Abstract
Importance
Schizophrenia is associated with widespread cognitive impairments. Although cognitive deficits are one of the factors most strongly associated with functional outcome in schizophrenia, current treatment strategies largely fail to ameliorate these impairments. To develop more efficient treatment strategies in patients with schizophrenia, a better understanding of the pathogenesis of these cognitive deficits is needed. Accumulating evidence indicates that genetic risk of schizophrenia may contribute to cognitive dysfunction.
Objective
To identify genomic regions jointly influencing schizophrenia and the cognitive domains of reaction time and verbal-numerical reasoning, as well as general cognitive function, a phenotype that captures the shared variation in performance across cognitive domains.
Design, Setting, and Participants
Combining data from genome-wide association studies from multiple phenotypes using conditional false discovery rate analysis provides increased power to discover genetic variants and could elucidate shared molecular genetic mechanisms. Data from the following genome-wide association studies, published from July 24, 2014, to January 17, 2017, were combined: schizophrenia in the Psychiatric Genomics Consortium cohort (n = 79 757 [cases, 34 486; controls, 45 271]); verbal-numerical reasoning (n = 36 035) and reaction time (n = 111 483) in the UK Biobank cohort; and general cognitive function in CHARGE (Cohorts for Heart and Aging Research in Genomic Epidemiology) (n = 53 949) and COGENT (Cognitive Genomics Consortium) (n = 27 888).
Main Outcomes and Measures
Genetic loci identified by conditional false discovery rate analysis. Brain messenger RNA expression and brain expression quantitative trait locus functionality were determined.
Results
Among the participants in the genome-wide association studies, 21 loci jointly influencing schizophrenia and cognitive traits were identified: 2 loci shared between schizophrenia and verbal-numerical reasoning, 6 loci shared between schizophrenia and reaction time, and 14 loci shared between schizophrenia and general cognitive function. One locus was shared between schizophrenia and 2 cognitive traits and represented the strongest shared signal detected (nearest gene TCF20; chromosome 22q13.2), and was shared between schizophrenia (z score, 5.01; P = 5.53 × 10−7), general cognitive function (z score, –4.43; P = 9.42 × 10−6), and verbal-numerical reasoning (z score, –5.43; P = 5.64 × 10−8). For 18 loci, schizophrenia risk alleles were associated with poorer cognitive performance. The implicated genes are expressed in the developmental and adult human brain. Replicable expression quantitative trait locus functionality was identified for 4 loci in the adult human brain.
Conclusions and Relevance
The discovered loci improve the understanding of the common genetic basis underlying schizophrenia and cognitive function, suggesting novel molecular genetic mechanisms.
This analysis of genome-wide association studies identifies genomic regions jointly influencing schizophrenia and the cognitive domains of reaction time and verbal-numerical reasoning, as well as general cognitive function.
Key Points
Question
What genetic loci jointly influence schizophrenia and cognitive function?
Findings
In this analysis of genome-wide association studies on schizophrenia and cognitive traits in more than 250 000 participants, 21 genomic regions were found to be shared between schizophrenia and cognitive traits.
Meaning
The findings provide new insights into the common genetic basis underlying schizophrenia and cognitive function, suggesting novel molecular genetic mechanisms.
Introduction
Schizophrenia is a severe, chronic psychiatric disorder that ranks among the leading causes of disability worldwide. Although the diagnosis of schizophrenia is based on the presence of positive and negative symptoms, cognitive dysfunction is regarded as a core component of the disorder. Compared with healthy individuals, patients with schizophrenia display widespread cognitive impairments including deficits in learning, memory, processing speed, attention, and executive functioning. Cognitive dysfunction often precedes the onset of psychosis by several years and is an important factor associated with functional outcomes in schizophrenia. Despite this fact, current treatment strategies largely fail to ameliorate the cognitive deficits in patients with schizophrenia. To develop more efficient treatment strategies for patients with schizophrenia, a better understanding of the pathogenesis underlying these cognitive deficits is needed.
Accumulating evidence indicates that the genetic risk of schizophrenia may contribute to cognitive impairment. Unaffected relatives of patients with schizophrenia display cognitive deficits, and family and twin studies find that the genetic liabilities of schizophrenia and cognitive abilities covary. Further evidence comes from analyses of genome-wide association study (GWAS) data. Polygenic risk scores based on GWAS data for schizophrenia are associated with decreased cognitive abilities in nonclinical cohorts, while polygenic risk for lower cognitive abilities is associated with increased likelihood of schizophrenia. Moreover, recent analyses of GWAS data estimated significant negative correlations between the genomic architectures of schizophrenia and different cognitive traits known to be affected in schizophrenia, including general cognitive function (GCF), verbal-numerical reasoning (VNR), and reaction time (RT). However, despite the robust evidence for a common genetic basis between cognitive dysfunction and schizophrenia, the specific gene variants jointly influencing schizophrenia and cognitive traits remain to be determined.
Analyses of GWAS data have estimated single-nucleotide polymorphism (SNP)–based heritabilities of 33% for schizophrenia, 28% for GCF, 31% for VNR, and 11% for RT. To date, 5 genome-wide significant loci are identified for GCF, 3 loci for VNR, and 2 loci for RT, while more than 100 loci are identified for schizophrenia. However, despite the assembly of very large GWAS cohorts, the identified genome-wide significant loci explain only a small fraction of the heritability of these phenotypes. To improve discovery of genetic variants in polygenic human disorders, we have developed a conditional false discovery rate (cFDR) statistical approach that includes all available variants in 2 independent GWASs. The cFDR method enables combined analysis of GWAS data with increased power to discover overlapping genetic variants and could elucidate shared molecular genetic mechanisms. Using this approach, studies have identified shared loci between schizophrenia, Alzheimer disease, immune-related diseases, and associated phenotypes and substantially increased the number of identified risk loci. Here, we used the same statistical approach, taking advantage of several large GWASs, to identify common genetic variants shared between schizophrenia and VNR, RT, and GCF.
Methods
Participant Samples
We obtained GWAS results in the form of summary statistics (P values and z scores). Data on schizophrenia were acquired from the Psychiatric Genomics Consortium (n = 79 757), data on GCF were from the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) (n = 53 949) and the Cognitive Genomics Consortium (COGENT) (n = 27 888), and data on VNR (n = 36 035), and RT (n = 111 483) were from the UK Biobank. These studies were published from July 24, 2014, to January 17, 2017. Details of the inclusion criteria, phenotype characteristics, and genotyping are described in the original publications. Overlapping cohorts (n = 7410) between CHARGE and COGENT were excluded from the COGENT GWAS (n = 35 298 in the full cohort). Participants from the Betula Study and the Hunter Community Study were involved in both the schizophrenia GWAS and the GCF GWAS by CHARGE, thus possibly implicating that 611 participants used as controls in the schizophrenia GWAS contributed to the CHARGE GWAS. To avoid any potential bias, we excluded the Betula Study and Hunter Community Study cohorts (n = 2558) from the schizophrenia data. All P values were corrected for inflation using a genomic inflation control procedure. All GWASs performed and investigated in the present study were approved by the local ethics committees, and informed consent was obtained from all participants. Furthermore, the Norwegian Institutional Review Board for the South-East Norway Region has evaluated the current protocol and found that no additional institutional review board approval was needed because no individual data were used.
Cognitive Phenotypes
We analyzed adequately powered GWASs for cognitive phenotypes known to be affected in schizophrenia: GCF, RT, and VNR. General cognitive function accounts for approximately 40% to 50% of the variation across cognitive domains. For each cohort contributing to the GWAS meta-analyses on GCF by CHARGE and COGENT, the GCF phenotype was constructed using the first unrotated component extracted from a principal components analysis of the individual cognitive test scores. The RT test was a computerized “Snap” game, in which participants were to press a button as quickly as possible when symbols of 2 “cards” on a computer screen were matching. There were 8 experimental trials, of which 4 had matching symbols. Each participant’s RT score was his or her mean time to press the button for these 4 matching trials. Verbal-numerical reasoning was measured using a 13-item test assessing verbal and arithmetical deduction. The test included 6 verbal and 7 numerical questions, all with multiple-choice answers, and had a total time limit of 2 minutes. For full details of each test, see the original publications.
Statistical Analysis
To assess for pleiotropic enrichment, we constructed conditional quantile-quantile plots, which compare the association with a primary trait (eg, schizophrenia) across all SNPs and within SNP strata determined by their association with a secondary trait (eg, GCF), and provide a visual pattern of overlap in SNP associations. For given associated phenotypes A and B, pleiotropic enrichment of phenotype A with phenotype B exists if the proportion of SNPs or genes associated with phenotype A increases as a function of increased association with phenotype B. The enrichment seen can be directly interpreted in terms of true discovery rate (1 − FDR). To identify shared loci between schizophrenia and cognitive traits, we used the cFDR framework. The cFDR is an extension of the standard FDR and incorporates information from GWAS summary statistics of a secondary phenotype to rerank the test statistics. We identified shared loci at a conjunctional FDR (conjFDR) less than .05, which is given by the maximum between the cFDRs for both phenotypes. The conjFDR analysis is a conservative approach requiring that loci exceed a cFDR significance threshold for 2 traits jointly. Because the cognitive traits investigated are not independent, we did not perform a Bonferroni correction. The risk loci were annotated to the closest gene. Given the long-range linkage disequilibrium (LD) within the extended major histocompatibility complex and its strong association with schizophrenia, we excluded SNPs in this region (genome build 19 location, 25652429-33368333) and SNPs in LD (r2>0.1) with such SNPs before fitting the cFDR model. For details, see the eAppendix in the Supplement. The significance threshold for identification of shared loci was conjFDR < .05; for conditional loci, cFDR < .01; for gene set enrichment, FDR < .05; and for expression quantitative trait locus (eQTL) functionality, FDR < .05.
Biological Context
We evaluated the biological context of the identified genetic variants. First, we determined the distribution of messenger RNA expression in the developing and adult human brain using data from the Human Brain Transcriptome Project and The UK Brain Expression Consortium (UKBEC). Second, we assessed whether the conjunctional SNPs have brain eQTL functionality using Genotype-Tissue Expression (GTEx) and UKBEC (UK Brain Expression Consortium) data. Finally, we determined whether genes in the loci shared between schizophrenia and GCF were enriched for reconstituted versions of gene sets using the computational tool Data-driven Expression Prioritized Integration for Complex Traits (DEPICT). For details, see the eAppendix in the Supplement.
Results
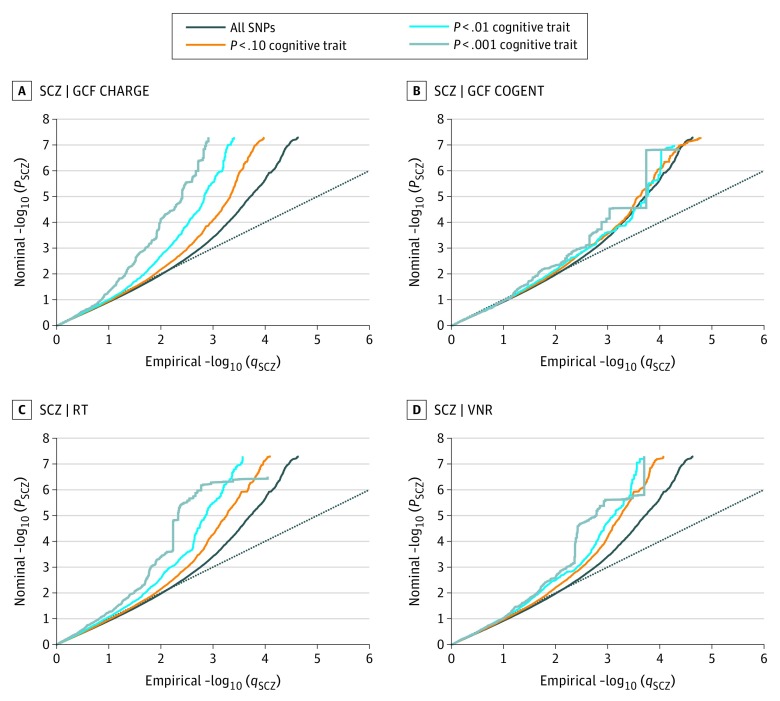
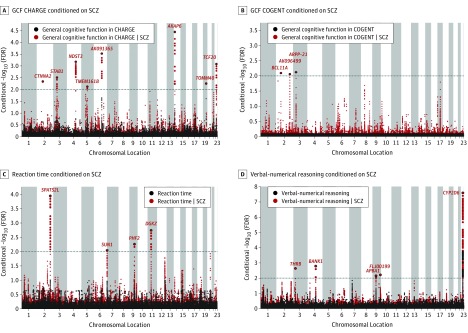
We observed SNP enrichment for schizophrenia with VNR, RT, and GCF in the CHARGE and COGENT cohorts, indicating polygenic overlap between these phenotypes (Figure 1), in line with results of previous work. The reverse conditional quantile-quantile plots demonstrate consistent enrichment in associations with GCF, VNR, and RT as a function of schizophrenia associations (eFigure 1 in the Supplement). To increase discovery of SNPs associated with cognitive traits, we ranked GCF, VNR, and RT SNPs conditional on their genetic association with schizophrenia (cFDR). At cFDR less than .01, we identified 8 loci associated with GCF in the CHARGE cohort, 3 loci associated with GCF in the COGENT cohort, 5 loci associated with VNR, and 4 loci associated with RT (eTable 1 in the Supplement). Figure 2 shows cFDR Manhattan plots for GCF, VNR, and RT conditional on schizophrenia, showing all SNPs with a cFDR less than .01 within an LD block in relation to their chromosomal location. The figures demonstrate the increased power for SNP discovery gained by conditioning on association with schizophrenia.
Figure 1. Polygenic Overlap Between Schizophrenia (SCZ) and General Cognitive Function (GCF), Reaction Time (RT), and Verbal-Numerical Reasoning (VNR).
Conditional quantile-quantile plots of nominal vs empirical −log10 P values (corrected for inflation) in SCZ below the standard genome-wide association study threshold of P < 5 × 10−8 as a function of significance of association with GCF in CHARGE (Cohorts for Heart and Aging Research in Genomic Epidemiology) (A), GCF in COGENT (Cognitive Genomics Consortium) (B), RT (C), and VNR (D), at the level of −log10 (P) ≥ 1, −log10 (P) ≥ 2, and −log10 (P) ≥ 3, corresponding to P ≤ .10, P ≤ .01, and P ≤ .001, respectively. The dashed lines indicate the null hypothesis.
Figure 2. Conditional False Discovery Rate (cFDR) Manhattan Plots of Conditional −log10 (FDR) Values.
A, General cognitive function in CHARGE (Cohorts for Heart and Aging Research in Genomic Epidemiology) conditioned on schizophrenia (SCZ). B, General cognitive function in COGENT (Cognitive Genomics Consortium) conditioned on SCZ. C, Reaction time conditioned on SCZ. D, Verbal-numerical reasoning conditioned on SCZ. Unconditioned FDR values are shown in black, cFDR values in red. Single-nucleotide polymorphisms (SNPs) with conditional −log10 (FDR) higher than 2.0 (horizontal dotted line) (ie, cFDR < .01) are shown with large points. A black line around the large points indicates the most significant SNP in each linkage disequilibrium block; this SNP is annotated with the closest gene.
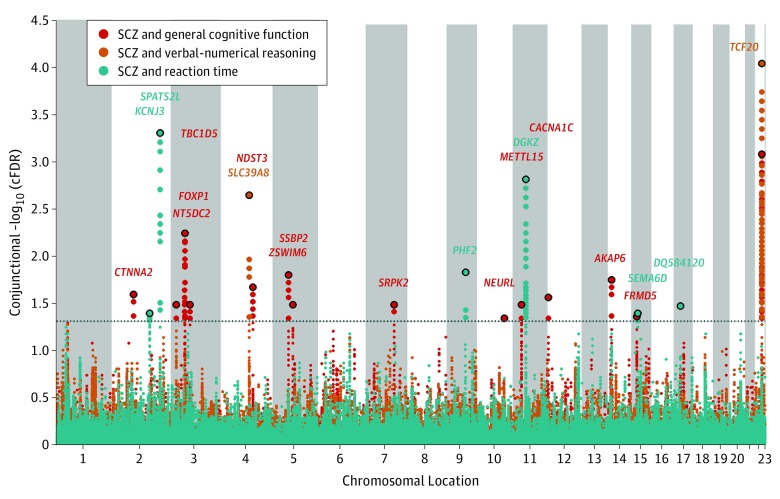
To provide a comprehensive, unselected map of shared loci between schizophrenia and GCF, VNR, and RT, we performed a conjFDR analysis (Figure 3). Based on a conjFDR less than .05, we identified 21 independent genetic loci shared between schizophrenia and cognitive traits. Specifically, we identified 14 loci shared between schizophrenia and GCF in the CHARGE cohort, 2 loci shared between schizophrenia and VNR, and 6 loci shared between schizophrenia and RT, on a total of 13 chromosomes (1 locus was shared between schizophrenia and 2 cognitive traits) (Table). We detected no loci shared between schizophrenia and GCF in the COGENT cohort. The strongest shared signal detected (nearest gene TCF20 [OMIM 603107]; chromosome 22q13.2) was shared between schizophrenia (z score, 5.01; P = 5.53 × 10−7), GCF (z score, –4.43; P = 9.42 × 10−6), and VNR (z score, –5.43; P = 5.64 × 10−8), demonstrating the importance of the locus for brain function. Thirteen of 21 conjunctional loci have P values less than .05 in at least 2 cognitive traits. To visualize the shared loci, we constructed a conjFDR Manhattan plot (Figure 3). All SNPs without pruning are shown, and the strongest signal in each LD block is encircled in black. The enlarged data points represent the SNPs at conjFDR less than .05, whereas the small points represent other SNPs. On the basis of 1000 Genomes Project LD structure, significant SNPs identified by conjFDR less than .05 were clustered into LD blocks at the LD level of r2 > 0.1. These blocks are numbered in the Table. Any block may contain more than 1 SNP. Genes close to each locus were obtained from the National Center for Biotechnology Information gene database (https://www.ncbi.nlm.nih.gov).
Figure 3. Conjunctional False Discovery Rate (FDR) Manhattan Plot of Conjunctional −log10 (FDR) Values for Schizophrenia (SCZ) and General Cognitive Function, Verbal-Numerical Reasoning, or Reaction Time.
Single-nucleotide polymorphisms (SNPs) with conjunctional −log10 (FDR) >1.3, ie, conjunctional FDR <.05, are shown with enlarged data points. A black circle around the enlarged data points indicates the most significant SNP in each linkage disequilibrium block; this SNP was annotated with the closest gene, which is listed above the symbols in each locus. The localization of the conjunctional loci is shown; further details are provided in the Table.
Table. Genetic Loci With Conjunctional False Discovery Rate Lower Than .05 Shared Between Schizophrenia and GCF, VNR, or RTa.
| Locus No. | SNP | Chr | Closest Gene (Region) | A1/A2 | z Score | Conjunctional False Discovery Rate | P Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCZ | GCF in CHARGE | VNR | RT | SCZ and GCF in CHARGE | SCZ and VNR | SCZ and RT | SCZ | GCF in CHARGE | VNR | RT | GCF in COGENT | |||||
| 1 | rs13024343 | 2p12 | CTNNA2 (intron) | A/T | NAb | NA | NA | NA | 2.61E−2c | 9.19E−1 | 6.98E−1 | 6.57E−4 | 2.92E−5d | 6.09E−1 | 2.67E−1 | 2.45E−2d |
| 2 | rs67338739 | 2q24.1 | KCNJ3 (intergenic) | C/T | 3.44 | 1.90 | 2.06 | −3.61 | 5.56E−1 | 4.67E−1 | 4.13E−2c | 5.84E−4 | 5.71E−2 | 3.98E−2d | 3.12E−4d | 2.28E−1 |
| 3 | rs6435048f,g | 2q33.1 | SPATS2L (intergenic) | T/C | 4.84 | 0.14 | −3.17 | 4.71 | >.99 | 1.40E−1 | 4.98E−4c | 1.31E−6 | 8.87E−1e | 1.54E−3d | 2.48E−6d | 8.57E−1 |
| 4 | rs1545424f | 3p24.3 | TBC1D5 (intron) | A/T | NAb | NA | NA | NA | 3.33E−2c | >.99 | 7.67E−1 | 1.04E−4 | 3.02E−4d | 9.94E−1 | 4.01E−1 | 5.46E−1 |
| 5 | rs4282054f | 3p21.1 | NT5DC2 (intron) | T/C | 3.88 | −4.29 | −2.36 | 0.81 | 5.82E−3c | 3.96E−1 | 7.67E−1 | 1.04E−4 | 1.82E−5d | 1.85E−2d | 4.18E−1 | 8.58E−1 |
| 6 | rs9842406 | 3p13 | FOXP1 (intron) | G/T | −4.08 | 3.58 | 1.85 | −1.98 | 3.33E−2c | 5.20E−1 | 4.98E−1 | 4.58E−5 | 3.49E−4d | 6.50E−2 | 4.72E−2d | 3.17E−2d |
| 7 | rs13107325f | 4q24 | SLC39A8 (missense) | T/C | 5.51 | −1.08 | −4.38 | 1.72 | 7.45E−1 | 2.28E−3c | 5.62E−1 | 3.52E−8 | 2.78E−1 | 1.17E−5d | 8.54E−2 | 5.56E−1 |
| 8 | rs4833558 | 4q26 | NDST3 (intergenic) | C/T | 3.48 | −4.45 | 0.77 | 0.92 | 2.18E−2c | 7.72E−1 | 7.67E−1 | 4.98E−4 | 8.42E−6d | 4.40E−1 | 3.58E−1 | 7.90E−1 |
| 9 | rs4391122f | 5q12.1 | ZSWIM6 (intergenic) | G/A | 5.89 | −3.86 | −2.40 | 0.55 | 1.61E−2c | 3.73E−1 | 9.10E−1 | 3.92E−9 | 1.13E−4d | 1.64E−2d | 5.84E−1 | 3.05E−2d |
| 10 | rs12521503 | 5q14.1 | SSBP2 (intergenic) | T/C | 3.52 | 3.61 | 1.58 | 2.19 | 3.33E−2c | 6.10E−1 | 4.34E−1 | 4.32E−4 | 3.09E−4d | 1.15E−1 | 2.83E−2d | 9.43E−1 |
| 11 | rs4266584 | 7q22.3 | SRPK2 (intergenic) | A/C | 4.07 | −3.62 | −1.54 | 0.25 | 3.33E−2c | 6.10E−1 | 9.99E−1 | 4.66E−5 | 2.96E−4d | 1.23E−1 | 8.03E−1 | 2.10E−1 |
| 12 | rs7857165 | 9q22.31 | PHF2 (intron) | A/T | NAb | NA | NA | NA | >.99 | 9.19E−1 | 1.51E−2c | 2.16E−4 | 7.48E−1 | 6.83E−1 | 3.00E−5d | 9.01E−3d |
| 13 | rs12253987 | 10q24.33 | NEURL (intron) | A/T | NAb | NA | NA | NA | 4.66E−2c | 7.30E−1 | 6.98E−1 | 9.31E−4 | 4.51E−4d | 3.07E−1 | 2.53E−1 | 2.60E−1 |
| 14 | rs10767734 | 11p14.1 | METT5D1 (intergenic) | T/C | −3.47 | 3.58 | 0.44 | −1.62 | 3.33E−2c | 9.19E−1 | 5.93E−1 | 5.21E−4 | 3.49E−4d | 6.62E−1 | 1.05E−1 | 5.41E−1 |
| 15 | rs2046768f | 11p11.2 | DGKZ (splice region variant) | C/T | 5.08 | −0.20 | −2.40 | 4.46 | >.99 | 3.73E−1 | 1.55E−3c | 3.74E−7 | 8.44E−1 | 1.62E−2d | 8.08E−6d | 2.99E−1 |
| 16 | rs2238057f | 12p13.33 | CACNA1C (intron) | G/T | 6.46 | −3.66 | −0.45 | −1.47 | 2.80E−2c | 9.19E−1 | 6.48E−1 | 1.02E−10 | 2.50E−4d | 6.54E−1 | 1.43E−1 | 9.46E−1 |
| 17 | rs12879159h | 14q12 | AKAP6 (3 prime UTR variant) | A/G | 3.52 | −4.96 | −2.77 | 3.39 | 1.82E−2c | 2.52E−1 | 6.82E−2 | 4.27E−4 | 6.98E−7d | 5.61E−3d | 6.92E−4d | 2.08E−2d |
| 18 | rs524908 | 15q15.3 | FRMD5 (intron) | C/A | −3.24 | 3.56 | 1.51 | 0.66 | 4.48E−2c | 6.21E−1 | 8.32E−1 | 1.20E−3 | 3.70E−4d | 1.32E−1 | 5.07E−1 | 1.12E−2d |
| 19 | rs1496897 | 15q21.1 | SEMA6D (intron) | C/T | 3.60 | −1.50 | −2.38 | 3.59 | 6.63E−1 | 3.73E−1 | 4.13E−2c | 3.22E−4 | 1.34E−1e | 1.73E−2d | 3.35E−4d | 7.33E−1 |
| 20 | rs216452 | 17q11.2 | DQ584120 (intron) | T/C | 3.66 | −1.60 | −1.20 | 3.65 | 6.31E−1 | 6.97E−1 | 3.46E−2c | 2.50E−4 | 1.10E−1 | 2.29E−1 | 2.59E−4d | 7.51E−1 |
| 21 | rs5758659f,i | 22q13.2 | TCF20 (intron) | T/C | 4.80 | −4.58 | −5.49 | −0.96 | 8.37E−4c | 2.87E−4c | 7.28E−1 | 1.55E−6 | 4.74E−6d | 4.13E−8d | 3.35E−1 | 6.91E−1 |
| 21 | rs134873f,i | 22q13.2 | TCF20 (intron) | G/T | 5.01 | −4.43 | −5.43 | −1.13 | 1.62E−3c | 9.12E−5c | 6.98E−1 | 5.53E−7 | 9.42E−6d | 5.64E−8d | 2.60E−1 | 5.78E−1 |
Abbreviations: A1, allele 1; A2, allele 2; CHARGE, Cohorts for Heart and Aging Research in Genomic Epidemiology; Chr, chromosome; COGENT, Cognitive Genomics Consortium; GCF, general cognitive function; GWAS, genome-wide association study; LD, linkage disequilibrium; NA, not available z score signs owing to A/T polymorphism; RT, reaction time; SCZ, schizophrenia; SNP, single-nucleotide polymorphism; VNR, verbal-numerical reasoning.
Independent complex or single-gene loci (r2 < 0.1) with SNPs with a conjunctional false discovery rate lower than .05 shared between SCZ and GCF in the CHARGE cohort, VNR, or RT. The significant SNPs are listed and sorted in each LD block, and independent loci are listed consecutively. All data were first corrected for genomic inflation, and SNPs within the major histocompatibility complex region and SNPs in LD (r2 > 0.1) with such SNPs were excluded from cFDR computation. The effect sizes are given with reference to A1.
Single-nucleotide polymorphisms in LD with rs13024343 show concordant associations in SCZ and GCF; SNPs in LD with rs1545424 and rs12253987 show inverse associations in SCZ and GCF; and SNPs in LD with rs7857165 show concordant associations in SCZ and RT (eTable 2 in the Supplement).
Indicates a conjunctional false discovery rate less than .05.
P < .05 in cognitive traits.
Summary statistics for rs67338739, rs6435048, and rs1496897 were not available in the GCF GWAS data set. Summary data for rs13002677 (in LD [r2 = 1] with rs67338739), rs1347551 (in LD [r2 = 0.964] with rs6435048), and rs13313462 (in LD [r2 = 1] with rs1496897) are shown instead.
Identified by the primary schizophrenia GWAS.
Identified by the primary reaction time GWAS.
Identified by the primary general cognitive function GWAS by CHARGE.
Identified by the primary verbal-numerical reasoning GWAS.
We evaluated the directionality of allelic effects in the loci shared between schizophrenia and cognitive traits by investigating their z scores (Table). For 10 loci shared between schizophrenia and GCF, we found opposite effect directions in the phenotypes, while rs12521503 (SSBP2 [OMIM 607389]) showed concordant effect directions. Owing to T/A polymorphisms, the effect directions were ambiguous for rs13024343 (CTNNA2 [OMIM 114025]), rs1545424 (TBC1D5 [OMIM 615740]), and rs12253987 (NEURL [OMIM 603804]). Single-nucleotide polymorphisms in LD with rs13024343 show concordant associations in schizophrenia and GCF, while SNPs in LD with rs1545424 and rs12253987 show inverse associations in the phenotypes (eTable 2 in the Supplement). Both loci shared between schizophrenia and VNR showed opposite effect directions in the phenotypes. For loci shared between schizophrenia and RT, 4 showed concordant associations in the phenotypes (ie, the schizophrenia risk alleles are associated with slower RT), while rs67338739 (KCNJ3 [OMIM 601534]) showed opposite effect directions. The effect directions were ambiguous for rs7857165 (PHF2 [OMIM 604351]; T/A polymorphism). Single-nucleotide polymorphisms in LD with rs7857165 show concordant associations in schizophrenia and RT (eTable 2 in the Supplement). The overall negative correlation between schizophrenia risk and cognitive performance was consistent among shared loci at conjFDR less than .10 (eTable 3 in the Supplement). Of note, identification of loci with conjFDR less than .10 implicated potential overlapping associations between schizophrenia and GCF located within the major histocompatibility complex region (eTable 3 in the Supplement). However, this subthreshold finding must be interpreted cautiously given the complex LD in this region.
Next, we determined the messenger RNA expression distribution in the human brain for genes implicated in the conjFDR analysis. Expression data provided by UKBEC demonstrate that the identified genes are globally expressed in the adult human brain (eFigure 2 in the Supplement). Data for RNA gene DQ584120 (GenBank DQ584120.1) were not available in this data set. Expression data from the Human Brain Transcriptome Project show that the genes are globally expressed in the developing and adult human brain (eFigure 3 in the Supplement). Data for ZSWIM6 (OMIM 615951) and DQ584120 were not available in this data set. We further investigated the eQTL functionality of the identified conjunctional loci. Using GTEx data, we identified significant eQTL associations for 5 SNPs in human brain tissue (eTable 4 in the Supplement). We assessed the replicability of these brain-specific eQTLs using UKBEC data and identified significant replicable eQTL functionality for the following 4 SNPs: rs12993822 for KCNJ3 in the cerebellum, rs4282054 for GNL3 (OMIM 608011) in the frontal cortex and cerebellum, rs524908 for STRC (OMIM 606440) in the frontal cortex, and rs134873 for both CYP2D6 (OMIM 124030) in several brain regions and for NAGA (OMIM 104170) in the cerebellum and frontal cortex (eTable 5 in the Supplement). Not all brain regions were jointly examined by GTEx and UKBEC.
The DEPICT analysis revealed multiple gene sets enriched for genes in loci shared between schizophrenia and GCF. However, the results did not remain significant after correction for multiple comparisons, which may reflect the relatively sparse number of analyzed loci and/or the subtlety of the biological signal. The top-ranked gene sets were “NFYA (OMIM 189903) subnetwork,” “increased neuron apoptosis,” and “chromatin remodeling complex” (eFigure 4 in the Supplement). NFYA encodes the sequence-specific DNA-binding subunit of the transcription factor NF-Y, a key regulator of differentiation in various proliferative cells. NF-Y is also active in mature neurons and may be involved in neurodegeneration.
Discussion
In the present study, we analyzed GWAS data using cFDR analysis and identified 21 genetic variants jointly influencing risk of schizophrenia and the cognitive traits of GCF, VNR, and RT. We found that genetic enrichment in cognitive traits based on SNP association with schizophrenia results in improved statistical power for gene discovery and increased gene discovery for these cognitive traits. Most of the loci found to be shared between schizophrenia and cognitive traits (18 of 21) show a negative correlation between risk of schizophrenia and cognitive performance, in line with the observed cognitive dysfunction in schizophrenia and prior genetic studies. Altogether, this study provides new insights into the common genetic basis of schizophrenia and cognitive traits, suggesting novel molecular genetic mechanisms.
Recent studies applying LD score regression reported significant negative correlations between the genomic architectures of schizophrenia and GCF, VNR, and RT. Here, we were able to dissect these coheritabilities by identifying multiple gene loci inversely associated with schizophrenia and these cognitive traits (Table). A total of 13 of 21 loci shared between schizophrenia and cognitive traits have a P value less than .05 in at least 2 cognitive traits, demonstrating consistent associations of the identified loci across cognitive domains (Table) and supporting the credibility of the cFDR approach. Among the shared loci, 13 are novel for schizophrenia. The overall negative association between allelic effect directions for schizophrenia and cognitive performance was consistent among conjunctional loci identified at a relaxed significance threshold (conjFDR < .10; eTable 3 in the Supplement). The low SNP enrichment observed with GCF in the COGENT cohort is likely attributable to the smaller sample size of this cohort (n = 27 888) compared with the CHARGE cohort (n = 53 949). We identified 3 loci with the same effect directions in schizophrenia and cognitive skills (rs13024343 and rs12521503 shared between schizophrenia and GCF, and rs67338739 shared between schizophrenia and RT), demonstrating that some schizophrenia risk loci may increase the likelihood of better cognitive performance. These results emphasize the complexity of the shared genetic effects influencing schizophrenia and cognitive function. Although many schizophrenia risk loci may not affect cognitive function, the findings may shed light on the positive genetic correlation between schizophrenia and measures of creativity and education, and why some patients with schizophrenia exhibit normal cognitive skills. Given that schizophrenia typically manifests in adolescence, while GCF, VNR, and RT were measured in adults older than 40 years of age, the conjunctional loci appear to influence brain function across a person’s life span. This finding is supported by the messenger RNA expression data showing that the implicated genes are globally expressed in the developing and adult human brain (eFigure 3 in the Supplement), and complies with previous work reporting genetic overlap between schizophrenia and cognitive abilities in middle and older age. Cognitive performance of participants in the CHARGE and UK Biobank GWASs may have been susceptible to age-associated cognitive decline or subclinical neurodegenerative conditions. Moreover, the genetics underlying cognitive variation in healthy individuals might differ across their life spans. Given that schizophrenia manifests in adolescence and cognitive decline often precedes the onset of psychosis by many years, an evaluation of shared genetic effects between schizophrenia and cognitive function measured in younger people is warranted.
The strongest signal of shared genetic effects between schizophrenia and cognitive traits was detected on chromosome 22q13.2, at a locus that contains many genes (nearest gene TCF20; Table). This locus was shared between schizophrenia, GCF, and VNR and was genome-wide significant in the primary GWASs on schizophrenia and VNR, but is a novel finding for GCF. TCF20 encodes a widely expressed transcriptional coregulator, and TCF20 mutations are associated with autism and intellectual disability. Using data from GTEx and UKBEC, we identified replicable eQTL functionality of the 22q13.2 locus for genes CYP2D6 and NAGA in several human brain regions (eTables 4 and 5 in the Supplement). The allele G of rs134873, which is associated with increased risk of schizophrenia and lower GCF and VNR scores, was associated with higher expression of NAGA and lower expression of CYP2D6. NAGA encodes a lysosomal enzyme that modifies glycoconjugates, and CYP2D6 encodes a cytochrome P450 enzyme that metabolizes a broad range of drugs, including antipsychotics, and may also be involved in the metabolism of neurotransmitters, including serotonin and dopamine. Another notable locus shared between schizophrenia and GCF is the intronic variant within CACNA1C (OMIM 114205) (rs2238057). Genetic variation in CACNA1C is robustly implicated in schizophrenia, and it is associated with cognitive impairment in patients with schizophrenia and in healthy individuals. We also found that a locus at AKAP6 (OMIM 604691) (rs12885467, 3′ UTR variant) jointly influences schizophrenia and GCF. This locus reached genome-wide significance for GCF in the CHARGE cohort and is replicated across all cognitive traits. The number of shared loci identified here is consistent with results of previous conjFDR analyses and depends on both the extent of genetic overlap between traits and the power of the investigated GWASs. More shared loci between schizophrenia and cognitive traits are expected to be uncovered when larger GWAS samples are available.
Limitations
As with all GWAS findings, any SNP represents through LD a genomic region including potentially many causal SNPs. Hence, further studies are required to determine the true causal variants underlying the shared associations detected here, and whether the same causal variants are involved in schizophrenia and cognitive traits.
Conclusions
We were able to increase discovery of genetic loci jointly influencing schizophrenia and cognitive traits using the cFDR approach. The findings provide new insights into the coheritability underlying schizophrenia and cognitive traits beyond their known genetic correlation. The discovered loci can be used as resources and to guide further efforts to disentangle the neurobiological basis underlying schizophrenia and cognitive function.
eAppendix. Methods
eFigure 1. Conditional Q-Q Plots of Cognitive Traits Given Association With Schizophrenia
eFigure 2. Averaged Regional mRNA Expression Distribution in the Adult Human Brain of Genes Implicated in Schizophrenia and General Cognitive Function, Verbal-Numerical Reasoning, and Reaction Time
eFigure 3. Expression Trajectories of Genes Annotated to Loci Shared Between Schizophrenia and General Cognitive Function, Verbal-Numerical Reasoning, and Reaction Time in the Developmental and Adult Human Brain
eFigure 4. Top-ranked Gene Sets Enriched for Genes in Loci Associated With Schizophrenia and General Cognitive Function Identified Using DEPICT Analysis
eTable 1. Loci With cFDR<.01 Associated With Cognitive Traits Given Association With Schizophrenia
eTable 2. Effect Sizes of SNPs in LD With Conjunctional Loci With Ambiguous Effect Directionality
eTable 3. List of Gene Loci (Unpruned) With Conjunctional False Discovery Rate (conjFDR<.10) Associated With Schizophrenia (SCZ) and General Cognitive Function in the CHARGE Cohort (COG CHARGE), COG in the COGENT Cohort (COG COGENT), Verbal-Numerical Reasoning (VNR) or Reaction Time (RT)
eTable 4. Significant eQTL Functionality of SNPs With Conjunction FDR < .05 Identified Using GTEx
eTable 5. Cis-eQTL Data in Human Brain Using UK Brain Expression Consortium Data
eReferences
References
- 1.Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575-1586. [DOI] [PubMed] [Google Scholar]
- 2.Green MF, Harvey PD. Cognition in schizophrenia: past, present, and future. Schizophr Res Cogn. 2014;1(1):e1-e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26(1):119-136. [DOI] [PubMed] [Google Scholar]
- 4.Kahn RS, Keefe RS. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. 2013;70(10):1107-1112. [DOI] [PubMed] [Google Scholar]
- 5.Dickinson D, Ragland JD, Gold JM, Gur RC. General and specific cognitive deficits in schizophrenia: Goliath defeats David? Biol Psychiatry. 2008;64(9):823-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aas M, Dazzan P, Mondelli V, Melle I, Murray RM, Pariante CM. A systematic review of cognitive function in first-episode psychosis, including a discussion on childhood trauma, stress, and inflammation. Front Psychiatry. 2014;4:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reichenberg A, Caspi A, Harrington H, et al. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am J Psychiatry. 2010;167(2):160-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fusar-Poli P, Deste G, Smieskova R, et al. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch Gen Psychiatry. 2012;69(6):562-571. [DOI] [PubMed] [Google Scholar]
- 9.Mohamed S, Rosenheck R, Swartz M, Stroup S, Lieberman JA, Keefe RS. Relationship of cognition and psychopathology to functional impairment in schizophrenia. Am J Psychiatry. 2008;165(8):978-987. [DOI] [PubMed] [Google Scholar]
- 10.Keefe RS, Buchanan RW, Marder SR, et al. Clinical trials of potential cognitive-enhancing drugs in schizophrenia: what have we learned so far? Schizophr Bull. 2013;39(2):417-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zai G, Robbins TW, Sahakian BJ, Kennedy JL. A review of molecular genetic studies of neurocognitive deficits in schizophrenia. Neurosci Biobehav Rev. 2017;72:50-67. [DOI] [PubMed] [Google Scholar]
- 12.Keshavan MS, Kulkarni S, Bhojraj T, et al. Premorbid cognitive deficits in young relatives of schizophrenia patients. Front Hum Neurosci. 2010;3:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McIntosh AM, Harrison LK, Forrester K, Lawrie SM, Johnstone EC. Neuropsychological impairments in people with schizophrenia or bipolar disorder and their unaffected relatives. Br J Psychiatry. 2005;186:378-385. [DOI] [PubMed] [Google Scholar]
- 14.Bora E, Lin A, Wood SJ, Yung AR, McGorry PD, Pantelis C. Cognitive deficits in youth with familial and clinical high risk to psychosis: a systematic review and meta-analysis. Acta Psychiatr Scand. 2014;130(1):1-15. [DOI] [PubMed] [Google Scholar]
- 15.Toulopoulou T, Goldberg TE, Mesa IR, et al. Impaired intellect and memory: a missing link between genetic risk and schizophrenia? Arch Gen Psychiatry. 2010;67(9):905-913. [DOI] [PubMed] [Google Scholar]
- 16.Fowler T, Zammit S, Owen MJ, Rasmussen F. A population-based study of shared genetic variation between premorbid IQ and psychosis among male twin pairs and sibling pairs from Sweden. Arch Gen Psychiatry. 2012;69(5):460-466. [DOI] [PubMed] [Google Scholar]
- 17.McIntosh AM, Gow A, Luciano M, et al. Polygenic risk for schizophrenia is associated with cognitive change between childhood and old age. Biol Psychiatry. 2013;73(10):938-943. [DOI] [PubMed] [Google Scholar]
- 18.Lencz T, Knowles E, Davies G, et al. Molecular genetic evidence for overlap between general cognitive ability and risk for schizophrenia: a report from the Cognitive Genomics Consortium (COGENT). Mol Psychiatry. 2014;19(2):168-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hubbard L, Tansey KE, Rai D, et al. Evidence of common genetic overlap between schizophrenia and cognition. Schizophr Bull. 2016;42(3):832-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liebers DT, Pirooznia M, Seiffudin F, Musliner KL, Zandi PP, Goes FS. Polygenic risk of schizophrenia and cognition in a population-based survey of older adults. Schizophr Bull. 2016;42(4):984-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatzimanolis A, Bhatnagar P, Moes A, et al. Common genetic variation and schizophrenia polygenic risk influence neurocognitive performance in young adulthood. Am J Med Genet B Neuropsychiatr Genet. 2015;168B(5):392-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagenaars SP, Harris SE, Davies G, et al. ; METASTROKE Consortium, International Consortium for Blood Pressure GWAS; SpiroMeta Consortium; CHARGE Consortium Pulmonary Group, CHARGE Consortium Aging and Longevity Group . Shared genetic aetiology between cognitive functions and physical and mental health in UK Biobank (N = 112 151) and 24 GWAS consortia. Mol Psychiatry. 2016;21(11):1624-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill WD, Davies G, Liewald DC, McIntosh AM, Deary IJ; CHARGE Cognitive Working Group . Age-dependent pleiotropy between general cognitive function and major psychiatric disorders. Biol Psychiatry. 2016;80(4):266-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trampush JW, Yang ML, Yu J, et al. GWAS meta-analysis reveals novel loci and genetic correlates for general cognitive function: a report from the COGENT consortium. Mol Psychiatry. 2017;22(3):336-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ripke S, O’Dushlaine C, Chambert K, et al. ; Multicenter Genetic Studies of Schizophrenia Consortium; Psychosis Endophenotypes International Consortium; Wellcome Trust Case Control Consortium 2 . Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet. 2013;45(10):1150-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies G, Armstrong N, Bis JC, et al. ; Generation Scotland . Genetic contributions to variation in general cognitive function: a meta-analysis of genome-wide association studies in the CHARGE consortium (N = 53 949). Mol Psychiatry. 2015;20(2):183-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies G, Marioni RE, Liewald DC, et al. Genome-wide association study of cognitive functions and educational attainment in UK Biobank (N = 112 151). Mol Psychiatry. 2016;21(6):758-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schizophrenia Working Group of the Psychiatric Genomics Consortium Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu JZ, Hov JR, Folseraas T, et al. ; UK-PSCSC Consortium; International PSC Study Group; International IBD Genetics Consortium . Dense genotyping of immune-related disease regions identifies nine new risk loci for primary sclerosing cholangitis. Nat Genet. 2013;45(6):670-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schork AJ, Wang Y, Thompson WK, Dale AM, Andreassen OA. New statistical approaches exploit the polygenic architecture of schizophrenia—implications for the underlying neurobiology. Curr Opin Neurobiol. 2016;36:89-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andreassen OA, Thompson WK, Dale AM. Boosting the power of schizophrenia genetics by leveraging new statistical tools. Schizophr Bull. 2014;40(1):13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andreassen OA, Thompson WK, Schork AJ, et al. ; Psychiatric Genomics Consortium (PGC); Bipolar Disorder and Schizophrenia Working Groups . Improved detection of common variants associated with schizophrenia and bipolar disorder using pleiotropy-informed conditional false discovery rate. PLoS Genet. 2013;9(4):e1003455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andreassen OA, Harbo HF, Wang Y, et al. ; Psychiatric Genomics Consortium (PGC) Bipolar Disorder and Schizophrenia Work Groups; International Multiple Sclerosis Genetics Consortium (IMSGC) . Genetic pleiotropy between multiple sclerosis and schizophrenia but not bipolar disorder: implications for immune-related gene loci. Mol Psychiatry. 2015;20(2):207-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andreassen OA, Djurovic S, Thompson WK, et al. ; International Consortium for Blood Pressure GWAS; Diabetes Genetics Replication and Meta-analysis Consortium; Psychiatric Genomics Consortium Schizophrenia Working Group . Improved detection of common variants associated with schizophrenia by leveraging pleiotropy with cardiovascular-disease risk factors. Am J Hum Genet. 2013;92(2):197-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Hellard S, Wang Y, Witoelar A, et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium . Identification of gene loci that overlap between schizophrenia and educational attainment. Schizophr Bull. 2017;43(3):654-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desikan RS, Schork AJ, Wang Y, et al. ; Inflammation Working Group and International Genomics of Alzheimer’s Disease Project (IGAP) and DemGene Investigators . Polygenic overlap between c-reactive protein, plasma lipids, and alzheimer disease. Circulation. 2015;131(23):2061-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andreassen OA, Desikan RS, Wang Y, et al. Abundant genetic overlap between blood lipids and immune-mediated diseases indicates shared molecular genetic mechanisms [published correction appears in PLoS One. 2015;10(5):e0128048]. PLoS One. 2015;10(4):e0123057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25(3):653-660. [DOI] [PubMed] [Google Scholar]
- 39.Schwartzman A, Lin X. The effect of correlation in false discovery rate estimation. Biometrika. 2011;98(1):199-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang HJ, Kawasawa YI, Cheng F, et al. Spatio-temporal transcriptome of the human brain. Nature. 2011;478(7370):483-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trabzuni D, Ryten M, Walker R, et al. Quality control parameters on a large dataset of regionally dissected human control brains for whole genome expression studies. J Neurochem. 2011;119(2):275-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.GTEx Consortium The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45(6):580-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pers TH, Karjalainen JM, Chan Y, et al. ; Genetic Investigation of ANthropometric Traits (GIANT) Consortium . Biological interpretation of genome-wide association studies using predicted gene functions. Nat Commun. 2015;6:5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sniekers S, Stringer S, Watanabe K, et al. Genome-wide association meta-analysis of 78,308 individuals identifies new loci and genes influencing human intelligence [published online May 22, 2017]. Nat Genet. doi: 10.1038/ng.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nardini M, Gnesutta N, Donati G, et al. Sequence-specific transcription factor NF-Y displays histone-like DNA binding and H2B-like ubiquitination. Cell. 2013;152(1-2):132-143. [DOI] [PubMed] [Google Scholar]
- 46.Yamanaka T, Tosaki A, Kurosawa M, et al. NF-Y inactivation causes atypical neurodegeneration characterized by ubiquitin and p62 accumulation and endoplasmic reticulum disorganization. Nat Commun. 2014;5:3354. [DOI] [PubMed] [Google Scholar]
- 47.Power RA, Steinberg S, Bjornsdottir G, et al. Polygenic risk scores for schizophrenia and bipolar disorder predict creativity. Nat Neurosci. 2015;18(7):953-955. [DOI] [PubMed] [Google Scholar]
- 48.Palmer BW, Heaton RK, Paulsen JS, et al. Is it possible to be schizophrenic yet neuropsychologically normal? Neuropsychology. 1997;11(3):437-446. [DOI] [PubMed] [Google Scholar]
- 49.Babbs C, Lloyd D, Pagnamenta AT, et al. ; International Molecular Genetic Study of Autism Consortium (IMGSAC) . De novo and rare inherited mutations implicate the transcriptional coregulator TCF20/SPBP in autism spectrum disorder. J Med Genet. 2014;51(11):737-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clark NE, Garman SC. The 1.9 Å structure of human α-N-acetylgalactosaminidase: the molecular basis of Schindler and Kanzaki diseases. J Mol Biol. 2009;393(2):435-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferguson CS, Tyndale RF. Cytochrome P450 enzymes in the brain: emerging evidence of biological significance. Trends Pharmacol Sci. 2011;32(12):708-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schyman P, Lai W, Chen H, Wang Y, Shaik S. The directive of the protein: how does cytochrome P450 select the mechanism of dopamine formation? J Am Chem Soc. 2011;133(20):7977-7984. [DOI] [PubMed] [Google Scholar]
- 53.Devor A, Andreassen OA, Wang Y, et al. Genetic evidence for role of integration of fast and slow neurotransmission in schizophrenia. Mol Psychiatry. 2017;22(6):792-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Q, Shen Q, Xu Z, et al. The effects of CACNA1C gene polymorphism on spatial working memory in both healthy controls and patients with schizophrenia or bipolar disorder. Neuropsychopharmacology. 2012;37(3):677-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dietsche B, Backes H, Laneri D, et al. The impact of a CACNA1C gene polymorphism on learning and hippocampal formation in healthy individuals: a diffusion tensor imaging study. Neuroimage. 2014;89:256-261. [DOI] [PubMed] [Google Scholar]
- 56.Solovieff N, Cotsapas C, Lee PH, Purcell SM, Smoller JW. Pleiotropy in complex traits: challenges and strategies. Nat Rev Genet. 2013;14(7):483-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Methods
eFigure 1. Conditional Q-Q Plots of Cognitive Traits Given Association With Schizophrenia
eFigure 2. Averaged Regional mRNA Expression Distribution in the Adult Human Brain of Genes Implicated in Schizophrenia and General Cognitive Function, Verbal-Numerical Reasoning, and Reaction Time
eFigure 3. Expression Trajectories of Genes Annotated to Loci Shared Between Schizophrenia and General Cognitive Function, Verbal-Numerical Reasoning, and Reaction Time in the Developmental and Adult Human Brain
eFigure 4. Top-ranked Gene Sets Enriched for Genes in Loci Associated With Schizophrenia and General Cognitive Function Identified Using DEPICT Analysis
eTable 1. Loci With cFDR<.01 Associated With Cognitive Traits Given Association With Schizophrenia
eTable 2. Effect Sizes of SNPs in LD With Conjunctional Loci With Ambiguous Effect Directionality
eTable 3. List of Gene Loci (Unpruned) With Conjunctional False Discovery Rate (conjFDR<.10) Associated With Schizophrenia (SCZ) and General Cognitive Function in the CHARGE Cohort (COG CHARGE), COG in the COGENT Cohort (COG COGENT), Verbal-Numerical Reasoning (VNR) or Reaction Time (RT)
eTable 4. Significant eQTL Functionality of SNPs With Conjunction FDR < .05 Identified Using GTEx
eTable 5. Cis-eQTL Data in Human Brain Using UK Brain Expression Consortium Data
eReferences