This randomized clinical trial evaluates the efficacy of internet-based guided self-help compared with traditional, face-to-face, individual cognitive behavior therapy.
Key Points
Question
Is an internet-based, guided self-help intervention noninferior to traditional, individual face-to-face cognitive behavioral therapy?
Findings
In this randomized clinical trial of 178 patients, face-to-face cognitive behavioral therapy was more efficacious in reducing binge eating days, in promoting abstinence from binge eating, and in reducing eating-related psychopathologic findings compared with internet-based guided self-help at the end of a 4-month treatment period and at 6-month follow-up. However, exploratory analysis in a smaller sample revealed that these differences disappeared by the 1.5-year follow-up.
Meaning
Internet-based guided self-help remains a viable, slower-acting, low-threshold treatment alternative compared with cognitive behavioral therapy for adults with binge eating disorder.
Abstract
Importance
Although cognitive behavioral therapy (CBT) represents the criterion standard for treatment of binge eating disorder (BED), most individuals do not have access to this specialized treatment.
Objective
To evaluate the efficacy of internet-based guided self-help (GSH-I) compared with traditional, individual face-to-face CBT.
Design, Setting, and Participants
The Internet and Binge Eating Disorder (INTERBED) study is a prospective, multicenter, randomized, noninferiority clinical trial (treatment duration, 4 months; follow-ups, 6 months and 1.5 years). A volunteer sample of 178 adult outpatients with full or subsyndromal BED were recruited from 7 university-based outpatient clinics from August 1, 2010, through December 31, 2011; final follow-up assessment was in April 2014. Data analysis was performed from November 30, 2014, to May 27, 2015.
Interventions
Participants received 20 individual face-to-face CBT sessions of 50 minutes each or sequentially completed 11 internet modules and had weekly email contacts.
Main Outcomes and Measures
The primary outcome was the difference in the number of days with objective binge eating episodes (OBEs) during the previous 28 days between baseline and end of treatment. Secondary outcomes included OBEs at follow-ups, eating disorder and general psychopathologic findings, body mass index, and quality of life.
Results
A total of 586 patients were screened, 178 were randomized, and 169 had at least one postbaseline assessment and constituted the modified intention-to-treat analysis group (mean [SD] age, 43.2 [12.3] years; 148 [87.6%] female); the 1.5-year follow-up was available in 116 patients. The confirmatory analysis using the per-protocol sample (n = 153) failed to show noninferiority of GSH-I (adjusted effect, 1.47; 95% CI, −0.01 to 2.91; P = .05). Using the modified intention-to-treat sample, GSH-I was inferior to CBT in reducing OBE days at the end of treatment (adjusted effect, 1.63; 95% CI, 0.17-3.05; P = .03). Exploratory longitudinal analyses also showed the superiority of CBT over GSH-I by the 6-month (adjusted effect, 0.36; 95% CI, 0.23-0.55; P < .001) but not the 1.5-year follow-up (adjusted effect, 0.91; 95% CI, 0.54-1.50; P = .70). Reductions in eating disorder psychopathologic findings were significantly higher in the CBT group than in the GSH-I group at 6-month follow-up (adjusted effect, −0.4; 95% CI, −0.68 to −0.13; P = .005). No group differences were found for body mass index, general psychopathologic findings, and quality of life.
Conclusions and Relevance
Face-to-face CBT leads to quicker and greater reductions in the number of OBE days, abstinence rates, and eating disorder psychopathologic findings and may be a better initial treatment option than GSH-I. Internet-based guided self-help remains a viable, slower-acting, low-threshold treatment alternative compared with CBT for adults with BED.
Trial Registration
isrctn.org Identifier: ISRCTN40484777 and germanctr.de Identifier: DRKS00000409
Introduction
On the basis of extensive research supporting the clinical validity of binge eating disorder (BED), BED has been included in DSM-5 as a distinct eating disorder diagnosis. Binge eating disorder is defined by recurrent objective binge eating (OBE) episodes that occur in the absence of inappropriate weight control behaviors. Binge eating disorder is the most common eating disorder, with a worldwide mean lifetime prevalence rate of 1.9%. The prevalence estimates substantially increase among individuals with obesity. In contrast to other eating disorders, the sex ratio for BED is less skewed. However, women are more often affected, with an odds ratio of 2.4. No ethnic or racial group is overrepresented. Binge eating disorder is associated with a significantly elevated risk of psychiatric and medical comorbidity and psychosocial impairment. Of importance, patients with subthreshold DSM-IV BED do not differ with regard to demographic characteristics, body mass index (BMI) (calculated as weight in kilograms divided by height in meters squared), and a wide range of eating disorder and general psychopathologic findings from individuals with full-syndrome BED. Binge eating disorder is associated with increases in health care use and costs.
Cognitive behavioral therapy (CBT) is the most well-established treatment of BED, significantly reducing binge eating and associated eating-related and general psychopathologic findings. Abstinence from binge eating can be achieved in approximately 50% to 60% of patients at the end of treatment and maintained during long-term follow-up. Psychological treatment approaches have generally not resulted in weight loss, although successfully eliminating binge eating might protect against future weight gain.
Although CBT is considered to be the criterion standard treatment of BED, this intervention is not offered areawide, leading to delayed delivery of adequate treatment. An alternative to traditional face-to-face CBT and a potential means to disseminate adequate treatment is structured self-help. In a review on manualized self-help interventions in eating disorders, randomized clinical trials including patients with BED were also included. So far, only a few randomized clinical trials of patients with BED have evaluated manualized self-help interventions. Most trials used book-based self-help, 1 study used a CD-ROM–based self-help intervention, and 1 study used an internet-based self-help intervention. Overall, specialist guidance improved adherence and outcomes compared with unguided interventions. Patients using self-help modalities did better than untreated control individuals in improving binge eating days and eating disorder psychopathologic findings and in achieving abstinence of binge eating. In the study that used an internet-based self-help intervention, 74 adults with full or subsyndromal BED were included. Abstinence rates were 35.1% in the self-help group and 8.1% in the waitlist group at the end of treatment, and these rates were maintained at the 6-month follow-up. Overall, little work has been performed on technology-enhanced delivery of CBT-based interventions for BED.
Only one study directly compared guided self-help (GSH) with face-to-face psychotherapy in patients with BED. In this trial, interpersonal psychotherapy (IPT) (n = 75) and GSH based on CBT using a book format (n = 66) were equally effective, with 4-week abstinence rates from OBEs in both treatment conditions of approximately 60% at the end of treatment, which was maintained at the 2-year follow-up. In addition, no differences were found for eating disorder and general psychopathologic findings. However, IPT was more successful in retaining patients in the trial than self-help.
An efficacy evaluation of internet-based GSH (GSH-I) compared with the criterion standard of face-to-face CBT is still outstanding. Thus, in this multicenter study, we aimed to evaluate the noninferiority of GSH-I compared with face-to-face CBT in a randomized clinical trial for individuals with full-syndrome or subsyndromal BED.
Methods
Study Design and Procedure
The Internet and Binge Eating Disorder (INTERBED) study was conducted at 7 trial sites with well-established, university-based eating disorder outpatient clinics. Details regarding design, methods, and treatments of the study have been published previously. The trial protocol can be found in Supplement 1. The study procedures and progress reports were regularly reviewed by an international advisory board, which also fulfilled the role of a data safety monitoring board. All patients provided written informed consent, and all data were deidentified. The institutional review boards of the Universities of Erlangen-Nuremberg, Tübingen, Heidelberg, Bochum, Freiburg, Fribourg, and Leipzig approved the trial (Ethikkommission der Medizinischen Fakultät der Ruhr-Universität Bochum, Germany; Ethik-Kommission der Friedrich-Alexander-Universität Erlangen-Nürnberg, Germany; Ethikkommission an der Medizinischen Fakultät der Universität Leipzig, Germany; Ethikkommission der Medizinischen Fakultät Heidelberg, Germany; Ethik-Kommission der Medizinischen Fakultät der Eberhard-Karls-Universität Tübingen, Germany; Ethik-Kommission der Albert-Ludwigs-Universität Freiburg, Germany; and Ethikkommission des Departments für Psychologie, Universität Fribourg, Switzerland).
A volunteer sample of 178 adult outpatients with full or subsyndromal BED were recruited from 7 university-based outpatient clinics from August 1, 2010, through December 31, 2011; final follow-up assessment was in April 2014. Data analysis was performed from November 30, 2014, to May 27, 2015. Patients were assessed at baseline (T0), at midtreatment (T1) (GSH-I: after 2 months; CBT: after 10 sessions), and after the completion of treatment (T2). Both treatments lasted 4 months, and maintenance of outcome was assessed 6 months after the end of treatment (T3). Halfway through the study, the funding agency agreed to sponsor a 1.5-year follow-up in addition to the 6-month follow-up (T4). During the follow-up period, patients had no further therapeutic contact with study personnel.
Participants
To be included in the study, participants had to be 18 years or older and German speaking, have a BMI between 27 and 40, and meet diagnostic criteria for BED according to DSM-IV-TR or subsyndromal BED, ascertained through the Eating Disorder Examination–Interview (EDE-I) (eAppendix in Supplement 2).
Treatment
Participants received 20 individual face-to-face CBT sessions with a therapist (CBT) that lasted 50 minutes each or had weekly email contacts (GSH-I) delivered during a period of 4 months. In addition, in the GSH-I group, coaches and participants met in person twice for 90 minutes before the beginning and after the end of treatment. The CBT was given in accordance with a validated manual. For the GSH-I, the Self-Help Guide (NetUnion & University Hospital of Geneva) was used (eAppendix in Supplement 2). Each therapist delivered both treatments.
Assessment, Randomization, Masking, and Sample Size Estimation
The primary outcome was assessed using the German version of the EDE-I, a validated expert interview. For secondary outcomes, validated observer and self-rating scales (in German) were applied, including the EDE-I, the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I/P), the Beck Depression Inventory II (BDI-II), the Rosenberg Self-Esteem Scale (RSES), the Impact of Weight on Quality of Life–Lite (IWQOL-Lite) scale, and the Clinical Impairment Assessment (CIA) scale. Independent raters coded digital audio files of the EDE-I and the SCID-I/P interviews and gave immediate feedback to ensure interrater reliability. Descriptions of the scales and reliability data are given in the eAppendix in Supplement 2. Details about randomization, masking, and power are given in the eAppendix in Supplement 2.
Statistical Analysis
All statistical analyses were performed using R 3.3.0 with a statistical analysis plan developed before study completion. A 2-sided P < .05 was considered to indicate statistical significance.
Confirmatory Analysis for Noninferiority
The confirmatory analysis for the noninferiority hypothesis regarding the primary outcome was based on the difference in the number of days with OBEs between T0 and T2. For sensitivity reasons, the analysis was performed in different ways: a per-protocol approach, a modified intention-to-treat (mITT) approach, and an intention-to-treat (ITT) approach. The mITT analysis included all patients with at least 1 postbaseline assessment (169 of 178). Imputations for missing values at T2 were performed with their last observation (eg, baseline value or T1) and with multiple imputations by chained equations. Per-protocol treatment completion was defined as attendance at 12 of 20 CBT treatment sessions. For GSH-I, attendance was counted if a participant had logged in until week 10. Effect sizes were calculated using the Cohen d.
The cross-sectional analysis was based on a linear mixed-effect model with the difference in the number of days with OBEs between T0 and T2 as the outcome variable and the treatment condition as the fixed effect. To adjust for possible confounders and dependencies, we additionally incorporated baseline values of OBE days, BMI, BDI-II score, and EDE-I global score as the fixed effects and the study center as the random effect. The resulting coefficient estimate for the treatment group thus represents an adjusted treatment effect for the difference in the number of days with OBEs between T0 and T2 between GSH-I and CBT and is reported with its 95% CI to compare it with the noninferiority margin. For sensitivity reasons, the confirmatory analysis was performed again, applying other imputation methods and incorporating the therapist as a random effect and the study center as a fixed effect (eAppendix and eTable 1 in Supplement 2).
Exploratory Longitudinal Analysis Testing for Superiority
In another step, exploratory longitudinal and multivariate mixed-effect regression analyses were performed for all end points, applying 2-sided testing for superiority. All longitudinal analyses were based on the ITT sample from T0 to T3 without imputations. For the number of days with OBEs, we used the zero-inflated negative binomial as outcome distribution; continuous secondary outcome measures were modeled applying classic linear mixed-effect modeling, whereas binary secondary outcomes were analyzed using generalized estimation equations. The models included the corresponding end point as outcome and treatment group, time (T0 to T3), and treatment × time interaction as fixed effects. In addition, all models included age, sex, baseline OBE days, BMI, BDI-II score, and EDE-I global score as fixed effects and the individual patients and the study center as random effects. Effect sizes were calculated using the Cohen d and are presented in eTable 2 in Supplement 2.
Safety
Adverse events were evaluated in all randomized patients who attended at least 1 treatment session (safety analysis set; 178 patients: 89 from the CBT group and 89 from the GSH-I group). Five severe adverse events occurred (eAppendix in Supplement 2).
Additional Post Hoc Analyses
In an exploratory longitudinal analysis, the primary outcome (OBE days) and abstinence rates were compared between treatment arms at the 1.5-year follow-up assessment (T4). Dropout rates were compared between treatment groups using the Fisher exact test for independence, applying different definitions of dropouts. Associations between BMI and abstinence rates at the end of treatment were analyzed using 2-sample, unpaired t tests. Finally, indicators of adherence with GSH-I were assessed, and correlations with the main outcome (the difference in the number of days with OBEs between T0 and T2 in GSH-I) were analyzed.
Results
Participants
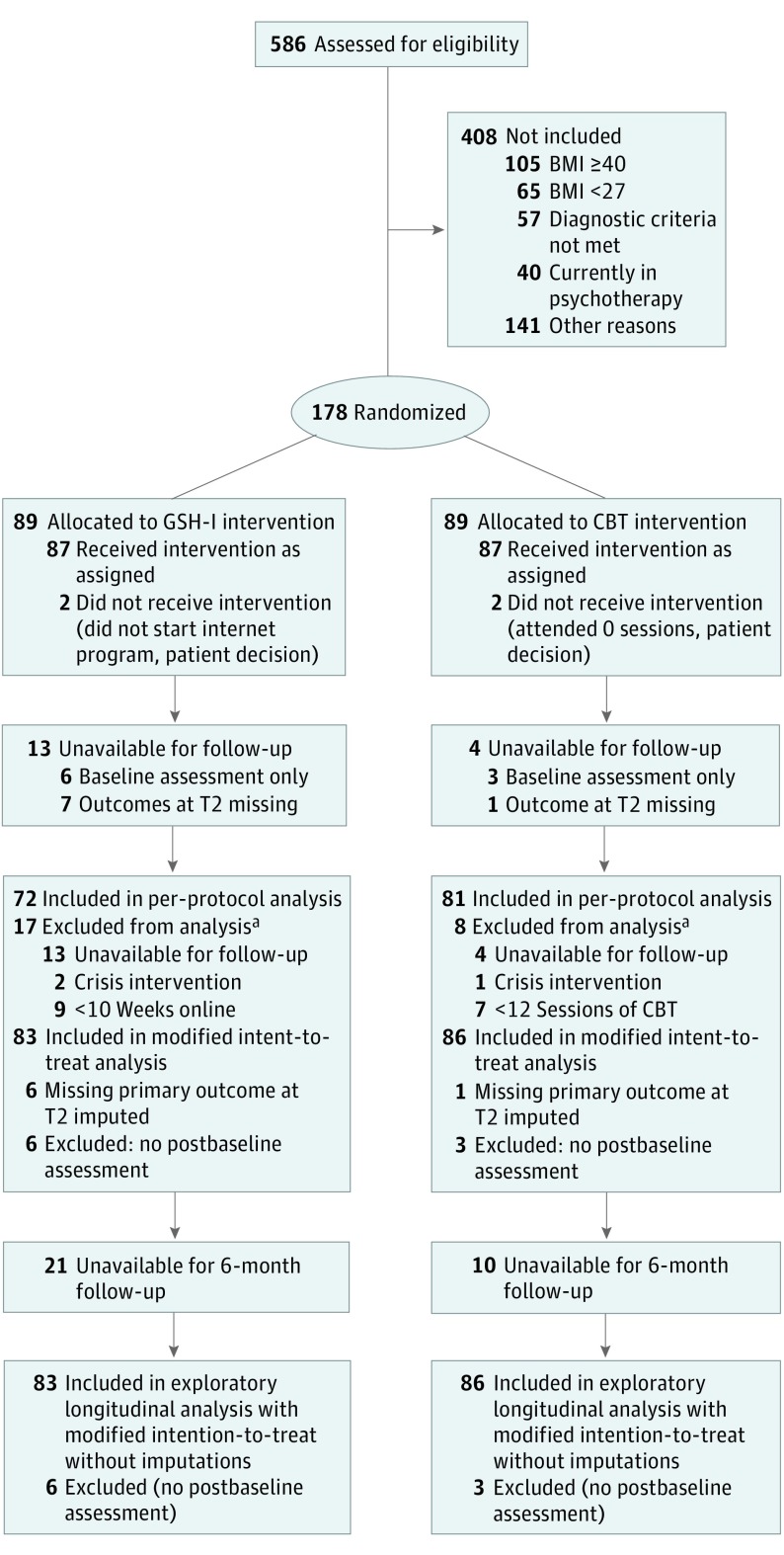
A total of 586 patients were screened, 178 were randomized, and 169 had at least 1 postbaseline assessment and constituted the mITT analysis group (mean [SD] age, 43.2 [12.3] years; 148 [87.6%] female); 1.5-year follow-up was available in 116 patients. The CONSORT flow diagram (Figure 1) summarizes participant enrollment and study flow, and Table 1 summarizes participant characteristics.
Figure 1. CONSORT Flow Diagram.
BMI indicates body mass index; CBT, cognitive behavioral treatment; GSH-I, internet-based guided self-help; and T2, completion of treatment.
aNot mutually exclusive categories.
Table 1. Baseline Sociodemographic and Clinical Characteristics of Individuals With BED in the GSH-I and CBT Groups in the mITT Analysisa .
| Characteristic | GSH-I (n = 83) |
CBT (n = 86) |
|---|---|---|
| Female | 74 (89.2) | 74 (86.0) |
| Age, mean (SD), y | 43.7 (12.7) | 42.7 (12.0) |
| Married | 30 (36.1) | 44 (51.2) |
| Employed | 57 (68.7) | 66 (76.7) |
| Education level ≥12 y of school attendance | 44 (53.0) | 43 (50.0) |
| BED diagnosis | ||
| Full syndrome | 77 (92.8) | 74 (86.0) |
| Subsyndromal | 6 (7.2) | 12 (14.0) |
| Duration of illness, mean (SD), y | 7.9 (9.3) | 10.4 (11.1) |
| Prior inpatient or day-patient treatment for BED | 11 (13.3) | 13 (15.1) |
| Prior outpatient treatment for BED | 18 (21.7) | 13 (15.1) |
| Current mental disorders | ||
| Any affective disorder | 37 (44.6) | 43 (50.0) |
| Any anxiety disorder | 19 (22.9) | 24 (27.9) |
| Current psychotropic medication | 8 (9.6) | 16 (18.6) |
| Type 2 diabetes | 7 (8.4) | 3 (3.5) |
| Hypertension | 16 (19.3) | 23 (26.7) |
| Body mass indexb | 33.4 (3.9) | 34.4 (3.9) |
| Frequency of OBE days (in the past 28 d), mean (SD) | 14.1 (7.8) | 13.5 (7.5) |
| Eating Disorder Examination–Interview global score, mean (SD) | 2.9 (1.0) | 2.9 (0.9) |
| Beck Depression Inventory II score, mean (SD) | 14.9 (11.1) | 13.1 (9.0) |
| Rosenberg Self-Esteem Scale score, mean (SD) | 18.5 (7.6) | 18.9 (6.2) |
| IWQOL-Lite total score, mean (SD) | 75.2 (22.9) | 76.4 (19.4) |
| Clinical Impairment Assessment score, mean (SD) | 20.3 (10.4) | 21.4 (9.1) |
Abbreviations: BED, binge eating disorder; CBT, cognitive behavioral therapy; GSH-I, internet-based guided self-help; IWQOL-Lite, Impact of Weight on Quality of Life–Lite; mITT, modified intention-to-treat; OBE, objective binge eating.
Data are presented as number (percentage) of patients unless otherwise indicated. The percentages reported are based on nonmissing data.
Calculated as weight in kilograms divided by height in meters squared.
Primary Outcome: Reduction in OBE Days
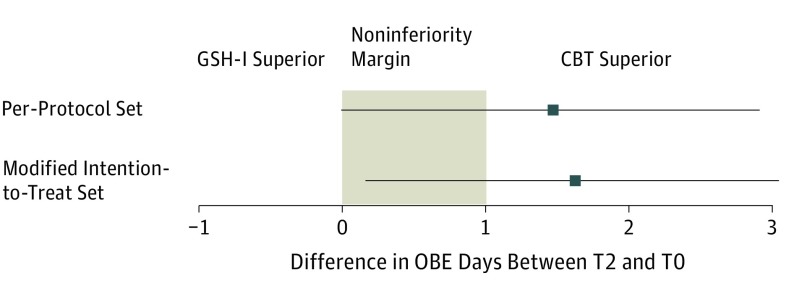
The confirmatory analysis using the per-protocol sample failed to show noninferiority of GSH-I compared with CBT with regard to the reduction of OBE days (mean [SD] difference, 10.4 [8.7] vs 11.7 [7.8] days; adjusted effect, 1.47 [95% CI, −0.01 to 2.91]; Cohen d, 0.16; P = .05). The corresponding null hypothesis (inferiority of GSH-I) was not rejected (Figure 2 and Table 2). The identical analyses on the mITT and ITT samples indicate that GSH-I was significantly inferior to CBT at the end of treatment and at 6-month follow-up (mITT: mean [SD] difference, 10.2 [8.8] vs 11.5 [7.7] days; adjusted effect, 1.63 [95% CI, 0.17 to 3.05]; Cohen d, 0.16; P = .03; ITT: mean [SD] difference, 9.8 [8.4] vs 11.4 [7.7] days; adjusted effect, 1.83 [95% CI, 0.30 to 3.37]; Cohen d, 0.20; P = .02). However, the lower 95% confidence limit included the noninferiority margin. Thus, the true difference in the reduction of OBE days between CBT and GSH-I could still be less than 1 day. Overall, OBE days decreased significantly in both groups at the end of treatment (GSH-I: adjusted effect, 0.29; 95% CI, 0.22-0.38; CBT: adjusted effect, 0.10; 95% CI, 0.07-0.15; P < .001) and by 6-month follow-up (GSH-I: adjusted effect, 0.45; 95% CI, 0.33-0.59; CBT: adjusted effect, 0.13; 95% CI, 0.09-0.19; P < .001) (eTable 3 and eFigure 1 in Supplement 2). Longitudinal exploratory analysis showed superiority of CBT at the end of treatment (T2) (mean [SD], 2.0 [4.1] vs 3.9 [5.5] days; adjusted effect, 0.41 [95% CI, 0.26 to 0.64]; P < .001) and at 6-month follow-up (T3) (mean [SD], 2.8 [5.2] vs 5.3 [6.9] days; adjusted effect, 0.36 [95% CI, 0.23 to 0.55]; P < .001) with small to medium effect sizes (Table 3 and eTable 2 in Supplement 2).
Figure 2. Adjusted Effect Estimates and Corresponding 95% CIs for the Difference in Objective Binge Eating (OBE) Days Between the Cognitive Behavioral Treatment (CBT) and Internet-Based Guided Self-help (GSH-I) Groups at the End of Treatment (T2) for the Per-Protocol and the Modified Intention-to-Treat Analyses.
Solid lines indicate 95% CIs; shaded section, noninferiority margin; and T0, baseline.
Table 2. Difference in the Number of OBE Days During the Previous 28 Days Between Baseline and the End of Treatment .
| Analysis | GSH-I | CBT | Adjusted Effecta (95% CI) | Effect Size (Cohen d) | P Value | ||
|---|---|---|---|---|---|---|---|
| No. of Patients | Mean (SD) Difference | No. of Patients | Mean (SD) Difference | ||||
| Per protocol (n = 153) | 72 | 10.4 (8.7) | 81 | 11.7 (7.8) | 1.47 (−0.01 to 2.91) | 0.16 | .05 |
| Modified intention-to-treat (n = 169) LOCF | 83 | 10.2 (8.8) | 86 | 11.5 (7.7) | 1.63 (0.17 to 3.05) | 0.16 | .03 |
| Modified intention-to-treat (n = 169) multiple imputationsb | 83 | 10.0 (8.6) | 86 | 11.4 (7.7) | 1.68 (0.15 to 3.21) | 0.17 | .04 |
| Intention-to-treat (n = 178) multiple imputationsb | 89 | 9.8 (8.4) | 89 | 11.4 (7.7) | 1.83 (0.30 to 3.37) | 0.20 | .02 |
Abbreviations: CBT, cognitive behavioral therapy; GSH-I, internet-based guided self-help; LOCF, last observation carried forward; OBE, objective binge eating.
Adjusted for age, sex, OBE days at baseline, body mass index, Beck Depression Inventory II score, and Eating Disorder Examination–Interview global score.
Multiple imputations with chained equations (Cohen d: 0.2 indicates small effect; 0.5, medium effect; and 0.8, large effect).
Table 3. Comparison of GSH-I and CBT on Primary and Secondary Outcomes Using the Intention-to-Treat Sample Without Imputations at End of Treatment and by 6-Month Follow-up .
| Outcome | End of Treatment (n = 162) |
6-Month Follow-up (n = 150) |
||||||
|---|---|---|---|---|---|---|---|---|
| GSH-I (n = 77) |
CBT (n = 85) |
Adjusted Effect Estimate for CBT (95% CI)a | P Value | GSH-I (n = 70) |
CBT (n = 80) |
Adjusted Effect Estimate for CBT (95% CI)a | P Value | |
| Primary outcome: OBE days, mean (SD) | 3.9 (5.5) | 2.0 (4.1) | 0.41 (0.26 to 0.64)b | <.001 | 5.3 (6.9) | 2.8 (5.2) | 0.36 (0.23 to 0.55)b | <.001 |
| Secondary outcomes, No./total patients (%) | ||||||||
| Abstinence from binge eating | 27/76 (36) | 52/85 (61) | 3.29 (1.68 to 6.43)c | <.001 | 26/68 (38) | 46/79 (58) | 2.54 (1.25 to 5.15)c | .01 |
| BED full diagnosis | 35/72 (49) | 24/83 (29) | 0.33 (0.15 to 0.69)c | .004 | 20/66 (30) | 20/78 (26) | 0.71 (0.30 to 1.64)c | .42 |
| Eating Disorder Examination score, mean (SD) | ||||||||
| Global | 2.0 (1.2) | 1.9 (1.2) | −0.22 (−0.48 to 0.04) | .10 | 2.0 (1.2) | 1.7 (1.1) | −0.40 (−0.68 to −0.13) | .005 |
| Restraint | 1.4 (1.4) | 1.5 (1.4) | 0.01 (−0.38 to 0.39) | .97 | 1.6 (1.4) | 1.3 (1.3) | −0.34 (−0.75 to 0.06) | .10 |
| Eating concern | 1.1 (1.2) | 0.9 (1.1) | −0.17 (−0.46 to 0.14) | .29 | 1.1 (1.3) | 0.9 (1.1) | −0.23 (−0.55 to 0.08) | .15 |
| Shape concern | 2.9 (1.6) | 2.6 (1.6) | −0.36 (−0.72 to 0.00) | .06 | 2.8 (1.5) | 2.4 (1.5) | −0.50 (−0.88 to −0.12) | .01 |
| Weight concern | 2.6 (1.5) | 2.3 (1.5) | −0.36 (−0.71 to −0.01) | .04 | 2.5 (1.4) | 2.1 (1.5) | −0.53 (−0.90 to −0.16) | .005 |
| BMI,d mean (SD) | 32.9 (3.9) | 34.2 (4.5) | −0.08 (−0.50 to 0.34) | .69 | 33.1 (4.2) | 33.5 (4.6) | −0.41 (−0.85 to 0.03) | .07 |
| Mental comorbidity | ||||||||
| Affective disorders, No./total patients (%) | 21/69 (30) | 16/85 (19) | 0.58 (0.26 to 1.30)c | .19 | 15/61 (25) | 18/76 (24) | 1.03 (0.43 to 2.45)c | .95 |
| BDI-II, mean (SD) | 11.7 (12.4) | 9.0 (10.8) | −1.42 (−3.70 to 0.87) | .23 | 10.0 (11.9) | 9.0 (10.0) | 0.28 (−2.11 to 2.67) | .67 |
| Anxiety disorders, No./total patients (%) | 9/69 (13) | 16/85 (19) | 1.47 (0.59 to 3.70)c | .41 | 9/61 (15) | 18/76 (18) | 1.13 (0.42 to 3.04)c | .81 |
| RSES score, mean (SD) | 20.4 (8.3) | 21.5 (6.9) | 0.74 (−0.98 to 2.44) | .40 | 21.4 (7.9) | 22.1 (7.1) | −0.10 (−1.68 to 1.86) | .92 |
| Quality of life | ||||||||
| IWQOL-Lite score, mean (SD) | 65.8 (25.6) | 63.8 (22.6) | −3.30 (−8.80 to 2.22) | .25 | 61.8 (26.3) | 59.5 (24.1) | −4.01 (−9.68 to 1.68) | .17 |
| CIA score, mean (SD) | 12.1 (10.9) | 11.9 (11.1) | 0.04 (−2.53 to 2.64) | .97 | 11.9 (11.5) | 10.5 (10.9) | −1.02 (−3.72 to 1.70) | .47 |
Abbreviations: BDI-II, Beck Depression Inventory II; BED, binge eating disorder; BMI, body mass index; CBT, cognitive behavioral therapy; CIA, Clinical Impairment Assessment; GSH-I, internet-based guided self-help; IWQOL-Lite, Impact of Weight on Quality of Life–Lite; OBE, objective binge eating, RSES, Rosenberg Self-Esteem Scale.
Effects are adjusted for baseline values of age and sex (fixed effects), OBE days, BMI, BDI-II score, and Eating Disorder Examination–Interview global score; the individual patient and the study center were incorporated as random intercepts.
Adjusted multiplicative effects from negative binomial model.
Adjusted odds ratios.
Calculated as weight in kilograms divided by height in meters squared.
Secondary Outcomes
Abstinence
Cognitive behavioral therapy was superior to GSH-I at the end of treatment (abstinence rate, 61% vs 36%) and at 6-month follow-up (abstinence rate, 58% vs 38%) (Table 3 and eTable 2 and eFigure 2 in Supplement 2).
Eating Disorder Psychopathologic Findings
Cognitive behavioral therapy was superior to GSH-I on the EDE-I global score and on shape and weight concern subscale scores at 6-month follow-up. Overall, values decreased in both groups (Table 3).
Body Mass Index
The BMI did not decrease in either treatment group, and there was no difference between groups at the end of treatment and at 6-month follow-up (Table 3). Patients who were abstinent at the end of treatment had a significantly higher BMI reduction compared with nonabstinent patients who experienced a slight BMI increase (mean [SD] BMI, −0.5 [1.6] vs +0.3 [1.4]; P = .01).
Comorbid Psychopathologic Findings and Quality of Life
The GSH-I and CBT groups did not differ with regard to BDI-II, RSES, IWQOL-Lite, and CIA scale scores at the end of treatment and 6-month follow-up (Table 3). In addition, the frequency of affective and anxiety disorders did not differ significantly between groups at both time points. Overall, values decreased in both treatment conditions.
Additional Post Hoc Analyses
Long-term Follow-up
Not all patients signed consent forms (40, including 4 study dropouts) and thus were not contacted again. Three additional patients dropped out of the study later, and 19 were unavailable for follow-up. Thus, at the 1.5-year follow-up, we were able to assess 116 patients (65.2%) (58 in the GSH-I group and 58 in the CBT group). At the 1.5-year follow-up, the number of OBE days did not differ between the treatment groups (eTable 4 and eFigure 1 in Supplement 2). This result did not change after including only those patients in the analyses for whom long-term data were available (n = 116) (eTable 5 in Supplement 2). The number of patients who were abstinent from binge eating during the previous 28 days was 27 of 58 (46.6%) in the CBT group and 25 of 58 (43.1%) in the GSH-I group (eFigure 2 in Supplement 2).
Dropout
Study dropout (n = 7) and treatment dropout were generally low. No group differences were found for the number of patients with insufficient treatment dose and the number of patients who were excluded from the per-protocol analysis. However, significantly fewer patients in the CBT group were unavailable at the end of treatment and at 6-month follow-up (eTable 6 in Supplement 2).
Adherence With GSH-I
Number of modules completed, number of days completed in the diary, and messages exchanged with the coach were used as indicators of adherence. Among the 83 participants in the mITT sample, 37 (44.6%) completed all 11 modules, and 67 (80.7%) reached module 6. In the per-protocol sample, 34 of 72 (47.2%) completed all 11 modules and 61 (84.7%) reached module 6. All associations between markers of adherence and the difference in the number of days with OBEs between T0 and T2 were nonsignificant in the per-protocol and the mITT samples (eAppendix and eTable 7 in Supplement 2).
Discussion
INTERBED represents, to our knowledge, the first multicenter randomized clinical trial to compare GSH-I with CBT in patients with BED. Confirmatory analysis did not prove noninferiority of GSH-I compared with CBT. On the contrary, exploratory analysis found that GSH-I was inferior to CBT in reducing OBE days and in producing abstinence from binge eating at the end of treatment and at 6-month follow-up. However, OBE days decreased significantly in both groups, with changes maintained at 6-month follow-up. In both groups, eating disorder and general psychopathologic findings, as well as quality of life and impairment caused by the disorder, were also improved. In addition, CBT was superior to GSH-I in reducing eating disorder psychopathologic findings but not in reducing general psychopathologic findings, quality of life, and impairment caused by the disorder.
Dropout rates were low; 91.0% of the CBT group and 80.9% of the GSH-I group were included into the per-protocol sample. However, CBT was more successful in retaining patients in the trial than was GSH-I. Similar results were reported by Wilson et al, who found dropout rates of 7% in individual IPT and 30% in book-based GSH at the end of treatment, although their definition of dropout was stricter. Adherence with the internet program was satisfactory and comparable to that in other studies using the same program. No association between indicators of adherence and the main outcome could be found.
As in other studies, mean BMI did not change over time in either treatment group; however, abstinent patients achieved a significantly larger weight loss, the magnitude of which, however, was small (BMI, −0.5). Masheb et al reported that 84 patients with BED (65%) gained a clinically significant amount of weight (≥5% body weight) in the year preceding treatment. Failure to produce weight loss may be reinterpreted as stabilization of weight and prevention of further weight gain.
Long-term follow-up (1.5 years after end of treatment) was conducted in a subsample of 116 patients (65.2% of the randomized sample); thus, the available data need to be carefully interpreted. At 1.5 years, no significant differences between treatments on any measure of eating behavior were found. This lack of difference is in line with the 2-year follow-up in the study by Wilson et al that compared IPT with book-based GSH. In our study, the number of OBE days slightly increased in both groups from the end of treatment to the 1.5-year follow-up. However, the number of OBE days was still significantly lower than baseline values, with abstinence rates of 47% in the CBT and 44% in the GSH-I condition, which is an acceptable long-term result in both groups.
The results are comparable to a recently published study that compared online chat groups with face-to-face group therapy in patients with bulimia nervosa. As in our study, the authors did not find noninferiority of the online chat group intervention compared with the face-to-face group intervention. Face-to-face treatment was superior to internet-based intervention. By 12-month follow-up, however, the differences were no longer present, which was mainly attributable to additional improvement in the online chat group intervention. In line with our results, the outcomes suggest a slower trajectory of improvement in internet-based self-help than in specialist therapy.
Strengths and Limitations
INTERBED is the first study, to our knowledge, to directly compare GSH-I with CBT in BED using rigorous methods. The study is adequately powered, used a noninferiority approach, was supported by an independent data center, and included manualized treatments, trained therapists, ongoing adherence ratings to ensure treatment fidelity, and independent assessors who conducted standardized outcome assessments. The inclusion of 7 centers increases the generalizability of the results. Treatment attrition and study dropout during treatment were low.
Among the limitations of our study is the loss of follow-up data by 1.5 years, which was mainly attributable to the late acquisition of funding for the extended follow-up. However, among those who consented (n = 138), the follow-up rate was 85%. Furthermore, treatment at all sites was delivered within the context of specialty university-based eating disorder clinics. Accordingly, the generalizability of our results to other populations and treatment in nonspecialist settings is limited. Only 12% of patients were men, and participants were highly educated. Future studies should specifically try to address men and those with low educational levels. Finally, therapist allegiance effects on outcome cannot be excluded; however, the allegiance outcome association has been reported to be weaker when the methodologic quality of a study is high.
Conclusions
Overall, our results suggest that face-to-face CBT is likely to be a better initial treatment option compared with GSH-I. Face-to-face CBT leads to quicker and greater reductions in the number of OBE days, abstinence rates, and eating disorder psychopathologic findings. However, given that improvements were significant in both treatment conditions, the effect size of the difference of the main outcome between treatment conditions was small, and there were no statistical differences between the treatment conditions at 1.5 years after treatment, GSH-I remains a viable, low-threshold treatment alternative for this patient population, for example, in a stepped-care approach. Cost-effectiveness analysis will show whether GSH-I was associated with lower costs compared with CBT. Communication technologies might offer significant benefits for delivering psychotherapy, including lowering barriers to access.
Trial Protocol.
eAppendix. Supplementary Information
eTable 1. Sensitivity Analyses for the Primary Outcome (Changes in OBE Days Between T0 and T2)
eTable 2. Effect Sizes for Secondary Outcomes
eTable 3. Within-Group Changes of the Primary Outcome (OBE Days) Using the Intention-to-Treat Sample Without Imputations
eTable 4. Exploratory Longitudinal Analysis for the Number of OBE Days During the Last 28 Days Also Containing the 1.5-Year Follow-up Data
eTable 5. Exploratory Longitudinal Analysis for the Number of OBE Days During the Last 28 Days Including Only the 116 Patients for Whom 1.5-Year Follow-up Data Are Available (Completer)
eTable 6. Comparison of Dropout Rates Using Different Definitions (Whole Sample n = 178)
eTable 7. Indicators of Adherence to GSH-I and Correlations of Adherence Measures With Primary Outcome (Change in OBE Days)
eFigure 1. Course of OBE Days Also Containing the Follow-up Data at T4
eFigure 2. Abstinence Rates (Percentages of Patients With Zero OBE Days During the Last 28 Days) by Time and Treatment Condition
eReferences
References
- 1.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 5th ed Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 2.Kessler RC, Berglund PA, Chiu WT, et al. . The prevalence and correlates of binge eating disorder in the World Health Organization World Mental Health Surveys. Biol Psychiatry. 2013;73(9):904-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohler-Kuo M, Schnyder U, Dermota P, Wei W, Milos G. The prevalence, correlates, and help-seeking of eating disorders in Switzerland. Psychol Med. 2016;46(13):2749-2758. [DOI] [PubMed] [Google Scholar]
- 4.Brownley KA, Berkman ND, Peat CM, et al. . Binge-eating disorder in adults: a systematic review and meta-analysis. Ann Intern Med. 2016;165(6):409-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crow SJ, Stewart Agras W, Halmi K, Mitchell JE, Kraemer HC. Full syndromal versus subthreshold anorexia nervosa, bulimia nervosa, and binge eating disorder: a multicenter study. Int J Eat Disord. 2002;32(3):309-318. [DOI] [PubMed] [Google Scholar]
- 6.Ágh T, Kovács G, Supina D, et al. . A systematic review of the health-related quality of life and economic burdens of anorexia nervosa, bulimia nervosa, and binge eating disorder. Eat Weight Disord. 2016;21(3):353-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vocks S, Tuschen-Caffier B, Pietrowsky R, Rustenbach SJ, Kersting A, Herpertz S. Meta-analysis of the effectiveness of psychological and pharmacological treatments for binge eating disorder. Int J Eat Disord. 2010;43(3):205-217. [DOI] [PubMed] [Google Scholar]
- 8.McElroy SL, Guerdjikova AI, Mori N, Munoz MR, Keck PE. Overview of the treatment of binge eating disorder. CNS Spectr. 2015;20(6):546-556. [DOI] [PubMed] [Google Scholar]
- 9.Hilbert A, Bishop ME, Stein RI, et al. . Long-term efficacy of psychological treatments for binge eating disorder. Br J Psychiatry. 2012;200(3):232-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson GT. Treatment of binge eating disorder. Psychiatr Clin North Am. 2011;34(4):773-783. [DOI] [PubMed] [Google Scholar]
- 11.Herpertz S, Hagenah U, Vocks S, von Wietersheim J, Cuntz U, Zeeck A; German Society of Psychosomatic Medicine and Psychotherapy; German College for Psychosomatic Medicine . The diagnosis and treatment of eating disorders. Dtsch Arztebl Int. 2011;108(40):678-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beintner I, Jacobi C, Schmidt UH. Participation and outcome in manualized self-help for bulimia nervosa and binge eating disorder: a systematic review and metaregression analysis. Clin Psychol Rev. 2014;34(2):158-176. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro JR, Reba-Harrelson L, Dymek-Valentine M, Woolson SL, Hamer RM, Bulik CM. Feasibility and acceptability of CD-ROM-based cognitive-behavioural treatment for binge-eating disorder. Eur Eat Disord Rev. 2007;15(3):175-184. [DOI] [PubMed] [Google Scholar]
- 14.Carrard I, Crépin C, Rouget P, Lam T, Golay A, Van der Linden M. Randomised controlled trial of a guided self-help treatment on the Internet for binge eating disorder. Behav Res Ther. 2011;49(8):482-491. [DOI] [PubMed] [Google Scholar]
- 15.Aardoom JJ, Dingemans AE, Spinhoven P, Van Furth EF. Treating eating disorders over the internet: a systematic review and future research directions. Int J Eat Disord. 2013;46(6):539-552. [DOI] [PubMed] [Google Scholar]
- 16.Kordy H. Internet- and media-assisted therapy. Psychother Psych Med. 2013;63:12-18. [DOI] [PubMed] [Google Scholar]
- 17.Berkman ND, Brownley KA, Peat CM, et al. , eds. Management and Outcomes of Binge-Eating Disorder Rockville, MD: Agency for Healthcare Research and Quality; 2015. Report 15(16)-EHC030-EF. AHRQ Comparative Effectiveness Reviews. [PubMed] [Google Scholar]
- 18.Wilson GT, Wilfley DE, Agras WS, Bryson SW. Psychological treatments of binge eating disorder. Arch Gen Psychiatry. 2010;67(1):94-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Zwaan M, Herpertz S, Zipfel S, et al. . INTERBED: internet-based guided self-help for overweight and obese patients with full or subsyndromal binge eating disorder: a multicenter randomized controlled trial. Trials. 2012;13:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hilbert A, Tuschen-Caffier B. Essanfälle und Adipositas: Ein Manual zur Kognitiv-Behavioralen Therapie der Binge-Eating-Störung. Göttingen, Germany: Hogrefe; 2011. [Google Scholar]
- 21.Hilbert A, Tuschen-Caffier B. Body image interventions in cognitive-behavioural therapy of binge-eating disorder: a component analysis. Behav Res Ther. 2004;42(11):1325-1339. [DOI] [PubMed] [Google Scholar]
- 22.Carrard I, Crépin C, Rouget P, Lam T, Van der Linden M, Golay A. Acceptance and efficacy of a guided internet self-help treatment program for obese patients with binge eating disorder. Clin Pract Epidemiol Ment Health. 2011;7:8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fairburn CG, Cooper Z. The Eating Disorder Examination In: Fairburn CG, Wilson GT, eds. Binge Eating: Nature, Assessment, and Treatment. 12th ed New York, NY: Guilford Press; 1993:317-360. [Google Scholar]
- 24.Hilbert A, Tuschen-Caffier B, Ohms M. Eating Disorder Examination: Deutschsprachige Version des Strukturierten Essstörungsinterviews. Diagnostica. 2004;50:98-106. [Google Scholar]
- 25.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 26.Wittchen HU, Zaudig M, Fydrich T. Strukturiertes Klinisches Interview für DSMIV (SKID). Göttingen, Germany: Hogrefe; 1997. [Google Scholar]
- 27.Beck AT, Steer RA, Brown GK. Beck Depression Inventory–II (BDI–II). San Antonio, TX: Harcourt Assessment Inc; 1996. [Google Scholar]
- 28.Hautzinger M, Keller F, Kühner C. Beck Depression Inventory (BDI-II) Revision. Frankfurt/Main. Germany: Harcourt Test Services; 2006. [Google Scholar]
- 29.Rosenberg M. Society and Adolescent Self-image. Princeton, NJ: Princeton University Press; 1965. [Google Scholar]
- 30.Ferring D, Filipp SH. Messung des selbstwertgefühls: befunde zu reliabilität, validität und stabilität der rosenberg-skala. Diagnostica. 1996;42:284-292. [Google Scholar]
- 31.Kolotkin RL, Crosby RD. Psychometric evaluation of the Impact of Weight on Quality of Life-Lite questionnaire (IWQOL-lite) in a community sample. Qual Life Res. 2002;11(2):157-171. [DOI] [PubMed] [Google Scholar]
- 32.Mueller A, Holzapfel C, Hauner H, et al. . Psychometric evaluation of the German version of the Impact of Weight on Quality of Life-Lite (IWQOL-Lite) questionnaire. Exp Clin Endocrinol Diabetes. 2011;119(2):69-74. [DOI] [PubMed] [Google Scholar]
- 33.Bohn K, Doll HA, Cooper Z, O’Connor M, Palmer RL, Fairburn CG. The measurement of impairment due to eating disorder psychopathology. Behav Res Ther. 2008;46(10):1105-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.R Development Core Team R: A Language and Environment for Statistical Computing Vienna, Austria: R Foundation for Statistical Computing; 2012. http://www.R-project.org/. Accessed May 27, 2015.
- 35.Snapinn SM. Noninferiority trials. Curr Control Trials Cardiovasc Med. 2000;1(1):19-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matilde Sanchez M, Chen X. Choosing the analysis population in non-inferiority studies: per protocol or intent-to-treat. Stat Med. 2006;25(7):1169-1181. [DOI] [PubMed] [Google Scholar]
- 37.van Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations. J Stat Softw. 2011;45(3):1-67. [Google Scholar]
- 38.Cohen J. A power primer. Psychol Bull. 1992;112(1):155-159. [DOI] [PubMed] [Google Scholar]
- 39.Masheb RM, White MA, Grilo CM. Substantial weight gains are common prior to treatment-seeking in obese patients with binge eating disorder. Compr Psychiatry. 2013;54(7):880-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zerwas SC, Watson HJ, Hofmeier SM, et al. . CBT4BN: a randomized controlled trial of online chat and face-to-face group therapy for bulimia nervosa. Psychother Psychosom. 2017;86(1):47-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munder T, Gerger H, Trelle S, Barth J. Testing the allegiance bias hypothesis: a meta-analysis. Psychother Res. 2011;21(6):670-684. [DOI] [PubMed] [Google Scholar]
- 42.Dragioti E, Dimoliatis I, Fountoulakis KN, Evangelou E. A systematic appraisal of allegiance effect in randomized controlled trials of psychotherapy. Ann Gen Psychiatry. 2015;14:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moessner M, Minarik C, Özer F, Bauer S. Can an internet-based program for the prevention and early intervention in eating disorders facilitate access to conventional professional healthcare? J Ment Health. 2016;25(5):441-447. [DOI] [PubMed] [Google Scholar]
- 44.Melioli T, Bauer S, Franko DL, et al. . Reducing eating disorder symptoms and risk factors using the internet: a meta-analytic review. Int J Eat Disord. 2016;49(1):19-31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
eAppendix. Supplementary Information
eTable 1. Sensitivity Analyses for the Primary Outcome (Changes in OBE Days Between T0 and T2)
eTable 2. Effect Sizes for Secondary Outcomes
eTable 3. Within-Group Changes of the Primary Outcome (OBE Days) Using the Intention-to-Treat Sample Without Imputations
eTable 4. Exploratory Longitudinal Analysis for the Number of OBE Days During the Last 28 Days Also Containing the 1.5-Year Follow-up Data
eTable 5. Exploratory Longitudinal Analysis for the Number of OBE Days During the Last 28 Days Including Only the 116 Patients for Whom 1.5-Year Follow-up Data Are Available (Completer)
eTable 6. Comparison of Dropout Rates Using Different Definitions (Whole Sample n = 178)
eTable 7. Indicators of Adherence to GSH-I and Correlations of Adherence Measures With Primary Outcome (Change in OBE Days)
eFigure 1. Course of OBE Days Also Containing the Follow-up Data at T4
eFigure 2. Abstinence Rates (Percentages of Patients With Zero OBE Days During the Last 28 Days) by Time and Treatment Condition
eReferences