Key Points
Question
How often does device-related reoperation occur after laparoscopic gastric band surgery and what are the associated expenditures for payers?
Findings
In this study of 25 042 Medicare beneficiaries who had the gastric band placed between 2006 and 2013, 4636 patients (18.5%) underwent 17 539 reoperations (an average of 3.8 procedures/patient). During the study period, Medicare paid $470 million for laparoscopic gastric band–associated procedures, of which $224 million (47.6%) of the payments were for reoperations.
Meaning
Reoperations after a gastric band placement are common and costly and raise concern about the safety, effectiveness, and value of the device.
Abstract
Importance
Following the US Food and Drug Administration approval for laparoscopic gastric band surgery in 2001, as many as 96 000 devices have been placed annually. The reported rates of reoperation range from 4% to 60% in short-term studies; however, to our knowledge, few long-term population-level data on outcomes or expenditures are known.
Objective
To describe the rate of device-related reoperations occurring after laparoscopic gastric band surgery as well as the associated payments in a longitudinal national cohort.
Design, Settings, and Participants
This retrospective review of 25 042 Medicare beneficiaries who underwent gastric band placement between 2006 and 2013 identifies gastric band–related reoperations, including device removal, device replacement, or revision to a different bariatric procedure (eg, a gastric bypass or sleeve gastrectomy). The rates of reoperation were risk adjusted using a multivariable logistic regression model that included patient age, sex, race/ethnicity, Elixhauser comorbidities, and the year that the operation was performed.
Main Outcomes and Measures
Rate of device-related reoperation nationally and across individual hospital referral regions. Thirty-day total episode Medicare payments to hospitals for the index operation and any subsequent reoperations.
Results
Of the 25 042 patients who underwent gastric band placement, 20 687 (82.61%) were white, 18 143 (72.45%) were women, and the mean age was 57.56 years. Patients (mean age, 57.5; 76.2% women) requiring reoperation had lower rates of hypertension (64.9% vs 73.4%; P < .001) and diabetes (40.4% vs 44.6%; P < .001) and were more likely to have their index operation at a for-profit hospital (34.6% vs 22.0%; P < .001). With an average of 4.5-year follow-up, 4636 patients (18.5%) underwent 17 539 reoperations (an average of 3.8 procedures/patient). Hospital referral regions demonstrated a 2.9-fold variation in risk- and reliability-adjusted rates of reoperation (lower quartile average, 13.3%; upper quartile average, 39.1%). During the study period, Medicare paid $470 million for laparoscopic gastric band associated procedures, of which $224 million (47.6%) of the payments were for reoperations. From 2006 to 2013, the proportion of payments from Medicare for reoperations increased from 16.4% to 77.3% of their annual spending on the gastric band device.
Conclusions and Relevance
Among Medicare beneficiaries undergoing gastric band surgery, device-related reoperation was common, costly, and varied widely across hospital referral regions. These findings suggest that payers should reconsider their coverage of the gastric band device.
This study of Medicare beneficiaries who had a laparoscopic gastric band placed explores how common reoperations were among this population and what costs were involved for payers.
Introduction
Following the approval of the laparoscopic gastric band to treat morbid obesity by the US Food and Drug Administration (FDA) in 2001, as many as 96 000 devices have been placed annually. When the gastric band malfunctions (eg, the band erodes into the stomach or slips down and causes obstruction) or the patient has not achieved the expected weight loss, a reoperation is indicated to replace or remove the band. Although these safety and effectiveness concerns have contributed to decreasing the device’s popularity, the American Society for Metabolic and Bariatric Surgery estimates that more than 11 000 devices were placed in 2015. In fact, the FDA expanded their indications for the device to patients with a body mass index (calculated as weight in kilograms divided by height in meters squared) of more than 30, making an estimated 19 million Americans able to have the gastric band placed who would not be eligible for other bariatric procedures.
To our knowledge, despite the continued use of the gastric band to treat morbid obesity, limited population-level data exist about the safety and costs of the device. Short-term trials to approve and expand indications for the device reported reoperation rates as low as 4%. Meanwhile, single-institution cohort studies and small trials vary widely in their results but have raised concerns by detecting reoperation rates as high as 60%. Each of these studies lack either an adequate sample size, long-term follow-up, or geographic representations to provide a population estimate. Moreover, none have assessed the downstream financial burden on payers for subsequent device-related procedures. Thus, whether these high rates of reoperation are representative throughout the country and what the costs are for payers remain unknown.
The purpose of this analysis was to describe reoperations that occur after a gastric band placement among a geographically representative cohort with long-term follow-up. By using longitudinal data from a national payer (ie, Medicare claims), this study also assessed the associated payer expenditures.
Methods
Data Sources
The Medicare Provider Analysis and Review file from the Centers for Medicare and Medicaid Services was used to obtain data on patients between 2006 and 2013. This represented 8 years of the most recent data while also providing adequate long-term follow-up. Moreover, this was a period after gastric band devices had already been modified to their second and third generation, representing the gastric bands that are currently used. The Centers for Medicare and Medicaid Services is the largest insurer in the United States and covers payments for approximately 15% of bariatric procedures.
Hospital-level data were obtained from the Annual Survey administered by the American Hospital Association. The 2 data sets were linked by their hospital provider number. This study was approved by the University of Michigan institutional review board and was deemed exempt from additional review because of the use of secondary data.
Identification of Procedures and Cohort
Procedure codes from the International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM) were used to identify patients who underwent laparoscopic gastric band surgery (ICD-9-CM code 44.95) who had also received a concurrent diagnosis code for obesity (ICD-9-CM codes 278.0, 278.01, 278.02, and V77.8). Patients who had gastric or small-intestine malignancies were excluded as they may undergo reoperation for reasons that are not device related.
Outcome Variables
Reoperation
The primary outcome variable in this study was gastric band device-related reoperation. The reoperation procedures were selected based on previous reports examining the same outcome. For this study, reoperation was defined as any surgery occurring after the index operation with Current Procedural Terminology codes for gastric band removal, gastric band replacement, or revision to a different bariatric procedure (eg, sleeve gastrectomy or Roux-en-Y gastric bypass). Notably, band size adjustment by accessing the port was not included in the reoperation definition, as this is often done in an outpatient clinic setting and is an expected aspect of managing the device. A full table of the Current Procedural Terminology codes used for this study can be found in the eTable in the Supplement.
Geographic Variation
This study also describes the geographic variation in gastric band device-related reoperation rates across the United States. Variations were assessed at the level of hospital referral regions as defined by the Dartmouth Atlas. Each hospital referral region represents a collection of zip codes where patients are most likely to be referred for hospital care. Patients were assigned to a hospital referral region based on where they underwent their index operation.
Medicare Payments
Medicare payments from the Medicare Provider Analysis and Review file were used to understand the financial implications of gastric band–related reoperations. Because wide variation exists in hospital charges, this analysis used actual payments to most accurately reflect the expense to Medicare. The total episode payments included payments for inpatient care, outpatient care, carrier costs (ie, physician), home health care, care in a skilled nursing facility, a long stay in a hospital, and durable medical equipment files. Following methods previously described to use claims data for surgical procedures, payments were grouped into the following discrete categories: index hospitalization, readmissions, physician services, and postdischarge ancillary care. Each total episode of care for the index operation and any subsequent reoperations included payments for the initial admission and care up to 30 days after discharge.
Statistical Analysis
The first step of this analysis was to compare the patient and hospital characteristics for patients who did and did not undergo a gastric band–related reoperation. Patient characteristics included age, sex, race/ethnicity, and comorbidities. Hospital characteristics included ownership status, size, geographic region, teaching status, and staffing ratios. They were compared using χ2and Wilcoxon rank sum tests as appropriate.
The next aim of this analysis was to evaluate the geographic variation in the rates of reoperation. Because patients may differ across hospital referral regions in ways that may influence their likelihood of having a reoperation, the rate of reoperation was risk-adjusted using a multivariable regression model that included age, sex, race/ethnicity, and comorbidities as described by Elixhauser and Southern et al. The year that the operation was performed was also included in the regression model to account for possible secular trends.
The rate of reoperation for each hospital referral region was also reliability-adjusted. Because hospital referral regions have different case volumes, the observed rate of reoperation in lower-volume regions is more likely to reflect “statistical noise” (ie, chance) compared with higher-volume regions. To account for these differences in volume per region (ie, the strength of the statistical signal), empirical Bayes estimates were applied to “shrink” lower-volume regions toward the overall population mean using the methods previously described. Thus, the final rate of reoperation for each hospital referral region used in this study was both risk- and reliability-adjusted, resulting in a conservative estimate of variation. Reliability adjustments were not used in the rest of our analysis beyond this specific national estimate of variation across referral regions.
The final aim of this analysis was to assess Medicare payments, which was done in 2 forms. First, the unadjusted payments were used to determine the bottom line to Medicare (ie, what was actually paid to providers for episodes of care). Next, the payments were assessed after a price standardization. This additional comparison was performed because payments from Medicare are partially based on geography (to account for the local cost of living and wage index) as well as the settings in which the care is provided (eg, if providers participate in graduate medical education or care for a disproportionate share of low-income patients.) By accounting for these intended payment adjustments, the price-standardized amounts provide more insight into the differences in resources associated with gastric band procedures. The methods described initially by the Medicare Payment Advisory Commission and subsequently by the Dartmouth Institute were used to perform price standardization. This same approach has also been applied in previous reports using Medicare Provider Analysis and Review data to examine payments for surgical procedures.
All reported P values were 2-sided and a value of less than .05 was used as threshold for significance. All statistical analyses were completed with Stata, version 14 (Stata Corp).
Results
A total of 25 042 patients undergoing a laparoscopic gastric band placement were included in this study (Table 1). Patients who did not undergo a reoperation, on average, were more likely to be men (28.4% vs 23.8%; P < .001) and white (82.9% vs 81.2%; P = .007). Patients requiring reoperation had lower rates of hypertension (64.9% vs 73.4%; P < .001) and diabetes (40.4% vs 44.6%; P < .001).
Table 1. Patient and Hospital Characteristics at the Time of the Index Operationa.
| Characteristic | All Patients | Patients Without Reoperation | Patients Undergoing Reoperation | P Value Without Reoperation vs Undergoing Reoperation |
|---|---|---|---|---|
| No. of patients | 25 042 | 20 406 | 4636 | NA |
| Age, mean, y | 57.56 | 57.51 | 57.75 | .22 |
| Men, % | 27.55 | 28.42 | 23.75 | <.001 |
| White, % | 82.61 | 82.92 | 81.22 | .01 |
| Comorbidities, %b | ||||
| Hypertension | 71.78 | 73.35 | 64.88 | <.001 |
| Diabetes | 43.83 | 44.61 | 40.38 | <.001 |
| Depression | 21.78 | 21.81 | 21.68 | .85 |
| Chronic pulmonary disease | 23.23 | 23.18 | 23.47 | .67 |
| Hypothyroidism | 13.50 | 13.33 | 14.24 | .11 |
| Liver disease | 6.59 | 7.05 | 4.57 | <.001 |
| Psychoses | 5.22 | 4.90 | 6.62 | <.001 |
| Deficiency anemias | 2.89 | 2.38 | 5.11 | <.001 |
| Renal failure | 4.29 | 4.25 | 4.44 | .57 |
| Fluid and electrolyte disorders | 3.33 | 2.18 | 8.43 | <.001 |
| Congestive heart failure | 5.24 | 5.24 | 5.22 | .95 |
| Rheumatoid arthritis | 2.58 | 2.61 | 2.44 | .49 |
| Valvular disease | 1.88 | 1.86 | 2.01 | .51 |
| Peripheral vascular disease | 1.41 | 1.38 | 1.55 | .39 |
| Coagulopathy | 0.46 | 0.38 | 0.82 | .00 |
| Paralysis | 0.47 | 0.46 | 0.52 | .62 |
| Elixhauser No. of comorbidities, % | ||||
| 0 | 5.83 | 5.52 | 7.20 | <.001 |
| 1 | 20.83 | 21.49 | 17.95 | <.001 |
| ≥2 | 73.33 | 72.99 | 74.85 | .01 |
| No. of hospitals | 732 | 631 | 564 | NA |
| Ownership, % | ||||
| For-profit | 24.37 | 22.01 | 34.64 | <.001 |
| Nonprofit | 66.37 | 68.59 | 56.73 | <.001 |
| Other | 9.26 | 9.40 | 8.63 | .09 |
| Bed size, % | ||||
| <250 beds | 29.02 | 29.83 | 26.24 | <.001 |
| >250 to <500 beds | 43.19 | 41.45 | 49.13 | <.001 |
| >500 beds | 28.31 | 29.24 | 24.23 | <.001 |
| Geographic region, % | ||||
| Northeast | 17.91 | 18.47 | 15.47 | <.001 |
| Midwest | 22.57 | 23.37 | 19.13 | <.001 |
| South | 42.86 | 41.03 | 50.84 | <.001 |
| West | 16.65 | 17.13 | 14.56 | <.001 |
| Teaching hospital, % | 60.23 | 61.72 | 54.44 | <.001 |
| No. of operating rooms | 22.78 | 22.66 | 23.27 | .08 |
| Nurse ratio | 7.13 | 7.10 | 7.28 | .01 |
Abbreviation: NA, not applicable.
Data source: Medicare claims, 2006 to 2013, and the American Hospital Association Annual Survey.
Comorbid diseases as defined by the Healthcare Cost and Utilization Project. Full definitions of the codes used can be found here: https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp.
Patients in this study underwent their index gastric band operations at 732 different hospitals. Compared with patients who underwent a reoperation, patients who did not undergo a reoperation were more likely to have their index operation at a nonprofit center (68.6% vs 56.7%; P < .01) or at a teaching hospital (61.7% vs 54.4%; P < .01). Most reoperations were performed during an elective admission (reoperation admission type: elective, 79.9%; urgent, 10.0%; and emergency, 10.1%).
A large proportion of patients who had a gastric band placed underwent a reoperation. With an average of a 4.5-year follow up, 4636 patients (18.5%) underwent 17 539 reoperations (an average of 3.8 procedures per patient in addition to their index operation). The frequency distribution of reoperations was right-skewed with a median of 2.5 reoperations. The most common reoperation was band removal, followed by band replacement (Table 2). Of the patients who had a reoperation, 19.1% underwent a subsequent different bariatric operation, including gastric bypass or sleeve gastrectomy.
Table 2. Laparoscopic Gastric Band-Associated Reoperationsa.
| Lap Band Specific Reoperation | Cumulative % |
|---|---|
| Any reoperation | 100.00 |
| Band removal | 41.8 |
| Band and port replacement | 28.6 |
| Conversion to Roux-en-Y gastric bypass (laparoscopic) | 13.1 |
| Band revision | 10.7 |
| Port replacement | 5.4 |
| Conversion to sleeve gastrectomy (laparoscopic) | 5.3 |
| Port revision | 5.2 |
| Port removal | 2.9 |
| Conversion to Roux-en-Y gastric bypass (open) | 0.7 |
| Mean No. of reoperations per patient | 3.78 |
Data source: Medicare claims, 2006 to 2013.
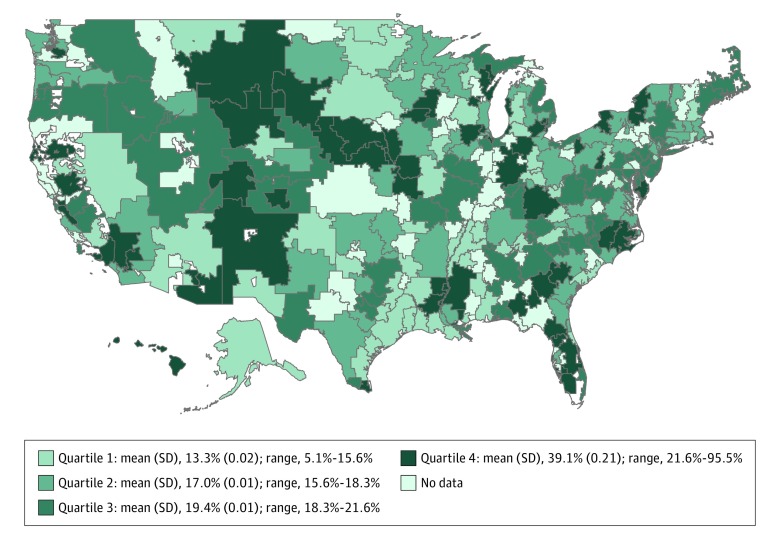
Geographic variation was observed in the rates of gastric band–related reoperation. The rates of reoperation across hospital referral regions ranged from 5.1% to 95.5%. The bottom quartile of hospital referral regions had a mean (SD) reoperation rate of 13.3% (0.03) while the top quartile had a mean (SD) reoperation rate of 39.1% (0.21), reflecting a 2.9-fold variation. Hospital referral regions divided into quartiles by rates of risk- and reliability-adjusted rates of reoperation are shown in Figure 1.
Figure 1. Variation in Reoperation Rates Across Hospital Referral Regions in the United States.
During the study period, Medicare paid $470 million for laparoscopic gastric band–associated procedures, of which $224 million (47.6%) of the payments were for reoperations. In 2006, reoperation procedures accounted for 16.4% of the total Medicare spending related to the gastric band device, which increased to 77.3% by 2013 (Figure 2).
Figure 2. Annual Medicare Spending on Gastric Band Procedures.
Price-standardized Medicare payments were assessed for the index operation to place the gastric band and then for any subsequent reoperations. The mean payment was $12 345 for the index operation and $19 657 for each reoperation. Compared with the episode payment to place the gastric band, payments for reoperations were more expensive across each measured category, including total amounts, index hospitalization, readmissions, physician services, and postdischarge ancillary care (Table 3).
Table 3. Payments for Laparoscopic Gastric Band Index Operations and Reoperationsa.
| Characteristic | Mean Cost of Index Operation (n = 20 406) |
Mean Cost of Each Reoperation Episode (n = 4636) |
Cost of Reoperation(s) per Patient,b $ (n = 4636) |
|---|---|---|---|
| Index hospitalization overall | 9950 | 13 420 | 49 290 |
| Diagnosis related group | 9933 | 13 170 | 48 634 |
| Outlier | |||
| Proportion with payment, % | 2.5 | 2.48 | 2.16 |
| Average payment when present | 5014 | 9612 | 25 914 |
| Average payment overall | 17 | 250 | 656 |
| Physician services | 2041 | 5034 | 16 617 |
| Readmission | |||
| Proportion with payment, % | 9.2 | 8.69 | 7.85 |
| Average payment when present | 3925 | 5932 | 18 304 |
| Average payment overall | 167 | 540 | 1687 |
| Post-discharge ancillary care | |||
| Proportion with payment, % | 33.0 | 41.63 | 39.78 |
| Average payment when present | 557 | 1540 | 4023 |
| Average payment overall | 188 | 671 | 1878 |
| Total episode | 12 345 | 19 657 | 68 896 |
Data source: Medicare claims, 2006 to 2013. Payments shown here are after price-standardization.
Includes the total costs of all reoperations (the average per patient was 3.8) for each patient who underwent at least 1 reoperation.
Discussion
This study has 3 important findings that improve our understanding of the laparoscopic gastric band used to treat patients with morbid obesity. First, a reoperation after gastric band placement is common, with nearly 1 in 5 patients requiring at least 1 additional gastric band–related surgical procedure within a median follow-up of 4.5 years. Second, there is wide geographic variation (2.9-fold) in the rates of reoperation across hospital referral regions after accounting for patient characteristics and secular trends. Third, although a substantial number of gastric bands are still being placed, as of 2013, more than 77% of payments related to the device were for reoperations, reflecting either complications related to the gastric band placement or weight loss failure. Taken together, these findings indicate that the gastric band is associated with high reoperation rates and considerable costs to payers, which raises concerns about its safety, effectiveness, and value.
Previous studies evaluating the rate of reoperation after a gastric band placement have been inconsistent. Initial trials that informed the FDA approval of the device reported reoperation rates as low as 4% with a 1- to 2-year follow-up. Subsequent similarly sized trials with longer follow-ups began reporting reoperation rates as high as 60%. The largest, long-term population-level estimates of the gastric band come from France, where a 7-year follow-up in a closed health care system detected a reoperation rate of 20%. To our knowledge, this study provides the first longitudinal, national population-based estimate in the United States and confirms the high reoperation rates seen elsewhere. Moreover, the finding of a 2.9-fold geographic variation may partially help explain the wide range of rates individually reported in previous smaller studies.
Whether the gastric band has been effective in facilitating weight loss has also been debated. A meta-analysis of 48 studies (9 randomized clinical trials and 39 observational trials) between 2003 and 2012 compared the gastric band with either a Roux-en-Y gastric bypass or a sleeve gastrectomy. At 1-, 3-, and 5-year follow-up, the gastric band was the least effective in facilitating weight loss compared with the other bariatric procedures. While this study did not specifically study weight loss as an outcome, the findings here do support concerns about the gastric band effectiveness, as nearly 1 in 5 patients who underwent a reoperation subsequently underwent a different bariatric procedure.
Although the present study cannot identify a mechanism to explain the wide variation in the rates of reoperation that were observed, the data suggest at least 2 possibilities. First, the cohort who underwent a reoperation had a higher rate of having received a psychiatric diagnosis at the time of their index operation. This finding is consistent with other previous reports that identified preoperative psychiatric illness as a risk factor for worse postoperative outcomes. Second, most of the reoperations were done in the elective setting. This suggests that the variable patient and clinician preferences about the gastric band may be important underlying drivers leading to a reoperation, rather than simply emergency scenarios in which a reoperation was required.
Strengths and Limitations
This study should be interpreted in the context of several limitations. First, using administrative claims data may not have captured all of the patient characteristics that could confound our results. The effect of unmeasured confounding on our results is likely minimal, as bariatric patients often have similar underlying comorbidities that make them eligible for the procedure. Second, administrative claims data also have potential limitations in capturing postoperative complications because of coding biases. This study, however, used episodes of care with a reoperation as a primary outcome that is significantly less susceptible to a coding bias compared with individual complications. Finally, focusing on the population receiving Medicare benefits may threaten the generalizability of the study to populations that are younger and not receiving Medicare benefits who frequently undergo bariatric procedures. Many patients included in this analysis qualified for Medicare through disabilities before age 65 years, which made our overall average age of the cohort closer to the general population. Furthermore, the reoperation rates in this study are within the range reported in smaller studies that included younger patients.
This study has several important practice and policy implications related to bariatric surgery. First, for patients considering bariatric surgery, these data help inform them about the long-term risks associated with placing the gastric band. These include the facts that not only is reoperation common, but also that those who underwent reoperation often underwent several additional procedures. This sharply contrasts with the lower reoperation rates of 3% to 9% that have been reported for other bariatric procedures. Second, for payers seeking out cost-containment strategies, this identifies an important opportunity to reduce the costs of bariatric procedures. Because approximately half of payments related to the gastric band went toward reoperations, payers may now have financial incentive to guide patients toward other bariatric operations as their first-line surgical treatment. Third, the findings of wide variation after controlling patient factors suggests that clinicians may have variable abilities to effectively place and manage the device. Those who continue to use the device may benefit from peer training to improve their operative technique or to develop a more judicious patient selection. Finally, policymakers and regulatory agencies approving future devices for bariatric patients may need a more longitudinal role beyond initial trials and the voluntary reporting of complications. Given our current ability to manage large data sets, the FDA may now have a feasible approach to prospectively track devices in claims data to ensure outcomes after broadly adopting a new technology mirror to those reported in early randomized clinical trials to approve the device.
Conclusions
Among Medicare beneficiaries undergoing laparoscopic adjustable gastric band surgery, reoperation was common, costly, and varied widely across hospital referral regions. These findings suggest that payers should reconsider their coverage of the gastric band device.
eTable. Current Procedural Terminology Codes Used to Identify Reoperation After Laparoscopic Gastric Band Surgery.
References
- 1.Food and Drug Administration The lap band adjustable gastric banding system summary of safety and effectiveness data. 2001; http://www.accessdata.fda.gov/cdrh_docs/pdf/P000008b.pdf. Accessed 19 April, 2016.
- 2.Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2008. Obes Surg. 2009;19(12):1605-1611. [DOI] [PubMed] [Google Scholar]
- 3.The American Society of Metabolic and Bariatric Surgery Estimate of bariatric surgery numbers, 2011-2015. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Accessed October 15, 2016.
- 4.Food and Drug Administration LAP-BAND; summary of safety and effectiveness (SSED). http://www.accessdata.fda.gov/cdrh_docs/pdf/P000008S017b.pdf. Accessed April 19, 2016.
- 5.Arthurs S, Abrahamian Y, Loughren EL, Hiatt JC, Cisneros R, Weissberg J. New technology review process: the laparoscopic adjustable gastric band. Perm J. 2011;15(4):54-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anwar M, Collins J, Kow L, Toouli J. Long-term efficacy of a low-pressure adjustable gastric band in the treatment of morbid obesity. Ann Surg. 2008;247(5):771-778. [DOI] [PubMed] [Google Scholar]
- 7.Himpens J, Cadière GB, Bazi M, Vouche M, Cadière B, Dapri G. Long-term outcomes of laparoscopic adjustable gastric banding. Arch Surg. 2011;146(7):802-807. [DOI] [PubMed] [Google Scholar]
- 8.Kasza J, Brody F, Vaziri K, et al. Analysis of poor outcomes after laparoscopic adjustable gastric banding. Surg Endosc. 2011;25(1):41-47. [DOI] [PubMed] [Google Scholar]
- 9.Lanthaler M, Aigner F, Kinzl J, Sieb M, Cakar-Beck F, Nehoda H. Long-term results and complications following adjustable gastric banding. Obes Surg. 2010;20(8):1078-1085. [DOI] [PubMed] [Google Scholar]
- 10.Stroh C, Hohmann U, Schramm H, Meyer F, Manger T. Fourteen-year long-term results after gastric banding. J Obes. 2011;2011:128451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Victorzon M, Tolonen P. Mean fourteen-year, 100% follow-up of laparoscopic adjustable gastric banding for morbid obesity. Surg Obes Relat Dis. 2013;9(5):753-757. [DOI] [PubMed] [Google Scholar]
- 12.Altieri MS, Yang J, Telem DA, et al. Lap band outcomes from 19 221 patients across centers and over a decade within the state of New York. Surg Endosc. 2016;30(5):1725-1732. [DOI] [PubMed] [Google Scholar]
- 13.Cobourn C, Degboe A, Super PA, et al. Safety and effectiveness of LAP-BAND AP System: results of Helping Evaluate Reduction in Obesity (HERO) prospective registry study at 1 year. J Am Coll Surg. 2013;217(5):907-918. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen NT, Masoomi H, Laugenour K, et al. Predictive factors of mortality in bariatric surgery: data from the Nationwide Inpatient Sample. Surgery. 2011;150(2):347-351. [DOI] [PubMed] [Google Scholar]
- 15.American Hospital Association AHA annual survey database. http://www.ahadataviewer.com/book-cd-products/AHA-Survey. Accessed June 6, 2016.
- 16.The Dartmouth Atlas of Health Care Research methods: defining hospital referral regions. http://www.dartmouthatlas.org/downloads/methods/research_methods.pdf. Accessed October 29, 2016.
- 17.Birkmeyer JD, Gust C, Dimick JB, Birkmeyer NJ, Skinner JS. Hospital quality and the cost of inpatient surgery in the United States. Ann Surg. 2012;255(1):1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibrahim AM, Ghaferi AA, Thumma JR, Dimick JB. Hospital quality and Medicare expenditures for bariatric surgery in the United States [published online September 6, 2016]. Ann Surg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. [DOI] [PubMed] [Google Scholar]
- 20.Southern DA, Quan H, Ghali WA. Comparison of the Elixhauser and Charlson/Deyo methods of comorbidity measurement in administrative data. Med Care. 2004;42(4):355-360. [DOI] [PubMed] [Google Scholar]
- 21.Krell RW, Finks JF, English WJ, Dimick JB. Profiling hospitals on bariatric surgery quality: which outcomes are most reliable? J Am Coll Surg. 2014;219(4):725-34.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones HE, Spiegelhalter DJ. The identification of “unusual” health-care providers from a hierarchical model. Am Stat. 2011;65(3):154-163. [Google Scholar]
- 23.Dimick JB, Staiger DO, Birkmeyer JD. Ranking hospitals on surgical mortality: the importance of reliability adjustment. Health Serv Res. 2010;45(6 pt 1):1614-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grenda TR, Krell RW, Dimick JB. Reliability of hospital cost profiles in inpatient surgery. Surgery. 2016;159(2):375-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottlieb DJ, Zhou W, Song Y, Andrews KG, Skinner JS, Sutherland JM. Prices don’t drive regional Medicare spending variations. Health Aff (Millwood). 2010;29(3):537-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller DC, Gust C, Dimick JB, Birkmeyer N, Skinner J, Birkmeyer JD. Large variations in Medicare payments for surgery highlight savings potential from bundled payment programs. Health Aff (Millwood). 2011;30(11):2107-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gottlieb DJ, Zhou W, Song Y, Andrews KG, Skinner J, Sutherland JM Technical report: a standardized method for adjusting Medicare expenditures for regional differences in prices. http://www.dartmouthatlas.org/downloads/papers/std_prc_tech_report.pdf. Accessed September 25, 2015.
- 28.Osborne NH, Nicholas LH, Ryan AM, Thumma JR, Dimick JB. Association of hospital participation in a quality reporting program with surgical outcomes and expenditures for Medicare beneficiaries. JAMA. 2015;313(5):496-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birkmeyer JD, Gust C, Baser O, Dimick JB, Sutherland JM, Skinner JS. Medicare payments for common inpatient procedures: implications for episode-based payment bundling. Health Serv Res. 2010;45(6, pt 1):1783-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibrahim AM, Hughes TG, Thumma JR, Dimick JB. Association of hospital critical access status with surgical outcomes and expenditures among Medicare beneficiaries. JAMA. 2016;315(19):2095-2103. [DOI] [PubMed] [Google Scholar]
- 31.Lazzati A, De Antonio M, Paolino L, et al. Natural history of adjustable gastric banding: lifespan and revisional rate: a nationwide study on administrative data on 53 000 patients. Ann Surg. 2017;265(3):439-445. [DOI] [PubMed] [Google Scholar]
- 32.Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014;149(3):275-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhatti JA, Nathens AB, Thiruchelvam D, Grantcharov T, Goldstein BI, Redelmeier DA. Self-harm emergencies after bariatric surgery: a population-based cohort study. JAMA Surg. 2016;151(3):226-232. [DOI] [PubMed] [Google Scholar]
- 34.Dawes AJ, Maggard-Gibbons M, Maher AR, et al. Mental health conditions among patients seeking and undergoing bariatric surgery: a meta-analysis. JAMA. 2016;315(2):150-163. [DOI] [PubMed] [Google Scholar]
- 35.Lagerros YT, Brandt L, Hedberg J, Sundbom M, Bodén R. Suicide, self-harm, and depression after gastric bypass surgery: a nationwide cohort study. Ann Surg. 2017;265(2):235-243. [DOI] [PubMed] [Google Scholar]
- 36.Morgan DJ, Ho KM. Incidence and risk factors for deliberate self-harm, mental illness, and suicide following bariatric surgery: a state-wide population-based linked-data cohort study. Ann Surg. 2017;265(2):244-252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Current Procedural Terminology Codes Used to Identify Reoperation After Laparoscopic Gastric Band Surgery.