Key Points
Question
Is treatment with methylphenidate associated with an increased risk of suicide attempts?
Findings
In this population-based, case series study of 154 patients with a suicide attempt identified from 25 629 patients who were receiving methylphenidate for treatment of attention-deficit/hyperactivity disorder, the risk of suicide attempts was 6.5-fold higher during the 90-day period before methylphenidate was initiated, remained elevated 4-fold during the first 90 days of treatment, and returned to baseline levels during ongoing treatment.
Meaning
The increased risk of suicide attempts preceding the initiation of methylphenidate is not causally related to the drug’s effects.
Abstract
Importance
Patients with attention-deficit/hyperactivity disorder (ADHD) are at an increased risk of attempting suicide. Stimulants, such as methylphenidate hydrochloride, are the most common treatment for ADHD, but the association between their therapeutic use and suicide is unclear.
Objective
To investigate the association between methylphenidate and the risk of suicide attempts.
Design, Setting, and Participants
A population-based, electronic medical records database from the Hong Kong Clinical Data Analysis & Reporting System was used to identify 25 629 individuals aged 6 to 25 years who were treated with methylphenidate between January 1, 2001, and December 31, 2015. Those who had attempted suicide were included in the analysis. A self-controlled case series design was used to control for time-invariant characteristics of the patients.
Main Outcomes and Measures
Relative incidence of suicide attempt during periods when patients were exposed to methylphenidate compared with nonexposed periods.
Results
Among 25 629 patients with methylphenidate prescriptions, 154 had their first recorded suicide attempt within the study period; of these individuals, 111 (72.1%) were male; mean (SD) age at baseline was 7.15 (2.19) years. The overall incidence of suicide attempts during methylphenidate treatment was 9.27 per 10 000 patient-years. An increased risk of suicide attempts was detected during the 90-day period before methylphenidate was initiated, with an incidence rate ratio (IRR) of 6.55 (95% CI, 3.37-12.72). The IRR remained elevated during the first 90 days of treatment (IRR, 3.91; 95% CI, 1.62-9.42) before returning to baseline levels during ongoing treatment (IRR, 1.35; 95% CI, 0.77-2.38). When the risk during the first 90 days of treatment was compared with the 90 days preceding first treatment, the incidence of suicide attempts was not elevated (IRR, 0.78; 95% CI, 0.26-2.35).
Conclusions and Relevance
The incidence of suicide attempts was higher in the period immediately before the start of methylphenidate treatment. The risk remained elevated immediately after the start of methylphenidate treatment and returned to baseline levels during continuation of methylphenidate treatment. The observed higher risk of suicide attempts before treatment may reflect emerging psychiatric symptoms that trigger medical consultations that result in a decision to begin ADHD treatment. Therefore, this study’s results do not support a causal association between methylphenidate treatment and suicide attempts.
This population-based, case series study examines the incidence of suicide attempts during treatment with methylphenidate in patients with attention-deficit/hyperactivity disorder.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a common neurodevelopmental disorder in children, with worldwide prevalence rates in school-aged children estimated at 5% to 7%. In Hong Kong (HK), the reported prevalence of ADHD is estimated to be 6.1% in schoolboys and 3.9% in early adolescents. Attention-deficit/hyperactivity disorder is associated with a diverse range of mental health comorbidities and adverse health, academic, and psychosocial outcomes. Individuals with ADHD are at increased risk of both attempted and completed suicide, even if comorbid psychiatric disorders are clinically treated.
National guidelines recommend psychostimulant medication for the treatment of ADHD. During the past 2 decades, the rate of medication use for ADHD has risen rapidly worldwide. The prevalence of ADHD medication use in HK is approximately 1% in school-aged children and adolescents and has increased 14-fold over the past decade. Methylphenidate hydrochloride, in particular, is commonly used as the first-line therapy. In 2009, the European Medicines Agency conducted a review of the safety of methylphenidate. The review concluded that further research on the association between methylphenidate and psychiatric adverse effects, including suicide risk, was needed.
Although there has been some concern about a potential association between methylphenidate and suicide-related events, few studies have addressed this issue directly. One Swedish register-based study investigated the risk of suicidal behavior among individuals receiving methylphenidate and other stimulants. When comparing treated and nontreated patients with ADHD, this study found that methylphenidate or other stimulant use was associated with a 31% increase in the rate of suicide-related events. However, this analysis did not adjust for potential confounding factors that may account for this association. For instance, a recent study on suicide in school-aged children and adolescents showed that ADHD was overrepresented in suicide victims. Previous studies have also suggested that comorbid disorders and familial/social factors may all play important roles in the association between ADHD and suicide. In a follow-up analysis in the abovementioned register study, when periods with and without treatment were compared within the same patient, no increased risk of suicide-related events during the treatment periods was found. Therefore, at present, there is still uncertainty around the potential effects of methylphenidate on the suicidal behavior of patients. Hence, the aim of the present study was to examine the association between methylphenidate and the risk of suicide attempts.
Methods
Data Source
This study used data from the Clinical Data Analysis & Reporting System (CDARS), an electronic health record database developed by the HK Hospital Authority, a statutory body that manages all public hospitals and their ambulatory clinics in HK. The service is available to all HK residents (>7.3 million) and covers approximately 80% of all hospital admissions in HK. Data from CDARS have been validated and used for various investigations of medication safety. Patient-specific data in CDARS include diagnosis, prescription, information on hospital admissions and discharges, payment method, and prescription and dispensing information. CDARS contains inpatient, outpatient, and emergency department admissions records, anonymized to protect patient confidentiality. The study protocol was approved by the institutional review board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster.
Self-controlled Case Series Design
We used the self-controlled case series (SCCS) study design to investigate the association between methylphenidate and suicide attempts. In this design, used previously to investigate the effects of methylphenidate on trauma and psychosis risk, patients serve as their own control. The SCCS design relies on within-person comparisons in a population of individuals who have experienced both the outcome and exposure of interest. Incidence rate ratios (IRRs) are derived by comparing the rate of events during periods of medication exposure with the rate during all other observed time periods (ie, without medication). A major advantage of this design over the classic design is that it controls for potential effects of measured and unmeasured time-invariant confounders that vary between individuals (ie, genetic factors, disease severity, and socioeconomic factors). Furthermore, we adjusted for time-varying factors, such as age and season, which are known to affect methylphenidate treatment prescribing. In addition to the standard SCCS analysis, the nonparametric SCCS approach was applied to investigate risk changes during the observation period.
Case Identification
Individuals aged 6 to 25 years who received at least 1 prescription for methylphenidate and who had made at least 1 suicide attempt during the study period (January 1, 2001, to December 31, 2015) were identified in CDARS. The suicide attempt codes were identified through the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic codes E950 to E959. The statistical modeling of the SCCS analysis requires incident cases to fulfill the model assumption; therefore, patients who had made a suicide attempt before the study period were excluded. Only methylphenidate and atomoxetine are licensed for the treatment of ADHD in HK; therefore, the observation periods were censored by atomoxetine treatment to avoid coprescribing situations that could affect comparisons.
We commenced follow-up at age 6 years, as methylphenidate is not recommended for younger children. There has been an increasing trend of methylphenidate use in college-aged young adults up to age 25 years. Observation periods began on January 1, 2001, or the sixth birthday of the patient (whichever was later) and ended on December 31, 2015, the 26th birthday of the patient, date of receiving atomoxetine treatment, or date of registered death (whichever was earlier). Because the aim of this study was to investigate the association between methylphenidate and suicide attempts, all methylphenidate users, regardless of the presence of a formal diagnosis of ADHD, were included.
Exposures and Outcomes
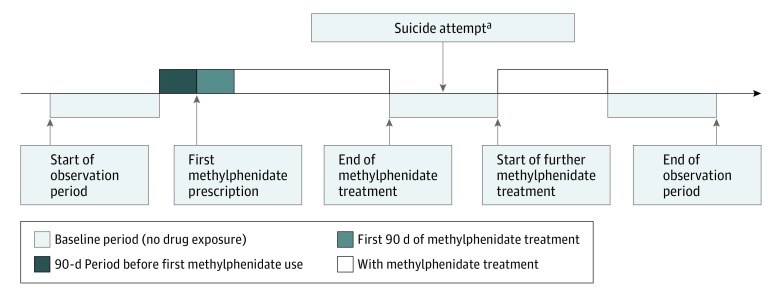
For each included participant, all methylphenidate prescriptions and suicide attempts were identified. All methylphenidate formulations (standard and extended release) and all strengths were included in the analysis. Exposed periods were defined as time receiving medication, with the duration between prescription start and end dates recorded in CDARS for each prescription. More than 99% of the prescriptions had the intended start and end dates as recorded in our data set. Daily dosages and the quantity prescribed were used to determine the duration of treatment if the prescription end date was not available. Median values for exposure duration were imputed when the above information was missing. We divided patient time into 4 discrete categories: absence of methylphenidate (baseline period, including patient-time before and after methylphenidate exposure), 90 days before the first methylphenidate exposure (pre-exposure period), first 90 days of methylphenidate use, and subsequent methylphenidate use. We did not assume that participants received continuous treatment on initiation of methylphenidate, because clinicians may offer drug holidays to patients with ADHD during school holidays and treatment may be stopped and started for various other reasons. The pre-exposure period was defined as the time before the first methylphenidate prescription; thus, there were no pre-exposure periods before the second or subsequent methylphenidate treatments. The study design and timeline for a single hypothetical participant are given in Figure 1. The corresponding date of a suicide attempt was identified as an event date, and only the first recorded suicide attempt for each patient was included in the analysis. In SCCS designs, there should be no censoring by the outcome of interest, as this would violate their assumption and invalidate the results. We conducted a validation analysis by reviewing the information in CDARS. Through doing this, we identified that, in 153 of 154 (99.4%) cases, the ICD-9-CM diagnosis code of a suicide attempt was confirmed in the medical records by an emergency department clinician, hospital pediatrician, and/or psychiatrist. Consequently, the risk of misclassification is considered to be low.
Figure 1. Illustration of Self-controlled Case Series Study Design.
aEvent can take place any time throughout the observation period; no censoring on the event date.
Statistical Analysis
The association between methylphenidate treatment and suicide attempts was calculated by comparing the rate of suicide attempts during exposure periods with that during baseline periods. Adjusted IRR and the corresponding 95% CIs were calculated using conditional Poisson regression, adjusted for age in 1-year bands and season. A 90-day pre-exposure period was added to take account of the possibility that the suicide attempt may affect the likelihood of methylphenidate treatment, which in turn may introduce bias into the risk estimate during treatment. We separated the first 90 days of methylphenidate use to allow the detection of any temporary change in the IRR of suicide attempts, and we also compared the rate of suicide attempts between the pre-exposure period and the methylphenidate-exposed periods. To investigate whether emergent psychiatric disorders could lead to methylphenidate treatment, post hoc analyses were conducted, using the same setting as the original analysis, to test the association between methylphenidate treatment and other psychiatric conditions (ICD-9-CM codes 290-319). A significance level of 5% was used in all statistical analyses. SAS, version 9.4 (SAS Institute Inc) and R, version 3.3.1 (http://www.R-project.org) were used for data manipulation and analysis.
Sensitivity Analyses
Sensitivity analyses were planned to test the validity and robustness of the initial study results. These analyses tested the effects of (1) different drug nonadherence scenarios; (2) restricting the sample to a 6-month age band; (3) more than 10 weeks of methylphenidate exposure; (4) removing patients with a diagnosis of substance misuse/dependence; (5) restricting observation periods to the date of prescription of any antidepressant or antipsychotic medications; (6) removing patients where the event occurred on the first day of prescription; (7) redefining the observation period to January 1, 2001, the sixth birthday of the patient, the first observed date of ADHD diagnosis, or the first date of methylphenidate treatment, whichever occurred last; and (8) restricting to incident patients receiving methylphenidate. The details of these variables can be found in eAppendix 1 in the Supplement.
Results
Among 25 629 patients with methylphenidate prescriptions, 19 had attempted suicide before the observation period and were therefore removed from the analysis, as per protocol. One hundred fifty-four patients had their first recorded suicide attempt within the observation period (eFigure 1 in the Supplement); of these, 111 (72.1%) were male and 43 (27.9%) were female. The mean (SD) age at commencement of observation was 7.15 (2.19) years (range, 6.0-16.47 years), and the mean duration of follow-up per participant was 12.15 (2.82) years. Mean methylphenidate exposure was 2.22 (2.33) years per participant. The median length of each prescription was 70 days (interquartile range [IQR], 35-105 days; range, 1-1023 days), and 3617 of 4300 (84.1%) of the prescriptions were for short-acting methylphenidate. Of 154 patients, 112 (72.7%) had an ADHD diagnosis, and the median age at diagnosis was 10.4 years (IQR, 8.3-13.4 years). Seventy-two (46.8%) patients had at least 1 prescription for an antidepressant or antipsychotic during the study period; 17 began receiving an antidepressant or antipsychotic before their first methylphenidate treatment, and 55 patients started after initiation of methylphenidate. Broader psychiatric comorbidities for these patients are reported in eTable 1 in the Supplement. Among patients without an ADHD diagnosis, 39 of 42 (92.9%) had at least 1 other psychiatric diagnosis (ICD-9-CM codes 290-319); 16 of 42 (38.1%) of these had a diagnosis of autism spectrum disorder (eTable 2 in the Supplement). Of the 154 suicide attempts, 44 occurred during methylphenidate treatment and 110 occurred during the off-treatment period (Table 1). The median age of the index suicide attempt group was 15.4 years (IQR, 12.7-18.1 years) (eFigure 2 in the Supplement). The overall incidence of suicide attempts during methylphenidate treatment was 9.27 per 10 000 patient-years. The crude incidence of suicide attempts in the different risk windows is summarized in Table 2. No participants in the SCCS died of completed suicide during the study period.
Table 1. Patient Characteristics.
| Characteristic | No. of Patients (%) | Age at Baseline, Mean (SD), y | Dose, Median (Range) [IQR], mg/d | Length of Prescription, Median (Range) [IQR], d | Exposed Period | Unexposed Period | ||
|---|---|---|---|---|---|---|---|---|
| Events, No. | Total Follow-up Time, Patient-years | Events, No. | Total Follow-up Time, Patient-years | |||||
| All | 154 (100) | 7.15 (2.19) | 20 (2.5-60) [15-35] | 70 (1-1023) [35-105] | 44 | 342.1 | 110 | 1529.6 |
| Male | 111 (72.1) | 7.13 (2.05) | 20 (2.5-60) [15-35] | 71 (1-838) [42-107] | 32 | 265.4 | 79 | 1085.4 |
| Female | 43 (27.9) | 7.20 (2.54) | 20 (5-60) [15-35] | 56 (1-1023) [28-96] | 12 | 76.7 | 31 | 444.2 |
Abbreviation: IQR, interquartile range.
Table 2. Incidence of Suicide Attempts Among Methylphenidate Users in Different Risk Windows.
| Risk Window | No. of Events | Patient-years | Incidence per 10 000 Patient-years (95% CI) |
|---|---|---|---|
| Before prerisk | 19 | 65 362 | 2.91 (1.86-4.54) |
| 90 d Before first methylphenidate treatment | 12 | 5594 | 21.45 (12.28-37.46) |
| First 90 d of methylphenidate treatment | 6 | 4687 | 12.80 (5.87-27.90) |
| Subsequent methylphenidate treatment | 36 | 42 728 | 8.43 (6.09-11.66) |
| After methylphenidate treatment | 81 | 68 636 | 11.80 (9.50-14.66) |
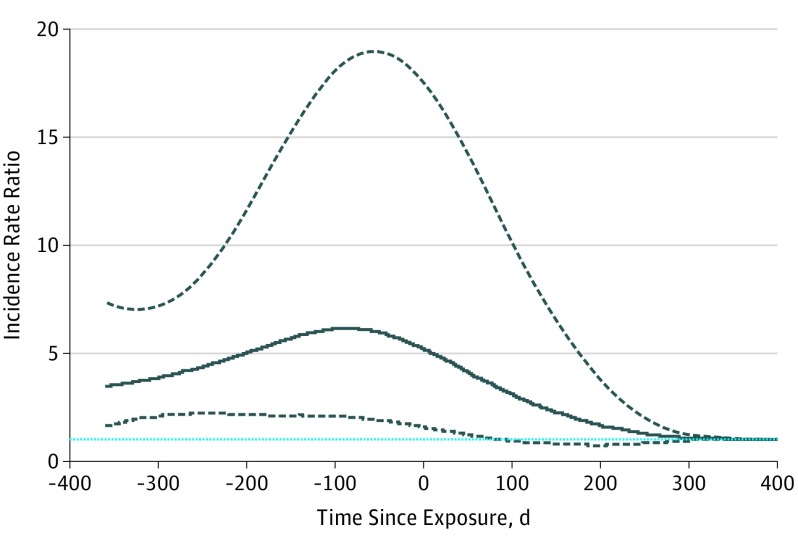
The analysis indicated some association between the decision to start methylphenidate treatment and suicide attempts (Table 3). After age and season were adjusted, an increased risk of suicide attempts was detected during the 90-day period before methylphenidate initiation (IRR, 6.55; 95% CI, 3.37-12.72). The IRR remained elevated during the first 90 days of methylphenidate treatment (IRR, 3.91; 95% CI, 1.62-9.42) before returning to baseline levels during prolonged treatment (IRR, 1.35; 95% CI, 0.77-2.38) (Table 3). A direct comparison between the risk of suicide attempts during the pre-exposure period and the methylphenidate treatment period showed that the corresponding risk was not increased during the first 90 days of methylphenidate treatment (IRR, 0.78; 95% CI, 0.26-2.35). However, a 72% lower risk was found in the subsequent period of methylphenidate treatment (IRR, 0.28; 95% CI, 0.08-0.94) compared with the pre-exposure period. Further analysis using nonparametric, spline-based SCCS showed that the risk of suicide attempts increased significantly before the initiation of methylphenidate treatment and reached a peak within 100 days before methylphenidate treatment (Figure 2). The age- and sex-stratified results showed a similar pattern to the overall analysis (eTable 3 in the Supplement). Post hoc analysis revealed that an increased risk of any psychiatric disorder was detected during the 90-day period before methylphenidate initiation (IRR, 22.14; 95% CI, 21.31-22.99). The risk remained elevated during the first 90 days of methylphenidate treatment (IRR, 9.40; 95% CI, 8.94-9.88) and during prolonged treatment (IRR, 1.53; 95% CI, 1.44-1.62) (eTable 4 in the Supplement). The additional sensitivity analyses did not change this general picture of results (eFigures 3-5 in the Supplement and Table 3).
Table 3. Results From the Self-Controlled Case Series Analyses.
| Risk Window | Adjusted IRR (95% CI) | P Value |
|---|---|---|
| Suicide Attempt (n = 154) | ||
| 90 d Before 1st methylphenidate treatment | 6.55 (3.37-12.72) | <.01 |
| First 90 d with methylphenidate treatment | 3.91 (1.62-9.42) | <.01 |
| Subsequent methylphenidate treatment | 1.35 (0.77-2.38) | .30 |
| Sensitivity Analyses | ||
| 6-mo Age band (n = 154) | ||
| 90 d Before 1st methylphenidate treatment | 5.36 (2.81-10.23) | <.01 |
| First 90 d with methylphenidate treatment | 4.04 (1.87-8.75) | <.01 |
| Subsequent methylphenidate treatment | 1.44 (0.88-2.36) | .15 |
| Patients with >10 wk methylphenidate exposure (n = 113) | ||
| 90 d Before 1st methylphenidate treatment | 5.05 (2.04-12.48) | <.01 |
| First 90 d with methylphenidate treatment | 3.38 (1.15-9.89) | .03 |
| Subsequent methylphenidate treatment | 1.38 (0.77-2.46) | .28 |
| Censor by antidepressants/antipsychotics (n = 154) | ||
| 90 d Before 1st methylphenidate treatment | 8.08 (3.88-16.85) | <.01 |
| First 90 d with methylphenidate treatment | 5.79 (2.28-14.73) | <.01 |
| Subsequent methylphenidate treatment | 1.14 (0.56-2.33) | .72 |
| Remove patients with substance dependence (n = 114) | ||
| 90 d Before 1st methylphenidate treatment | 6.91 (3.44-13.87) | <.01 |
| First 90 d with methylphenidate treatment | 3.69 (1.42-9.6) | <.01 |
| Subsequent methylphenidate treatment | 1.42 (0.77-2.61) | .26 |
| Remove cases with event on the 1st day of treatment (n = 137) | ||
| 90 d Before 1st methylphenidate treatment | 5.54 (2.72-11.29) | <.01 |
| First 90 d with methylphenidate treatment | 3.76 (1.56-9.05) | <.01 |
| Subsequent methylphenidate treatment | 1.32 (0.75-2.33) | .33 |
| Start of observation at January 1, 2001; the 6th birthday of the patient; the first observed date of ADHD diagnosis; or first date of methylphenidate treatment, whichever occurred last (n = 126) | ||
| 90 d Before 1st methylphenidate treatment | 7.28 (3.22-16.5) | <.01 |
| First 90 d with methylphenidate treatment | 3.65 (1.45-9.18) | <.01 |
| Subsequent methylphenidate treatment | 1.25 (0.69-2.28) | .46 |
| Incident methylphenidate users only (n = 140) | ||
| 90 d Before 1st methylphenidate treatment | 6.57 (3.37-12.8) | <.01 |
| First 90 d with methylphenidate treatment | 4.02 (1.66-9.73) | <.01 |
| Subsequent methylphenidate treatment | 1.32 (0.73-2.4) | .36 |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; IRR, incidence rate ratio.
Figure 2. Results From the Spline-Based Self-controlled Case Series Analysis.
Incidence rate ratio (IRR) of suicide attempts throughout the time before and after methylphenidate exposure. The solid line is the estimated IRR, the dashed lines indicate the 95% CI, and the blue dashed line indicates baseline IRR.
Discussion
In this population-based, retrospective study, the incidence of methylphenidate-related suicide attempts demonstrated a 6.5-fold and 4-fold elevation during the 90-day periods before and after the start of treatment, respectively. This finding suggests that the decision to start methylphenidate treatment follows the period of increasing risk for suicide attempts, with the risk remaining elevated and then beginning to fall after initiation of methylphenidate. The most parsimonious interpretation of this pattern of temporal association is that the observed increased risk of suicide attempts is not due to methylphenidate but precedes it, perhaps reflecting changes in behavioral and mental health symptoms or associated impairment that lead to a medical consultation, which in turn may contribute to the decision to prescribe methylphenidate. This hypothesis fits with the finding that the incidence of suicide attempts just after treatment initiation was comparable to that just before it, while after more than 90 days of methylphenidate use, the incidence was similar to that during the baseline period. In addition, the spline-based SCCS analysis showed a decreasing incidence of suicide attempts on the initiation of methylphenidate treatment. However, our results cannot be interpreted as demonstrating that methylphenidate has an immediate effect on lowering the risk of suicide attempts. The increased risk of suicide attempts before treatment may have been missed in a classic cohort study in which patients with either events or exposures before the commencement of the study are usually excluded. To our knowledge, this is the first study investigating the risk of suicide attempts before and after the start of methylphenidate treatment. The study results thus provide new evidence with which to interpret reports of an elevated risk of suicide attempts after initiation of methylphenidate treatment.
Several factors may explain why the initiation of methylphenidate treatment tends to coincide with the times of increased risk of suicide attempts. The initiation of new medication often occurs at a time of specific concerns about patients’ health. Patients with ADHD are at higher risk of suicide-related events. The decision to start treatment with methylphenidate may be a response to changes in behavioral or related psychiatric problems. These problems could be transient psychiatric disorders with ADHD or clinical observation in the period leading to initiation of methylphenidate. It is also well recognized that patients with ADHD are prone to cognitive, emotional, and behavioral comorbidities, for example, depression or disruptive behavioral disorders. These comorbidities may increase the likelihood of suicide attempts, which may consequently increase both the likelihood of medical and psychiatric consultations and receiving a methylphenidate prescription. This position is further supported by the post hoc analysis that found an increased risk of other psychiatric disorders before methylphenidate initiation. A previous study investigating the association between antidepressant medication and suicide also found the peak incidence of suicide attempts to be immediately before initiation of an antidepressant, suggesting that the attempt was a precipitant for initiation of antidepressant treatment. Only 2 patients in our sample died within the study period, and neither of them was receiving methylphenidate treatment at the time of death. Furthermore, the cause of death was not recorded as suicide. Therefore, in our cohort, death by suicide while receiving methylphenidate treatment is a rare outcome.
The suicidal ideation that precedes a suicide attempt may not be an acute event; patients with suicidal ideation may not carry out the attempt immediately. Therefore, there may be a time lag between the beginning of suicidal ideation and the suicide attempt event. This lag could possibly contribute to the increased risk during the first 90 days of methylphenidate treatment (IRR, 3.91; 95% CI, 1.62-9.42).
To our knowledge, only 1 published report evaluated the risk of suicidal behavior in patients receiving methylphenidate and/or other stimulant users. Chen et al identified an increased risk of suicidal behavior in patients receiving ADHD medications (hazard ratio [HR], 1.31; 95% CI, 1.19-1.44) compared with nontreated patients with ADHD. Chen et al further applied a within-individual methodology (ie, comparing patient time with and without medication) and found no increased risk of suicide-related events (HR, 0.89; 95% CI, 0.79-1.00). Among stimulant users, a reduced within-patient rate of suicide-related events was seen during treatment periods (HR, 0.81; 95% CI, 0.70-0.94). Chen et al assumed the risk of suicidal behavior to be constant in nontreatment periods. However, in the present study, we found an increased risk of suicide attempts before the initiation of treatment. This finding suggests, therefore, that the estimate of risk derived by Chen et al may need to be reevaluated.
Limitations
There are a number of limitations to our study. First, CDARS does not have linkage to data from private medical practitioners. However, in HK, the public sector is the main provider of specialist care and there are very few private child and adolescent psychiatrists. As a consequence, the vast majority of patients receiving methylphenidate are likely to have been included in this study, and our sample should be highly representative of the HK population. In addition, our cohort included only clinically referred patients who had sufficiently severe ADHD symptoms and/or impairment to have received methylphenidate treatment. Therefore, our cohort may have a higher baseline risk compared with nonmedicated patients. However, since we applied an SCCS design, individual baseline risk does not affect our study results and conclusion. Second, CDARS provides data on drug prescriptions, but not on adherence, which may lead to misclassification of exposure periods. Third, because we had a comparatively long follow-up time, other time-varying cofounding factors may affect the study results. Therefore, we conducted various sensitivity analyses to explore the potential effects of nonadherence and other confounding factors, and the results were consistent.
Conclusions
The incidence of suicide attempts peaked before the start of methylphenidate treatment, remained high immediately after the start of methylphenidate treatment, and declined during continuation of treatment. Our data, therefore, do not support a causal association between methylphenidate treatment and suicide attempts.
eAppendix 1. Sensitivity Analyses
eFigure 1. Flowchart of Patient Identification
eFigure 2. Histogram of Age at the First Suicide Attempt
eFigure 3. Sensitivity Analysis on Exposure Periods by Adding 1 to 10 Weeks After the End of an Exposed Period: Incidence Rate Ratio (IRR) of Suicide Attempt in the 90-Day Pre-Exposure Period
eFigure 4. Sensitivity Analysis on Exposure Periods by Adding 1 to 10 Weeks After the End of an Exposed Period: Incidence Rate Ratio (IRR) of Suicide Attempt in the First 90-Days MPH Exposure Period
eFigure 5. Sensitivity Analysis on Exposure Periods By Adding 1 To 10 Weeks After The End Of An Exposed Period: Incidence Rate Ratio (IRR) of Suicide Attempt in MPH Treatment After the First 90 Days
eTable 1. Psychiatric Comorbidities of Patients With Suicide Attempt
eTable 2. Psychiatric Comorbidities of Patients With Suicide Attempt but Without ADHD Diagnosis
eTable 3. Result of Age and Gender Stratified Analyses
eTable 4. Result of Post Hoc Analysis
References
- 1.Polanczyk GV, Willcutt EG, Salum GA, Kieling C, Rohde LA. ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int J Epidemiol. 2014;43(2):434-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas R, Sanders S, Doust J, Beller E, Glasziou P. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics. 2015;135(4):e994-e1001. [DOI] [PubMed] [Google Scholar]
- 3.Leung PW, Luk SL, Ho TP, Taylor E, Mak FL, Bacon-Shone J. The diagnosis and prevalence of hyperactivity in Chinese schoolboys. Br J Psychiatry. 1996;168(4):486-496. [DOI] [PubMed] [Google Scholar]
- 4.Leung PW, Hung SF, Ho TP, et al. Prevalence of DSM-IV disorders in Chinese adolescents and the effects of an impairment criterion: a pilot community study in Hong Kong. Eur Child Adolesc Psychiatry. 2008;17(7):452-461. [DOI] [PubMed] [Google Scholar]
- 5.Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366(9481):237-248. [DOI] [PubMed] [Google Scholar]
- 6.Harstad E, Levy S; Committee on Substance Abuse . Attention-deficit/hyperactivity disorder and substance abuse. Pediatrics. 2014;134(1):e293-e301. [DOI] [PubMed] [Google Scholar]
- 7.Connor DF, Doerfler LA. ADHD with comorbid oppositional defiant disorder or conduct disorder: discrete or nondistinct disruptive behavior disorders? J Atten Disord. 2008;12(2):126-134. [DOI] [PubMed] [Google Scholar]
- 8.Lambek R, Tannock R, Dalsgaard S, Trillingsgaard A, Damm D, Thomsen PH. Executive dysfunction in school-age children with ADHD. J Atten Disord. 2011;15(8):646-655. [DOI] [PubMed] [Google Scholar]
- 9.Ljung T, Chen Q, Lichtenstein P, Larsson H. Common etiological factors of attention-deficit/hyperactivity disorder and suicidal behavior: a population-based study in Sweden. JAMA Psychiatry. 2014;71(8):958-964. [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Clinical Excellence Attention deficit hyperactivity disorder: diagnosis and management. https://www.nice.org.uk/guidance/cg72/chapter/recommendations. Accessed June 20, 2017. [PubMed]
- 11.Wolraich M, Brown L, Brown RT, et al. ; Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management . ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pliszka S; AACAP Work Group on Quality Issues . Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894-921. [DOI] [PubMed] [Google Scholar]
- 13.Garfield CF, Dorsey ER, Zhu S, et al. Trends in attention deficit hyperactivity disorder ambulatory diagnosis and medical treatment in the United States, 2000-2010. Acad Pediatr. 2012;12(2):110-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brault MC, Lacourse É. Prevalence of prescribed attention-deficit hyperactivity disorder medications and diagnosis among Canadian preschoolers and school-age children: 1994-2007. Can J Psychiatry. 2012;57(2):93-101. [DOI] [PubMed] [Google Scholar]
- 15.McCarthy S, Wilton L, Murray ML, Hodgkins P, Asherson P, Wong IC. The epidemiology of pharmacologically treated attention deficit hyperactivity disorder (ADHD) in children, adolescents and adults in UK primary care. BMC Pediatr. 2012;12(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong IC, Murray ML, Camilleri-Novak D, Stephens P. Increased prescribing trends of paediatric psychotropic medications. Arch Dis Child. 2004;89(12):1131-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garbe E, Mikolajczyk RT, Banaschewski T, et al. Drug treatment patterns of attention-deficit/hyperactivity disorder in children and adolescents in Germany: results from a large population-based cohort study. J Child Adolesc Psychopharmacol. 2012;22(6):452-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Man KK, Ip P, Hsia Y, et al. ADHD drug prescribing trend is increasing among children and adolescents in Hong Kong [published online July 3, 2014]. J Atten Disord. doi: 10.1177/1087054714536047 [DOI] [PubMed] [Google Scholar]
- 19.EMA European Medicines Agency Makes Recommendations for Safer Use of Ritalin and Other Methylphenidate-Containing Medicines in the EU. London: European Medicines Agency Press Office; 2009. [Google Scholar]
- 20.Cortese S, Holtmann M, Banaschewski T, et al. ; European ADHD Guidelines Group . Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. J Child Psychol Psychiatry. 2013;54(3):227-246. [DOI] [PubMed] [Google Scholar]
- 21.Chen Q, Sjölander A, Runeson B, D’Onofrio BM, Lichtenstein P, Larsson H. Drug treatment for attention-deficit/hyperactivity disorder and suicidal behaviour: register based study. BMJ. 2014;348:g3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheftall AH, Asti L, Horowitz LM, et al. Suicide in elementary school-aged children and early adolescents. Pediatrics. 2016;138(4):e20160436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chronis-Tuscano A, Molina BSG, Pelham WE, et al. Very early predictors of adolescent depression and suicide attempts in children with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2010;67(10):1044-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Impey M, Heun R. Completed suicide, ideation and attempt in attention deficit hyperactivity disorder. Acta Psychiatr Scand. 2012;125(2):93-102. [DOI] [PubMed] [Google Scholar]
- 25.Meza JI, Owens EB, Hinshaw SP. Response inhibition, peer preference and victimization, and self-harm: longitudinal associations in young adult women with and without ADHD. J Abnorm Child Psychol. 2016;44(2):323-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan EW, Lau WCY, Leung WK, et al. Prevention of dabigatran-related gastrointestinal bleeding with gastroprotective agents: a population-based study. Gastroenterology. 2015;149(3):586-95.e3. [DOI] [PubMed] [Google Scholar]
- 27.Chui CSL, Man KKC, Cheng CL, et al. An investigation of the potential association between retinal detachment and oral fluoroquinolones: a self-controlled case series study. J Antimicrob Chemother. 2014;69(9):2563-2567. [DOI] [PubMed] [Google Scholar]
- 28.Wong AYS, Root A, Douglas IJ, et al. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ. 2016;352:h6926. [DOI] [PubMed] [Google Scholar]
- 29.He Y, Chan EW, Man KKC, et al. Dosage effects of histamine-2 receptor antagonist on the primary prophylaxis of non-steroidal anti-inflammatory drug (NSAID)-associated peptic ulcers: a retrospective cohort study. Drug Saf. 2014;37(9):711-721. [DOI] [PubMed] [Google Scholar]
- 30.Man KK, Chan EW, Coghill D, et al. Methylphenidate and the risk of trauma. Pediatrics. 2015;135(1):40-48. [DOI] [PubMed] [Google Scholar]
- 31.Man KK, Coghill D, Chan EW, et al. Methylphenidate and the risk of psychotic disorders and hallucinations in children and adolescents in a large health system. Transl Psychiatry. 2016;6(11):e956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chui CS, Chan EW, Wong AY, Root A, Douglas IJ, Wong IC. Association between oral fluoroquinolones and seizures: a self-controlled case series study. Neurology. 2016;86(18):1708-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Man KKC, Chan EW, Ip P, et al. Prenatal antidepressant use and risk of attention-deficit/hyperactivity disorder in offspring: population based cohort study. BMJ. 2017;357:j2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hospital Authority Head Office IT Department. Clinical Data Analysis & Reporting System (CDARS) User's Manual: 2.0 ed. Hong Kong, Hong Hospital Authority; 2003:3.
- 35.Lao KS, Chui CS, Man KK, Lau WC, Chan EW, Wong IC. Medication safety research by observational study design. Int J Clin Pharm. 2016;38(3):676-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med. 2006;25(10):1768-1797. [DOI] [PubMed] [Google Scholar]
- 37.Oner O, Yilmaz ES, Karadag H, et al. ADHD medication trends in Turkey: 2009-2013 [published online February 19, 2014]. J Atten Disord. doi: 10.1177/1087054714523129 [DOI] [PubMed] [Google Scholar]
- 38.Suhail K, Cochrane R. Seasonal variations in hospital admissions for affective disorders by gender and ethnicity. Soc Psychiatry Psychiatr Epidemiol. 1998;33(5):211-217. [DOI] [PubMed] [Google Scholar]
- 39.Ghebremichael-Weldeselassie Y, Whitaker HJ, Farrington CP. Self-controlled case series method with smooth age effect. Stat Med. 2014;33(4):639-649. [DOI] [PubMed] [Google Scholar]
- 40.Ghebremichael-Weldeselassie Y, Whitaker HJ, Farrington CP. Flexible modelling of vaccine effect in self-controlled case series models. Biom J. 2016;58(3):607-622. [DOI] [PubMed] [Google Scholar]
- 41.Thomas KH, Davies N, Metcalfe C, Windmeijer F, Martin RM, Gunnell D. Validation of suicide and self-harm records in the Clinical Practice Research Datalink. Br J Clin Pharmacol. 2013;76(1):145-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.National Institute for Health and Clinical Excellence Attention deficit hyperactivity disorder: pharmacological and psychological interventions in children, young people and adults. London: The British Psychological Society and the Royal College of Psychiatrists; 2009. [Google Scholar]
- 43.Lakhan SE, Kirchgessner A. Prescription stimulants in individuals with and without attention deficit hyperactivity disorder: misuse, cognitive impact, and adverse effects. Brain Behav. 2012;2(5):661-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruchkin V, Koposov RA, Koyanagi A, Stickley A. Suicidal behavior in juvenile delinquents: the role of ADHD and other comorbid psychiatric disorders [published online October 12, 2016]. Child Psychiatry Hum Dev. doi: 10.1007/s10578-016-0693-9 [DOI] [PubMed] [Google Scholar]
- 45.Simon GE, Savarino J, Operskalski B, Wang PS. Suicide risk during antidepressant treatment. Am J Psychiatry. 2006;163(1):41-47. [DOI] [PubMed] [Google Scholar]
- 46.Carrigan CG, Lynch DJ. Managing suicide attempts: guidelines for the primary care physician. Prim Care Companion J Clin Psychiatry. 2003;5(4):169-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leung GM, Tin KY, O’Donnell O. Redistribution or horizontal equity in Hong Kong’s mixed public-private health system: a policy conundrum. Health Econ. 2009;18(1):37-54. [DOI] [PubMed] [Google Scholar]
- 48.Chan CW, Lam C, Lau J, et al. Attention Deficit/Hyperactivity Disorder in Children: 2007 Position Paper. Hong Kong: The Hong Kong Society of Child Neurology & Developmental Paediatrics; 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Sensitivity Analyses
eFigure 1. Flowchart of Patient Identification
eFigure 2. Histogram of Age at the First Suicide Attempt
eFigure 3. Sensitivity Analysis on Exposure Periods by Adding 1 to 10 Weeks After the End of an Exposed Period: Incidence Rate Ratio (IRR) of Suicide Attempt in the 90-Day Pre-Exposure Period
eFigure 4. Sensitivity Analysis on Exposure Periods by Adding 1 to 10 Weeks After the End of an Exposed Period: Incidence Rate Ratio (IRR) of Suicide Attempt in the First 90-Days MPH Exposure Period
eFigure 5. Sensitivity Analysis on Exposure Periods By Adding 1 To 10 Weeks After The End Of An Exposed Period: Incidence Rate Ratio (IRR) of Suicide Attempt in MPH Treatment After the First 90 Days
eTable 1. Psychiatric Comorbidities of Patients With Suicide Attempt
eTable 2. Psychiatric Comorbidities of Patients With Suicide Attempt but Without ADHD Diagnosis
eTable 3. Result of Age and Gender Stratified Analyses
eTable 4. Result of Post Hoc Analysis