bias for all the analyses performed. These results are shown in eFigure 8 and eFigure 9 in the Supplement.
Discussion
To our knowledge, this study is the first comprehensive meta-analysis that includes a large number of studies on survival and cardiovascular outcomes in patients with ischemic or dilated cardiomyopathies and SMR. The results of this study demonstrate the following findings: (1) patients seen with SMR have higher rates of all-cause mortality, HF hospitalization, cardiac mortality, and a composite end point of death, HF hospitalization, and cardiac transplant; (2) this observation held true independent of how SMR had been detected (left ventriculography or echocardiography) or graded (qualitatively or quantitatively); (3) even patients with mild SMR have a worse prognosis; (4) higher grades of SMR severity are associated with worse LV dysfunction; (5) higher all-cause mortality persisted in patients with SMR and ischemic vs nonischemic cardiomyopathy; and (6) in studies with a higher prevalence of NYHA class III or IV HF symptoms and in studies with longer follow-up, the relationship between SMR and all-cause mortality was attenuated.
Secondary mitral regurgitation is the most common valve disease worldwide; however, its relationship with prognosis and its treatment have been a matter of debate. Although many studies have shown a significant worsening of survival in patients with SMR, other studies did not. Controversy exists regarding the survival of patients with mild SMR, with some studies demonstrating a lower survival in patients with mild SMR but other studies not showing a mortality difference.
Secondary mitral regurgitation is dynamic and strongly dependent on loading conditions; moreover, all methods to quantify SMR have inherent strengths and weaknesses, rendering accurate quantification of SMR challenging. Current guidelines recommend integration of multiple qualitative and quantitative parameters to determine severity as opposed to simple eyeballing of MR jet size. However, this recommendation is seldom followed in practice and was not specified in the Methods section of most of the studies included in this meta-analysis, which could explain the high disparity in the results. In the present meta-analysis, there was considerable diversity in the methods used to grade SMR severity (eTable 4 in the Supplement). Most of the included studies used echocardiography to detect SMR and a variable spectrum of parameters to grade it; SMR was detected by left ventriculography only in a few studies.
In our meta-analysis that pooled together 53 studies and 45 900 patients with ischemic or nonischemic cardiomyopathies, patients with SMR displayed an increased risk of all-cause mortality, hospitalization for HF, cardiac death, and a composite end point of death, HF hospitalization, and cardiac transplant. Even patients with mild SMR experienced a 1.36-fold increase in all-cause mortality (Figure 3). Although there was fairly high heterogeneity in our analyses, our results were confirmed by the leave-one-out sensitivity analysis, by the additional sensitivity analyses, and by repeating the main analysis with a fixed-effects model. Moreover, no matter how SMR was detected or graded, patients with ischemic or idiopathic cardiomyopathies and any degree of SMR appear to have worsened survival (Figure 4). Notably, as a result of our meta-regression analysis, the association between SMR and all-cause mortality was attenuated in the studies with longer follow-up. A possible explanation might be that patients with ischemic or dilated cardiomyopathies will eventually die of their disease regardless of the presence or absence of SMR if followed up for many years. Moreover, in patients with more symptomatic HF (NYHA III-IV), the presence of SMR seemed to be less predictive of all-cause mortality. A potential explanation is that, in highly symptomatic patients, HF or worsened LV systolic and diastolic function may dominate the prognosis relative to MR severity (eFigure 7A in the Supplement). This is a critical point because, by definition, SMR is a consequence of LV dysfunction. Therefore, SMR may be a surrogate for more extensive LV dysfunction or HF symptoms rather than causal. Indeed, it has been previously shown that, in patients with HF and low LVEF, the severity of SMR is related to the severity of LV dysfunction. The present meta-analysis confirms this observation; in 26 of the 36 studies that reported LVEF according to SMR grade, LV systolic function was significantly worse in patients having moderate or severe SMR compared with patients having only mild or absent SMR. In the remaining 10 studies, the association was not statistically significant (Figure 2 and eTable 3 in the Supplement). Because the hemodynamic effects of MR are known to augment LVEF, the observed decline in LVEF with increasing MR grades is somewhat unexpected and suggests that patients with SMR have worse LV myocardial function than implied from LVEF measurement alone. Therefore, it is important to acknowledge that SMR may be a marker of risk due to greater LV dysfunction rather than a cause of mortality.
Limitations
This meta-analysis has several limitations. Secondary mitral regurgitation is a dynamic condition and may change over time in individual patients. Moreover, SMR detection and grading differed across the included studies, which could have led to an interpretation bias. However, given the large number of studies and patients included, this limitation may be irrelevant. Various populations are included in this meta-analysis, and this factor might account for the differences in outcomes. This is why we performed sensitivity and subgroup analyses to rule out most of the differences in subgroup populations. There is a fairly high degree of heterogeneity in the performed meta-analysis. However, the consistency of our results was confirmed by both the sensitivity analyses (eTable 6 and eTable 7 in the Supplement) and by using a fixed-effects model for the primary outcome of interest (eFigure 4 in the Supplement).
Despite the association of SMR with mortality, there is no convincing evidence that surgical or percutaneous correction of SMR is superior to medical therapy. The results of randomized clinical trials, such as the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation (COAPT), may finally answer this important clinical question. Nevertheless, SMR occurs along a broad spectrum of LV dysfunction. At one extreme, patients with a markedly dilated spherical LV, very low LVEF, and advanced HF might be best treated with transplant, assist devices, or even hospice. At the other extreme, patients with severe SMR due to focal tethering by an inferobasal infarct with preserved LVEF and LV geometry might have symptomatic improvement with mitral valve repair or replacement. The present meta-analysis could not distinguish those patients because accurate data on LV volumes and sphericity and regional wall motion were not available in most of the studies.
The observation that even mild SMR is associated with an adverse prognosis had been used as a reason to lower the threshold values for defining severe SMR by echocardiographic measurements of effective regurgitant orifice area and regurgitant volumes. The recent update to the guidelines has reiterated that the threshold for defining severe SMR is the same as for primary MR, partly to prevent unnecessary operation due to overestimation of the severity of SMR and the lack of proof that surgery improves outcomes. Grading of SMR severity is complicated by the fact that the amount of SMR resulting in significant loss of forward stroke volume is related to LV volumes and LVEF and thus may be different in smaller patients, particularly women.
Conclusions
The large number of studies and patients in this meta-analysis demonstrate clear findings that SMR may be a marker of adverse outcomes independent of how it has been detected or graded. However, because SMR is an intrinsic consequence of LV dysfunction, causality between SMR and mortality should not be implied.
eTable 1. Baseline Characteristics of the Included Studies
eTable 2. Data Extraction and Relative Adjustment for the Primary Outcome (All-Cause Mortality)
eTable 3. Values of LVEF and LVESV According to SMR Grade in the Included Studies
eTable 4. SMR Detection and Quantitation Methods Used in the Included Studies and Relative Study Population
eTable 5. Newcastle-Ottawa Quality Assessment Scale
eTable 6. Leave-One-Out Sensitivity Analysis for the Primary End Point (Results After Removing 1 Study at a Time)
eTable 7. Additional Sensitivity Analysis for the Primary Outcome
eTable 8. Meta-regression Analysis for All-Cause Mortality
eFigure 1. Secondary Outcome: Hospitalization for Heart Failure
eFigure 2. Secondary Outcome: Cardiac Mortality
eFigure 3. Secondary Outcome: Composite Outcome of Death, Hospitalization for Heart Failure, and Transplant
eFigure 4. Fixed-Effects Analysis for All-Cause Mortality
eFigure 5. Analysis of All-Cause Mortality, Including Only Studies Reporting Adjusted Estimates. A, SMR detected at echocardiography and qualitatively graded. B, SMR detected at echocardiography and quantitatively graded. C, SMR present vs absent detected either at echocardiography or ventriculography.
eFigure 6. Subgroup Analysis for All-Cause Mortality According to SMR Pathogenesis
eFigure 7. Meta-regression Plots. A, NYHA class III-IV. B, Follow-up length in studies reporting SMR as present vs absent. C, Follow-up length in studies qualitatively reporting SMR.
eFigure 8. Funnel Plot for the Primary Outcome
eFigure 9. Funnel Plot for the Secondary Outcomes
References
- 1.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005-1011. [DOI] [PubMed] [Google Scholar]
- 2.Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103(13):1759-1764. [DOI] [PubMed] [Google Scholar]
- 3.Pellizzon GG, Grines CL, Cox DA, et al. Importance of mitral regurgitation in patients undergoing percutaneous coronary intervention for acute myocardial infarction: the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) trial. J Am Coll Cardiol. 2004;43(8):1368-1374. [DOI] [PubMed] [Google Scholar]
- 4.Ellis SG, Whitlow PL, Raymond RE, Schneider JP. Impact of mitral regurgitation on long-term survival after percutaneous coronary intervention. Am J Cardiol. 2002;89(3):315-318. [DOI] [PubMed] [Google Scholar]
- 5.Lamas GA, Mitchell GF, Flaker GC, et al. ; Survival and Ventricular Enlargement Investigators . Clinical significance of mitral regurgitation after acute myocardial infarction. Circulation. 1997;96(3):827-833. [DOI] [PubMed] [Google Scholar]
- 6.Deja MA, Grayburn PA, Sun B, et al. Influence of mitral regurgitation repair on survival in the Surgical Treatment for Ischemic Heart Failure trial. Circulation. 2012;125(21):2639-2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calafiore AM, Mazzei V, Iacò AL, et al. Impact of ischemic mitral regurgitation on long-term outcome of patients with ejection fraction above 0.30 undergoing first isolated myocardial revascularization. Ann Thorac Surg. 2008;86(2):458-464. [DOI] [PubMed] [Google Scholar]
- 8.Aronson D, Goldsher N, Zukermann R, et al. Ischemic mitral regurgitation and risk of heart failure after myocardial infarction. Arch Intern Med. 2006;166(21):2362-2368. [DOI] [PubMed] [Google Scholar]
- 9.Mallidi HR, Pelletier MP, Lamb J, et al. Late outcomes in patients with uncorrected mild to moderate mitral regurgitation at the time of isolated coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2004;127(3):636-644. [DOI] [PubMed] [Google Scholar]
- 10.Bursi F, Enriquez-Sarano M, Nkomo VT, et al. Heart failure and death after myocardial infarction in the community: the emerging role of mitral regurgitation. Circulation. 2005;111(3):295-301. [DOI] [PubMed] [Google Scholar]
- 11.Hillis GS, Møller JE, Pellikka PA, Bell MR, Casaclang-Verzosa GC, Oh JK. Prognostic significance of echocardiographically defined mitral regurgitation early after acute myocardial infarction. Am Heart J. 2005;150(6):1268-1275. [DOI] [PubMed] [Google Scholar]
- 12.Grayburn PA, Appleton CP, DeMaria AN, et al. ; BEST Trial Echocardiographic Substudy Investigators . Echocardiographic predictors of morbidity and mortality in patients with advanced heart failure: the Beta-blocker Evaluation of Survival Trial (BEST). J Am Coll Cardiol. 2005;45(7):1064-1071. [DOI] [PubMed] [Google Scholar]
- 13.Cioffi G, Tarantini L, De Feo S, et al. Functional mitral regurgitation predicts 1-year mortality in elderly patients with systolic chronic heart failure. Eur J Heart Fail. 2005;7(7):1112-1117. [DOI] [PubMed] [Google Scholar]
- 14.Rossi A, Dini FL, Faggiano P, et al. Independent prognostic value of functional mitral regurgitation in patients with heart failure: a quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart. 2011;97(20):1675-1680. [DOI] [PubMed] [Google Scholar]
- 15.Patel JB, Borgeson DD, Barnes ME, Rihal CS, Daly RC, Redfield MM. Mitral regurgitation in patients with advanced systolic heart failure. J Card Fail. 2004;10(4):285-291. [DOI] [PubMed] [Google Scholar]
- 16.Boriani G, Gasparini M, Landolina M, et al. ; InSync/InSync ICD Italian Registry Investigators . Impact of mitral regurgitation on the outcome of patients treated with CRT-D: data from the InSync ICD Italian Registry. Pacing Clin Electrophysiol. 2012;35(2):146-154. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Published 2009. Accessed July 26, 2017.
- 19.Higgins JP, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med. 2004;23(11):1663-1682. [DOI] [PubMed] [Google Scholar]
- 20.Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37(5):1148-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gargiulo G, Sannino A, Capodanno D, et al. Transcatheter aortic valve implantation versus surgical aortic valve replacement: a systematic review and meta-analysis. Ann Intern Med. 2016;165(5):334-344. [DOI] [PubMed] [Google Scholar]
- 22.Sannino A, Schiattarella GG, Toscano E, et al. Meta-analysis of effect of body mass index on outcomes after transcatheter aortic valve implantation. Am J Cardiol. 2017;119(2):308-316. [DOI] [PubMed] [Google Scholar]
- 23.Sannino A, Gargiulo G, Schiattarella GG, et al. A meta-analysis of the impact of pre-existing and new-onset atrial fibrillation on clinical outcomes in patients undergoing transcatheter aortic valve implantation. EuroIntervention. 2016;12(8):e1047-e1056. [DOI] [PubMed] [Google Scholar]
- 24.Abate E, Hoogslag GE, Al Amri I, et al. Time course, predictors, and prognostic implications of significant mitral regurgitation after ST-segment elevation myocardial infarction. Am Heart J. 2016;178:115-125. [DOI] [PubMed] [Google Scholar]
- 25.Agricola E, Ielasi A, Oppizzi M, et al. Long-term prognosis of medically treated patients with functional mitral regurgitation and left ventricular dysfunction. Eur J Heart Fail. 2009;11(6):581-587. [DOI] [PubMed] [Google Scholar]
- 26.Agricola E, Stella S, Figini F, et al. Non-ischemic dilated cardiopathy: prognostic value of functional mitral regurgitation. Int J Cardiol. 2011;146(3):426-428. [DOI] [PubMed] [Google Scholar]
- 27.Barra S, Providência R, Paiva L, et al. Mitral regurgitation during a myocardial infarction: new predictors and prognostic significance at two years of follow-up. Acute Card Care. 2012;14(1):27-33. [DOI] [PubMed] [Google Scholar]
- 28.Bruch C, Klem I, Breithardt G, Wichter T, Gradaus R. Diagnostic usefulness and prognostic implications of the mitral E/E′ ratio in patients with heart failure and severe secondary mitral regurgitation. Am J Cardiol. 2007;100(5):860-865. [DOI] [PubMed] [Google Scholar]
- 29.Bursi F, Barbieri A, Grigioni F, et al. Prognostic implications of functional mitral regurgitation according to the severity of the underlying chronic heart failure: a long-term outcome study. Eur J Heart Fail. 2010;12(4):382-388. [DOI] [PubMed] [Google Scholar]
- 30.Engström AE, Vis MM, Bouma BJ, et al. Mitral regurgitation is an independent predictor of 1-year mortality in ST-elevation myocardial infarction patients presenting in cardiogenic shock on admission. Acute Card Care. 2010;12(2):51-57. [DOI] [PubMed] [Google Scholar]
- 31.Faris R, Coats AJ, Henein MY. Echocardiography-derived variables predict outcome in patients with nonischemic dilated cardiomyopathy with or without a restrictive filling pattern. Am Heart J. 2002;144(2):343-350. [DOI] [PubMed] [Google Scholar]
- 32.Fattouch K, Sampognaro R, Speziale G, et al. Impact of moderate ischemic mitral regurgitation after isolated coronary artery bypass grafting. Ann Thorac Surg. 2010;90(4):1187-1194. [DOI] [PubMed] [Google Scholar]
- 33.Feinberg MS, Schwammenthal E, Shlizerman L, et al. Prognostic significance of mild mitral regurgitation by color Doppler echocardiography in acute myocardial infarction. Am J Cardiol. 2000;86(9):903-907. [DOI] [PubMed] [Google Scholar]
- 34.García-Cosío Carmena MD, Roig Minguell E, Ferrero-Gregori A, Vázquez García R, Delgado Jiménez J, Cinca J; investigators of the REDINSCOR research network . Prognostic implications of functional mitral regurgitation in patients with heart failure and reduced ejection fraction [published online January 23, 2017]. Rev Esp Cardiol (Engl Ed). 2017;S1885-5857(16)30456-X. doi: 10.1016/j.rec.2016.12.033 [DOI] [PubMed] [Google Scholar]
- 35.Grigioni F, Detaint D, Avierinos JF, Scott C, Tajik J, Enriquez-Sarano M. Contribution of ischemic mitral regurgitation to congestive heart failure after myocardial infarction. J Am Coll Cardiol. 2005;45(2):260-267. [DOI] [PubMed] [Google Scholar]
- 36.Hickey MS, Smith LR, Muhlbaier LH, et al. Current prognosis of ischemic mitral regurgitation: implications for future management. Circulation. 1988;78(3, pt 2):I51-I59. [PubMed] [Google Scholar]
- 37.Kajimoto K, Takano T. Role of functional mitral regurgitation in heart failure with preserved ejection fraction: an unrecognized protagonist?: reply. Eur J Heart Fail. 2017;19(2):291. [DOI] [PubMed] [Google Scholar]
- 38.Karatolios K, Holzendorf V, Richter A, Schieffer B, Pankuweit S; Competence Network Heart Failure Germany . Long-term outcome and predictors of outcome in patients with non-ischemic dilated cardiomyopathy. Int J Cardiol. 2016;220:608-612. [DOI] [PubMed] [Google Scholar]
- 39.Koelling TM, Aaronson KD, Cody RJ, Bach DS, Armstrong WF. Prognostic significance of mitral regurgitation and tricuspid regurgitation in patients with left ventricular systolic dysfunction. Am Heart J. 2002;144(3):524-529. [DOI] [PubMed] [Google Scholar]
- 40.Lancellotti P, Gérard PL, Piérard LA. Long-term outcome of patients with heart failure and dynamic functional mitral regurgitation. Eur Heart J. 2005;26(15):1528-1532. [DOI] [PubMed] [Google Scholar]
- 41.Lehmann KG, Francis CK, Dodge HT; TIMI Study Group . Mitral regurgitation in early myocardial infarction: incidence, clinical detection, and prognostic implications. Ann Intern Med. 1992;117(1):10-17. [DOI] [PubMed] [Google Scholar]
- 42.López-Pérez M, Estévez-Loureiro R, López-Sainz A, et al. Long-term prognostic value of mitral regurgitation in patients with ST-segment elevation myocardial infarction treated by primary percutaneous coronary intervention. Am J Cardiol. 2014;113(6):907-912. [DOI] [PubMed] [Google Scholar]
- 43.MacHaalany J, Bertrand OF, O’Connor K, et al. Predictors and prognosis of early ischemic mitral regurgitation in the era of primary percutaneous coronary revascularisation. Cardiovasc Ultrasound. 2014;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mentias A, Raza MQ, Barakat AF, et al. Prognostic significance of ischemic mitral regurgitation on outcomes in acute ST-elevation myocardial infarction managed by primary percutaneous coronary intervention. Am J Cardiol. 2017;119(1):20-26. [DOI] [PubMed] [Google Scholar]
- 45.Naser N, Dzubur A, Kusljugic Z, et al. Echocardiographic assessment of ischaemic mitral regurgitation, mechanism, severity, impact on treatment strategy and long term outcome. Acta Inform Med. 2016;24(3):172-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nesković AN, Marinković J, Bojić M, Popović AD. Early mitral regurgitation after acute myocardial infarction does not contribute to subsequent left ventricular remodeling. Clin Cardiol. 1999;22(2):91-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Núñez-Gil IJ, Estrada I, Pérez de Isla L, et al. Functional mitral regurgitation after a first non–ST segment elevation acute coronary syndrome: very-long-term follow-up, prognosis and contribution to left ventricular enlargement and atrial fibrillation development. Heart. 2013;99(20):1502-1508. [DOI] [PubMed] [Google Scholar]
- 48.Pastorius CA, Henry TD, Harris KM. Long-term outcomes of patients with mitral regurgitation undergoing percutaneous coronary intervention. Am J Cardiol. 2007;100(8):1218-1223. [DOI] [PubMed] [Google Scholar]
- 49.Pérez de Isla L, Zamorano J, Quezada M, et al. Functional mitral regurgitation after a first non–ST-segment elevation acute coronary syndrome: contribution to congestive heart failure. Eur Heart J. 2007;28(23):2866-2872. [DOI] [PubMed] [Google Scholar]
- 50.Persson A, Hartford M, Herlitz J, Karlsson T, Omland T, Caidahl K. Long-term prognostic value of mitral regurgitation in acute coronary syndromes. Heart. 2010;96(22):1803-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rihal CS, Nishimura RA, Hatle LK, Bailey KR, Tajik AJ. Systolic and diastolic dysfunction in patients with clinical diagnosis of dilated cardiomyopathy: relation to symptoms and prognosis. Circulation. 1994;90(6):2772-2779. [DOI] [PubMed] [Google Scholar]
- 52.Tcheng JE, Jackman JD Jr, Nelson CL, et al. Outcome of patients sustaining acute ischemic mitral regurgitation during myocardial infarction. Ann Intern Med. 1992;117(1):18-24. [DOI] [PubMed] [Google Scholar]
- 53.Trichon BH, Felker GM, Shaw LK, Cabell CH, O’Connor CM. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Cardiol. 2003;91(5):538-543. [DOI] [PubMed] [Google Scholar]
- 54.Uddin AM, Henry TD, Hodges JS, Haq Z, Pedersen WR, Harris KM. The prognostic role of mitral regurgitation after primary percutaneous coronary intervention for acute ST-elevation myocardial infarction. Catheter Cardiovasc Interv. 2012;80(5):779-786. [DOI] [PubMed] [Google Scholar]
- 55.Upadhyay GA, Chatterjee NA, Kandala J, et al. Assessing mitral regurgitation in the prediction of clinical outcome after cardiac resynchronization therapy. Heart Rhythm. 2015;12(6):1201-1208. [DOI] [PubMed] [Google Scholar]
- 56.Kaneko H, Suzuki S, Uejima T, et al. Prevalence and the long-term prognosis of functional mitral regurgitation in Japanese patients with symptomatic heart failure. Heart Vessels. 2014;29(6):801-807. [DOI] [PubMed] [Google Scholar]
- 57.Kwon DH, Kusunose K, Obuchowski NA, et al. Predictors and prognostic impact of progressive ischemic mitral regurgitation in patients with advanced ischemic cardiomyopathy: a multimodality study. Circ Cardiovasc Imaging. 2016;9(7):pii:e004577. [DOI] [PubMed] [Google Scholar]
- 58.Di Mauro M, Di Giammarco G, Vitolla G, et al. Impact of no-to-moderate mitral regurgitation on late results after isolated coronary artery bypass grafting in patients with ischemic cardiomyopathy. Ann Thorac Surg. 2006;81(6):2128-2134. [DOI] [PubMed] [Google Scholar]
- 59.Okura H, Takada Y, Kubo T, et al. Functional mitral regurgitation predicts prognosis independent of left ventricular systolic and diastolic indices in patients with ischemic heart disease. J Am Soc Echocardiogr. 2008;21(4):355-360. [DOI] [PubMed] [Google Scholar]
- 60.Stolfo D, Tonet E, Barbati G, et al. Acute hemodynamic response to cardiac resynchronization in dilated cardiomyopathy: effect on late mitral regurgitation. Pacing Clin Electrophysiol. 2015;38(11):1287-1296. [DOI] [PubMed] [Google Scholar]
- 61.Verhaert D, Popović ZB, De S, et al. Impact of mitral regurgitation on reverse remodeling and outcome in patients undergoing cardiac resynchronization therapy. Circ Cardiovasc Imaging. 2012;5(1):21-26. [DOI] [PubMed] [Google Scholar]
- 62.Yalonetsky S, Eden H, Lessick J, et al. Impact of functional mitral regurgitation on right ventricular function and outcome in patients with right ventricular infarction. Am J Cardiol. 2014;114(1):36-41. [DOI] [PubMed] [Google Scholar]
- 63.Acker MA, Parides MK, Perrault LP, et al. ; CTSN . Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med. 2014;370(1):23-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30(4):303-371. [DOI] [PubMed] [Google Scholar]
- 65.clinicaltrials.gov. Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation (The COAPT Trial) (COAPT). NCT01626079. https://clinicaltrials.gov/ct2/show/NCT01626079. Accessed July 24, 2017.
- 66.Nishimura RA, Otto CM, Bonow RO, et al. AHA/ACC focused update of the 2014 AHA/ACC guidelines for the management of patients with valvular heart disease. J Am Coll Cardiol. 2017;70(2):252-289. [DOI] [PubMed] [Google Scholar]
- 67.Grayburn PA, Carabello B, Hung J, et al. Defining “severe” secondary mitral regurgitation: emphasizing an integrated approach. J Am Coll Cardiol. 2014;64(25):2792-2801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline Characteristics of the Included Studies
eTable 2. Data Extraction and Relative Adjustment for the Primary Outcome (All-Cause Mortality)
eTable 3. Values of LVEF and LVESV According to SMR Grade in the Included Studies
eTable 4. SMR Detection and Quantitation Methods Used in the Included Studies and Relative Study Population
eTable 5. Newcastle-Ottawa Quality Assessment Scale
eTable 6. Leave-One-Out Sensitivity Analysis for the Primary End Point (Results After Removing 1 Study at a Time)
eTable 7. Additional Sensitivity Analysis for the Primary Outcome
eTable 8. Meta-regression Analysis for All-Cause Mortality
eFigure 1. Secondary Outcome: Hospitalization for Heart Failure
eFigure 2. Secondary Outcome: Cardiac Mortality
eFigure 3. Secondary Outcome: Composite Outcome of Death, Hospitalization for Heart Failure, and Transplant
eFigure 4. Fixed-Effects Analysis for All-Cause Mortality
eFigure 5. Analysis of All-Cause Mortality, Including Only Studies Reporting Adjusted Estimates. A, SMR detected at echocardiography and qualitatively graded. B, SMR detected at echocardiography and quantitatively graded. C, SMR present vs absent detected either at echocardiography or ventriculography.
eFigure 6. Subgroup Analysis for All-Cause Mortality According to SMR Pathogenesis
eFigure 7. Meta-regression Plots. A, NYHA class III-IV. B, Follow-up length in studies reporting SMR as present vs absent. C, Follow-up length in studies qualitatively reporting SMR.
eFigure 8. Funnel Plot for the Primary Outcome
eFigure 9. Funnel Plot for the Secondary Outcomes