Key Points
Question
Are patients with glaucoma able to measure their own intraocular pressure using a rebound tonometer?
Findings
This study of 100 patients found that 73% could perform self-tonometry using a rebound tonometer and obtain intraocular pressure measurements within 5 mm Hg of a clinician. Self-tonometry was judged easy and comfortable by most patients; most were happy to perform self-tonometry in the future.
Meaning
These data suggest that self-tonometry offers a feasible method for measuring intraocular pressure fluctuations in glaucoma.
Abstract
Importance
The ability of patients to measure their own intraocular pressure (IOP) would allow more frequent measurements and better appreciation of peak IOP and IOP fluctuation.
Objective
To examine whether patients with glaucoma can perform self-tonometry using a rebound tonometer and examine patient acceptability.
Design, Setting, and Participants
An observational study in which IOP was assessed using Goldmann applanation tonometry and a rebound tonometer. Consecutive patients were provided with a patient information sheet and those consenting to take part in the study received standardized self-tonometry training and were then instructed to measure their own IOP under observation. This study was conducted at a glaucoma clinic at a university hospital from March 1, 2016, to December 30, 2016, and included both eyes of 100 patients with glaucoma or ocular hypertension.
Main Outcomes and Measures
The percentage of patients who could successfully perform self-tonometry. Complete success was defined by a good technique and an IOP reading within 5 mm Hg of that obtained by a clinician using the same device. A 3-item questionnaire was used to examine perceptions of self-tonometry among patients.
Results
Among the 100 patients, the mean (SD) age was 67.5 (10.9) years (53% female). A total 73 of 100 patients (73%) met the complete success criteria. An additional 6 patients could use the device but had IOP readings greater than 5 mm Hg different from those obtained by the clinician. On average, IOP by the rebound tonometer was 2.66 mm Hg lower than Goldmann applanation tonometry (95% limits of agreement, −3.48 to 8.80 mm Hg). The IOPs with the rebound tonometer were similar whether obtained by self-tonometry or investigator, with excellent reproducibility with an intraclass correlation coefficient of 0.903 (95% CI, 0.867-0.928). A total of 56 of 79 successful or partially successful patients (71%) felt self-tonometry was easy, with 73 of 79 (92%) reporting self-tonometry to be comfortable, and a similar number happy to perform self-tonometry in the future.
Conclusions and Relevance
Most patients could perform self-tonometry and the method was acceptable to patients. Self-tonometry has the potential to improve patient engagement, while also providing a more complete picture of IOP changes over time.
This study examines whether patients with glaucoma can perform self-tonometry using a rebound tonometer and examines patient acceptability.
Introduction
The major risk factor for development and progression of glaucoma is elevated intraocular pressure (IOP), with Goldmann applanation tonometry (GAT) the reference standard for measurement. However, conventional tonometry has limitations because the patient must go to their clinician for measurements and thus readings tend to be obtained infrequently. The result is poor understanding of peak IOP and IOP fluctuation. Although the significance of diurnal and long-term IOP fluctuations remains uncertain, it is unsatisfactory to base treatment decisions on a small number of measurements of what is a highly dynamic variable.
A fuller understanding of IOP fluctuation can be assessed by admitting patients for repeat measurements; however, this is logistically difficult, especially as up to 75% of individuals have peak IOP outside office hours. A potential, more accessible, solution is for patients to measure their own IOP by self-tonometry. Home monitoring and application of patient-generated data are not new and have been successfully used in other conditions (eg, hypertension and diabetes). Recently, a novel device designed for home IOP monitoring has become available, the Icare HOME (TA022; Icare Finland Oy).
The Icare HOME is a rebound tonometer that quantifies IOP by measuring the deceleration of a magnetized disposable probe as it rebounds from the surface of the cornea. The device does not require instillation of topical anesthesia and has been reported to provide repeatable readings with good agreement with GAT. However, testing one’s own IOP may be technically difficult, and it is not certain whether self-tonometry is acceptable to patients. The aim of this study was to determine whether patients with glaucoma can measure their own IOP and to evaluate patients’ perceptions of self-tonometry.
Methods
Conducted from March 1, 2016, to December 30, 2016, this was an observational study involving both eyes of 100 patients with glaucoma or ocular hypertension recruited from the glaucoma clinic at Princess Alexandra Eye Pavilion, University of Edinburgh, Scotland. The study methods, which adhered to the tenets of the Declaration of Helsinki, were prospectively approved by West Scotland Research Ethics Committee. Written informed consent was obtained prospectively from all patients. The sample size was chosen based on a previous study reporting 75% of patients could perform self-tonometry. A sample size of 75 was calculated as sufficient to demonstrate this proportion of successful patients (±10%) with 95% confidence.
Patients and Study Procedures
Consecutive patients attending the glaucoma clinic were provided with a patient information sheet and those consenting to take part in the study had a comprehensive ophthalmologic examination including best-corrected visual acuity, slitlamp biomicroscopy, gonioscopy, measurement of central corneal thickness (CCT) (Accutome PachPen; Keeler), and dilated fundus examination and standard automated perimetry (SAP) (Humphrey Field Analyzer; Carl Zeiss Meditec) using Swedish interactive threshold algorithm (SITA standard 24-2). Intraocular pressure was measured using GAT.
Glaucoma was defined by the presence of a repeatable visual field defect (>2 consecutive) on SAP, defined as a pattern SD outside 95% normal confidence limits or an abnormal glaucoma hemifield test, in conjunction with glaucomatous appearance of the optic nerve on fundoscopic examination. Visual fields with more than 33% fixation losses or false-negative errors, or more than 15% false-positive errors, were excluded. Ocular hypertension was defined as IOP greater than 21 mm Hg on GAT with a normal SAP and optic nerve appearance. Only patients with no previous experience of self-tonometry were included. Exclusion criteria included a known diagnosis of dementia or inability to hold the Icare HOME owing to upper limb weakness or disability. Patients with corneal disease that might affect tonometry measurements were also excluded.
All patients had IOP measured in both eyes using GAT by an experienced glaucoma specialist followed by Icare HOME measurements performed by investigators masked to the results of previous tests. Patients were then taught to measure their own IOP using Icare HOME using a standardized training protocol (eFigure in the Supplement). Briefly, the training session consisted of familiarizing the patient with the Icare HOME tonometer, explaining the user interface, loading a disposable probe, demonstrating adjustment of the measurement distance from the instrument to the eye, demonstrating tonometry in both eyes, and guiding the patient to obtain a measurement from their own eye, with steps repeated until the patient could demonstrate consistent and reliable use of the device. Training was limited to a maximum of 30 minutes with the time recorded at which point technique was deemed acceptable. The patient was then asked to measure their own IOP. Icare HOME calculates IOP as the average of 4 readings from 6 measurements, with the highest and lowest measurements discarded. Measurement quality was graded automatically by the device from 1 to 4, with 1 being the highest quality and measurements with a quality score greater than 3 automatically rejected. All patients were masked to all IOP measurements.
Complete success was defined as correct use of the Icare HOME and obtaining an IOP within 5 mm Hg of that obtained by the investigator using the same device in both eyes. The 5–mm Hg value was chosen as the cutoff for success in accordance with the manufacturer’s guideline and, as the International Organization for Standardization recommends, tonometers should be accurate to within 5 mm Hg of measurements obtained by GAT. Although we compared Icare HOME and GAT measurements, rather than define a patient’s ability to use the Icare HOME based on comparison with GAT, we opted to compare with Icare HOME measurements obtained by the clinician. Partial success was defined when the patient was deemed to have a good technique for self-tonometry but obtained an IOP measurement greater than 5 mm Hg from the investigator. Patients were then asked to complete a 3-item 5-point Likert scale questionnaire regarding their perception of home tonometry including the questions: (1) How easy was the device to use? (2) How comfortable was it to take measurements? (3) Would you be happy to use this device in the future?
Statistical Analysis
Differences between successful and unsuccessful patients were assessed using the t test and Mann-Whitney U test for continuous parametric and nonparametric variables, respectively. Univariable and multivariable logistic regression was used to analyze possible predictors of successful self-tonometry. Data from paired eyes are likely to be correlated so an adjustment was made in the regression analysis for the inclusion of both eyes to generate cluster-robust standard errors. Bland-Altman plots were used to evaluate agreement between Icare HOME and GAT IOP measurements, with analyses conducted to determine 95% limits of agreement throughout the range of measurements. The presence of proportional bias was determined by regression of the difference, with the average of the 2 methods. Reproducibility of measurements was examined using the intraclass correlation coefficient (ICC). All statistical analyses were performed with commercially available software (STATA, version 14.2; StataCorp LP). The α level (type I error) was set at .05.
Results
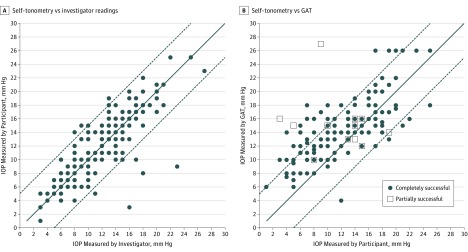
Demographic and clinical characteristics of patients included in the study are summarized in Table 1. A total of 73 of 100 patients (73%) achieved complete success, with an additional 6 partially successful, leaving 21 of 100 patients (21%) unsuccessful. For those partially or completely successful, the mean (SD) IOP by self-tonometry was 11.74 (4.89) mm Hg compared with 11.62 (4.61) mm Hg when the investigator used Icare HOME (Figure, A) and 14.22 (3.96) mm Hg with GAT (Figure, B). Comparison of Icare HOME IOP measurements by self-tonometry and investigator indicated excellent reproducibility, with an ICC of 0.903 (95% CI, 0.867-0.928). The mean (SD) Icare HOME quality score of accepted measures was 1 (0.7).
Table 1. Demographic and Clinic Characteristics of Participants Included in the Study.
| Characteristic | Mean (SD) | P Valuea | |||
|---|---|---|---|---|---|
| All Participants (n = 100) |
Self-tonometry Performance | ||||
| Partially Successful (n = 79) |
Completely Successful (n = 73) |
Unsuccessful (n = 21) |
|||
| Age, y | 67.5 (10.9) | 65.5 (10.2) | 65.5 (10.4) | 75.1 (9.9) | <.001 |
| Female | 53 (53) | 38 (48) | 36 (49) | 15 (71) | .06 |
| Right-handedness, No. (%) | 91 (91) | 70 (89) | 64 (88) | 21 (100) | .11 |
| SAP MD, No. (%), dB | |||||
| Better eye | −4.75 (6.04) | −4.65 (6.15) | −4.72 (6.25) | −5.15 (5.78) | .74 |
| Worse eye | −10.52 (8.28) | −10.15 (8.33) | −10.15 (8.04) | −11.88 (8.26) | .40 |
| Visual acuity, logMar | |||||
| Better eye | −0.04 (0.13) | −0.06 (0.12) | −0.05 (0.12) | 0.06 (0.13) | <.001 |
| Worse eye | 0.16 (0.29) | 0.12 (0.28) | 0.12 (0.28) | 0.32 (0.32) | .006 |
| CCT, um | 534 (37) | 536 (37) | 536 (37) | 530 (40) | .40 |
| IOP GAT, mm Hg | 14.3 (3.9) | 14.3 (4.0) | 14.2 (4.0) | 14.5 (3.8) | .75 |
| IOP Icare HOME reading, mm Hg | |||||
| Self-tonometry | 11.8 (4.9) | 11.7 (4.9) | 11.7 (4.9) | 13.7 (5.9) | .30 |
| Investigator | 11.7 (4.7) | 11.8 (4.6) | 11.6 (4.6) | 11.3 (4.8) | .58 |
Abbreviations: CCT, central corneal thickness; GAT, Goldmann applanation tonometry; IOP, intraocular pressure; MD, mean deviation; SAP, standard automatic perimetry.
t Test or Fisher exact test (2-tailed) of partially or completely successful groups vs unsuccessful group.
Figure. Participant and Investigator Icare HOME Readings and Goldmann Applanation Tonometry (GAT) .
A, Scatterplot of Icare HOME readings obtained by self-tonometry (successful and partially successful participants) and by the investigators. B, Scatterplot of Icare HOME readings obtained by self-tonometry vs GAT readings. The solid lines represent the reference line of perfect agreement and the dashed lines, 5 mm Hg limits of agreement. IOP indicates intraocular pressure.
The mean (SD) error between Icare HOME readings taken by the investigator and GAT was 2.66 (3.13) mm Hg, indicating a systemic underestimation of 2.66 mm Hg with Icare HOME, very similar to that between GAT and Icare HOME IOP readings from self-tonometry (Table 2). However, there was also evidence of proportional bias (coefficient = −0.194; 95% CI, −0.088 to −0.300; R2 = 0.062; P < .001), with a greater difference between readings found in eyes with lower IOP. Icare HOME self-tonometry readings from the 165 eyes with partial or complete success had a similar underestimation compared with GAT (mean [SD], 2.58 [3.72] mm Hg; 95% limits of agreement of −4.71 mm Hg [95% CI, −5.28 to −4.14 mm Hg] to 9.88 mm Hg [95% CI, 9.31 to 10.45 mm Hg], also with proportional bias [coefficient = −0.234; 95% CI, −0.098 to −0.370; R2 = 0.066; P < .001]). Thinner CCT was associated with lower IOP measurements using GAT (0.29 mm Hg lower IOP [95% CI, 0.14-0.43] for each 10-μm thinner CCT; R2 = 0.077; P < .001); however, Icare HOME measurements were affected more (0.48 mm Hg lower IOP [95% CI, 0.30-0.63]) for each 10-μm thinner CCT; R2 = 0.135; P < .001).
Table 2. Bland-Altman Analysis of Intraocular Pressure Measurements Comparing Results From GAT and Icare HOME Performed by the Investigator and by Self-tonometry.
| GAT vs Icare HOME | LoA (95% CI), mm Hg | Width, mm Hg | Fixed Bias, mm Hg | SE (95% CI) | |
|---|---|---|---|---|---|
| Lower 95% | Upper 95% | ||||
| Participant (n = 165) | −4.71 (−4.14 to −5.28) | 9.88 (9.31 to 10.45) | 14.59 | 2.58 | 0.29 (2.01 to 3.15) |
| Investigator (n = 198) | −3.48 (−3.91 to −3.05) | 8.8 (8.37 to 9.23) | 12.28 | 2.66 | 0.22 (2.22 to 3.10) |
Abbreviations: GAT, Goldmann applanation tonometer; LoA, limit of agreement.
Of 100 patients, 4 declined to complete the questionnaire regarding perceptions of self-tonometry, all of whom had been unsuccessful at self-tonometry (Table 3). A total of 56 of 79 partial or completely successful patients (71%) felt Icare HOME was easy to use compared with only 4 of 17 (24%) of those unsuccessful. A total of 73 of 79 successful or partially successful patients (92%) agreed self-tonometry was comfortable compared with 15 of 17 unsuccessful patients (88%). A total of 73 of 79 successful or partially successful patients (92%) stated they would be happy to perform self-tonometry in the future.
Table 3. Participants’ Perceptions of Self-tonometry Following the Training Session and Assessment.
| Perception | No. Responding | Likert Rating, No. (%) | Score, Mean (SD) | ||||
|---|---|---|---|---|---|---|---|
| 1: Strongly Disagree | 2: Disagree | 3: Neutral | 4: Agree | 5: Strongly Agree | |||
| “How Easy Was the Device to Use?” | |||||||
| Overall | 96 | 3 (3) | 8 (8) | 25 (26) | 35 (36) | 25 (26) | 3.75 (1.02) |
| Partially successful | 79 | 0 (0) | 4 (5) | 19 (24) | 34 (43) | 22 (28) | 3.96 (0.84) |
| Unsuccessful | 17 | 3 (18) | 4 (24) | 6 (35) | 2 (12) | 2 (12) | 2.89 (1.26) |
| “How Comfortable Was It to Take Measurements?” | |||||||
| Overall | 96 | 0 (0) | 2 (2) | 6 (6) | 29 (30) | 59 (61) | 4.51 (0.71) |
| Partially successful | 79 | 0 (0) | 1 (1) | 5 (6) | 20 (25) | 53 (67) | 4.58 (0.67) |
| Unsuccessful | 17 | 0 (0) | 1 (6) | 1 (6) | 9 (53) | 6 (35) | 4.00 (0.81) |
| “Would You Be Happy to Use the Device in the Future?” | |||||||
| Overall | 95 | 9 (9) | 3 (3) | 4 (4) | 18 (19) | 61 (64) | 4.25 (1.27) |
| Partially successful | 78 | 1 (1) | 1 (1) | 4 (5) | 15 (19) | 58 (73) | 4.62 (0.76) |
| Unsuccessful | 16 | 8 (50) | 2 (13) | 0 (0) | 3 (19) | 3 (19) | 2.61 (1.71) |
Patients took a mean (SD) of 20.3 (6.8) minutes to learn how to perform self-tonometry, with no significant difference in teaching time between the unsuccessful and partially or completely successful groups (mean [SD], 18.3 [9.0] vs 20.8 [6.1] minutes, respectively; P = .14). Those unable to use Icare HOME were older than completely or partially successful patients (mean [SD], 75.2 [9.9] vs 65.5 [10.2] years, respectively; P < .001) and had worse visual acuity in the better eye (mean [SD], 0.1 [0.13] vs −0.1 [0.12] LogMAR, respectively; P < .001).
Using the partial definition of success, older age (odds ratio [OR], 0.89; 95% CI, 0.83-0.95; P < .001) and worse visual acuity in the better eye (OR, 0.001; 95% CI, 0-0.06; P < .001) were associated with inability to perform self-tonometry (Table 4). Neither sex nor SAP MD were associated with the ability to use the device. In the multivariable analysis, older age (OR, 0.91; 95% CI, 0.85-0.98; P = .01) and worse visual acuity in the better eye (OR, 0.007; 95% CI, 0-0.58; P = .03) remained associated with failure. Using the complete success definition, multivariable analysis showed older age (OR, 0.93; 95% CI, 0.88-0.99; P = .02) remained significant but visual acuity in the better eye did not (OR, 0.12; 95% CI, 0.002-5.44; P = .27). Handedness had no influence on success and there was no association between handedness and the ability to use the device in right or left eye.
Table 4. Univariable and Multivariable Logistic Regression Analyses Examining the Relationship Between Age, Sex, Visual Acuity, and SAP MD and Success With Self-tonometry.
| Variable | Univariable Model | Multivariable Model | ||||||
|---|---|---|---|---|---|---|---|---|
| Partial Success | Complete Success | Partial Success | Complete Success | |||||
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
| Age | 0.89 (0.83-0.95) | <.001 | 0.92 (0.87-0.97) | .003 | 0.91 (0.85-0.98) | .01 | 0.93 (0.88-0.99) | .02 |
| Female | 0.37 (0.13-1.05) | .06 | 0.57 (0.23-1.42) | .23 | NA | NA | NA | NA |
| Visual acuity in the better eye | 0.001 (0-0.06) | <.001 | 0.02 (0-0.56) | .02 | 0.007 (0-0.58) | .03 | 0.12 (0-5.44) | .27 |
| SAP MD in the better eye | 1.01 (0.94-1.10) | .74 | 1.00 (0.93-1.08) | .94 | NA | NA | NA | NA |
Abbreviations: MD, mean deviation; NA, not applicable; SAP, standard automatic perimetry.
Discussion
This study demonstrates that most patients with glaucoma can perform self-tonometry using Icare HOME and obtain IOP measurements close to those of clinicians using the same device. After a short period of training, 73% of patients could perform self-tonometry and obtain measurements within 5 mm Hg of a clinician. Previous studies have reported a similarly high proportion are able to perform self-tonometry. For example, Dabasia et al found that 74% of patients could successfully use Icare HOME, and Mudie et al reported a similar success rate. However, the present study goes further by examining patient perceptions of self-tonometry and factors associated with the ability to perform self-tonometry. The results demonstrate self-tonometry is acceptable to patients, with 92% able to perform self-tonometry reporting the Icare HOME to be easy to use and a similar proportion expressing interest in using self-tonometry in the future. This suggests self-tonometry may be ready to become a mainstream test, with the potential to add a new dimension to glaucoma care by providing patient-generated out-of-office data.
However, for self-tonometry to be useful, it must be reproducible and preferably show good agreement to conventional tonometry. We found patients could obtain highly reproducible measurements compared with clinicians, with excellent agreement between Icare HOME IOP measurements obtained by self-tonometry and by clinicians within 5 mm Hg of each other in 150 of 158 eyes (94.9%), for an ICC of 0.903, similar to the within-patient ICC of 0.91 (95% CI, 0.89-0.94) reported by Mudie et al. In contrast, we found only 88 of 158 self-tonometry Icare HOME measurements (56%) were within 5 mm Hg of GAT, which was substantially less than the 84% reported by Mudie et al and less than the 84% of Icare HOME measurements within 3 mm Hg of GAT reported by Dabasia et al. Reasons for these differences likely include differences in patient characteristics, including CCT and biomechanical properties of the cornea, which may affect agreement between GAT and Icare HOME. We found thinner CCT was associated with lower IOP measurements using GAT and Icare HOME. However, there was a stronger relationship between CCT and Icare HOME measurements. Evaluations of other rebound tonometers have found a similar trend, suggesting rebound tonometers are affected more by properties of the cornea, which is a topic that warrants further investigation. Whether IOP measurements are obtained in the central or peripheral cornea may also be important, with at least 1 study showing measurements from the peripheral cornea underestimated IOP; however, this has not been found by all.
We examined factors associated with the ability to perform self-tonometry and found older patients were less likely to be able to use Icare HOME successfully. It is important to acknowledge that age itself is not a factor that intrinsically inhibits self-tonometry, rather it is likely a surrogate for other factors such as loss of fine motor function or impaired coordination. Further studies are needed to determine such factors and evaluate whether modifications to training or tonometer design might facilitate self-tonometry in these patients. From observing participants and listening to their feedback, 3 obstacles to successful tonometry were identified: (1) difficulty seeing the device when held close to the eye, (2) difficulty holding the device steady, and (3) difficulty applying enough force to press the button to obtain a measurement. These problems might be overcome with modifications to the tonometer, for example, by including an audible cue to correct alignment or automatic measurement when the device is correctly aligned. We found visual acuity and severity of visual field loss were not strongly associated with ability to perform self-tonometry; however, this is likely due to the relatively good visual acuity of included patients.
Limitations
The study has several limitations. Although we found teaching patients to use Icare HOME can be performed in a relatively short time, the method only assessed ability to perform self-tonometry immediately after teaching; therefore, we cannot comment on whether the skill is maintained if the device is taken home. It is possible that patients may forget how to use the device once taken home or, conversely, become better with further practice. It is also possible that those who struggled to measure their own IOPs may have benefited from further teaching as the session was limited to 30 minutes. It may be impractical to have supervised teaching of a longer duration in a busy clinical environment, and we found no relationship between longer teaching time and ability to perform self-tonometry. Also, the study design was not randomized and it is possible that selection bias was introduced by including patients already attending the glaucoma clinic. We chose to define successful self-tonometry based on the patient obtaining an IOP measurement within 5 mm Hg of that obtained by a clinician using the same device. However, previous investigators have suggested that IOP with self-tonometry should be within 5 mm Hg of IOP with GAT for a patient to be “certified” as competent. Owing to confounding factors influencing the relationship between GAT and Icare HOME readings, we would suggest that instead it would be reasonable to “compare like with like” and certify based on agreement to Icare HOME measurements obtained by the clinician. Although in clinical practice a difference of 5 mm Hg between devices could be considered too great, the good reproducibility of the Icare HOME measurements could overcome this limitation by allowing multiple measurements to be taken over time.
Conclusions
The results of this study demonstrate the feasibility of self-tonometry in patients with glaucoma, with most patients being able to accurately measure their own IOP after a short training session. Self-tonometry was deemed comfortable and relatively easy to perform and has the potential to improve patient engagement in their own care, while also providing a more complete picture of IOP changes over time, which may have significant implications for disease management.
eFigure. The Icare HOME Tonometer is a Hand-Held Rebound Tonometer.
References
- 1.Sit AJ. Intraocular pressure variations: causes and clinical significance. Can J Ophthalmol. 2014;49(6):484-488. [DOI] [PubMed] [Google Scholar]
- 2.Bliziotis IA, Destounis A, Stergiou GS. Home versus ambulatory and office blood pressure in predicting target organ damage in hypertension: a systematic review and meta-analysis. J Hypertens. 2012;30(7):1289-1299. [DOI] [PubMed] [Google Scholar]
- 3.Sakamoto M, Kanamori A, Fujihara M, Yamada Y, Nakamura M, Negi A. Assessment of IcareONE rebound tonometer for self-measuring intraocular pressure. Acta Ophthalmol. 2014;92(3):243-248. [DOI] [PubMed] [Google Scholar]
- 4.Rosentreter A, Jablonski KS, Mellein AC, Gaki S, Hueber A, Dietlein TS. A new rebound tonometer for home monitoring of intraocular pressure. Graefes Arch Clin Exp Ophthalmol. 2011;249(11):1713-1719. [DOI] [PubMed] [Google Scholar]
- 5.Moreno-Montañés J, Martínez-de-la-Casa JM, Sabater AL, Morales-Fernandez L, Sáenz C, Garcia-Feijoo J. Clinical evaluation of the new rebound tonometers Icare PRO and Icare ONE compared with the Goldmann tonometer. J Glaucoma. 2015;24(7):527-532. [DOI] [PubMed] [Google Scholar]
- 6.Halkiadakis I, Stratos A, Stergiopoulos G, et al. . Evaluation of the Icare-ONE rebound tonometer as a self-measuring intraocular pressure device in normal subjects. Graefes Arch Clin Exp Ophthalmol. 2012;250(8):1207-1211. [DOI] [PubMed] [Google Scholar]
- 7.Gandhi NG, Prakalapakorn SG, El-Dairi MA, Jones SK, Freedman SF. Icare ONE rebound versus Goldmann applanation tonometry in children with known or suspected glaucoma. Am J Ophthalmol. 2012;154(5):843-849.e1. [DOI] [PubMed] [Google Scholar]
- 8.Dabasia PL, Lawrenson JG, Murdoch IE. Evaluation of a new rebound tonometer for self-measurement of intraocular pressure. Br J Ophthalmol. 2016;100(8):1139-1143. [DOI] [PubMed] [Google Scholar]
- 9.Mudie LI, LaBarre S, Varadaraj V, et al. . The Icare HOME (TA022) study: performance of an intraocular pressure measuring device for self-tonometry by glaucoma patients. Ophthalmology. 2016;123(8):1675-1684. [DOI] [PubMed] [Google Scholar]
- 10.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 11.McAlinden C, Khadka J, Pesudovs K. Statistical methods for conducting agreement (comparison of clinical tests) and precision (repeatability or reproducibility) studies in optometry and ophthalmology. Ophthalmic Physiol Opt. 2011;31(4):330-338. [DOI] [PubMed] [Google Scholar]
- 12.Icare Finland Oy Icare HOME tonometer Instruction manual for health care professionals. http://www.icaretonometer.com/wp-content/uploads/2014/06/Icare_HOME_instruction_manual_TA022-036_EN-3-1_lo.pdf. Published 2014. Accessed July 22, 2017.
- 13.International Organization for Standardization (ISO) Standards Catalogue ISO 8612:2009 Ophthalmic Instruments: Tonometers. Geneva, Switzerland: International Organization for Standardization; 2009. [Google Scholar]
- 14.Brusini P, Salvetat ML, Zeppieri M, Tosoni C, Parisi L. Comparison of ICare tonometer with Goldmann applanation tonometer in glaucoma patients. J Glaucoma. 2006;15(3):213-217. [DOI] [PubMed] [Google Scholar]
- 15.Pakrou N, Gray T, Mills R, Landers J, Craig J. Clinical comparison of the Icare tonometer and Goldmann applanation tonometry. J Glaucoma. 2008;17(1):43-47. [DOI] [PubMed] [Google Scholar]
- 16.Salim S, Du H, Wan J. Comparison of intraocular pressure measurements and assessment of intraobserver and interobserver reproducibility with the portable ICare rebound tonometer and Goldmann applanation tonometer in glaucoma patients. J Glaucoma. 2013;22(4):325-329. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura M, Darhad U, Tatsumi Y, et al. . Agreement of rebound tonometer in measuring intraocular pressure with three types of applanation tonometers. Am J Ophthalmol. 2006;142(2):332-334. [DOI] [PubMed] [Google Scholar]
- 18.Rao A, Kumar M, Prakash B, Varshney G. Relationship of central corneal thickness and intraocular pressure by iCare rebound tonometer. J Glaucoma. 2014;23(6):380-384. [DOI] [PubMed] [Google Scholar]
- 19.Muttuvelu DV, Baggesen K, Ehlers N. Precision and accuracy of the ICare tonometer: peripheral and central IOP measurements by rebound tonometry. Acta Ophthalmol. 2012;90(4):322-326. [DOI] [PubMed] [Google Scholar]
- 20.Chui WS, Lam A, Chen D, Chiu R. The influence of corneal properties on rebound tonometry. Ophthalmology. 2008;115(1):80-84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. The Icare HOME Tonometer is a Hand-Held Rebound Tonometer.