Key Points
Question
Are there distinctive phenotypical characteristics of patients with age-related macular degeneration carrying a rare variant in the complement factor H gene?
Findings
This cross-sectional study found that larger drusen area within the Early Treatment Diabetic Retinopathy Study grid, a crystalline appearance of some drusen, and drusen nasal to the optic disc were associated with patients with age-related macular degeneration carrying a rare pathogenic complement factor H variant.
Meaning
These phenotypical characteristics might aid ophthalmologists in the identification of complement factor H variant carriers, which may be increasingly important if complement-inhibiting therapies are shown to be beneficial.
Abstract
Importance
Rare variants in the complement factor H (CFH) gene and their association with age-related macular degeneration (AMD) have been described. However, there is limited literature on the phenotypes accompanying these rare variants. Phenotypical characteristics could help ophthalmologists select patients for additional genetic testing.
Objective
To describe the phenotypical characteristics of patients with AMD carrying a rare variant in the CFH gene.
Design, Setting, and Participants
In this cross-sectional study, we searched the genetic database of the department of ophthalmology at the Radboudumc (tertiary ophthalmologic referral center) and the European Genetic Database for patients with AMD with a rare genetic variant in the CFH gene. Patient recruitment took place from March 30, 2006, to February 18, 2013, and data were analyzed from November 30, 2015, to May 8, 2017. Phenotypical features on fundus photographs of both eyes of patients were graded by 2 independent reading center graders masked for carrier status.
Main Outcomes and Measures
Differences in phenotypical characteristics between rare variant carriers and noncarriers were analyzed using univariable generalized estimated equations logistic regression models accounting for intereye correlation.
Results
Analyses included 100 eyes of 51 patients with AMD carrying a CFH variant (mean [SD] age, 66.7 [12.1] years; 64.7% female) and 204 eyes of 102 age-matched noncarriers (mean [SD] age, 67.1 [11.8] years; 54.9% female). Carrying a rare pathogenic CFH variant was associated with larger drusen area (odds ratio range, 6.98 [95% CI, 2.04-23.89] to 18.50 [95% CI, 2.19-155.99]; P = .002), presence of drusen with crystalline appearance (odds ratio, 3.24; 95% CI, 1.24-8.50; P = .02), and drusen nasal to the optic disc (odds ratio range, 4.03 [95% CI, 1.70-9.56] to 7.42 [95% CI, 0.65-84.84]; P = .003).
Conclusions and Relevance
Identification of rare CFH variant carriers may be important for upcoming complement-inhibiting therapies. Patients with an extensive drusen area, drusen with crystalline appearance, and drusen nasal to the optic disc are more likely to have a rare variant in the CFH gene. However, it is not likely that carriers can be discriminated from noncarriers based solely on phenotypical characteristics from color fundus images. Therefore, ophthalmologists should consider genetic testing in patients with these phenotypic characteristics in combination with other patient characteristics, such as early onset, cuticular drusen on fluorescein angiography, and family history of AMD.
This cross-sectional study describes the phenotypical characteristics of patients with age-related macular degeneration carrying a rare variant in the complement factor H gene.
Introduction
Age-related macular degeneration (AMD) is a common multifactorial eye disease in Western countries; however, the exact pathophysiology of the disease is not yet completely understood. Environmental factors, such as age and smoking, and both common and rare genetic variants have been identified as risk factors for AMD. A large number of these genetic variants are located in genes encoding components of the complement system. Additionally, higher local and systemic complement activity has been reported in patients with AMD compared with control individuals. Together, these findings implicate a pivotal role of the complement system in AMD.
Rare genetic variants located in the complement factor H (CFH) gene are among the variants that confer the highest risk for AMD. The CFH gene encodes factor H (FH), a regulator of the alternative pathway of the complement system. Factor H inhibits the C3-convertase (C3bBb) and also acts as cofactor for factor I–mediated inactivation of C3b, leading to decreased activity and thereby preventing overactivation of the complement system. Several studies showed lower systemic FH levels in patients carrying a rare CFH variant. Furthermore, functional studies have reported an altered function of FH in patients carrying a rare variant in CFH resulting in increased complement activation despite normal systemic FH levels.
While antivascular endothelial growth factor treatment is available for neovascular AMD, there is currently no effective treatment available for the early and atrophic stages of AMD. Because the complement system plays an important role in AMD pathogenesis, therapies targeting different components of the complement system are being developed. Currently, a number of phase 2/3 clinical trials are in progress and so far, 2 phase 2 trials have been completed with mixed results. The Complement Inhibition With Eculizumab for the Treatment of Nonexudative Age-Related Macular Degeneration Study did not show decreased atrophy progression after administration of eculizumab, while the MAHALO study showed a beneficial effect of lampalizumab treatment on reducing atrophy progression.
With upcoming therapies targeting the complement system, it may be important to identify the patients who will most likely benefit from these therapies. Patients carrying a rare variant in the CFH gene seem to be a very suitable patient group for complement-inhibiting therapies because of the associated functional consequences on complement activation. However, it is expensive to genetically screen every patient with AMD in a diagnostic setting; therefore, it is desirable to preselect cases for genotyping based on phenotype. Unfortunately, there is limited literature on the phenotypes accompanying these CFH variants. Previously, a higher burden of extramacular drusen was reported in families carrying rare CFH variants compared with unrelated AMD cases; however, other distinct phenotypical characteristics were not described. Another study described phenotypical characteristics in a more detailed manner, but only included individuals carrying the rare p.Arg1210Cys variant in CFH. We hypothesize that all pathogenic CFH variants share phenotypical characteristics owing to their functional influences on FH. Detailed characterization of phenotypes caused by a broad spectrum of rare CFH variants is lacking. Therefore, we aim to describe the phenotypical characteristics of patients with AMD carrying a rare variant in the CFH gene. A distinct phenotype description of these CFH carriers will enable ophthalmologists to select patients for additional genetic testing and complement-inhibiting therapies more efficiently.
Methods
Study Population
In this retrospective cross-sectional study, we searched the genetic database of the department of ophthalmology at the Radboud university medical center, Nijmegen, the Netherlands (Radboudumc), and the European Genetic Database (EUGENDA), a multicenter database for clinical and molecular analysis of AMD, for individuals with a rare genetic variant in the CFH gene. Patient recruitment took place from March 30, 2006, to February 18, 2013. We selected AMD cases carrying protein-altering variants with a population frequency of less than 1%. We defined AMD as the presence of at least 10 small drusen (<63 μm) and pigmentary changes, intermediate or large drusen (≥63 μm), or late AMD, including subfoveal geographic atrophy and/or choroidal neovascularization in at least 1 eye on color fundus images. Details of this classification are described elsewhere. In total, 51 patients, with 33 different CFH variants, were identified and included in this study, hereafter referred to as carriers. Additionally, for each carrier, we selected from the European Genetic Database, 2 similarly aged AMD cases (±2 years) without a rare genetic variant associated with AMD; these cases were defined as noncarriers (n = 102). For 2 carriers, color fundus images of only 1 eye were available; therefore, final analyses included 100 eyes of 51 carriers and 204 eyes of 102 noncarriers. All participants indicated to be of European descent. Written informed consent was provided by all participants. The study was approved by the local ethics committee on research involving human participants, Commissie Mensgebonden Onderzoek Regio Arnhem–Nijmegen, and the local committee of University Hospital Cologne and was performed in accordance with the tenets of the Declaration of Helsinki.
Genotyping
Whole-exome sequencing (WES) and/or Sanger sequencing was previously performed. For both approaches, DNA was extracted from venous blood using standard procedures.
Most CFH carriers (n = 42) were identified through WES. Preparation and sequencing of the DNA samples were done as previously described. In short, exome capture Nimblegen SeqCap EZ V2 kit (Roche) paired-end sequencing was performed on an Illumina HiSeq2000 sequencer using TruSeq V3 chemistry (Illumina) followed by downstream quality control and genotyping of the samples. For this study, WES data were filtered specifically for the CFH gene (HUGO Gene Nomenclature Committee ID: 4883; NM_000186). Additional filtering steps on the data were implemented to select genetic variants that result in a splice-site or protein change (nonsynonymous) as these variants are more likely to be pathogenic. We focused on rare genetic variants only (minor allele frequency <1%) as based on the Exome Aggregation Consortium database, specifically the non-Finnish European population. Individual variants were confirmed with Sanger sequencing using primers designed by Primer3 software. The remainder of CFH carriers (n = 9) was identified through conventional Sanger sequencing of the entire CFH gene as described in detail previously. We excluded rare CFH variants with a described protective effect in case-control analyses (c.2850G>T p.Gln950His) or a likely benign effect in functional studies (c.2669G>T p.Ser890Ile, c.2867C>T p.Thr956Met, c.3019G>T p.Val1007Leu). All CFH variants included in this study are described in the eTable in the Supplement.
For all noncarriers, WES data were available and screened for rare genetic variants in the CFH, CFI, C3, and C9 genes associated with AMD. Only individuals without any rare variant in the CFH gene or a described pathogenic rare variant in the other AMD-associated genes were included in this study as noncarriers.
Image Assessment
Digital 35° or 40° field of view color fundus photographs centered on the fovea were performed with a Topcon TRC 50IX camera (Topcon Corporation) or Canon 60 UVi fundus camera (Canon), respectively. Color fundus photographs were analyzed for this study by 2 senior graders from an independent reading center (Moorfields Eye Hospital, London, England) according to a standardized grading protocol. The following fundus features were assessed: predominant type of drusen, largest type of drusen in the central field, percentage of the area of the Early Treatment Diabetic Retinopathy Study (ETDRS) grid covered with drusen, presence of extramacular drusen (defined as drusen outside the ETDRS grid), drusen nasal to the optic disc, reticular drusen, drusen with crystalline appearance, serogranular/serous drusen pigment epithelium detachment, pigmentary abnormalities, geographic atrophy, or signs of neovascularization.
Statistical Analysis
Data were analyzed from November 30, 2015, to May 8, 2017. Demographic characteristics of the 2 study groups were compared using 1-way analysis of variance or the χ2 test. Phenotypical characteristics were individually assessed using binary logistic regression models. Generalized estimating equations procedures were used to correct for the fellow eye. To compare the frequencies of late AMD subtypes between carriers and noncarriers, we performed a χ2 test based on the more severely affected eye of each patient. In case both geographic atrophy and choroidal neovascularization were present in an individual, it was classified as mixed late AMD. A phenotypic risk score for each eye was calculated as the sum of regression coefficients of all individual phenotypical characteristics resulting from univariable generalized estimating equations logistic regression analyses. A receiver operating characteristic curve was obtained and the area under the curve was measured for this risk score. Finally, symmetry between eyes was calculated as follows: number of equal phenotypical characteristics between right and left eyes divided by the number of phenotypical characteristics graded times 100%. All statistical analyses were performed using SPSS statistics software (released 2013; IBM SPSS Statistics for Windows, Version 22.0; IBM Corp).
Results
In total, 100 eyes of 51 carriers and 204 eyes of 102 noncarriers were included for analyses. Demographic and environmental characteristics were comparable between carriers and noncarriers (Table 1). The frequency of common genetic variants in CFH, ARMS2, and C3 seems to be slightly higher in noncarriers compared with carriers. However, the minor allele frequencies of these common variants in noncarriers are comparable with the general AMD population. This may imply that carriers of rare CFH variants are less burdened by common AMD risk variants and that their AMD risk is rather attributable to the rare variants.
Table 1. General Characteristics of the Study Groups.
| Characteristic | No. (%) | |
|---|---|---|
| Noncarriers (n = 102; 204 Eyes) |
Carriers (n = 51; 100 Eyes) |
|
| Age at participation, mean (SD), y | 67.1 (11.8) | 66.7 (12.1) |
| Sex | ||
| Male | 46 (45.1) | 18 (35.3) |
| Female | 56 (54.9) | 33 (64.7) |
| Smoking status | ||
| Never | 21 (22.3) | 16 (39.0) |
| Past | 53 (56.4) | 17 (41.5) |
| Current | 20 (21.3) | 8 (19.5) |
| BMI, mean (SD) | 26.1 (4.1) | 26.5 (4.2) |
| Family history for AMD | ||
| Yes | 52 (58.4) | 27 (67.5) |
| No | 37 (41.6) | 13 (32.5) |
| Common genetic variants, No. of minor alleles/total No. of alleles (MAF %) | ||
| ARMS2, rs10490923, T | 75/176 (42.6) | 17/74 (23.0) |
| CFH, rs1061170, C | 99/176 (56.3) | 29/76 (38.2) |
| C3, rs2230199, G | 49/174 (28.2) | 29/76 (38.2) |
Abbreviations: AMD, age-related macular degeneration; ARMS2, age-related maculopathy susceptibility 2; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CFH, complement factor H; C3, complement component 3; MAF, minor allele frequency.
When comparing the fundus features by carrier status, the odds of carrying any rare CFH variant increases with increasing drusen area within the ETDRS grid (odds ratio [OR] up to 6.85 when more than 50% of the ETDRS grid is covered with drusen, P = .004) and with the presence of serogranular/serous drusen pigment epithelium detachment (OR, 4.74; 95% CI, 1.30-17.31; P = .02). Additionally, drusen deposition in rare variant carriers is often not limited to the central retina; these carriers tend to have extramacular drusen (80.8%) and drusen nasal to the optic disc (43.8%) more frequently than noncarriers (73.4% and 35.1%, respectively), although these differences were not significant. The association of all assessed fundus features of carriers and noncarriers are shown in Table 2.
Table 2. Phenotypical Characteristics of Carriers and Noncarriers of Rare CFH Variants.
| Phenotypic Characteristic | No. of Eyes (%) | Odds Ratio (95% CI)a | P Value | |
|---|---|---|---|---|
| Noncarrier (n = 204) |
Carrier (n = 100) |
|||
| Predominant drusen type within ETDRS grid | ||||
| None or small drusen (<63 µm) | 32 (15.7) | 12 (12.0) | 1 [Reference] | .31 |
| Intermediate drusen (63-125 µm) | 107 (52.5) | 45 (45.0) | 1.12 (0.47-2.68) | |
| Large drusen (>125 µm) | 65 (31.9) | 43 (43.0) | 1.76 (0.71-4.38) | |
| Largest drusen type within central field | ||||
| None or small drusen (<63 µm) | 106 (52.5) | 44 (44.9) | 1 [Reference] | .58 |
| Intermediate drusen (63-125 µm) | 77 (38.1) | 44 (44.9) | 1.38 (0.75-2.52) | |
| Large drusen (>125 µm) | 19 (9.4) | 10 (10.2) | 1.27 (0.46-3.48) | |
| Proportion of grid area covered by drusen, % | ||||
| 0-10 | 111 (54.4) | 27 (27.3) | 1 [Reference] | .004b |
| 10-25 | 61 (29.9) | 41 (41.4) | 2.76 (1.36-5.63) | |
| 25-50 | 29 (14.2) | 26 (26.3) | 3.69 (1.58-8.58) | |
| >50 | 3 (1.5) | 5 (5.1) | 6.85 (1.37-34.37) | |
| Extramacular drusen | ||||
| Absent | 54 (26.6) | 19 (19.2) | 1 [Reference] | .27 |
| Present | 149 (73.4) | 80 (80.8) | 1.53 (0.72-3.24) | |
| Drusen nasal to the optic disc | ||||
| None or small drusen (<63 µm) | 89 (65.0) | 45 (56.3) | 1 [Reference] | .47 |
| Intermediate drusen (63-125 µm) | 46 (33.6) | 32 (40.0) | 1.38 (0.69-2.74) | |
| Large drusen (>125 µm) | 2 (1.5) | 3 (3.8) | 2.97 (0.30-29.51) | |
| Reticular drusen | ||||
| Absent | 163 (86.7) | 83 (93.3) | 1 [Reference] | .14 |
| Present | 25 (13.3) | 6 (6.7) | 0.47 (0.18-1.27) | |
| Drusen with crystalline appearance | ||||
| Absent | 178 (89.4) | 76 (81.7) | 1 [Reference] | .15 |
| Present | 21 (10.6) | 17 (18.3) | 1.90 (0.80-4.48) | |
| SPED | ||||
| Absent | 199 (97.5) | 84 (89.4) | 1 [Reference] | .02 |
| Present | 5 (2.5) | 10 (10.6) | 4.74 (1.30-17.31) | |
| RPE pigmentation | ||||
| Absent | 84 (45.4) | 31 (32.3) | 1 [Reference] | .08 |
| Present | 101 (54.6) | 65 (67.7) | 1.74 (0.93-3.27) | |
| Geographic atrophy | ||||
| Absent | 154 (76.2) | 65 (70.7) | 1 [Reference] | .42 |
| Present | 48 (23.8) | 27 (29.3) | 1.33 (0.66-2.69) | |
| Neovascular AMD | ||||
| Absent | 144 (72.4) | 79 (82.3) | 1 [Reference] | .12 |
| Present | 55 (27.6) | 17 (17.7) | 0.56 (0.28-1.15) | |
Abbreviations: AMD, age-related macular degeneration; CFH, complement factor H; ETDRS, Early Treatment Diabetic Retinopathy Study; RPE, retinal pigment epithelium; SPED, serogranular/serous drusen pigment epithelium detachment.
The presented odds ratios result from univariable generalized estimating equations logistic regression analyses.
P value remained significant after Bonferroni correction for multiple testing.
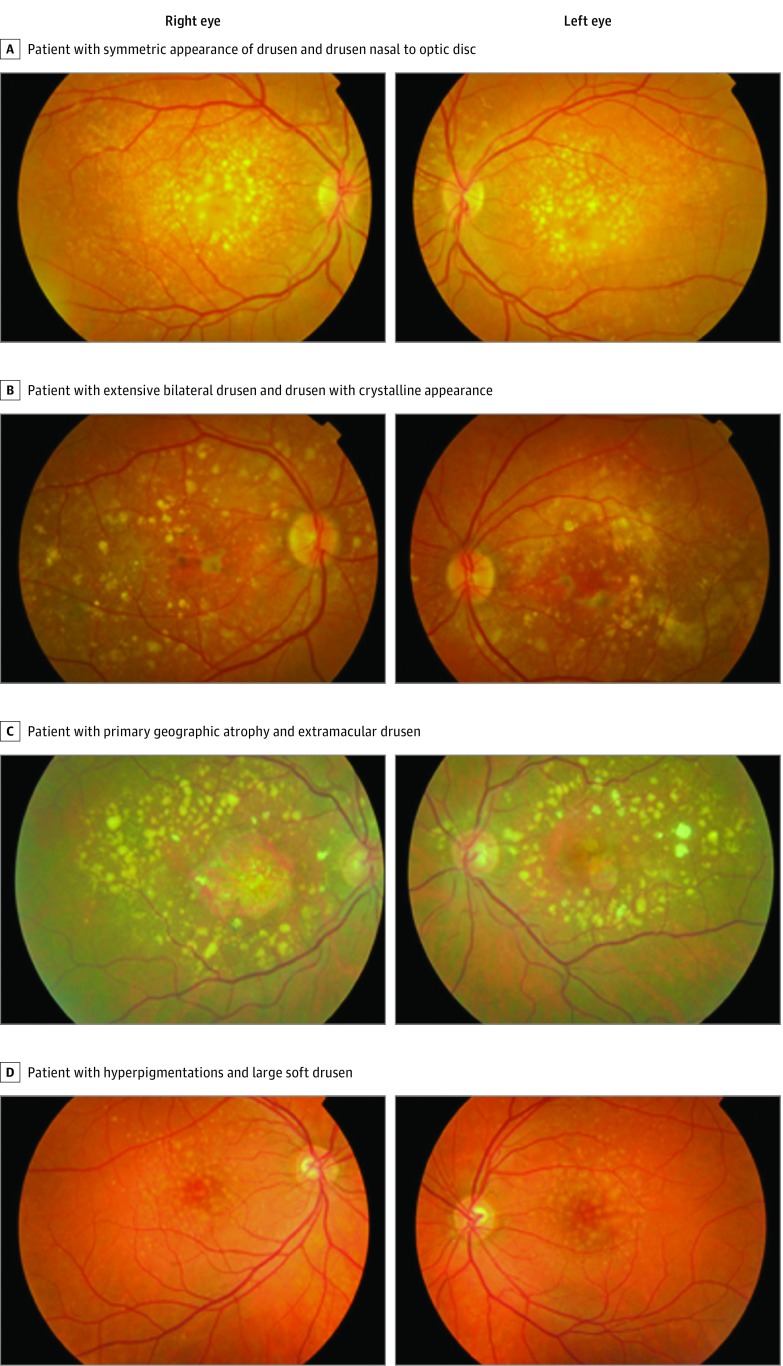
Because the carrier group contains both rare variants known to be associated with AMD and rare variants of unknown clinical significance, we repeated the analyses with stricter inclusion criteria comparing only cases carrying a known pathogenic variant (n = 25) with noncarriers (Table 3). Known pathogenic variants included rare CFH variants associated with AMD in case-control or segregation analyses or with a described functional effect. This subanalysis showed an even higher association between drusen area within the ETDRS grid and rare pathogenic CFH variant carriers (OR range, 6.98 [95% CI, 2.04-23.89] to 18.50 [95% CI, 2.19-155.99]; P = .002). Additionally, intermediate and large drusen located nasal to the optic disc (OR range, 4.03 [95% CI, 1.70-9.56] to 7.42 [95% CI, 0.65-84.84]; P = .003) and the presence of drusen with crystalline appearance (OR, 3.24; 95% CI, 1.24-8.50; P = .02) were significantly associated with carrying a rare pathogenic CFH variant. Subanalysis of late AMD cases only (n = 71) showed a higher frequency of late atrophic AMD in rare pathogenic variant carriers (57.1%) compared with noncarriers (28.1%), although this was not significantly different (P = .12). Notably, the association between serogranular/serous drusen pigment epithelium detachment and carrier status did not remain significant but still tended to increase the odds of carrying a rare CFH variant. Examples of color fundus photographs of carriers of rare CFH variants with the associated fundus features are displayed in the Figure.
Table 3. Associations of Phenotypical Characteristics With Confirmed Pathogenic Rare CFH Variants.
| Phenotypic Characteristic | No. of Eyes (%) | Odds Ratio (95% CI)a | P Value | |
|---|---|---|---|---|
| Noncarrier (n = 204) |
Carrier (n = 48) |
|||
| Predominant drusen type within ETDRS grid | ||||
| None or small drusen (<63 µm) | 32 (15.7) | 4 (8.3) | 1 [Reference] | .06 |
| Intermediate drusen (63-125 µm) | 107 (52.5) | 18 (37.5) | 1.35 (0.34-5.38) | |
| Large drusen (>125 µm) | 65 (31.9) | 26 (54.2) | 3.20 (0.80-12.75) | |
| Largest drusen type within central field | ||||
| None or small drusen (<63 µm) | 106 (52.5) | 24 (50.0) | 1 [Reference] | .97 |
| Intermediate drusen (63-125 µm) | 77 (38.1) | 19 (39.6) | 1.16 (0.29-4.66) | |
| Large drusen (>125 µm) | 19 (9.4) | 5 (10.4) | 1.09 (0.50-2.39) | |
| Proportion of grid area covered by drusen, % | ||||
| 0-10 | 111 (54.4) | 6 (12.5) | 1 [Reference] | .002b |
| 10-25 | 61 (29.9) | 23 (47.9) | 6.98 (2.04-23.89) | |
| 25-50 | 29 (14.2) | 16 (33.3) | 10.21 (2.85-36.59) | |
| >50 | 3 (1.5) | 3 (6.3) | 18.50 (2.19-155.99) | |
| Any drusen outside grid | ||||
| Absent | 54 (26.6) | 6 (12.5) | 1 [Reference] | .11 |
| Present | 149 (73.4) | 42 (87.5) | 2.54 (0.80-8.04) | |
| Drusen nasal to the optic disc | ||||
| None or small drusen (<63 µm) | 89 (65.0) | 12 (30.8) | 1 [Reference] | .003b |
| Intermediate drusen (63-125 µm) | 46 (33.6) | 25 (64.1) | 4.03 (1.70-9.56) | |
| Large drusen (>125 µm) | 2 (1.5) | 2 (5.1) | 7.42 (0.65-84.84) | |
| Reticular drusen | ||||
| Absent | 163 (86.7) | 43 (93.5) | 1 [Reference] | .23 |
| Present | 25 (13.3) | 3 (6.5) | 0.46 (0.13-1.64) | |
| Drusen with crystalline appearance | ||||
| Absent | 178 (89.4) | 34 (72.3) | 1 [Reference] | .02 |
| Present | 21 (10.6) | 13 (27.7) | 3.24 (1.24-8.50) | |
| SPED | ||||
| Absent | 199 (97.5) | 43 (91.5) | 1 [Reference] | .11 |
| Present | 5 (2.5) | 4 (8.5) | 3.70 (0.74-18.63) | |
| RPE pigmentation | ||||
| Absent | 84 (45.4) | 13 (28.3) | 1 [Reference] | .10 |
| Present | 101 (54.6) | 33 (71.7) | 2.11 (0.87-5.14) | |
| Geographic atrophy | ||||
| Absent | 154 (76.2) | 28 (65.1) | 1 [Reference] | .23 |
| Present | 48 (23.8) | 15 (34.9) | 1.72 (0.71-4.14) | |
| Neovascular AMD | ||||
| Absent | 144 (72.4) | 38 (82.6) | 1 [Reference] | .23 |
| Present | 55 (27.6) | 8 (17.4) | 0.55 (0.21-1.47) | |
Abbreviations: AMD, age-related macular degeneration; CFH, complement factor H; ETDRS, Early Treatment Diabetic Retinopathy Study; RPE, retinal pigment epithelium; SPED, serogranular/serous drusen pigment epithelium detachment.
The presented odds ratios result from univariable generalized estimating equations logistic regression analyses.
P value remained significant after Bonferroni correction for multiple testing.
Figure. Color Fundus Photographs of Carriers of Rare Variants in the Complement Factor H (CFH) Gene.
A, A woman in her 50s (CFH c.2537A>G, p.Gln846Arg) with a symmetric appearance of extensive drusen deposition within the Early Treatment Diabetic Retinopathy Study grid extending beyond the inferior and superior retinal arcades and nasal to the optic disc in both eyes. B, A man in his 60s (CFH c.550delA, p.Ile184Leufs*33) with extensive bilateral drusen deposition inside and outside the Early Treatment Diabetic Retinopathy Study grid and nasal to the optic disc, presence of drusen with crystalline appearance, and hypopigmentations and hyperpigmentations. C, Woman in her 70s (CFH c.524G>A, p.Arg175Gln) with primary geographic atrophy surrounded by predominantly large drusen, some with crystalline appearance, beyond the retinal arcades and the optic disc. D, A man in his 40s (CFH c.1198C>A, p.Gln400Lys) with hyperpigmentations and mainly centrally located large soft drusen but also extending to the peripheral retina.
Overall, rare CFH variant carriers tend to have more and larger drusen, and drusen are more often located outside the ETDRS grid. However, not all of these analyzed phenotypical characteristics individually reach statistical significance. Based on the findings in Table 3, we calculated a phenotypic risk score for each eye including all assessed phenotypical characteristics (eFigure 1 in the Supplement). The mean (SD) phenotypic risk score in carriers (4.35 [2.0]) was significantly higher compared with noncarriers (2.32 [2.5]), although the ability to accurately discriminate between eyes of carriers of pathogenic CFH variants and noncarriers based on the phenotypic risk score was limited (area under the curve, 0.75; 95% CI, 0.65-0.85; eFigure 2 in the Supplement). Similar results were obtained when including only the highest phenotypic risk score for each patient (area under the curve, 0.75; 95% CI, 0.61-0.88).
Finally, for every patient, the grade of symmetry between eyes was determined based on the number of equal characteristics. Each study group showed a high grade of symmetry between the eyes (79.9% in noncarriers vs 79.1% in carriers of pathogenic variants) and the groups were not significantly different (P = .85).
Discussion
In this study, we aimed to describe the phenotypical characteristics of patients with AMD carrying a rare variant in the CFH gene. Overall, rare CFH variant carriers have a more severe phenotype with more and larger drusen, often extending to the peripheral retina. Larger drusen area within the ETDRS grid and drusen located nasal to the optic disc were significantly associated with patients with AMD carrying a rare pathogenic CFH variant. These findings are in line with previous studies reporting extensive macular drusen accumulation and presence of extramacular drusen in patients with AMD carrying the CFH p.Arg1210Cys variant and other rare CFH variants.
In addition, we report an association between the presence of drusen with crystalline appearance and carrying a rare variant in CFH. Drusen with crystalline appearance, also known as refractile or calcified drusen, have a characteristic glistening appearance on color fundus imaging and have been associated with the development of geographic atrophy. Thus, these patients might be at higher risk for developing geographic atrophy compared with noncarriers. In the current study, rare CFH variant carriers seem to develop geographic atrophy more often than choroidal neovascularization, as was already observed in rare variant carriers of other complement genes (CFI, C3, and C9). However, probably owing to the small number of patients with late AMD, this difference was not significant.
From literature, it is known that CFH carriers usually have an earlier age at onset. Owing to our study design, a lower age at onset in rare variant carriers could not be analyzed. Our study was merely designed to analyze phenotypical differences between rare CFH carriers and noncarriers; therefore, age-matched noncarriers were selected. As a consequence, no difference in age at onset could be observed. However, assessing the age at onset remains an important clue for ophthalmologists when considering (rare) genetic variants in a patient.
Familial burden is also known to be associated with rare CFH variant carriers. Although the number of carriers with a family history of AMD (64.7%) was not significantly different from noncarriers (53.9%), it must be emphasized that family history was obtained through interviewer-assisted questionnaires. From previous studies, it is known that CFH carriers often have asymptomatic family members and, therefore, it is plausible that the percentage of carriers with a family history of AMD is underestimated.
Assuming that rare protein-altering variants located in the CFH gene lead to similar phenotype, this study was not restricted to 1 or more specific CFH variants but included a wide variety of rare protein-altering CFH variants identified by WES or Sanger sequencing in our cohort. Therefore, our analyses also included some variants that were not described before in the literature. However, when limiting the analyses to confirmed pathogenic variants only, the associations between rare variant carriers and phenotypical characteristics become more pronounced. More information on pathogenicity of variants is therefore desirable. As prediction tools do not always correctly predict a genetic variant to be functionally impaired, other large sequencing or functional studies are needed to confirm the clinical significance of these variants.
Limitations
Because of an overlap in phenotypical characteristics between carriers and noncarriers, even when including only confirmed pathogenic variants, the sample size of our study might be insufficient to detect small to moderate associations or associations with relatively infrequent features, such as serogranular/serous drusen pigment epithelium detachment and the presence of geographic atrophy. Additionally, when correcting for multiple comparisons, only drusen area remained significantly associated with rare variant carriers, which is most likely the result of our small sample size.
Our study was also restricted by its retrospective design, therefore, for the analyses, we were limited to the images that were captured in the past. Peripheral fundus images and other image modalities were often lacking and therefore not taken into account in the current study. To assess to what extent drusen are located outside the central retina, imaging should preferably be extended to the peripheral retina. Additionally, certain phenotypical characteristics are better visualized with other imaging techniques (eg, cuticular drusen). Previously, CFH variants were identified in patients with the cuticular drusen subtype of AMD, and fluorescein angiography is considered the best modality to diagnose these type of drusen. Furthermore, optical coherence tomography enables detailed visualization of the different retinal layer structures that are not visible on color fundus images and has the advantage of 3-dimensional image assessment. Future prospective studies could therefore benefit from assessing multiple image modalities and imaging of the peripheral retina.
Conclusions
Because patients with AMD carrying a rare CFH variant seem a very suitable group for upcoming complement-inhibiting therapies, identification of this subpopulation may be very important to direct choice of treatment. Our results indicate that patients with an extensive drusen area, drusen with crystalline appearance, and drusen nasal to the optic disc are more likely to have a rare genetic variant in the CFH gene. These phenotypical characteristics could aid ophthalmologists to select patients for genetic screening. However, it is unlikely that carriers can be discriminated from noncarriers based solely on phenotypical characteristics. Therefore, ophthalmologists should consider genetic testing in patients with extensive drusen deposition, drusen with crystalline appearance, and/or drusen nasal to the optic disc in combination with other patient characteristics, such as an early age at onset, cuticular drusen on fluorescein angiography, and a positive family history for AMD.
eTable. Rare Genetic Variants Identified in the CFH Gene.
eFigure 1. Distribution of Phenotypic Risk Scores in Eyes of Rare Pathogenic CFH Variant Carriers and Noncarriers.
eFigure 2. Receiver Operating Characteristic Curve of the Phenotypic Risk Score.
eReferences
References
- 1.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106-e116. [DOI] [PubMed] [Google Scholar]
- 2.Chakravarthy U, Wong TY, Fletcher A, et al. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol. 2010;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358(24):2606-2617. [DOI] [PubMed] [Google Scholar]
- 4.Fritsche LG, Igl W, Bailey JN, et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016;48(2):134-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ristau T, Paun C, Ersoy L, et al. Impact of the common genetic associations of age-related macular degeneration upon systemic complement component C3d levels. PLoS One. 2014;9(3):e93459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smailhodzic D, Klaver CC, Klevering BJ, et al. Risk alleles in CFH and ARMS2 are independently associated with systemic complement activation in age-related macular degeneration. Ophthalmology. 2012;119(2):339-346. [DOI] [PubMed] [Google Scholar]
- 7.Schick T, Steinhauer M, Aslanidis A, et al. Local complement activation in aqueous humor in patients with age-related macular degeneration. Eye (Lond). 2017;31(5):810-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raychaudhuri S, Iartchouk O, Chin K, et al. A rare penetrant mutation in CFH confers high risk of age-related macular degeneration. Nat Genet. 2011;43(12):1232-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geerlings MJ, de Jong EK, den Hollander AI. The complement system in age-related macular degeneration: a review of rare genetic variants and implications for personalized treatment. Mol Immunol. 2017;84:65-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Triebwasser MP, Roberson ED, Yu Y, et al. Rare variants in the functional domains of complement factor H are associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015;56(11):6873-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner EK, Raychaudhuri S, Villalonga MB, et al. Mapping rare, deleterious mutations in factor H: association with early onset, drusen burden, and lower antigenic levels in familial AMD. Sci Rep. 2016;6:31531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geerlings MJ, Kremlitzka M, Bakker B, et al. The functional effect of rare variants in complement genes on C3b degradation in patients with age-related macular degeneration. JAMA Ophthalmol. 2017;135(1):39-46. [DOI] [PubMed] [Google Scholar]
- 13.Yu Y, Triebwasser MP, Wong EK, et al. Whole-exome sequencing identifies rare, functional CFH variants in families with macular degeneration. Hum Mol Genet. 2014;23(19):5283-5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyer DS, Schmidt-Erfurth U, van Lookeren Campagne M, Henry EC, Brittain C. The pathophysiology of geographic atrophy secondary to age-related macular degeneration and the complement pathway as a therapeutic target. Retina. 2017;37(5):819-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volz C, Pauly D. Antibody therapies and their challenges in the treatment of age-related macular degeneration. Eur J Pharm Biopharm. 2015;95(pt B):158-172. [DOI] [PubMed] [Google Scholar]
- 16.Yehoshua Z, de Amorim Garcia Filho CA, Nunes RP, et al. Systemic complement inhibition with eculizumab for geographic atrophy in age-related macular degeneration: the COMPLETE study. Ophthalmology. 2014;121(3):693-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yaspan BL, Williams DF, Holz FG, et al. ; MAHALO Study Investigators . Targeting factor D of the alternative complement pathway reduces geographic atrophy progression secondary to age-related macular degeneration. Sci Transl Med. 2017;9(395):eaaf1443. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara D, Seddon JM. Phenotypic characterization of complement factor H R1210C rare genetic variant in age-related macular degeneration. JAMA Ophthalmol. 2015;133(7):785-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ristau T, Ersoy L, Lechanteur Y, et al. Allergy is a protective factor against age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55(1):210-214. [DOI] [PubMed] [Google Scholar]
- 20.Lek M, Karczewski KJ, Minikel EV, et al. ; Exome Aggregation Consortium . Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536(7616):285-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Untergasser A, Cutcutache I, Koressaar T, et al. Primer3: new capabilities and interfaces. Nucleic Acids Res. 2012;40(15):e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duvvari MR, Saksens NT, van de Ven JP, et al. Analysis of rare variants in the CFH gene in patients with the cuticular drusen subtype of age-related macular degeneration. Mol Vis. 2015;21:285-292. [PMC free article] [PubMed] [Google Scholar]
- 23.Saksens NT, Lechanteur YT, Verbakel SK, et al. Analysis of risk alleles and complement activation levels in familial and non-familial age-related macular degeneration. PLoS One. 2016;11(6):e0144367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oishi A, Thiele S, Nadal J, et al. Prevalence, natural course, and prognostic role of refractile drusen in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017;58(4):2198-2206. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki M, Curcio CA, Mullins RF, Spaide RF. Refractile drusen: clinical imaging and candidate histology. Retina. 2015;35(5):859-865. [DOI] [PubMed] [Google Scholar]
- 26.Saksens NT, Geerlings MJ, Bakker B, et al. Rare genetic variants associated with development of age-related macular degeneration. JAMA Ophthalmol. 2016;134(3):287-293. [DOI] [PubMed] [Google Scholar]
- 27.Hughes AE, Meng W, Bridgett S, Bradley DT. Rare CFH mutations and early-onset age-related macular degeneration. Acta Ophthalmol. 2016;94(3):e247-e248. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman JD, Cooke Bailey JN, D’Aoust L, et al. Rare complement factor H variant associated with age-related macular degeneration in the Amish. Invest Ophthalmol Vis Sci. 2014;55(7):4455-4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boon CJ, Klevering BJ, Hoyng CB, et al. Basal laminar drusen caused by compound heterozygous variants in the CFH gene. Am J Hum Genet. 2008;82(2):516-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van de Ven JP, Boon CJ, Fauser S, et al. Clinical evaluation of 3 families with basal laminar drusen caused by novel mutations in the complement factor H gene. Arch Ophthalmol. 2012;130(8):1038-1047. [DOI] [PubMed] [Google Scholar]
- 31.Jaffe A, Wojcik G, Chu A, et al. Identification of functional genetic variation in exome sequence analysis. BMC Proc. 2011;5(suppl 9):S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flanagan SE, Patch AM, Ellard S. Using SIFT and PolyPhen to predict loss-of-function and gain-of-function mutations. Genet Test Mol Biomarkers. 2010;14(4):533-537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Rare Genetic Variants Identified in the CFH Gene.
eFigure 1. Distribution of Phenotypic Risk Scores in Eyes of Rare Pathogenic CFH Variant Carriers and Noncarriers.
eFigure 2. Receiver Operating Characteristic Curve of the Phenotypic Risk Score.
eReferences