Key Points
Question
Are temporal trends in vena cava filter placement and pulmonary embolism changing over time?
Findings
In this cohort study using data from patients with traumatic injury from 3 databases, rates of vena cava filter placement showed an initial upward trend followed by a precipitous decline. Rates of pulmonary embolism demonstrated an initial increase and were followed by a reduction in the Pennsylvania Trauma Outcome Study and National Trauma Data Bank data sets, with no change in the National (Nationwide) Inpatient Sample data set.
Meaning
Vena cava filter use is not associated with rates of pulmonary embolism.
Abstract
Importance
Vena cava filter (VCF) placement for pulmonary embolism (PE) prophylaxis in trauma is controversial. Limited research exists detailing trends in VCF use and occurrence of PE over time.
Objective
To analyze state and nationwide temporal trends in VCF placement and PE occurrence from 2003 to 2015 using available data sets.
Design, Setting, and Participants
A retrospective trauma cohort study was conducted using data from the Pennsylvania Trauma Outcome Study (PTOS) (461 974 patients from 2003 to 2015), the National Trauma Data Bank (NTDB) (5 755 095 patients from 2003 to 2014), and the National (Nationwide) Inpatient Sample (NIS) (24 449 476 patients from 2003 to 2013) databases.
Main Outcomes and Measures
Temporal trends in VCF placement and PE rates, filter type (prophylactic or therapeutic), and established predictors of PE (obesity, pregnancy, cancer, deep vein thrombosis, major procedure, spinal cord paralysis, venous injury, lower extremity fracture, pelvic fracture, central line, intracranial hemorrhage, and blood transfusion). Prophylactic filters were defined as VCFs placed before or without an existing PE, while therapeutic filters were defined as VCFs placed after a PE.
Results
Of the 461 974 patients in PTOS, the mean (SD) age was 47.2 (26.4) and 61.6% (284 621) were men; of the 5 755 095 patients in NTDB, the mean age (SD) was 42.0 (24.3) and 63.7% (3 666 504) were men; and of the 24 449 476 patients in NIS, the mean (SD) age was 58.0 (25.2) and 49.7% (12 160 231) were men. Of patients receiving a filter (11 405 in the PTOS, 71 029 in the NTDB, and 189 957 in the NIS), most were prophylactic VCFs (93.6% in the PTOS, 93.5% in the NTDB, and 93.3% in the NIS). Unadjusted and adjusted temporal trends for the PTOS and NTDB showed initial increases in filter placement followed by significant declines (unadjusted reductions in VCF placement rates, 76.8% in the PTOS and 53.3% in the NTDB). The NIS demonstrated a similar unadjusted trend, with a slight increase and modest decline (22.2%) in VCF placement rates over time; however, adjusted trends showed a slight but significant increase in filter rates. Adjusted PE rates for the PTOS and NTDB showed significant initial increases followed by slight decreases, with limited variation during the declining filter use periods. The NIS showed an initial increase in PE rates followed by a period of stagnation.
Conclusions and Relevance
Despite a precipitous decline of VCF use in trauma, PE rates remained unchanged during this period. Taking this association into consideration, VCFs may have limited utility in influencing rates of PE. More judicious identification of at-risk patients is warranted to determine individuals who would most benefit from a VCF.
This cohort study analyzes state and nationwide temporal trends in vena cava filter placement and pulmonary embolism occurrence in patients with traumatic injury from 2003 to 2015.
Introduction
Despite advances in trauma care, venous thromboembolism (VTE) remains a significant complication among hospitalized trauma patients. As many as 600 000 Americans are affected by VTE each year, including deep vein thrombosis (DVT) and pulmonary embolism (PE), resulting in more than 100 000 deaths. Although guidelines recommend VTE prophylaxis with low-molecular-weight heparin in trauma, there is a small proportion of high-risk patients who have contraindications for heparin therapy due to an ongoing risk of life-threatening bleeding. For this patient population, the use of prophylactic vena cava filters (VCFs) is implemented as an alternate means of DVT and PE prevention, although their utility is debated. While some studies have found that VCFs prevent dramatic and life-threatening PE, other evidence suggests that this approach is ineffective and should be curtailed. A meta-analysis published in 2014 demonstrated modest support for the association between VCF placement and decreased incidence of PE and mortality, further fueling the controversy. The presence of contradictory evidence has also resulted in conflicting professional guidelines regarding the use of VCFs in trauma patients. Although management guidelines published by the Eastern Association for the Surgery of Trauma and the Society of Interventional Radiology promote VCF use in certain patient populations, the American College of Chest Physicians suggests that VCFs should not be used for VTE prophylaxis in trauma.
A paucity of level I randomized clinical trial research and epidemiological analyses addressing the influence of VCF placement on PE development likely explains many of the conflicting recommendations for VCF use in trauma. The goal of this investigation was to add to the literature on this understudied issue by providing a comprehensive state and nationwide objective view of temporal trends in VCF placement and PE occurrence across a 13-year study period. Specifically, our aims were (1) to determine if any significant variation in filter placement was observed over time and (2) to test whether these trends were influencing rates of PE. We hypothesized that rates of VCF placement would decline at the state and national levels while rates of PE would increase over time.
Methods
After approval by the institutional review boards of Chandler Regional Medical Center and Penn Medicine Lancaster General Health, a retrospective trauma cohort study was conducted using data from the Pennsylvania Trauma Outcome Study (PTOS) (2003-2015), National Trauma Data Bank (NTDB) (2003-2014), and the National (Nationwide) Inpatient Sample (NIS) (2003-2013) databases. Because patients were not recruited to participate in this study and the nature of this work was retrospective, no participant informed consent was applicable. The rationale for including each of these data sets in the analysis was to gain the telescoping perspectives of (1) a single, well-organized trauma system in geographic proximity (PTOS); (2) a broader convenience sample limited to trauma centers from across the country (NTDB); and (3) a comprehensive, population-based national hospital sample (NIS) that includes trauma centers and non–trauma centers. Each data set adds information that the others do not. The PTOS is the statewide trauma registry of the Pennsylvania Trauma Systems Foundation, the accrediting body for trauma centers within the Commonwealth of Pennsylvania. To remain an accredited institution, trained registrars from the 38 level I through level IV trauma centers throughout the state are required to extract and submit deidentified hospital data to the PTOS for all patients meeting specified trauma criteria. Developed by the American College of Surgeons in 1989, the NTDB is the largest aggregate sample of voluntarily submitted US and Canadian trauma registry data. The NIS contains a population-based sample of US inpatient data. As such, the NIS data set allows for inferences to be made regarding the application of VCF in trauma centers and non–trauma centers. The selected time frames analyzed in this study represent the most recently available data from these databases while maintaining the maximum number of overlapping years.
The cohort was restricted to patients whose primary diagnosis was traumatic injury classified in the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code range of 800 to 959.9 and who had an ICD-9-CM Supplemental Classification of External Causes of Injury and Poisoning (E-code) code. In addition, the patient’s type of admission was required to be emergent.
After identification of the cohort, VCF placement was identified for all patients across the 3 databases using ICD-9-CM procedure code 38.7. The published risk factors for VTE or indications for VCF placement were identified for each patient using the respective ICD-9-CM codes for PE, obesity, pregnancy, cancer, DVT, major procedure, spinal cord paralysis, venous injury, lower extremity fracture, pelvic fracture, central line, intracranial hemorrhage, and blood transfusion. In the PTOS and NTDB data sets, PE was identified in the complications files and the diagnoses files. Prophylactic filters were defined as VCFs placed before or without an existing PE. Therapeutic filters were defined as VCFs placed after a PE in the PTOS or in patients with the diagnosis of PE in the NTDB and NIS. Injury severity was estimated using the trauma mortality prediction model for the ICD-9-CM lexicon. Patient characteristics were compared by VCF status among each of the 3 data sets using parametric and nonparametric methods as appropriate.
Hierarchical logistic regression models were developed for each data set. The models included predictors significantly associated with VCF placement in univariate analysis. Each model included year as an index variable and an individual facility identifier variable as the random-effects parameter. Model discrimination was measured using the area under the receiver operating characteristic curve. Unadjusted annual rates of VCF placement for the PTOS, NTDB, and NIS data sets with 95% CIs were plotted over years on a single graph for comparison. Similarly, odds ratios for each year from the hierarchical models of VCF placement with 95% CIs were plotted over years for each of the 3 data sets for comparison of risk-adjusted trends. Differences with P < .05 were considered statistically significant. All data manipulation and statistical computation were performed using a software program (Stata/MP, version 14.2; StataCorp LP).
Results
A total of 24 449 476 patients from 17 512 hospitals met inclusion criteria from the NIS data set for the 2003 to 2013 time frame. From 2003 to 2014, the NTDB produced a cohort of 5 755 095 trauma patients from 1359 trauma centers. From 2003 to 2015, data on 461 974 trauma patients were submitted to the PTOS database from 38 centers. The overall VCF placement rate (2.5% for the PTOS, 1.2% for the NTDB, and 0.8% for the NIS) and PE rate (0.5% for the PTOS, 0.3% for the NTDB, and 0.6% for the NIS) showed minor variation across the 3 data sets. Among patients receiving a filter, most were coded as prophylactic VCFs (93.6% for the PTOS, 93.5% for the NTDB, and 93.3% for the NIS). The characteristics of the study population, including risk factors for VTE and indications for VCF placement, mechanism of injury, and injury severity in the form of the trauma mortality prediction model, are listed in Table 1.
Table 1. Total Study Population Demographics.
| Variable | PTOS (2003-2015) (n = 461 974) | NTDB (2003-2014) (n = 5 755 095) | NIS (2003-2013) (n = 24 449 476) | |||
|---|---|---|---|---|---|---|
| VCF | No VCF | VCF | No VCF | VCF | No VCF | |
| Total, No./total No. (%) | 11 405/461 974 (2.5) | 450 569/461 974 (97.5) | 71 029/5 755 095 (1.2) | 5 684 066/5 755 095 (98.8) | 189 957/24 449 476 (0.8) | 24 259 519/24 449 476 (99.2) |
| VCF placement, No./total No. (%) | ||||||
| Prophylactic | 10 676/11 405 (93.6) | NA | 66 413/71 029 (93.5) |
NA | 177 217/189 957 (93.3) |
NA |
| Therapeutic | 729/11 405 (6.4) | NA | 4616/71 029 (6.5) |
NA | 12 740/189 957 (6.7) |
NA |
| PE, No./total No. (%) | 869/2088 (41.6) | 1219/2088 (58.4) | 4656/15 857 (29.2) | 11 201/15 857 (70.8) | 35 296/154 051 (22.9) | 118 755/154 051 (77.1) |
| PE predictor, No./total No. (%) | ||||||
| Obesity | 1279/24 643 (5.2) | 23 364/24 643 (94.8) | 586/15 400 (3.8) | 14 814/15 400 (96.2) |
8292/1 055 435 (0.8) | 1 047 143/1 055 435 (99.2) |
| Pregnancy | 25/1341 (1.9) | 1316/1341 (98.1) | 14/2744 (0.5) | 2730/2744 (99.5) | 5/1478 (0.3) | 1473/1478 (99.7) |
| Cancer | 224/5847 (3.8) | 5603/5847 (96.2) | 90/4011 (2.2) | 3921/4011 (97.8) | 7194/592 357 (1.2) | 585 163/592 357 (98.8) |
| Deep vein thrombosis | 2384/5784 (41.2) | 3400/5784 (58.8) | 1782/4689 (38.0) | 2907/4689 (62.0) | 67 458/273 729 (24.6) | 206 271/273 729 (75.4) |
| Major procedure | 4350/35 203 (12.4) | 30 853/35 203 (87.6) | 28 935/304 250 (9.5) | 275 315/304 250 (90.5) | 39 557/276 304 (14.3) | 236 747/276 304 (85.7) |
| Spinal cord paralysis | 366/1098 (33.3) | 732/1098 (66.7) | 842/4317 (19.5) | 3475/4317 (80.5) | 695/3510 (19.8) | 2815/3510 (80.2) |
| Venous injury | 210/565 (8.2) | 2355/2565 (91.8) | 1398/25 404 (5.5) | 24 006/25 404 (94.5) | 3854/74 461 (5.2) | 70 607/74 461 (94.8) |
| Lower extremity fracture | 4259/95 773 (4.5) | 91 514/95 773 (95.6) | 20 062/936 663 (2.1) |
916 601/936 663 (97.9) |
54 856/5 865 895 (0.9) | 5 811 039/5 865 895 (99.1) |
| Pelvic fracture | 3212/33 007 (9.7) | 29 795/33 007 (90.3) | 17 684/316 758 (5.6) |
299 074/316 758 (94.4) |
34 216/1 045 458 (3.3) | 1 011 242/1 045 458 (96.7) |
| Central line | 4398/42 775 (10.3) | 38 377/42 775 (89.7) | 25 789/386 583 (6.7) | 360 794/386 583 (93.3) | 9425/113 533 (8.3) | 104 108/113 533 (91.7) |
| Intracranial hemorrhage | 3713/77 055 (4.8) | 73 342/77 055 (95.2) | 19 171/585 319 (3.3) |
566 148/585 319 (96.7) |
36 952/1 303 156 (2.8) | 1 266 204/1 303 156 (97.2) |
| Blood transfusion | 104/1378 (7.6) | 1274/1378 (92.5) | 18 190/281 144 (6.5) | 262 954/281 144 (93.5) | 5398/33 868 (15.9) | 28 470/33 868 (84.1) |
| Trauma mortality prediction model, median (IQR) | 0.13 (0.40) | 0.02 (0.05) | 0.10 (0.20) | 0.02 (0.03) | 0.03 (0.10) | 0.01 (0.01) |
| Age, mean (95% CI), y | 51.3 (50.9-51.7) | 47.2 (47.1-47.3) | 47.1 (47.0-47.3) | 41.1 (41.1-41.2) | 57.9 (57.8-57.9) | 57.9 (57.6-58.1) |
| Male sex, No./total No. (%) | 7606/284 621 (2.7) | 277 015/284 621 (97.3) | 48 821/3 666 504 (1.3) | 3 617 683/3 666 504 (98.7) | 113 714/12 160 231 (0.9) | 12 046 517/12 160 231 (99.4) |
| Race/ethnicity, No./total No. (%) | ||||||
| White | 9276/357 198 (2.6) | 347 922/357 198 (97.4) | 50 960/3 873 011 (1.3) | 3 822 051/3 873 011 (98.7) | 113 786/14 725 652 (0.8) | 14 611 866/14 725 652 (99.2) |
| Black | 1442/66 180 (2.2) | 64 738/66 180 (97.8) | 10 322/870 307 (1.2) |
859 985/870 307 (98.8) |
19 734/1 987 139 (1.0) | 1 967 405/1 987 139 (99.0) |
| Hispanic | NA | NA | 5605/633 946 (0.9) |
628 341/633 946 (99.1) |
14 696/1 881 988 (0.8) | 1 867 292/1 881 988 (99.2) |
| Asian/Pacific Islander | 85/370 (2.0) | 4285/4370 (98.0) | 873/95 306 (0.9) | 94 469/95 306 (99.1) | 2230/330 935 (0.7) | 328 705/330 935 (99.3) |
| Native American | NA | NA | 416/44 249 (1.0) | 43 834/44 249 (99.1) | 798/133 294 (0.6) | 132 496/133 294 (99.4) |
| Other or unknown | 602/34 226 (1.8) | 33 624/34 226 (98.2) | 2889/238 275 (1.1) |
235 386/238 275 (98.9) |
38 713/5 390 468 (0.7) | 5 351 755/5 390 468 (99.3) |
| Mechanism of injury, No./total No. (%) | ||||||
| Penetrating | 630/35 553 (1.8) | 34 923/35 553 (98.3) | 4178/472 502 (0.9) | 468 324/472 502 (99.1) | 4635/630 364 (0.7) | 625 729/360 364 (99.3) |
| Blunt | 10 629/404 352 (2.6) | 393 723/404 352 (97.4) | 65 499/5 003 040 (1.3) | 4 937 541/5 003 040 (98.7) | 116 401/13 573 069 (0.9) | 13 456 668/13 573 069 (99.1) |
| Neither | 146/22 069 (0.7) | 21 923/22 069 (99.3) | 1352/279 553 (0.5) |
278 201/279 553 (99.5) |
68 921/10 246 043 (0.7) | 10 177 122/10 246 043 (99.3) |
| Length of stay, median (IQR), d | 16 (16) | 3 (4) | 19 (20) | 3 (5) | NA | NA |
| Died, No./total No. (%) | 954/22 369 (4.3) | 21 415/22 369 (95.7) | 3917/121 573 (3.2) | 117 656/121 573 (96.8) | 12 836/623 227 (2.1) | 610 391/623 227 (97.9) |
Abbreviations: IQR, interquartile range; NA, not applicable; NIS, National (Nationwide) Inpatient Sample; NTDB, National Trauma Data Bank; PE, pulmonary embolism; PTOS, Pennsylvania Trauma Outcome Study; VCF, vena cava filter.
Within the total VCF population (11 405 for the PTOS, 71 029 for the NTDB, and 189 957 for the NIS), most patients were found to have at least one established predictor of PE (92.9% for the PTOS, 86.7% for the NTDB, and 88.6% for the NIS). In total, 815 patients (7.1%) in the PTOS, 11 143 patients (15.7%) in the NTDB, and 23 469 patients (12.4%) in the NIS received a filter without any established predictors of PE development. Conversely, within the non-VCF population (450 569 for the PTOS, 5 684 066 for the NTDB, and 24 259 519 for the NIS), 203 784 patients (45.2%) in the PTOS, 2 883 503 patients (50.7%) in the NTDB, and 10 511 902 patients (43.3%) in the NIS were diagnosed as having at least one established predictor of PE without receiving a VCF. Of patients who developed a PE, most had at least one established predictor (83.1% of the PTOS, 86.7% of the NTDB, and 88.7% of the NIS). A total of 354 patients in the PTOS, 4463 patients in the NTDB, and 61 964 patients in the NIS developed a PE without documented risk factors.
Pennsylvania Trauma Outcome Study
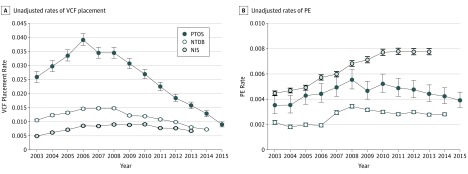
Within the PTOS database, unadjusted annual rates of filter placement increased from 2003 until 2006. After the peak VCF placement rate, a decline in filter placement was observed, with filter rates decreasing from 3.9% in 2006 to 0.9% in 2015 (76.8% reduction). Unadjusted temporal trends in PE rates were similar, reaching a maximum in 2008 within the PTOS. During the period of declining VCF placement rates (2006-2015), a modest reduction in unadjusted PE rates was observed (Figure 1).
Figure 1. Unadjusted Rates of Vena Cava Filter (VCF) Placement and Pulmonary Embolism (PE) Occurrence in the PTOS, NTDB, and NIS From 2003 to 2015.
NIS indicates National (Nationwide) Inpatient Sample; NTDB, National Trauma Data Bank; and PTOS, Pennsylvania Trauma Outcome Study.
After modeling VCF placement and PE occurrence using multivariable hierarchical logistic regression techniques, similar temporal trends were observed. A significant adjusted increase in VCF placement rates was found until 2006, followed by a similar steady decline in filter rates until the end of available data in 2015 (Table 2 and Figure 2). Regarding adjusted PE rates, significant increased odds ratios for PE were observed from 2005 to 2015 compared with the 2003 reference year. The adjusted odds ratio for PE was highest in 2008 at 1.9. After the 2008 peak, a 30% reduction in the odds ratio for PE was found by 2015 (Table 3 and Figure 2). In terms of fatal PE trends in the PTOS data set, 195 fatal PEs were identified during the study period. Similar to trends for the total study population, no significant adjusted change in fatal PE rates was observed.
Table 2. Adjusted Odds Ratios for VCF Placement in the PTOS, NTDB, and NIS.
| Variable | Adjusted Odds Ratio (95% CI) | ||
|---|---|---|---|
| PTOS (n = 461 974) |
NTDBa (n = 5 755 095) |
NISb (24 449 476) |
|
| PE | 13.1 (11.6-14.7) | 17.2 (16.5-17.9) | 18.7 (18.0-19.5) |
| Deep vein thrombosis | 12.4 (11.5-13.3) | 14.1 (13.0-15.4) | 48.8 (47.3-50.3) |
| Male sex | 1.0 (1.0-1.1)c | 1.1 (1.1-1.2) | 1.2 (1.2-1.3) |
| Obesity | 1.7 (1.6-1.8) | 1.7 (1.5-1.8) | 1.3 (1.2-1.3) |
| Major procedure | 2.8 (2.7-3.0) | 3.9 (3.9-4.0) | 6.9 (6.7-7.2) |
| Central catheter | 2.2 (2.1-2.3) | 2.9 (2.9-3.0) | 3.1 (2.9-3.3) |
| Blood transfusion | 1.0 (0.7-1.2)c | 2.1 (2.0-2.1) | 3.2 (2.9-3.5) |
| Pelvic fracture | 3.7 (3.5-3.9) | 3.0 (2.9-3.1) | 3.7 (3.6-3.8) |
| Lower extremity fracture | 2.6 (2.4-2.7) | 2.0 (2.0-2.1) | 1.9 (1.8-1.9) |
| Spinal cord paralysis | 10.7 (9.1-12.5) | 6.8 (6.2-7.4) | 4.9 (3.8-6.2) |
| Trauma mortality prediction model | 1.4 (1.4-1.4) | 1.4 (1.4-1.5) | 1.7 (1.7-1.7) |
| Age group, y | |||
| ≤20s | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 30s | 1.4 (1.3-1.5) | 1.4 (1.4-1.4) | 1.2 (1.2-1.3) |
| 40s | 1.5 (1.4-1.6) | 1.6 (1.5-1.6) | 1.3 (1.2-1.3) |
| 50s | 1.7 (1.6-1.8) | 1.7 (1.7-1.8) | 1.4 (1.4-1.5) |
| 60s | 1.9 (1.8-2.1) | 1.9 (1.8-1.9) | 1.4 (1.4-1.5) |
| 70s | 2.0 (1.9-2.2) | 1.8 (1.7-1.8) | 1.4 (1.3-1.5) |
| 80s | 1.9 (1.7-2.0) | 1.5 (1.4-1.5) | 1.3 (1.2-1.3) |
| ≥90s | 1.3 (1.1-1.5) | 0.8 (0.7-0.9) | 1.0 (1.0-1.1)c |
| Year | |||
| 2003 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 2004 | 1.4 (1.3-1.6) | 1.2 (1.2-1.3) | 1.1 (0.9-1.2)c |
| 2005 | 1.6 (1.4-1.8) | 1.7 (1.6-1.8) | 1.3 (1.1-1.5) |
| 2006 | 1.9 (1.7-2.2) | 1.8 (1.6-1.8) | 1.4 (1.2-1.6) |
| 2007 | 1.6 (1.4-1.8) | 1.7 (1.6-1.8) | 1.4 (1.2-1.6) |
| 2008 | 1.7 (1.5-1.9) | 1.7 (1.6-1.8) | 1.4 (1.2-1.6) |
| 2009 | 1.7 (1.5-1.8) | 1.4 (1.3-1.5) | 1.4 (1.2-1.6) |
| 2010 | 1.3 (1.2-1.5) | 1.4 (1.3-1.4) | 1.6 (1.4-1.9) |
| 2011 | 1.0 (0.9-1.1)c | 1.1 (1.0-1.1) | 1.5 (1.3-1.8) |
| 2012 | 0.8 (0.7-0.9) | 0.9 (0.9-1.0) | 1.7 (1.5-1.9) |
| 2013 | 0.7 (0.6-0.8) | 0.7 (0.7-0.8) | 1.5 (1.3-1.7) |
| 2014 | 0.6 (0.5-0.6) | 0.6 (0.6-0.7) | NA |
| 2015 | 0.4 (0.3-0.4) | NA | NA |
| Overall AUROC | 0.90 | 0.92 | 0.94 |
Abbreviations: AUROC, area under the receiver operating characteristic curve; NA, not applicable; NIS, National (Nationwide) Inpatient Sample; NTDB, National Trauma Data Bank; PE, pulmonary embolism; PTOS, Pennsylvania Trauma Outcome Study; VCF, vena cava filter.
From 2003 to 2014 only.
From 2003 to 2013 only.
P > .05.
Figure 2. Adjusted Odds Ratios for Vena Cava Filter (VCF) Placement and Pulmonary Embolism (PE) Occurrence in the PTOS, NTDB, and NIS From 2004 to 2015.
NIS indicates National (Nationwide) Inpatient Sample; NTDB, National Trauma Data Bank; and PTOS, Pennsylvania Trauma Outcome Study.
Table 3. Adjusted Odds Ratios for PE in the PTOS, NTDB, and NIS.
| Variable | Adjusted Odds Ratio (95% CI) | ||
|---|---|---|---|
| PTOS (n = 461 974) |
NTDBa (n = 5 755 095) |
NISb (24 449 476) |
|
| Deep vein thrombosis | 8.6 (7.6-9.8) | 11.7 (10.4-13.1) | 32.9 (32.0-33.9) |
| Male sex | 1.3 (1.2-1.5) | 1.3 (1.2-1.3) | 1.1 (1.0-1.1) |
| Obesity | 1.7 (1.5-2.0) | 1.8 (1.5-2.1) | 1.7 (1.6-1.8) |
| Major procedure | 2.2 (1.9-2.5) | 2.6 (2.5-2.7) | 1.9 (1.8-2.1) |
| Central catheter | 2.2 (2.0-2.5) | 2.4 (2.3-2.5) | 2.0 (1.8-2.1) |
| Blood transfusion | 1.2 (0.7-1.9)c | 1.6 (1.6-1.7) | 1.5 (1.3-1.8) |
| Pelvic fracture | 1.6 (1.4-1.8) | 1.6 (1.6-1.7) | 1.1 (1.1-1.2) |
| Lower extremity fracture | 2.3 (2.1-2.5) | 2.1 (2.1-2.2) | 1.2 (1.1-1.2) |
| Spinal cord paralysis | 2.5 (1.8-3.6) | 2.1 (1.8-2.6) | 1.2 (0.7-2.1)c |
| Trauma mortality prediction model | 1.1 (1.1-1.1) | 1.2 (1.2-1.2) | 1.1 (1.1-1.1) |
| Age group, y | |||
| ≤20s | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 30s | 1.5 (1.3-1.8) | 1.8 (1.8-2.0) | 1.9 (1.8-2.1) |
| 40s | 1.5 (1.3-1.7) | 2.3 (2.2-2.4) | 2.4 (2.2-2.5) |
| 50s | 1.7 (1.5-2.0) | 2.7 (2.5-2.8) | 2.8 (2.6-3.0) |
| 60s | 2.0 (1.7-2.4) | 3.1 (3.0-3.3) | 3.3 (3.1-3.5) |
| 70s | 1.9 (1.6-2.3) | 2.9 (2.8-3.1) | 3.4 (3.2-3.6) |
| 80s | 1.4 (1.2-1.7) | 2.4 (2.3-2.6) | 3.0 (2.8-3.2) |
| ≥90s | 1.0 (0.7-1.4)c | 1.9 (1.5-2.4) | 2.4 (2.2-2.5) |
| Year | |||
| 2003 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 2004 | 1.2 (0.9-1.6)c | 0.9 (0.8-1.0) | 1.0 (0.9-1.1)c |
| 2005 | 1.4 (1.1-1.9) | 1.0 (0.9-1.2)c | 1.1 (1.0-1.2)c |
| 2006 | 1.5 (1.1-2.0) | 1.0 (0.9-1.1)c | 1.2 (1.1-1.3) |
| 2007 | 1.6 (1.2-2.1) | 1.1 (1.0-1.2)c | 1.2 (1.1-1.3) |
| 2008 | 1.9 (1.5-2.5) | 1.3 (1.2-1.4) | 1.3 (1.2-1.4) |
| 2009 | 1.8 (1.4-2.3) | 1.2 (1.1-1.3) | 1.4 (1.3-1.5) |
| 2010 | 1.9 (1.5-2.5) | 1.1 (1.0-1.3) | 1.6 (1.5-1.8) |
| 2011 | 1.8 (1.4-2.3) | 1.0 (0.9-1.1)c | 1.6 (1.5-1.7) |
| 2012 | 1.7 (1.3-2.2) | 1.1 (1.0-1.2)c | 1.6 (1.5-1.7) |
| 2013 | 1.6 (1.3-2.1) | 1.0 (0.9-1.1)c | 1.6 (1.5-1.7) |
| 2014 | 1.6 (1.3-2.1) | 1.0 (0.9-1.1)c | NA |
| 2015 | 1.6 (1.2-2.0) | NA | NA |
| Overall AUROC | 0.83 | 0.84 | 0.78 |
Abbreviations: AUROC, area under the receiver operating characteristic curve; NA, not applicable; NIS, National (Nationwide) Inpatient Sample; NTDB, National Trauma Data Bank; PE, pulmonary embolism; PTOS, Pennsylvania Trauma Outcome Study.
From 2003 to 2014 only.
From 2003 to 2013 only.
P > .05.
National Trauma Data Bank
Like the PTOS, the NTDB showed similar unadjusted trends in VCF placement and PE rates. Filter placement and PE rates increased from the beginning of the study period until peaking in 2008. After this point, VCF placement rates decreased by more than half, from 1.5% in 2008 to 0.7% in 2014 (53.3% reduction). A minor reduction in PE rates was observed after this time until the end of available data in 2014 (Figure 1).
In adjusted analysis, significant increases in VCF placement were observed from 2004 to 2006, followed by a period of steady decline until the end of available data in 2014 (Table 2 and Figure 2). Significant increases in adjusted odds ratios for PE were found within the NIS from 2008 to 2010 compared with the reference, with the highest odds ratio of 1.3 occurring in 2008. Minor reductions in adjusted odds ratios for PE occurred from 2009 to 2014 (Table 3 and Figure 2).
National (Nationwide) Inpatient Sample
Within the NIS database, unadjusted annual rates of VCF placement increased from 2003 until 2010. After this point, a modest reduction in VCF placement rates was observable, from 0.9% in 2010 to 0.7% in 2013 (22.2% reduction). Unadjusted rates of PE increased from 2003 to 2011 and remained constant after this point (Figure 1).
After multivariable modeling, the NIS showed a marked increase in the odds of VCF placement over time (Table 2 and Figure 2). The adjusted odds of PE significantly increased from 2006 to a peak of 1.63 in 2010 relative to 2003. Beginning in 2010, odds ratios for PE in the NIS increased over baseline but stayed stable through the end of 2013 (Table 3 and Figure 2).
Multivariable modeling produced strong support for many previously cited predictors of PE development across the 3 data sets analyzed. Male sex, age, obesity, DVT, major procedure, lower extremity fracture, pelvic fracture, and central line were all found to be significant predictors of PE within the PTOS, NTDB, and NIS. Blood transfusion was a significant predictor of PE in all but the PTOS data set, and spinal cord paralysis was a significant predictor in all but the NIS data set (Table 3).
Discussion
The results of this investigation suggest that, while rates of VCF placement are declining throughout the nation, rates of PE (including fatal PE) are unchanged or are also decreasing in some populations. Although these findings support our hypothesis with respect to decreased VCF placement over time, we must reject our other hypothesis in terms of an expected rise in PE rates. Viewing these results in composite, it is reasonable to discern that decreasing trends in practice patterns for VCF placement are not accompanied by changes in the incidence of PE. While this finding may suggest that filters have limited utility in preventing PE, supporting a modest amount of literature on the subject, it is important to note that VCFs are designed to prevent fatal PEs and not all PEs. Although analyses within the PTOS found no change in fatal PE rates over time, it is likely that this investigation failed to identify all fatal PE cases because routine autopsies are no longer performed on most trauma patients who die of unspecified causes. However, what this finding alarmingly suggests and advocates is more judicious identification and management of patients at risk for developing a PE, an area in need of reform based on the results of this investigation.
As evidenced in this study, a large percentage of trauma patients across all 3 data sets had at least one predictor of PE without receiving a filter. However, due to limitations in the NTDB and NIS data, we are unable to report the use of chemoprophylaxis. In addition, a modest percentage of patients received a filter without any documented predictors of PE development. While VCF placement is at the discretion of the attending physician, it is likely that many patients with risk factors who did not receive a VCF received chemoprophylaxis. During the time frame of the study, the previously considered contradictions to chemoprophylaxis have been found to pose acceptable risk for subcutaneous heparinoid VTE prophylaxis. It is also possible that a number of PEs could have been prevented had these at-risk patients received a VCF. However, despite a plethora of research detailing factors associated with PE development, predicting which patients will develop a PE is a difficult task. Even in recent years, measures that were designed to determine the relative weight of risk factors for VTE (eg, the Trauma Embolic Scoring System by Rogers et al) have not gained widespread traction in the trauma community.
On review of the major trends observed in this investigation, the initial rise in VCF placement and PE rates seen throughout the first years of this investigation is likely due to multiple interacting factors. As suggested in other temporal analyses, the rise in VCF use was likely fueled by the introduction of the retrievable filter in 2003 as well as advancing radiographic technology, a factor that likely also resulted in the increased incidence of PE. With the advent of retrievable VCFs, physicians no longer had to be concerned about the long-term influence of permanently implanted VCFs, making them an attractive option for the prophylaxis of at-risk trauma patients, although these retrievable filters are never retrieved in many cases. As detailed by Wiener et al, the introduction of computed tomographic pulmonary angiography resulted in a rising incidence of PE diagnosis (an 81% increase from 62.1 to 112.3 cases per 100 000) but a minimal change in mortality. In addition to leading to the overdiagnosis of venous thromboembolic events, this increased use of computed tomographic pulmonary angiography also inevitably led to an increase in VCF placement as evidenced by the initial increase in VCF use, mirroring the rise in PE diagnosis found in this study.
We can only speculate that, after the initial rise in PE rates, the stagnant (or in some cases, declining) trend in PE seen in this study represents the new normal regarding its incidence relative to these changes in radiographic technology. During this time, declining VCF use seemed to have no association with changes in PE rates. The decrease in VCF use may be in part due to the 2010 statement released by the US Food & Drug Administration detailing complications with retrievable filters.
Changes in rates of VCF placement observed in this study, following the trend of the PTOS, NTDB, and NIS, are likely a result of increasing variation in the practice of VCF use among the trauma centers in the data sets. As one of the most mature trauma systems in the nation, the Pennsylvania system is composed of a variety of centers that were founded in the mid- to late 1980s. As such, this system is likely at the forefront of trends pertaining to the optimal management of trauma patients. As the present investigation shifted to account for the national trauma population (NTDB) and all hospitalized patients meeting trauma classification (NIS), it is feasible to suggest that these groups, which contained patients managed in less established trauma systems as well as patients managed at non–trauma centers, were likely not on the cutting edge of trends pertaining to VCF use.
Limitations
This study is not without its limitations. Although we analyzed the PTOS, NTDB, and NIS as 3 separate data sets, they represent nested cohorts for the overlapping years. The PTOS patients are included in and represent 7.3% of the NTDB for the years 2003 through 2014. Similarly, most (if not all) of the patients in the NTDB are in the NIS data set from 2003 through 2013. Because many states do not contribute data to the NIS and given the anonymous nature of all 3 data sets, it is not possible to estimate the number of patients shared by the PTOS, NTDB, and NIS with any degree of certainty. However, if the inclusion was complete, the NTDB would represent 23.5% of the cohort and 37.5% of the VCFs in the NIS during the overlapping years of 2003 through 2013. This limitation leaves most of the variance observed in the NIS unaccounted for by that from the NTDB for the 11 years of overlap. Furthermore, the ICD-9-CM lexicon lacks a code for VTE chemoprophylaxis; therefore, we were unable to include it as a competing treatment choice. Certainly, there are patients with risk factors for PE who did not receive a VCF and were treated with subcutaneous enoxaparin sodium or unfractionated heparin instead. Finally, this study included almost 25 million patients during 13 years. In such a large sample of patients from different settings of care, a wide range of data quality is to be expected. In addition, widespread implementation of the electronic medical record took place, which may have influenced documentation of predictors, such as comorbid conditions. As such, the data included in this study are primarily administrative, not clinical. Like other studies of administrative data, ours is subject to the same attendant imperfections, including variation in diagnosis accuracy and data completeness, resulting in some degree of surveillance bias. Measuring the degree to which such bias influenced our inferences is beyond the scope of this study. Taking these limitations into account, our study provides a robust depiction of the temporal trends of VCF placement in the care of traumatically injured patients.
Conclusions
The practice of VCF use in trauma has shifted over time, although the trend in recent years is toward decreasing use. Meanwhile, rates of PE (including fatal PE) have remained stable. In addition, a large percentage of patients with risk factors for PE are not undergoing VCF placement, while a modest percentage of patients are receiving VCFs without identified risk factors. More investigation is needed to optimize patient selection for VCF use when the risks of chemoprophylaxis are prohibitive.
References
- 1.Geerts WH, Code KI, Jay RM, Chen E, Szalai JP. A prospective study of venous thromboembolism after major trauma. N Engl J Med. 1994;331(24):1601-1606. [DOI] [PubMed] [Google Scholar]
- 2.Haut ER, Chang DC, Pierce CA, et al. . Predictors of posttraumatic deep vein thrombosis (DVT): hospital practice versus patient factors: an analysis of the National Trauma Data Bank (NTDB). J Trauma. 2009;66(4):994-999. [DOI] [PubMed] [Google Scholar]
- 3.Knudson MM, Gomez D, Haas B, Cohen MJ, Nathens AB. Three thousand seven hundred thirty-eight posttraumatic pulmonary emboli: a new look at an old disease. Ann Surg. 2011;254(4):625-632. [DOI] [PubMed] [Google Scholar]
- 4.Office of the Surgeon General The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville, MD: Office of the Surgeon General; 2008. [PubMed] [Google Scholar]
- 5.Rogers FB, Cipolle MD, Velmahos G, Rozycki G, Luchette FA. Practice management guidelines for the prevention of venous thromboembolism in trauma patients: the EAST Practice Management Guidelines Work Group. J Trauma. 2002;53(1):142-164. [DOI] [PubMed] [Google Scholar]
- 6.Gould MK, Garcia DA, Wren SM, et al. ; American College of Chest Physicians . Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. [published correction appears in Chest. 2012;141(5):1369]. Chest. 2012;141(2)(suppl):e227S-e277S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malinoski D, Ewing T, Patel MS, et al. . Risk factors for venous thromboembolism in critically ill trauma patients who cannot receive chemical prophylaxis. Injury. 2013;44(1):80-85. [DOI] [PubMed] [Google Scholar]
- 8.Carlin AM, Tyburski JG, Wilson RF, Steffes C. Prophylactic and therapeutic inferior vena cava filters to prevent pulmonary emboli in trauma patients. Arch Surg. 2002;137(5):521-525. [DOI] [PubMed] [Google Scholar]
- 9.McMurtry AL, Owings JT, Anderson JT, Battistella FD, Gosselin R. Increased use of prophylactic vena cava filters in trauma patients failed to decrease overall incidence of pulmonary embolism. J Am Coll Surg. 1999;189(3):314-320. [DOI] [PubMed] [Google Scholar]
- 10.Haut ER, Garcia LJ, Shihab HM, et al. . The effectiveness of prophylactic inferior vena cava filters in trauma patients: a systematic review and meta-analysis. JAMA Surg. 2014;149(2):194-202. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman JA, Kinney TB, Streiff MB, et al. . Guidelines for the use of retrievable and convertible vena cava filters: report from the Society of Interventional Radiology multidisciplinary consensus conference. J Vasc Interv Radiol. 2006;17(3):449-459. [DOI] [PubMed] [Google Scholar]
- 12.Khansarinia S, Dennis JW, Veldenz HC, Butcher JL, Hartland L. Prophylactic Greenfield filter placement in selected high-risk trauma patients. J Vasc Surg. 1995;22(3):231-235. [DOI] [PubMed] [Google Scholar]
- 13.Leach TA, Pastena JA, Swan KG, Tikellis JI, Blackwood JM, Odom JW. Surgical prophylaxis for pulmonary embolism. Am Surg. 1994;60(4):292-295. [PubMed] [Google Scholar]
- 14.Rodriguez JL, Lopez JM, Proctor MC, et al. . Early placement of prophylactic vena caval filters in injured patients at high risk for pulmonary embolism. J Trauma. 1996;40(5):797-802. [DOI] [PubMed] [Google Scholar]
- 15.Rogers FB, Shackford SR, Wilson J, Ricci MA, Morris CS. Prophylactic vena cava filter insertion in severely injured trauma patients: indications and preliminary results. J Trauma. 1993;35(4):637-641. [DOI] [PubMed] [Google Scholar]
- 16.Rogers FB, Shackford SR, Ricci MA, Wilson JT, Parsons S. Routine prophylactic vena cava filter insertion in severely injured trauma patients decreases the incidence of pulmonary embolism. J Am Coll Surg. 1995;180(6):641-647. [PubMed] [Google Scholar]
- 17.Greenfield LJ, Proctor MC, Michaels AJ, Taheri PA. Prophylactic vena caval filters in trauma: the rest of the story. J Vasc Surg. 2000;32(3):490-495. [DOI] [PubMed] [Google Scholar]
- 18.Allen TL, Carter JL, Morris BJ, Harker CP, Stevens MH. Retrievable vena cava filters in trauma patients for high-risk prophylaxis and prevention of pulmonary embolism. Am J Surg. 2005;189(6):656-661. [DOI] [PubMed] [Google Scholar]
- 19.Morris CS, Rogers FB, Najarian KE, Bhave AD, Shackford SR. Current trends in vena caval filtration with the introduction of a retrievable filter at a level I trauma center. J Trauma. 2004;57(1):32-36. [DOI] [PubMed] [Google Scholar]
- 20.Hoff WS, Hoey BA, Wainwright GA, et al. . Early experience with retrievable inferior vena cava filters in high-risk trauma patients. J Am Coll Surg. 2004;199(6):869-874. [DOI] [PubMed] [Google Scholar]
- 21.Gosin JS, Graham AM, Ciocca RG, Hammond JS. Efficacy of prophylactic vena cava filters in high-risk trauma patients. Ann Vasc Surg. 1997;11(1):100-105. [DOI] [PubMed] [Google Scholar]
- 22.Rogers FB, Shackford SR, Ricci MA, Huber BM, Atkins T. Prophylactic vena cava filter insertion in selected high-risk orthopaedic trauma patients. J Orthop Trauma. 1997;11(4):267-272. [DOI] [PubMed] [Google Scholar]
- 23.Rosenthal D, McKinsey JF, Levy AM, Lamis PA, Clark MD. Use of the Greenfield filter in patients with major trauma. Cardiovasc Surg. 1994;2(1):52-55. [PubMed] [Google Scholar]
- 24.Gorman PH, Qadri SF, Rao-Patel A. Prophylactic inferior vena cava (IVC) filter placement may increase the relative risk of deep venous thrombosis after acute spinal cord injury. J Trauma. 2009;66(3):707-712. [DOI] [PubMed] [Google Scholar]
- 25.Decousus H, Leizorovicz A, Parent F, et al. ; Prévention du Risque d’Embolie Pulmonaire par Interruption Cave Study Group . A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. N Engl J Med. 1998;338(7):409-415. [DOI] [PubMed] [Google Scholar]
- 26.Rajasekhar A, Lottenberg R, Lottenberg L, Liu H, Ang D. Pulmonary embolism prophylaxis with inferior vena cava filters in trauma patients: a systematic review using the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines. J Thromb Thrombolysis. 2011;32(1):40-46. [DOI] [PubMed] [Google Scholar]
- 27.American College of Surgeons National Trauma Data Bank report 2014. https://www.facs.org/quality%20programs/trauma/ntdb/docpub. Accessed November 10, 2015.
- 28.Healthcare Cost and Utilization Project. NIS database documentation. http://www.hcup-us.ahrq.gov//db/nation/nis/nisdbdocumentation.jsp. Accessed July 10, 2016.
- 29.Glance LG, Osler TM, Mukamel DB, Meredith W, Wagner J, Dick AW. TMPM-ICD9: a trauma mortality prediction model based on ICD-9-CM codes. Ann Surg. 2009;249(6):1032-1039. [DOI] [PubMed] [Google Scholar]
- 30.Rogers FB, Shackford SR, Horst MA, et al. . Determining venous thromboembolic risk assessment for patients with trauma: the Trauma Embolic Scoring System. J Trauma Acute Care Surg. 2012;73(2):511-515. [DOI] [PubMed] [Google Scholar]
- 31.Yunus TE, Tariq N, Callahan RE, et al. . Changes in inferior vena cava filter placement over the past decade at a large community-based academic health center. J Vasc Surg. 2008;47(1):157-165. [DOI] [PubMed] [Google Scholar]
- 32.Kuy S, Dua A, Lee CJ, et al. . National trends in utilization of inferior vena cava filters in the United States, 2000-2009. J Vasc Surg Venous Lymphat Disord. 2014;2(1):15-20. [DOI] [PubMed] [Google Scholar]
- 33.Kaufman JA. Retrievable vena cava filters. Tech Vasc Interv Radiol. 2004;7(2):96-104. [DOI] [PubMed] [Google Scholar]
- 34.Dixon A, Stavropoulos SW. Improving retrieval rates for retrievable inferior vena cava filters. Expert Rev Med Devices. 2013;10(1):135-141. [DOI] [PubMed] [Google Scholar]
- 35.Ray CE Jr, Mitchell E, Zipser S, Kao EY, Brown CF, Moneta GL. Outcomes with retrievable inferior vena cava filters: a multicenter study. J Vasc Interv Radiol. 2006;17(10):1595-1604. [DOI] [PubMed] [Google Scholar]
- 36.Kim HS, Young MJ, Narayan AK, Hong K, Liddell RP, Streiff MB. A comparison of clinical outcomes with retrievable and permanent inferior vena cava filters. J Vasc Interv Radiol. 2008;19(3):393-399. [DOI] [PubMed] [Google Scholar]
- 37.Dabbagh O, Nagam N, Chitima-Matsiga R, Bearelly S, Bearelly D. Retrievable inferior vena cava filters are not getting retrieved: where is the gap? Thromb Res. 2010;126(6):493-497. [DOI] [PubMed] [Google Scholar]
- 38.Karmy-Jones R, Jurkovich GJ, Velmahos GC, et al. . Practice patterns and outcomes of retrievable vena cava filters in trauma patients: an AAST multicenter study. J Trauma. 2007;62(1):17-24. [DOI] [PubMed] [Google Scholar]
- 39.Imberti D, Bianchi M, Farina A, Siragusa S, Silingardi M, Ageno W. Clinical experience with retrievable vena cava filters: results of a prospective observational multicenter study. J Thromb Haemost. 2005;3(7):1370-1375. [DOI] [PubMed] [Google Scholar]
- 40.Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 2011;171(9):831-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.US Food & Drug Administration Removing retrievable inferior vena cava filters: initial communication. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm396377.htm. Published August 9, 2010. Accessed July 12, 2016.