Abstract
Importance
Sensitive outcome measures for disease progression are needed for treatment trials of Stargardt disease.
Objective
To describe the yearly progression rate of atrophic lesions in the retrospective Progression of Stargardt Disease study.
Design, Setting, and Participants
A multicenter retrospective cohort study was conducted at tertiary referral centers in the United States and Europe. A total of 251 patients aged 6 years or older at baseline, harboring disease-causing variants in ABCA4 (OMIM 601691), enrolled in the study from 9 centers between August 2, 2013, and December 12, 2014; of these patients, 215 had at least 2 gradable fundus autofluorescence images with atrophic lesion(s) present in at least 1 eye.
Exposures
Areas of definitely decreased autofluorescence (DDAF) and questionably decreased autofluorescence were quantified by a reading center. Progression rates were estimated from linear mixed models with time as the independent variable.
Main Outcomes and Measures
Yearly rate of progression using the growth of atrophic lesions measured by fundus autofluorescence.
Results
A total of 251 participants (458 study eyes) were enrolled. Images from 386 eyes of 215 participants (126 females and 89 males; mean [SD] age, 29.9 [14.7] years; mean [SD] age of onset of symptoms, 21.9 [13.3] years) showed atrophic lesions present on at least 2 visits and were graded for 2 (156 eyes), 3 (174 eyes), or 4 (57 eyes) visits. A subset of 224 eyes (123 female participants and 101 male participants; mean [SD] age, 33.0 [15.1] years) had areas of DDAF present on at least 2 visits; these eyes were included in the estimation of the progression of the area of DDAF. At the first visit, DDAF was present in 224 eyes (58.0%), with a mean (SD) lesion size of 2.2 (2.7) mm2. The total mean (SD) area of decreased autofluorescence (DDAF and questionably decreased autofluorescence) at first visit was 2.6 (2.8) mm2. Mean progression of DDAF was 0.51 mm2/y (95% CI, 0.42-0.61 mm2/y), and of total decreased fundus autofluorescence was 0.35 mm2/y (95% CI, 0.28-0.43 mm2/y). Rates of progression depended on the initial size of the lesion.
Conclusions and Relevance
In Stargardt disease with DDAF lesions, fundus autofluorescence may serve as a monitoring tool for interventional clinical trials that aim to slow disease progression. Rates of progression depended mainly on initial lesion size.
This multicenter cohort study describes the yearly progression rate of atrophic lesions as determined by fundus autofluorescence in the retrospective Progression of Stargardt Disease study.
Key Points
Question
What is the growth rate of atrophic lesions in patients with Stargardt disease?
Findings
In this multicenter cohort study, mean progression of definitely decreased autofluorescence lesions was 0.51 mm2/y, and of total decreased fundus autofluorescence was 0.35 mm2/y. Rates of progression depended on initial lesion size.
Meaning
The growth rate of atrophic lesions as determined by fundus autofluorescence in patients with Stargardt disease may be a suitable outcome measure for treatment trials.
Introduction
Autosomal recessive Stargardt macular dystrophy (STGD1; OMIM 248200) is the most common form of juvenile macular degeneration and is caused by mutations in ABCA4 (OMIM 601691). Several treatment trials, including stem cell therapy, gene therapy, and medical therapy, are in early clinical phase trials. Visual acuity appears to be inadequate to capture the disease progression in STGD1 for most patients. The multicenter Natural History of the Progression of Atrophy Secondary to Stargardt Disease (ProgStar) studies were therefore designed to characterize the natural history of progression and to establish sensitive and meaningful end points for interventional trials. From the perspective of the US Food and Drug Administration, the rate or extent of the anatomical progression of atrophy, similar to geographical atrophy secondary to age-related macular degeneration, can be such a possible end point beyond visual acuity. Fundus autofluorescence (FAF) imaging is well established in clinical practice as a convenient, fast, and noninvasive imaging procedure, and has been shown to identify and delineate areas of atrophy in STGD1. Furthermore, FAF has been demonstrated to allow tracking disease progression successfully in smaller cohorts of patients with STGD1 at single centers.
The purpose of this study is to report the growth rates of atrophic lesions as determined by FAF as the primary outcome measure in the retrospective ProgStar study.
Methods
The design, inclusion and exclusion criteria, and patients of the retrospective ProgStar study were described in detail previously; the ProgStar studies were designed between August 8, 2012, and March 14, 2013. Briefly, patients with at least 2 pathogenic mutations in the ABCA4 gene (or 1 mutation, but with the clinical phenotype of flecks at the level of the retinal pigment epithelium typical for STGD1) were eligible. A total of 251 patients aged 6 years or older at baseline, harboring disease-causing variants in ABCA4, enrolled in the study from 9 centers between August 2, 2013, and December 12, 2014. Minimum age was 6 years at the most recent visit. The minimum total observation period was 24 months, and the maximum period between single visits was 60 months. Inclusion criteria required the presence of a well-defined atrophic lesion at the most recent visit of at least 300 µm in diameter; the area of all lesions together must total 12 mm2 or less and must represent no more than 5 disc areas in at least 1 eye. The presence of an atrophic lesion at the first retrospective visit was not required. The study was conducted according to the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Good Clinical Practice Guidelines, the applicable regulatory requirements, the current Declaration of Helsinki and in compliance with the Health Insurance Portability and Accountability Act. Ethics committee approval was granted by the Western Institutional Review Board, the local institutional review boards (Cleveland Clinic Institutional Review Board; the University of Texas Southwestern Institutional Review Board; the Greater Baltimore Medical Center Institutional Review Board; the Johns Hopkins University School of Medicine Office of Human Subjects Research Institutional Review Boards; University of Pennsylvania, Office of Regulatory Affairs, Institutional Review Board; University of Utah Institutional Review Board; National Health Service Health Research Authority, London–Queen Square Research Ethics Committee; French National Commission of Data Processing and Liberties; and the Ethics Committee of the Medical Faculty of the Eberhard-Karls University and of the University Hospitals, Tübingen), and the Human Research Protection Office of the US Army Medical Research and Materiel Command prior to enrollment of the first patient. Most local institutional review boards exempted the centers from an informed consent process; if an exemption was not granted, written informed consent was obtained from patients prior to enrollment. The study has been registered at https://www.clinicaltrials.gov (NCT01977846).
Fundus autofluorescence images were eligible if they were obtained with a Heidelberg Engineering device such as the Heidelberg Retina Angiograph 2 or Heidelberg Spectralis (excitation light, 488 nm; barrier filter, 500 nm). Fundus autofluorescence images were graded at a central reading center (eAppendix in the Supplement). The field of view was 30° × 30° in 367 of 386 eyes (95.1%), 35° × 35° and 20° × 20° in 2 eyes each (0.5%), 34° × 30° in 1 eye (0.3%), and 55° in diameter in 10 eyes (2.6%). The magnification could not be graded in 4 eyes. Fundus autofluorescence image grading protocols were developed within the ProgStar consortium based on preliminary discussions and previous publications on FAF imaging in STGD1 and were validated in a preliminary pilot projects.
Qualitative Grading Parameters
Qualitative grading parameters included the absence or presence of flecks both within and outside the arcades, the presence of increased FAF at the edge of a lesion of decreased FAF, and FAF background uniformity based on a previously published grading scheme: a homogeneous background signal was defined as an even distribution of background autofluorescence, while a heterogeneous background signal was defined as widespread small foci of increased or reduced autofluorescence.
Quantitative Grading Parameters
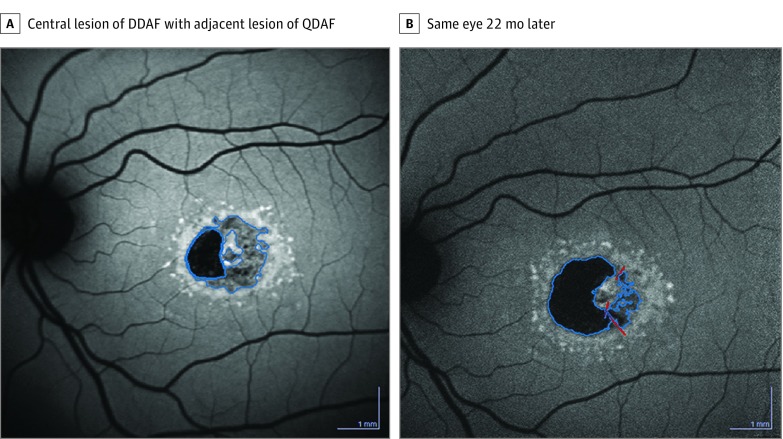
Different types of decreased areas of autofluorescence were defined based on the qualitative measures of darkness (Figure 1): definitely decreased autofluorescence (DDAF) and questionably decreased autofluorescence (QDAF). The optic nerve head (or, if not present in the image, the vessels) was the reference for 100% blackness on the gray scale. The decreased autofluorescence pattern had to appear darker than background gray level and the lesion diameter had to be 125 µm or more. Definitely decreased autofluorescence was more than 90% black in reference to the optic nerve head, and questionably decreased autofluorescence was 50% to 90% black.
Figure 1. Examples of Progression of Lesion of Definitely Decreased Autofluorescence (DDAF) and Questionably Decreased Autofluorescence (QDAF).
A, Central lesion of DDAF (0.82 mm2) with a temporal adjacent lesion of QDAF (1.53 mm2). B, Same eye after 22 months: the lesion of DDAF is enlarged (2.09 mm2), whereas the lesion of QDAF (0.45 mm2) is decreased, indicating the transformation from QDAF into DDAF; the total area enlarged from 2.35 to 2.54 mm2.
The area of the respective lesions was semiautomatically evaluated using the RegionFinder module of the Heidelberg Eye Explorer (Heidelberg Engineering) with grading conventions previously described and provided in the eAppendix in the Supplement.
Statistical Analysis
The yearly increase in the size of DDAF or total lesion area (sum of DDAF and QDAF) was assessed using eyes in which the specific lesion type was present in at least 2 visits. Exploratory inspection of lesion growth over time showed a strong dependency on lesion size at the first visit. For both DDAF and total lesion area, stratified analysis by lesion size at first visit was performed using the 33rd and 66th percentiles of the distribution of lesion size to create 3 categories.
The yearly progression rate was similar in the first 2 categories; hence, the analyses presented here use only a single cutoff: for the DDAF baseline area of 1.92 mm2 or less (66th percentile) and greater than 1.92 mm2 and for the total lesion size (DDAF and QDAF) baseline total area of 2.50 mm2 or less (66th percentile) and baseline total area greater than 2.50 mm2 (Figure 2). Linear mixed models stratified by lesion size, with time as the independent variable, were used to estimate the yearly change for each outcome. The models include random effects for the intercept, the slope for time, and the eye. Parallel analyses using the square root of the area were also performed but did not eliminate the dependency of progression on initial lesion size. Statistical analyses were conducted using SAS, version 9.3 (SAS Institute Inc) and R project for statistical computing, version 2.15.1 (Institute for Statistics and Mathematics).
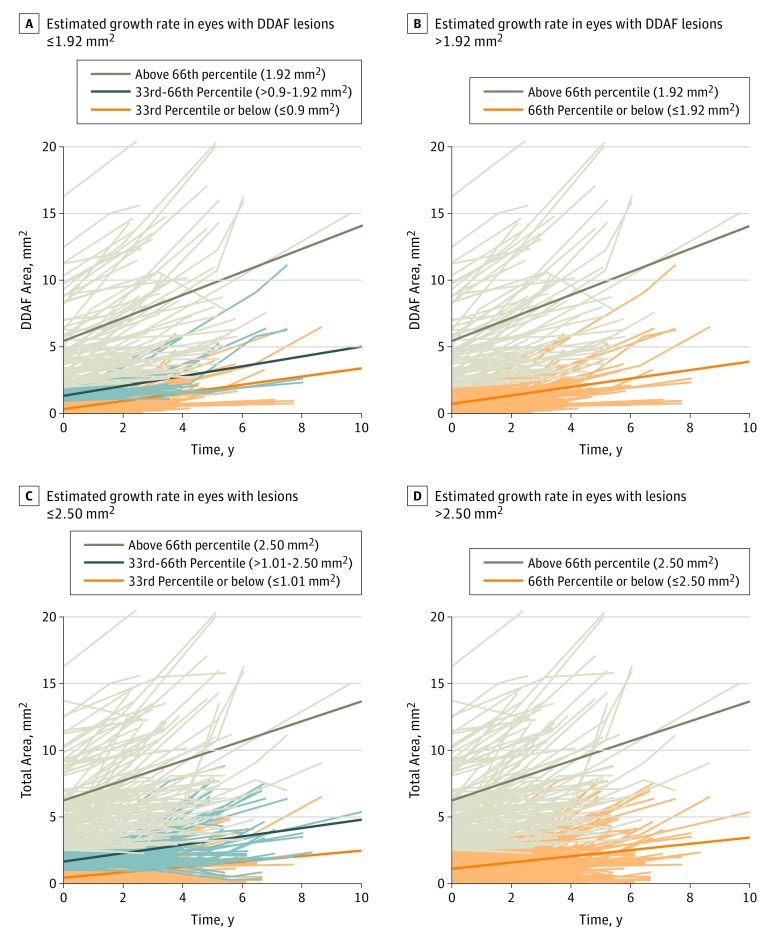
Figure 2. Estimated Yearly Progression of Areas Based on Lesion Size at the First Included Visit.
A, Estimated growth rate in eyes with definitely decreased autofluorescence (DDAF) lesion sizes ≤1.92 mm2 (66th percentile) was 0.32 mm2/y (95% CI, 0.24-0.39 mm2/y) (brown line). B, Estimated growth rate in eyes with DDAF lesion sizes >1.92 mm2 was estimated as 0.86 mm2/y (95% CI, 0.67-1.06 mm2/y) (orange line). C, For total area of decreased areas of autofluorescence, eyes with lesion sizes ≤2.50 mm2 (66th percentile) showed an estimated growth rate of 0.26 mm2/y (95% CI, 0.21-0.32 mm2/y) (brown line). D, For total area of decreased areas of autofluorescence, eyes with lesions sizes >2.50 mm2 showed an estimated growth rate of 0.74 mm2/y (95% CI, 0.57-0.91 mm2/y) (orange line). Diagonal lines in parts A-D indicate estimated growth rates.
Results
A total of 251 patients were enrolled at 9 centers in the United States and Europe between August 2, 2013, and December 12, 2014; FAF images for at least 2 visits were available for 217 patients, of which 173 patients (79.7%) were enrolled with both eyes and 44 (20.3%) with 1 eye, for a total of 390 study eyes. Images of 4 eyes of 2 patients had to be excluded during the grading process owing to low quality. Images of 386 study eyes of 215 patients with a total of 1075 eye visits (mean, 2.8 visits per eye) had atrophic lesions present on at least 2 visits. Of these patients, 146 (67.9%) were white and 126 (58.6%) were female, the mean (SD) age was 29.9 (14.7) years and the mean (SD) age of onset of symptoms was 21.9 (13.3) years, and lesions were graded for 2 study visits (156 eyes), 3 study visits (173 eyes), or 4 study visits (57 eyes) (eTable 1 in the Supplement). The mean (SD) follow-up time was 3.9 (1.6) years (range, 0.44-12.1 years).
eTable 1 in the Supplement summarizes patient characteristics. For DDAF analyses, 90 of 133 patients (67.7%) contributed both eyes to analyses, and for any lesion, 170 of 215 patients (79.1%) contributed both eyes (eTable 1 in the Supplement). The presence or absence of the qualitative grading parameters of flecks, increased FAF at the lesion edge of DDAF lesions, and background uniformity are presented in Table 1.
Table 1. Baseline Characteristics and DDAF and Total Area of the Lesions (DDAF + QDAF).
| Characteristic | First Visit With DDAF Present (224 Eyes) | DDAF + QDAF (386 Eyes) | ||||
|---|---|---|---|---|---|---|
| No. | DDAF Area, Mean (SD), mm2 |
P Value | No. | Total Area, Mean (SD), mm2 |
P Value | |
| Demographics | ||||||
| Age at first visit, y | ||||||
| <18 | 38 | 1.8 (1.8) | .02a | 93 | 2.2 (2.6) | .009a |
| 18-29 | 67 | 1.6 (1.6) | 123 | 2.0 (2.1) | ||
| ≥30 | 119 | 2.6 (3.3) | 170 | 3.1 (3.1) | ||
| Age at onset of symptoms, y | ||||||
| <18 | 89 | 2.3 (2.8) | .72b | 162 | 2.6 (2.8) | .44b |
| 18-29 | 50 | 1.8(2.2) | 85 | 2.4 (2.2) | ||
| ≥30 | 51 | 2.7 (3.4) | 81 | 3.0 (3.4) | ||
| Unknown | 34 | 1.7 (1.9) | 58 | 2.3 (2.4) | ||
| Duration, y | ||||||
| <10 | 103 | 1.6 (2.2) | .004 | 230 | 2.1 (2.6) | <.001 |
| ≥10 | 87 | 3.0 (3.2) | 98 | 3.1 (2.9) | ||
| Unknown | 34 | 1.7 (1.9) | 58 | 2.3 (2.4) | ||
| Sex | ||||||
| Male | 101 | 2.6 (2.9) | .12 | 160 | 2.8 (2.7) | .29 |
| Female | 123 | 1.9 (2.4) | 226 | 2.4 (2.8) | ||
| Qualitative FAF Grading Parameters | ||||||
| Flecks | ||||||
| Absent | 6 | 3.2 (1.6) | .24 | 57 | 1.2 (1.1) | <.001 |
| Present | 214 | 2.2 (2.7) | 329 | 2.8 (2.9) | ||
| Questionable | 4 | 1.1 (0.9) | 0 | NA | ||
| Flecks beyond the arcades | ||||||
| Absent | 72 | 1.4 (1.5) | .001 | 195 | 1.5 (1.7) | <.001 |
| Present | 151 | 2.6 (3.0) | 186 | 3.7 (3.2) | ||
| Questionable | 1 | 0.6c | 0 | NA | ||
| Cannot grade | 0 | NA | 5 | 0.9 (1.7) | ||
| Increased FAF at DDAF lesion edge | ||||||
| Absent | 122 | 2.2 (2.8) | .75 | 189 | 2.8 (2.8) | .46 |
| Present | 80 | 1.9 (2.3) | 157 | 2.3 (2.7) | ||
| Questionable | 22 | 2.9 (3.4) | 39 | 2.7 (2.7) | ||
| Background | ||||||
| Homogeneous | 121 | 1.5 (2.1) | .001 | 259 | 1.9 (2.0) | <.001 |
| Heterogeneous | 103 | 2.9 (3.1) | 127 | 4.0 (3.4) | ||
| No. of lesions of DDAF | ||||||
| Unifocal | 161 | 2.0 (2.5) | .25 | 131 | 3.9 (3.1) | <.001 |
| Multifocal | 63 | 2.6 (2.9) | 54 | 3.9 (3.7) | ||
| NA | 0 | NA | 201 | 1.4 (1.4) | ||
| Background signal and flecks beyond the arcades | ||||||
| Homogeneous with no flecks beyond the arcades | 72 | 1.4 (1.5) | <.001b | 195 | 1.5 (1.7) | <.001b |
| Homogeneous with flecks beyond the arcades | 48 | 1.8 (2.7) | 60 | 3.0 (2.6) | ||
| Heterogeneousd | 103 | 2.9 (3.1) | 127 | 4.0 (3.4) | ||
| Cannot grade flecks | 1 | 0.6c | 4 | 1.0 (1.0) | ||
| Overall | 224 | 2.2 (2.7) | NA | 386 | 2.6 (2.8) | NA |
Abbreviations: DDAF, definitely decreased autofluorescence; FAF, fundus autofluorescence; NA, not applicable; QDAF, questionably decreased autofluorescence.
Using age at first visit.
Test for trend and categories not included in the statistical testing, accounting for within-eye correlation.
No SD provided because the value is from only 1 eye.
All images graded as heterogeneous background were graded as having flecks beyond the arcades.
Characteristics of Eyes With DDAF
A total of 224 of the 386 eyes (58.0%) showed at least 1 lesion of DDAF that could be tracked over at least 2 study visits during the follow-up period, either alone or in combination with other lesions (Figure 1). Overall mean (SD) lesion size at the first visit included for analysis was 2.2 (2.7) mm2. Patients younger than 18 years at the first visit had larger lesions (mean [SD], 1.8 [1.8] mm2) than patients between 18 and 29 years (mean [SD], 1.6 [1.6] mm2), but smaller than patients 30 years or older (mean [SD], 2.6 [3.3] mm2) (Table 1). Patients with disease duration of 10 years or more had larger lesions than patients with a disease duration of less than 10 years (mean [SD], 3.0 [3.2] vs 1.6 [2.2] mm2; P = .004). All except 6 of these 224 eyes with DDAF showed the presence of flecks. In 151 eyes (67.4%), flecks were present beyond the arcades; these had larger lesions than did eyes without flecks beyond the arcades (mean [SD], 2.6 [3.0] vs 1.4 [1.5] mm2; P = .001). A total of 121 of the eyes (54.0%) with DDAF lesions presented with a homogeneous background and 103 (46.0%) with a heterogeneous background. Eyes with a heterogeneous background had larger lesions (mean [SD], 2.9 [3.1] vs 1.5 [2.1] mm2; P = .001). Also, eyes with a homogeneous background without flecks beyond the arcades had smaller lesions than did eyes with a homogeneous background with flecks beyond the arcades (mean [SD], 1.4 [1.5] vs 1.8 [2.7] mm2; P < .001).
Estimated Growth Rates of Lesions of DDAF
Overall, mean progression of DDAF was 0.51 mm2/y (95% CI, 0.42-0.61 mm2/y). However, the growth rates were strongly dependent on initial lesion size. Thus, it is important to take into consideration initial lesion size when reporting progression rates in terms of mm2 per year. As described in the Methods, we analyzed the growth rate within lesion size, using the 66th percentile of the distribution of lesion size at baseline (1.92 mm2) to divide the cohort into subgroups. The estimated growth rates in eyes with lesion sizes of 1.92 mm2 or less was 0.32 mm2/y (95% CI, 0.24-0.39 mm2/y). The estimated growth rate in eyes with lesions larger than 1.92 mm2 was estimated as 0.86 mm2/y (95% CI, 0.67-1.06 mm2/y) (Figure 2A and B; eTable 2 in the Supplement).
After stratifying by lesion size, neither duration of disease (<10 vs ≥10 years) nor background heterogeneity (homogeneous vs heterogeneous background) was associated with differences in progression (Table 2). Within lesion size categories, growth rates were age dependent, with lower rates observed in the younger age group. However, stratification for the number of foci of DDAF revealed differences, with lower growth rates for unifocal lesions regardless of lesion size at baseline.
Table 2. Estimates of Yearly Growth Rates for DDAF and the Total Area (DDAF + QDAF) by Baseline Lesion Size and Baseline Characteristics.
| Characteristic | Estimated Progression Rate (mm2/y [Slope of Time]) (95% CI) |
P Value | Interaction P Value |
|---|---|---|---|
| DDAF | |||
| Age at first visit with lesion ≤1.92 mm2 | |||
| <30 y | 0.25 (0.14-0.36) | <.001 | .002 |
| ≥30 y | 0.39 (0.27-0.50) | <.001 | |
| Age at first visit with lesion >1.92 mm2 | |||
| <30 y | 0.63 (0.33-0.93) | <.001 | .03 |
| ≥30 y | 1.04 (0.77-1.30) | <.001 | |
| Duration with lesion ≤1.92 mm2 | |||
| <10 y | 0.30 (0.18-0.42) | <.001 | .24 |
| ≥10 y | 0.37 (0.24-0.49) | <.001 | |
| Duration with lesion >1.92 mm2 | |||
| <10 y | 0.96 (0.53-1.39) | <.001 | .45 |
| ≥10 y | 0.80 (0.57-1.03) | <.001 | |
| Background with lesion ≤1.92 mm2 | |||
| Homogeneous | 0.34 (0.24-0.43) | <.001 | .72 |
| Heterogeneous | 0.32 (0.21-0.44) | <.001 | |
| Background with lesion >1.92 mm2 | |||
| Homogeneous | 1.00 (0.44-1.55) | <.001 | .06 |
| Heterogeneous | 1.03 (0.67-1.38) | <.001 | |
| No. of lesions ≤1.92 mm2 | |||
| Unifocal | 0.26 (0.19-0.33) | <.001 | <.001 |
| Multifocal | 0.52 (0.35-0.68) | <.001 | |
| No. of lesions >1.92 mm2 | |||
| Unifocal | 0.72 (0.51-0.94) | <.001 | .02 |
| Multifocal | 1.16 (0.83-1.49) | <.001 | |
| Total Area | |||
| Age at first visit with lesion ≤2.50 mm2 | |||
| <30 y | 0.12 (0.09-0.16) | <.001 | .003 |
| ≥30 y | 0.24 (0.15-0.33) | <.001 | |
| Age at first visit with lesion >2.50 mm2 | |||
| <30 y | 0.68 (0.42-0.95) | <.001 | .99 |
| ≥30 y | 0.68 (0.48 -0.89) | <.001 | |
| Duration with lesion ≤2.50mm2 | |||
| <10 y | 0.27 (0.21-0.33) | <.001 | .56 |
| ≥10 y | 0.32 (0.21-0.43) | <.001 | |
| Duration with lesion >2.50 mm2 | |||
| <10 y | 0.75 (0.46-1.04) | <.001 | .96 |
| ≥10 y | 0.77 (0.51-1.02) | <.001 | |
| Background with lesion ≤2.50 mm2 | |||
| Homogeneous | 0.26 (0.19-0.32) | <.001 | .05 |
| Heterogeneous | 0.32 (0.24-0.41) | <.001 | |
| Background with lesion >2.50 mm2 | |||
| Homogeneous | 0.70 (0.35-1.05) | <.001 | .73 |
| Heterogeneous | 0.77 (0.45-1.09) | <.001 | |
Abbreviation: DDAF, definitely decreased autofluorescence; QDAF, questionably decreased autofluorescence.
Characteristics of Eyes With Any Lesion (DDAF and QDAF)
Overall mean (SD) lesion size in 386 eyes of decreased areas of autofluorescence (sum of DDAF and QDAF) at the first visit included for analysis was 2.6 (2.8) mm2. Mean (SD) lesion size was larger in patients 30 years of age or older compared with patients younger than 18 years (3.1 [3.1] vs 2.2 [2.6] mm2; P = .009) (Table 1). Patients with duration of disease of 10 years or more had larger lesions than those with a duration of disease less than 10 years (mean [SD], 3.1 [2.9] vs 2.1 [2.6] mm2; P < .001). Most eyes had flecks present (329 of 386 eyes [85.2%]); in 186 eyes (48.2%), flecks were present outside the arcades. Eyes with flecks had larger lesions than eyes without flecks (mean [SD], 2.8 [2.9] vs 1.2 [1.1] mm2; P < .001), and eyes with flecks outside the arcades had larger lesions than eyes without flecks outside the lesions (mean [SD], 3.7 [3.2] vs 1.5 [1.7] mm2; P < .001). Most eyes (259 [67.1%]) had a homogeneous background, which was associated with smaller mean (SD) total area of decreased autofluorescence (1.9 [2.0] vs 4.0 [3.4] mm2; P < .001), although, as noted for DDAF, because all heterogeneous eyes had flecks, it was not possible to determine which of the characteristics was more associated with larger area.
Estimated Growth Rates of Lesions of Total Area of Decreased Autofluorescence Over Time
Mean progression of total decreased FAF was 0.35 mm2/y (95% CI, 0.28-0.43 mm2/y). As described in the Methods, the following 2 subgroups were defined using the 66th percentile of the distribution of lesion size at first visit: eyes with lesion areas of 2.50 mm2 or less and eyes with lesion areas greater than 2.50 mm2 (Figure 2C and D). There was a difference in estimated growth rate of lesions: eyes with smaller lesions had an estimated growth rate of 0.26 mm2/y (95% CI, 0.21-0.32 mm2/y), whereas eyes with larger lesions had an estimated growth rate of 0.74 mm2/y (95% CI, 0.57-0.91 mm2/y; P < .001 for the interaction of lesion size and growth rate; eTable 2 in the Supplement).
There was no difference in growth rate by duration of symptoms or background heterogeneity. However, in eyes with growth of lesion areas of 2.50 mm2 or less, rates were age dependent, with higher rates in patients 30 years of age or older (Table 2).
Discussion
Other natural history studies and interventional trials have reported progression rates that suggest wide variation in starting sizes of lesions. With a mean (SD) follow up of 3.9 (1.6) years, we found a yearly progression for both DDAF and total area (DDAF and QDAF). The growth of these lesions depended on the baseline lesion, with greater rates seen in lesions with a larger initial size. Thus, we think it is not accurate to report an overall growth rate, as the rate will depend on the starting size, which is an important point for clinicians in determining the rate of progression for their patients.
The choice of model to characterize the growth rates was challenging. We tried different models, including linear, log linear, and square root transformation. However, to estimate yearly progression rates, linear mixed models stratified by initial lesion size showed the best fit, and characterized the rate with reasonable complexity. However, we provide addition results using the square root transformation of the lesion area as the outcome (eTable 3 in the Supplement), as previously done in age-related macular degeneration.
In ProgStar, we defined another type of reduced, although not absent, autofluorescence as QDAF: previous studies either defined 2 types called absent FAF and areas of abnormal FAF, or profound hypofluorescence vs less profound hypoautofluorescence. In these studies, a semiquantitative approach was used by analyzing the FAF images using levels of gray. One limitation of our study is that the grading did not permit use of an absolute quantitative gray scale. This limitation may have contributed to the variability in the grading. One challenge when grading the lesions of QDAF is the macular pigment that, when present, could be misinterpreted as reduced FAF. To counteract this confounder, especially in foveal-sparing cases, the graders used the constraint tool to exclude the foveal center from measurements, and could consult infrared images obtained at the same visit (if available). If deemed necessary, graders could consult images from a previous visit, although this was discouraged until completion of an independent assessment of the current images. However, it may have biased the grading process to an unknown extent.
Lesions of QDAF may be of interest for future therapeutic intervention because tissue may still be amenable to rescue. In exploratory analyses, the areas of QDAF showed 3 different patterns in respect to lesion size: increase, stable, or decrease. The observation that areas of decreased autofluorescence tend to be unstable and can vary with respect to the temporal sequence of autofluorescence changes has been previously described. Questionably decreased autofluorescence may be considered a transition state between healthy retina and later stages of the disease process (ie, DDAF); hence, we report the total area of lesions (sum of DDAF and QDAF). Future analysis and correlation of FAF with findings in spectral domain optical coherence tomography as part of ProgStar will shed further light on the temporal sequence.
Previous reports have found a higher rate of lesion growth for all eyes with a heterogeneous background compared with a homogeneous background. However, these studies did not adjust for mean lesion size at baseline, and while we found an association of heterogeneous background with mean area at baseline, we did not find an association with progression of lesions. If eyes with a heterogeneous background represent a more advanced stage of the disease with larger lesions, then by adjusting for lesion size, we have adjusted out the effect of the background heterogeneity. Further comparisons of our results to the ones from previous studies are limited owing to differences including image acquisition and analysis, missing confirmation of disease-causing mutations, and categorization of phenotypes and statistical methods applied. However, similar characteristics are observed with geographic atrophy lesions in age-related macular degeneration, such as faster rates of progression in multifocal lesions, even though the mechanisms in the 2 diseases are incompletely understood and may be very different, as illustrated by different growth rates when comparing late-onset STGD1 with age-related macular degeneration.
There is evidence from several studies that the FAF signal originates from lipofuscin in retinal pigment epithelium cells and that excessive accumulation of lipofuscin causes an increased FAF; higher concentrations of lipofuscin are also thought to have harmful effects on normal cell function. We therefore investigated the possible influence on rates of progression. There was no association of area of lesion with the presence of increased FAF at the lesion edge for DDAF. We did not quantify the extent of increased FAF, which may explain some of this difference. Also, Smith et al reported that focally increased autofluorescence deposits with FAF levels elevated above the initial macular background were less likely in the short term (2 years) to transform to geographic atrophy than were any random areas, suggesting additional mechanisms beyond direct lipofuscin toxicity. We did find that flecks and a heterogeneous background were associated with lesion area for DDAF and total area, but were not associated with progression. Our data suggest that the flecks and heterogeneous background develop with, rather than precede, the growth of the lesion. Our prospective study will be able to confirm or refute this hypothesis.
Limitations and Strengths
Our study has inherent limitations based on the retrospective design, including absence of standardization of image acquisition both across and within participating sites, and data collected on the basis of medical record review. However, a strength is the longer observational period in this large cohort, as is the multicenter setting that provided data from a broad range of patients. Another strength is the use of standardized grading procedures by certified, experienced reading center graders.
Conclusions
We have shown that tracking the size of lesions of DDAF can be a useful tool to monitor disease progression in STGD1, while for earlier stages of STGD1 exhibiting lesions of QDAF only, structural analysis derived from spectral domain optical coherence tomography might be more reliable. Furthermore, growth rates depended on initial lesion size, and this should be accounted for in upcoming interventional trials that aim to slow down disease progression.
eAppendix. Methods
eTable 1. Demographic Characteristics at First Included Visit ( = Baseline) of Patients With FAF Images of Sufficient Quality and DDAF and/or Any Lesion in at Least 2 Study Visits
eTable 2. Estimates of Yearly Growth Rates DDAF and the Total Area (DDAF+QDAF) by Baseline Lesion Size
eTable 3. Estimated Progression Rate (Slope of Time) Using the Square Root Transformation for Areas of Definitely Decreased Autofluorescence (DDAF) and Total Area of Decreased Autofluorescence
References
- 1.Allikmets R, Singh N, Sun H, et al. . A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15(3):236-246. [DOI] [PubMed] [Google Scholar]
- 2.Tanna P, Strauss RW, Fujinami K, Michaelides M. Stargardt disease: clinical features, molecular genetics, animal models and therapeutic options. Br J Ophthalmol. 2017;101(1):25-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scholl HP, Strauss RW, Singh MS, et al. . Emerging therapies for inherited retinal degeneration. Sci Transl Med. 2016;8(368):368rv6. [DOI] [PubMed] [Google Scholar]
- 4.Kong X, Strauss RW, Michaelides M, et al. ; ProgStar Study Group . Visual acuity loss and associated risk factors in the retrospective Progression of Stargardt Disease Study (ProgStar report no. 2). Ophthalmology. 2016;123(9):1887-1897. [DOI] [PubMed] [Google Scholar]
- 5.Strauss RW, Ho A, Muñoz B, et al. ; Progression of Stargardt Disease Study Group . The natural history of the progression of atrophy secondary to Stargardt disease (ProgStar) studies: design and baseline characteristics: ProgStar report no. 1. Ophthalmology. 2016;123(4):817-828. [DOI] [PubMed] [Google Scholar]
- 6.Csaky KG, Richman EA, Ferris FL III. Report from the NEI/FDA Ophthalmic Clinical Trial Design and Endpoints Symposium. Invest Ophthalmol Vis Sci. 2008;49(2):479-489. [DOI] [PubMed] [Google Scholar]
- 7.Fishman GA, Jacobson SG, Alexander KR, et al. . Outcome measures and their application in clinical trials for retinal degenerative diseases: outline, review, and perspective. Retina. 2005;25(6):772-777. [DOI] [PubMed] [Google Scholar]
- 8.Sparrow JR, Gregory-Roberts E, Yamamoto K, et al. . The bisretinoids of retinal pigment epithelium. Prog Retin Eye Res. 2012;31(2):121-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cukras CA, Wong WT, Caruso R, Cunningham D, Zein W, Sieving PA. Centrifugal expansion of fundus autofluorescence patterns in Stargardt disease over time. Arch Ophthalmol. 2012;130(2):171-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujinami K, Lois N, Mukherjee R, et al. . A longitudinal study of Stargardt disease: quantitative assessment of fundus autofluorescence, progression, and genotype correlations. Invest Ophthalmol Vis Sci. 2013;54(13):8181-8190. [DOI] [PubMed] [Google Scholar]
- 11.Chen B, Tosha C, Gorin MB, Nusinowitz S. Analysis of autofluorescent retinal images and measurement of atrophic lesion growth in Stargardt disease. Exp Eye Res. 2010;91(2):143-152. [DOI] [PubMed] [Google Scholar]
- 12.McBain VA, Townend J, Lois N. Progression of retinal pigment epithelial atrophy in Stargardt disease. Am J Ophthalmol. 2012;154(1):146-154. [DOI] [PubMed] [Google Scholar]
- 13.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH harmonized tripartite guideline: guideline for good clinical practice. J Postgrad Med. 2001;47(1):45-50. [PubMed] [Google Scholar]
- 14.Malik AY, Foster C. The revised Declaration of Helsinki: cosmetic or real change? J R Soc Med. 2016;109(5):184-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strauss RW, Muñoz B, Jha A, et al. . Comparison of short-wavelength reduced-illuminance and conventional autofluorescence imaging in Stargardt macular dystrophy. Am J Ophthalmol. 2016;168:269-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuehlewein L, Hariri AH, Ho A, et al. . Comparison of manual and semiautomated fundus autofluorescence analysis of macular atrophy in Stargardt disease phenotype. Retina. 2016;36(6):1216-1221. [DOI] [PubMed] [Google Scholar]
- 17.Schmitz-Valckenberg S, Brinkmann CK, Alten F, et al. . Semiautomated image processing method for identification and quantification of geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52(10):7640-7646. [DOI] [PubMed] [Google Scholar]
- 18.Strauss RW, Muñoz B, Ho A, et al. ; ProgStar Study Group . Incidence of atrophic lesions in Stargardt disease in the Progression of Atrophy Secondary to Stargardt Disease (ProgStar) Study: report no. 5. JAMA Ophthalmol. 2017;135(7):687-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sparrow JR, Marsiglia M, Allikmets R, et al. . Flecks in recessive Stargardt disease: short-wavelength autofluorescence, near-infrared autofluorescence, and optical coherence tomography. Invest Ophthalmol Vis Sci. 2015;56(8):5029-5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piri N, Nesmith BL, Schaal S. Choroidal hyperreflective foci in Stargardt disease shown by spectral-domain optical coherence tomography imaging: correlation with disease severity. JAMA Ophthalmol. 2015;133(4):398-405. [DOI] [PubMed] [Google Scholar]
- 21.Greenstein VC, Schuman AD, Lee W, et al. . Near-infrared autofluorescence: its relationship to short-wavelength autofluorescence and optical coherence tomography in recessive Stargardt disease. Invest Ophthalmol Vis Sci. 2015;56(5):3226-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parker MA, Choi D, Erker LR, et al. . Test-retest variability of functional and structural parameters in patients with Stargardt disease participating in the SAR422459 Gene Therapy Trial. Transl Vis Sci Technol. 2016;5(5):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feuer WJ, Yehoshua Z, Gregori G, et al. . Square root transformation of geographic atrophy area measurements to eliminate dependence of growth rates on baseline lesion measurements: a reanalysis of age-related eye disease study report no. 26. JAMA Ophthalmol. 2013;131(1):110-111. [DOI] [PubMed] [Google Scholar]
- 24.Smith RT, Gomes NL, Barile G, Busuioc M, Lee N, Laine A. Lipofuscin and autofluorescence metrics in progressive STGD. Invest Ophthalmol Vis Sci. 2009;50(8):3907-3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomes NL, Greenstein VC, Carlson JN, et al. . A comparison of fundus autofluorescence and retinal structure in patients with Stargardt disease. Invest Ophthalmol Vis Sci. 2009;50(8):3953-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duncker T, Marsiglia M, Lee W, et al. . Correlations among near-infrared and short-wavelength autofluorescence and spectral-domain optical coherence tomography in recessive Stargardt disease. Invest Ophthalmol Vis Sci. 2014;55(12):8134-8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teussink MM, Lee MD, Smith RT, et al. . The effect of light deprivation in patients with Stargardt disease. Am J Ophthalmol. 2015;159(5):964-72.e2. [DOI] [PubMed] [Google Scholar]
- 28.Schmitz-Valckenberg S, Sahel JA, Danis R, et al. . Natural history of geographic atrophy progression secondary to age-related macular degeneration (Geographic Atrophy Progression Study). Ophthalmology. 2016;123(2):361-368. [DOI] [PubMed] [Google Scholar]
- 29.Lindner M, Lambertus S, Mauschitz MM, et al. ; Foveal Sparing Atrophy Study Team (FAST) . Differential disease progression in atrophic age-related macular degeneration and late-onset Stargardt disease. Invest Ophthalmol Vis Sci. 2017;58(2):1001-1007. [DOI] [PubMed] [Google Scholar]
- 30.Sparrow JR, Boulton M. RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res. 2005;80(5):595-606. [DOI] [PubMed] [Google Scholar]
- 31.Schmitz-Valckenberg S, Bindewald-Wittich A, Dolar-Szczasny J, et al. ; Fundus Autofluorescence in Age-Related Macular Degeneration Study Group . Correlation between the area of increased autofluorescence surrounding geographic atrophy and disease progression in patients with AMD. Invest Ophthalmol Vis Sci. 2006;47(6):2648-2654. [DOI] [PubMed] [Google Scholar]
- 32.Strauss RW, Muñoz B, Wolfson Y, et al. . Assessment of estimated retinal atrophy progression in Stargardt macular dystrophy using spectral-domain optical coherence tomography. Br J Ophthalmol. 2016;100(7):956-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Methods
eTable 1. Demographic Characteristics at First Included Visit ( = Baseline) of Patients With FAF Images of Sufficient Quality and DDAF and/or Any Lesion in at Least 2 Study Visits
eTable 2. Estimates of Yearly Growth Rates DDAF and the Total Area (DDAF+QDAF) by Baseline Lesion Size
eTable 3. Estimated Progression Rate (Slope of Time) Using the Square Root Transformation for Areas of Definitely Decreased Autofluorescence (DDAF) and Total Area of Decreased Autofluorescence