This case series quantifies the microstructural changes of the retina in infants with congenital Zika syndrome following Zika exposure in utero.
Key Points
Question
What are the microstructural changes of the retina in congenital Zika syndrome?
Findings
In this case series of 8 patients with congenital Zika syndrome and 8 individuals with cobalamin C deficiency, retinal regions without chorioretinal atrophy demonstrated ganglion cell layer loss on spectral-domain optical coherence tomography that was disproportionately more severe than coexisting changes in the photoreceptor and inner nuclear layers.
Meaning
Consistent with a murine model of congenital Zika syndrome, this study provides in vivo evidence of depletion of a specific neuronal population (ganglion cells) in this condition, which may constitute the primary event that ultimately leads to foveal maldevelopment and central chorioretinal atrophy.
Abstract
Importance
A better pathophysiologic understanding of the neurodevelopmental abnormalities observed in neonates exposed in utero to Zika virus (ZIKV) is needed to develop treatments. The retina as an extension of the diencephalon accessible to in vivo microcopy with spectral-domain optical coherence tomography (SD-OCT) can provide an insight into the pathophysiology of congenital Zika syndrome (CZS).
Objective
To quantify the microstructural changes of the retina in CZS and compare these changes with those of cobalamin C (cblC) deficiency, a disease with potential retinal maldevelopment.
Design, Setting, and Participants
This case series included 8 infants with CZS and 8 individuals with cblC deficiency. All patients underwent ophthalmologic evaluation at 2 university teaching hospitals and SD-OCT imaging in at least 1 eye. Patients with cblC deficiency were homozygous or compound heterozygotes for mutations in the methylmalonic aciduria and homocystinuria type C (MMACHC) gene. Data were collected from January 1 to March 17, 2016, for patients with CZS and from May 4, 2015, to April 23, 2016, for patients with cblC deficiency.
Main Outcomes and Measures
The SD-OCT cross-sections were segmented using automatic segmentation algorithms embedded in the SD-OCT systems. Each retinal layer thickness was measured at critical eccentricities using the position of the signal peaks and troughs on longitudinal reflectivity profiles.
Results
Eight infants with CZS (5 girls and 3 boys; age range, 3-5 months) and 8 patients with cblC deficiency (3 girls and 5 boys; age range, 4 months to 15 years) were included in the analysis. All 8 patients with CZS had foveal abnormalities in the analyzed eyes (8 eyes), including discontinuities of the ellipsoid zone, thinning of the central retina with increased backscatter, and severe structural disorganization, with 3 eyes showing macular pseudocolobomas. Pericentral retina with normal lamination showed a thinned (<30% of normal thickness) ganglion cell layer (GCL) that colocalized in 7 of 8 eyes with a normal photoreceptor layer. The inner nuclear layer was normal or had borderline thinning. The central retinal degeneration was similar to that of cblC deficiency.
Conclusions and Relevance
Congenital Zika syndrome showed a central retinal degeneration with severe GCL loss, borderline inner nuclear layer thinning, and less prominent photoreceptor loss. The findings provide the first, to date, in vivo evidence in humans for possible retinal maldevelopment with a predilection for retinal GCL loss in CZS, consistent with a murine model of the disease and suggestive of in utero depletion of this neuronal population as a consequence of Zika virus infection.
Introduction
Zika virus (ZIKV) infection has reached pandemic proportions. A devastating consequence of this otherwise frequently benign disease in adults has been the emergence of severe congenital central nervous system (CNS) malformations in infants exposed to ZIKV in utero. The increase in the number of newborns with microcephaly and neurologic disorders in adults during ZIKV outbreaks has triggered a worldwide emergency response from the scientific community, with substantial efforts focusing on determining ZIKV neurotropism and potential links between the viral infection and disrupted neurodevelopmental processes.
Zika virus is a flavivirus that is transmitted to humans primarily by the bite of infected Aedes aegypti mosquitoes. Although the exact mechanisms mediating neuronal maldevelopment in this disease are not fully understood, evidence supports a selective tropism of the virus to glial cells and neuronal elements after prenatal exposure, leading to impaired survival of neuronal progenitors in the developing brain. The eye as an extension of the brain has been scrutinized in patients with microcephaly and documented maternal ZIKV infection. As many as 55% of the patients in the 2015-2016 Brazilian ZIKV epidemic had obvious central retinal abnormalities and ocular changes. The neurodevelopmental defects associated with ZIKV infections are now grouped together as congenital Zika syndrome (CZS).
Retinal changes in CZS generally involve the macula, with lesions ranging from subtle pigmentary changes to overt retinal atrophy. Glial and neuronal elements present in the CNS are well represented in the retina and can be viewed noninvasively in vivo at high resolution with spectral-domain optical coherence tomography (SD-OCT), which was recently used to qualitatively describe the retinal disease in CZS. In the present study, we used SD-OCT to quantify the changes in the neuronal organization of the retina in a cohort of patients with CZS, hoping to contribute to a better understanding of the neurologic disease.
Methods
This retrospective analysis consisted of data recorded from 8 infants with CZS who were examined at the Altino Ventura Foundation, Recife, Brazil, from January 1 to March 17, 2016, and 8 patients with cobalamin C (cblC) deficiency examined at The Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania, from May 4, 2015, to April 23, 2016. Patients underwent comprehensive ophthalmologic evaluations and imaging of at least 1 retina with SD-OCT; when both eyes were available, the eye better aligned with respect to the fovea and/or with the best signal was chosen for quantitative analysis for a total of 8 eyes (Table 1). Details of the clinical examinations and SD-OCT methods have been published elsewhere. Procedures adhered to the Declaration of Helsinki, and the study was approved by the institutional review boards of both participating institutions. Parents of patients with CZS provided written informed consent before study enrollment; data from patients with cblC deficiency were obtained as a retrospective review of records under a waiver of consent approved by the institutional review board of The Children’s Hospital of Philadelphia.
Table 1. Clinical Characteristics of the Patients With CZS.
| Patient No./Sex/Age at Examination, mo | Eye Analyzed | Visual Acuitya | Refractiona | Fundus Examination Result | Foveal Thickness, μm | Neurologic and Developmental Findings | |||
|---|---|---|---|---|---|---|---|---|---|
| Eye Analyzed | Cycles/Degree | Optic Nerve | Macula | Periphery | |||||
| 1/F/5 | Right | 0.32 | 20/2700 | +3.00 | Normal | Normal | Normal | 171 | Developmental delay, hypotony, arthrogryposis, bilateral hip luxation, severe dysphagia, laryngomalacia, and epilepsy |
| 2/M/4 | Right | LP | LP | Plano | Hypoplasia | Pseudocoloboma | Normal | 32b | Developmental delay, hypertonia, mild dysphagia, pyramidal syndrome, and epilepsy |
| 3/F/3 | Right | 1.6 | 20/540 | +1.00 | Normal | Pseudocoloboma | Normal | 36b | Developmental delay, hyperexcitability, hypertonia, extrapyramidal syndrome, pyramidal syndrome, dysphagia, epilepsy |
| 4/M/4 | Right | NLP | NLP | +0.5 | Cupping | Atrophy | Normal | 142 | Developmental delay, hypertonia, hyperexcitability, pyramidal syndrome, dysphagia, and epilepsy |
| 5/F/4 | Left | 1.6 | 20/540 | -1.00 | Pallor | Atrophy | Normal | 63 | Developmental delay, hypertonia, mild dysphagia, arthrogryposis (bilateral hip luxation), and epilepsy |
| 6/M/4 | Left | 1.6 | 20/540 | +1.00 | Normal | Atrophy, pigmentary changec | Normal | 186d | Developmental delay, hypertonia, pyramidal syndrome, extrapyramidal syndrome, dysphagia, epilepsy |
| 7/F/4 | Right | 0.32 | 20/2700 | +2.00 | Normal | Pseudocoloboma | Normal | 30b | NA |
| 8/F/5 | Left | NLP | NLP | -0.50 | Normal | Atrophy | Normal | 67 | Developmental delay, hyperexcitability, hypertonia, pyramidal syndrome, extrapyramidal syndrome, dysphagia, and epilepsy |
Abbreviations: CZS, congenital Zika syndrome; LP, light perception; NA, not available; NLP, no light perception without tracking.
Visual acuity (measured using Teller Acuity Cards II; Stereo Optical Co, Inc) and refraction (spherical equivalent) were similar in both eyes, unless specified. Visual acuity was abnormal in all patients compared with healthy infants (within the 90% limits) (Salomão and Ventura).
Indicates thickness of remnant tissue at the center of the pseudocoloboma.
Includes mottling and depigmentation.
Measured nasal to an area of apparent subretinal separation (Ventura et al).
For reference, the patient identification used throughout this report corresponds to that used in previous publications. The following landmarks were inspected to confirm the centration of the SD-OCT scans in relation to the foveal center: location of the fovea on fundus photographs, position of the scanning line in the infrared reflectance image acquired during SD-OCT, and the shape and position of the parafoveal ridge in relation to areas of retinal thinning and increased posterior backscattering from chorioretinal atrophy. Scans were aligned and rotated to compensate for head rotation. Regions of interest were magnified for analysis; the full extent of these SD-OCT scans have been published elsewhere. Segmentation of SD-OCT images was performed automatically with the built-in software of the OCT systems and with ImageJ imaging analysis software (https://imagej.nih.gov/ij/links.html). Each retinal layer thickness was measured at specific eccentricities using the distance between the signal peaks (maxima) and troughs (minima) on longitudinal reflectivity profiles (LRP) according to published criteria.
Results
Eight infants with CZS (5 girls and 3 boys; age range, 3-5 months) and 8 with cblC deficiency (3 girls and 5 boys; age range, 4 months to 15 years) were included in the analysis. Patients with cblC deficiency were homozygous or compound heterozygotes for mutations in the methylmalonic aciduria and homocystinuria type C (MMACHC [OMIM 609831]) gene. An SD-OCT cross-section from the fovea into nasal retina in a healthy 4-month-old infant showed the expected contour and lamination of the retina near the foveal center (Figure 1A). The hyporeflective bands correspond to nuclear layers (outer nuclear layer [ONL], inner nuclear layer [INL], and ganglion cell layer [GCL]), separated by thin hyperreflective bands that correspond to the inner plexiform layer (IPL) and outer plexiform layer; the retinal nerve fiber layer is the superficial hyperreflective band (Figure 1A). Lateral displacement of the inner retinal neurons causes the central foveal depression. With increasing distance from the foveal center, the INL can be seen first, followed by the GCL. The GCL was maximal in thickness at approximately 0.5 to 1.5 mm from the foveal center, where the overall thickness from the plexiform layer to the GCL–retinal nerve fiber layer interface approximates that of the ONL, creating the foveal ridge. At greater eccentricities, the GCL declined rapidly in thickness; the ONL did this gradually (Figure 1A).
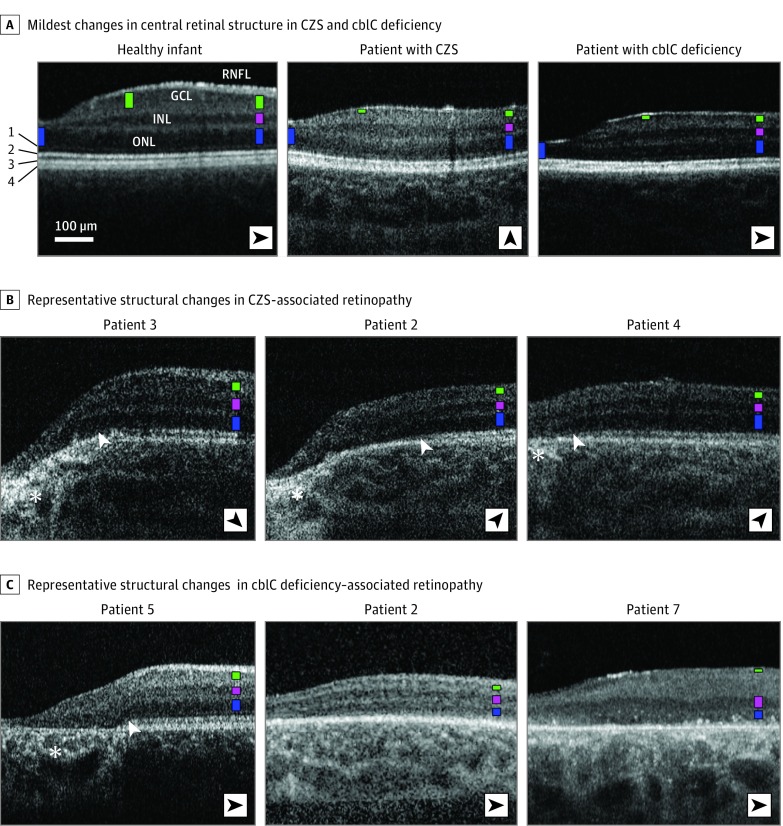
Figure 1. Spectral-Domain Optical Coherence Tomography (SD-OCT) Findings in Study Participants.
A, Nonstraightened, magnified, SD-OCT cross-sections extending 2.7 mm from the foveal center in patients with the mildest retinal abnormalities in congenital Zika syndrome (CZS)– and cobalamin C (cblC) deficiency–associated retinopathies compared with a healthy infant. Patients’ identification corresponds to that used in recent articles; fundus images have been published in Ventura et al and Bonafede et al. Nuclear layers are labeled in the healthy infant; structures distal to the outer nuclear layer (ONL) are numbered to the left as external limiting membrane (1), inner-outer segment boundary zone or ellipsoid zone (2), interdigitation between the tip of the photoreceptors and the apical retinal pigment epithelium (RPE; 3), and RPE (4). To visualize comparisons in thicknesses of the different nuclear layers, colored bars are overlaid on each nuclear layer at 2 mm of eccentricity (ONL, blue; inner nuclear layer [INL], pink; and ganglion cell layer [GCL], green) and at locations where maximal thickness values for ONL (foveal center) and GCL thickness (approximately 1 mm from the foveal center) are approached. Orientation of the scans varied (black arrowheads). B and C, Examples of cross-sections in patients with CZS– and cblC deficiency–associated retinopathy. White arrowheads indicate regions of transition with loss of the ellipsoid zone signal. Asterisks mark increased posterior backscattering from RPE demelanization or loss. RNFL indicates retinal nerve fiber layer.
All patients with CZS in this study had neurologic disease, ocular findings, and abnormal visual acuity compared with healthy infants (Table 1 and Table 2). All patients with CZS had positive serologic findings for ZIKV and negative findings for toxoplasmosis, rubella virus, cytomegalovirus, herpes simplex virus, syphilis, and human immunodeficiency virus. Testing for cblC disease was not pursued in patients with CZS, and no perinatal metabolic screening for cblC deficiency was in place; the disease was not suspected on clinical grounds.
Table 2. Brain Imaging Characteristics of the Patients With CZS.
| Patient No. | Neuroimaging Findings |
|---|---|
| 1 | Volumetric reduction of the brain, parenchymal thinning, subcortical calcifications, ventriculomegaly, and cerebellar and brainstem atrophy |
| 2 | Occipital bone protuberance, volumetric reduction of the brain, parenchymal thinning, subcortical and basal ganglia calcifications, and ventriculomegaly |
| 3 | Volumetric reduction of the brain, parenchymal thinning, multiple cerebral calcifications distributed mainly around the periventricular region, ventriculomegaly, and corpus callosum agenesis |
| 4 | Volumetric reduction of the brain, parenchymal thinning, periventricular calcifications, and ventriculomegaly |
| 5 | Volumetric reduction of the brain, parenchymal thinning, ventriculomegaly, corpus callosum hypoplasia, and periventricular, basal ganglia, and brainstem calcifications |
| 6 | Parenchymal thinning, ventriculomegaly, and calcifications in the basal ganglia |
| 7 | Volumetric reduction of the brain, parenchymal thinning, ventriculomegaly, subcortical calcifications, brainstem, and cerebellum atrophy |
| 8 | Volumetric reduction of the brain, parenchymal thinning, ventriculomegaly, and subcortical, basal ganglia, thalamus, and periventricular calcifications |
Abbreviation: CZS, congenital Zika syndrome.
An SD-OCT image of a patient with CZS and relatively mild retinal changes in his right eye is shown in Figure 1; his left eye had severe central thinning. At a distance from the foveal center where changes are not clinically apparent, the lamination pattern appeared to be normal. However, close inspection revealed that the GCL was barely detectable as a thin hyporeflective band, whereas the ONL was clearly visible. The interdigitation zone was not detectable across the entire cross-section, and the ellipsoid zone was closer to the retinal pigmented epithelium (RPE)–Bruch membrane band, suggestive of photoreceptor outer segment loss and/or shortening. Closer to the fovea, loss of the ellipsoid zone was noted in the rest of the patients with CZS (Figure 1B, white arrowheads), and there were sharp transitions to severe ONL thinning with disorganization of the foveal anatomy. The foveal center was hyperreflective, which suggests intraretinal glial changes, and hyperreflectivities were observed posterior to a thinned RPE, likely representing RPE loss and/or demelanization (Figure 1B, asterisks). At the most distant location from the fovea (2.0-2.7 mm), where little lateral displacement of the inner neurons is observed relative to the photoreceptors from which they receive the synaptic input, the appearance of severe GCL thinning but spared ONL was almost identical to that observed in cblC deficiency (Figure 1A; patient 1 with cblC deficiency).
Cross-sections from patients 2, 3, and 4 with CZS were used to inquire whether the above pattern of disease expression was common in CZS (Figure 1B). Although a spectrum of severity exists, with patient 4 having the least severe expression, all 3 patients recapitulated the same pattern of GCL loss and relative preservation of the ONL. Widening and approximation of the IPL to the superficial retinal nerve fiber layer with intervening hyperreflectivities was observed, which confers the retina superficial to the INL as the appearance of a single hyperreflective band, obscuring a thinned GCL. The interdigitation zone band was nondetectable. In all 3 patients, a steep transition to severe structural outer retinal abnormalities was found (Figure 1B, white arrowheads), with increasing proximity to the foveal center with ellipsoid zone loss followed by severe ONL thinning. At the fovea, thinning and posterior displacement of the ocular layers were observed in patients 2 and 3 with CZS (Figure 1B), a pattern termed pseudocoloboma. We found no apparent relation between the degree of structural abnormalities in the perifovea and this foveal feature. Patient 3 with CZS, who had the least severe parafoveal abnormalities, still showed severe foveal thinning and distortion, whereas patient 4 with CZS, who had an overall thinner perifovea, showed no posterior displacement at the fovea; GCL loss in this eye confers the fovea as a flattened appearance from loss of the foveal ridge.
Patients 2, 5, and 7 with cblC deficiency with a different underlying primary abnormality (metabolic) had a similar central retinal structural outcome (Figure 1C). Patient 5 with cblC deficiency had the mildest disease, with an intact perifovea but severe foveal changes with retinal atrophy and RPE loss. Patient 2 with cblC deficiency had overall retinal thinning with an almost indistinguishable GCL and a flattened foveal contour, as in patient 4 with CZS. Amalgamation of the ONL and INL as a result of severe photoreceptor loss and a thickened superficial hyperreflective band without a detectable GCL in patient 7 with cblC deficiency resembled the appearance in patient 4 with CZS and the pericentral retina of patient 2 with CZS (Figure 1C).
The SD-OCT cross-sections were quantified to better understand the structural abnormalities. Included were locations far enough from the fovea (2 mm) where photoreceptor and ganglion cell contributions to the nuclear layer thicknesses were expected to be near maximal, where minimal displacement of the neurons in the inner retina was relative to the photoreceptors from which the ganglion cells receive their synaptic input, and a location where variability of the topography around the foveal center was minimal. This last feature was required to allow comparisons between scans that departed from the conventional horizontal and vertical directions. Cross-sectional SD-OCT images are built by combining a series of depth scans (A-scans) that contain information about the amplitude of the OCT signal. The resulting waveforms or LRPs contain information that can be used to quantitatively ascertain changes in retinal structure that may not be apparent by inspection (Figure 2).
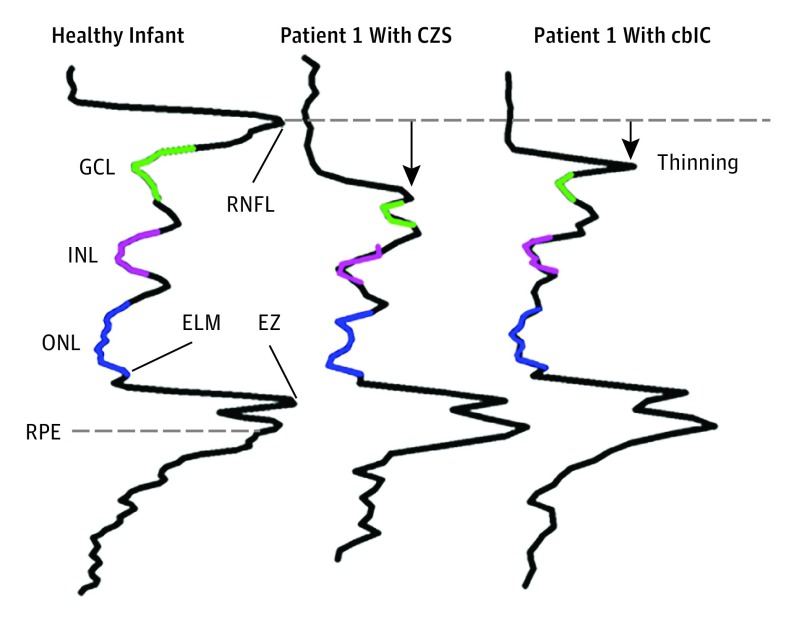
Figure 2. Longitudinal Reflectivity Profiles.
Longitudinal reflectivity profiles from a healthy infant 4 months of age were compared with representative patients with congenital Zika syndrome (CZS) and cobalamin C (cblC) deficiency. Colored segments denote signal trough for each of the nuclear layers following the color code given in Figure 1. A dashed line perpendicular to the longitudinal reflectivity profile extending from the retinal nerve fiber layer (RNFL) peak in the healthy infant provides a reference to better visualize thinning in the patients. Black arrow indicates the direction of thinning relative to normal. ELM indicates external limiting membrane; EZ, ellipsoid zone; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; and RPE, retinal pigment epithelium.
We compared an LRP from a heathy infant aged 4 months with LRPs from patient 1 with CZS and patient 1 with cblC deficiency (Figure 2). A normal LRP shows signal peaks corresponding to the hyperreflectivities on the SD-OCT cross-sections and troughs associated with lower signal amplitudes in each of the nuclear layers. The LRPs aligned by the peak that corresponds to the RPE demonstrated retinal thinning as the signal peak that corresponds to the retinal nerve fiber layer moved closer to the peak that corresponds to the RPE in both patients. Of the 3 troughs that correspond to the nuclear layers, the GCL showed the larger change compared with the normal profile. The LRPs from each of the patients were used to estimate the thickness of each of the nuclear layers.
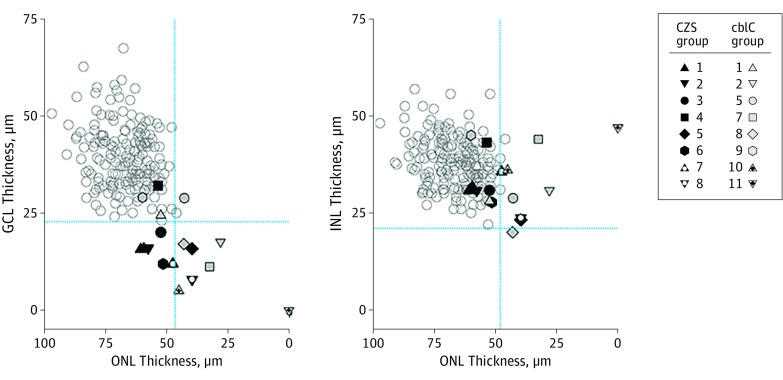
Next, the interrelationships between the changes observed in each of the retinal layers were explored by plotting GCL and INL thicknesses against photoreceptor (ONL) thickness (Figure 3). Measurements were obtained at 2 mm from the foveal center, where minimal lateral displacement of the inner retina related to the distal photoreceptors from which they receive their input exists; the GCL thickness topography at this location is rather symmetric around the circumference of the parafoveal retina. Significant GCL thinning and nonsignificant INL and ONL thinning in 6 of 8 patients with CZS was found; the 2 other patients showed thinning of all nuclear layers. In CZS and cblC deficiency, the most notable abnormality was the GCL thinning and the presence of near-normal ONL thickness in most patients, suggesting primary susceptibility of GCL and possible interference with foveal development (Figure 3). The structural disorganization in CZS prevented accurate quantitation of the different layers near the foveal center in all but patient 1 with CZS (Figure 1A). Qualitatively, however, we found no obvious association between the level of structural disruption at the fovea and the level of GCL thinning in the pericentral retina.
Figure 3. Quantitation of Nuclear Layer Thickness in Congenital Zika Syndrome (CZS).
Thicknesses of ganglion cell layer (GCL) and inner nuclear layer (INL) were plotted as a function of outer nuclear layer (ONL) thickness in all patients. Blue dotted lines indicate normal limits (normal mean [2 SDs] of 51 individuals aged 1-72 years). Gray unfilled symbols indicate data from health individuals. cblC indicates cobalamin C.
Discussion
The association of severe congenital CNS malformations, stillbirth, neonatal deaths, and the increased recognition of neurologic disease in adults with ZIKV infections are cause for concern and emphasize the need for a better understanding of the mechanisms underlying this disease. Histopathologic and experimental evidence now supports ZIKV neurotropism toward neuronal progenitors in CZS. In this study, we used SD-OCT to quantify in vivo the microscopic changes in a group of patients with CZS. By focusing on regions distanced from areas of histologic disorganization where neuronal layers are not easily discernible, we were able to identify a predilection for cell loss within the GCL. Quantitative analyses demonstrated that thinning was below reported lower limits for any foveal developmental stage and was proportionally greater in the GCL than in distal photoreceptors. The findings suggest that ganglion cells and perhaps surrounding glia are the primary cellular targets in the retina of patients with CZS, consistent with histologic observations in a murine model of ZIKV infection.
The involvement of the astrocyte-rich GCL is intriguing because astrocytes and/or glia have been hypothesized to be central to the spread of ZIKV in the developing CNS. Retinal ganglion cells, their axons, and their synaptic partners are most dense in the central retina. The regional predilection of the lesions for the central retina may reflect neuronal death as a consequence of peripheral ZIKV spread through retinal ganglion cell axons from infected CNS targets, a pathway documented in murine CNS infections. Axonal loss after ganglion cell death helps explain reports of optic nerve hypoplasia and atrophy in CZS. Studies in milder cases of CZS compared with age-matched controls are needed to confirm these observations overcoming the limitation of the normative data in this study.
The retina, an extension of the forebrain accessible to exploration in vivo SD-OCT, provides a unique platform that can be used to explore the pathophysiologic features of the CNS disease in CZS. For example, whether the retinal findings in CZS are a simple consequence of an infection and subsequent death of terminally differentiated retinal neurons and/or glia, are driven by disturbed neurodevelopmental processes after the death of retinal progenitor cells, or are a combination of both remains unclear. Retinal changes noted during the CZS epidemic include the presence of central regions of well-delimited chorioretinal atrophy with posterior displacement of the ocular layers in some patients that resemble congenital ocular lesions known as macular pseudocolobomas. The retinal findings in CZS resemble the structural abnormalities associated with cblC deficiency, in which a neurodevelopmental abnormality is also suspected. We confirmed that both diseases share abnormalities at the level of the GCL exceeding those in the outer retina and atrophic foveal lesions; this finding may indicate interference with common developmental processes, a mechanism that has been proposed to explain CNS abnormalities in CZS. Other ocular findings in CZS, such as iris coloboma, optic nerve hypoplasia, and lenticular changes in the absence of active ocular inflammation, appear to favor ocular maldevelopment.
Localized central chorioretinal atrophies and pseudocolobomas have been associated with early-onset retinal degenerations and diseases in the spectrum of TORCH syndrome (toxoplasmosis, other agents, rubella, cytomegalovirus, and herpes simplex), pseudo-TORCH syndrome, and complex genetic syndromes. The association of chorioretinal atrophy and microcephaly in CZS is particularly interesting because similar associations exist within this etiologically heterogenous group of diseases. Documented cases of preserved retinal structure despite total absence of the GCL in anencephaly or hydranencephaly argue against an obligatory causal relationship between the CNS malformations and central chorioretinal degenerations. We speculate that heterogeneous insults that cause ganglion cell (and/or glia) loss and the death of their synaptic partners during a specific stage in development may lead to foveal maldevelopment and to a common phenotype of sharply delineated central chorioretinal atrophy. Additional studies in larger cohorts of patients are needed to confirm whether this specific pattern of neuronal loss is consistently associated with this nonspecific congenital retinal lesion. Electroretinography and/or visual evoked potentials may help by providing objective measures of the magnitude and relative contributions of the different neuronal populations to the visual dysfunction, as well as the effect of the disease on the processing and transmission of the visual signals along the visual pathway.
The structural detail of the lesions observed in our patients with CZS differs from that of retinal lesions described so far in adults infected with ZIKV, where abnormalities are predominantly found in the outer retina, RPE, and choroid. Although CZS has a similar predilection for the central retina, GCL loss has not been observed, suggesting a peculiar vulnerability of the inner retina in CZS. The differences in retinal disease expression may be caused by a different molecular phenotype of the vascular barriers, the susceptible neuronal populations, or the immunologic environment of the mature retina.
Limitations
This study is limited by the small sample size. Future studies including large numbers of patents representing a full spectrum of retinal disease severity are required to determine the relevance of our findings.
Conclusions
CZS showed a central retinal degeneration with severe GCL loss, borderline INL thinning, and less prominent photoreceptor loss. The findings provide the first, to date, in vivo evidence in humans for possible maldevelopment with a predilection of retinal GCL loss in CZS, consistent with a murine model of the disease and suggestive of in utero depletion of this neuronal population as a consequence of Zika infection. Patient 1 with CZS showed interocular asymmetry with mild clinical changes in 1 eye but obvious retinopathy on SD-OCT; these findings raise the possibility of subclinical but functionally important retinal changes that may be present even in cases without obvious microcephaly. Retinal and CNS neurodevelopment is a continuous process that does not end at birth. We hope that detection of such subtle abnormalities will lead to interventions that may help patients achieve the best possible functional outcome. By alerting the clinician to a subclinical form of CZS, SD-OCT has the potential to become an additional tool in the neurologic surveillance of infants with serologic evidence of prenatal exposure to ZIKV but with a normal head circumference. As such, SD-OCT promises to provide quantitative structural biomarkers with which to define the impact that future interventions may have in preventing the complications of CZS.
References
- 1.Paixão ES, Barreto F, Teixeira MdaG, Costa MdaC, Rodrigues LC. History, epidemiology, and clinical manifestations of Zika: a systematic review. Am J Public Health. 2016;106(4):606-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarado MG, Schwartz DA. Zika virus infection in pregnancy, microcephaly, and maternal and fetal health: what we think, what we know, and what we think we know. Arch Pathol Lab Med. 2017;141(1):26-32. [DOI] [PubMed] [Google Scholar]
- 3.de Araújo TVB, Rodrigues LC, de Alencar Ximenes RA, et al. ; Microcephaly Epidemic Research Group; Brazilian Ministry of Health; Pan American Health Organization; Instituto de Medicina Integral Professor Fernando Figueira; State Health Department of Pernambuco . Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case-control study. Lancet Infect Dis. 2016;16(12):1356-1363. [DOI] [PubMed] [Google Scholar]
- 4.França GVA, Schuler-Faccini L, Oliveira WK, et al. . Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet. 2016;388(10047):891-897. [DOI] [PubMed] [Google Scholar]
- 5.Mlakar J, Korva M, Tul N, et al. . Zika virus associated with microcephaly. N Engl J Med. 2016;374(10):951-958. [DOI] [PubMed] [Google Scholar]
- 6.Tang H, Hammack C, Ogden SC, et al. . Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell. 2016;18(5):587-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qian X, Nguyen HN, Song MM, et al. . Brain-region–specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 2016;165(5):1238-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Paula Freitas B, de Oliveira Dias JR, Prazeres J, et al. . Ocular findings in infants with microcephaly associated with presumed Zika virus congenital infection in Salvador, Brazil. JAMA Ophthalmol. 2016;134(5):529-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ventura CV, Fernandez MP, Gonzalez IA, et al. . First travel-associated congenital Zika syndrome in the US: ocular and neurological findings in the absence of microcephaly. Ophthalmic Surg Lasers Imaging Retina. 2016;47(10):952-955. [DOI] [PubMed] [Google Scholar]
- 10.Ventura CV, Ventura LO, Bravo-Filho V, et al. . Optical coherence tomography of retinal lesions in infants with congenital Zika syndrome. JAMA Ophthalmol. 2016;134(12):1420-1427. [DOI] [PubMed] [Google Scholar]
- 11.Ventura CV, Maia M, Ventura BV, et al. . Ophthalmological findings in infants with microcephaly and presumable intra-uterus Zika virus infection. Arq Bras Oftalmol. 2016;79(1):1-3. [DOI] [PubMed] [Google Scholar]
- 12.Ventura CV, Maia M, Bravo-Filho V, Góis AL, Belfort R Jr. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet. 2016;387(10015):228. [DOI] [PubMed] [Google Scholar]
- 13.Ventura CV, Maia M, Dias N, Ventura LO, Belfort R Jr. Zika: neurological and ocular findings in infant without microcephaly. Lancet. 2016;387(10037):2502. [DOI] [PubMed] [Google Scholar]
- 14.Campos AG de M, Lira RP, Arantes TE. Optical coherence tomography of macular atrophy associated with microcephaly and presumed intrauterine Zika virus infection. Arq Bras Oftalmol. 2016;79(6):400-401. [DOI] [PubMed] [Google Scholar]
- 15.Miranda HA II, Costa MC, Frazão MA, Simão N, Franchischini S, Moshfeghi DM. Expanded spectrum of congenital ocular findings in microcephaly with presumed Zika infection. Ophthalmology. 2016;123(8):1788-1794. [DOI] [PubMed] [Google Scholar]
- 16.Culjat M, Darling SE, Nerurkar VR, et al. . Clinical and imaging findings in an infant with Zika embryopathy. Clin Infect Dis. 2016;63(6):805-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Oliveira Dias JR, Ventura CV, Borba PD, et al. . Infants with congenital Zika syndrome and ocular findings from Sao Paulo, Brazil: spread of infection [published online January 2, 2017]. Retin Cases Brief Rep. doi: 10.1097/ICB.0000000000000518 [DOI] [PubMed] [Google Scholar]
- 18.Salomão SR, Ventura DF. Large sample population age norms for visual acuities obtained with Vistech-Teller Acuity Cards. Invest Ophthalmol Vis Sci. 1995;36(3):657-670. [PubMed] [Google Scholar]
- 19.Bonafede L, Ficicioglu CH, Serrano L, et al. . Cobalamin C deficiency shows a rapidly progressing maculopathy with severe photoreceptor and ganglion cell loss. Invest Ophthalmol Vis Sci. 2015;56(13):7875-7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 21.Huang Y, Cideciyan AV, Papastergiou GI, et al. . Relation of optical coherence tomography to microanatomy in normal and RD chickens. Invest Ophthalmol Vis Sci. 1998;39(12):2405-2416. [PubMed] [Google Scholar]
- 22.Cideciyan AV, Hufnagel RB, Carroll J, et al. . Human cone visual pigment deletions spare sufficient photoreceptors to warrant gene therapy. Hum Gene Ther. 2013;24(12):993-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curcio CA, Allen KA. Topography of ganglion cells in human retina. J Comp Neurol. 1990;300(1):5-25. [DOI] [PubMed] [Google Scholar]
- 24.Drasdo N, Millican CL, Katholi CR, Curcio CA. The length of Henle fibers in the human retina and a model of ganglion receptive field density in the visual field. Vision Res. 2007;47(22):2901-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz DA. Autopsy and postmortem studies are concordant: pathology of Zika virus infection is neurotropic in fetuses and infants with microcephaly following transplacental transmission. Arch Pathol Lab Med. 2017;141(1):68-72. [DOI] [PubMed] [Google Scholar]
- 26.Roach T, Alcendor DJ. Zika virus infection of cellular components of the blood-retinal barriers: implications for viral associated congenital ocular disease. J Neuroinflammation. 2017;14(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martines RB, Bhatnagar J, de Oliveira Ramos AM, et al. . Pathology of congenital Zika syndrome in Brazil: a case series. Lancet. 2016;388(10047):898-904. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Saucedo-Cuevas L, Regla-Nava JA, et al. . Zika virus infects neural progenitors in the adult mouse brain and alters proliferation. Cell Stem Cell. 2016;19(5):593-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Retallack H, Di Lullo E, Arias C, et al. . Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc Natl Acad Sci U S A. 2016;113(50):14408-14413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dang J, Tiwari SK, Lichinchi G, et al. . Zika virus depletes neural progenitors in human cerebral organoids through activation of the innate immune receptor TLR3. Cell Stem Cell. 2016;19(2):258-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vajzovic L, Hendrickson AE, O’Connell RV, et al. . Maturation of the human fovea: correlation of spectral-domain optical coherence tomography findings with histology. Am J Ophthalmol. 2012;154(5):779-789.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maldonado RS, O’Connell RV, Sarin N, et al. . Dynamics of human foveal development after premature birth. Ophthalmology. 2011;118(12):2315-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dubis AM, Costakos DM, Subramaniam CD, et al. . Evaluation of normal human foveal development using optical coherence tomography and histologic examination. Arch Ophthalmol. 2012;130(10):1291-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miner JJ, Sene A, Richner JM, et al. . Zika virus infection in mice causes panuveitis with shedding of virus in tears. Cell Rep. 2016;16(12):3208-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Pol AN, Mao G, Yang Y, Ornaghi S, Davis JN. Zika virus targeting in the developing brain. J Neurosci. 2017;37(8):2161-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Provis JM, Dubis AM, Maddess T, Carroll J. Adaptation of the central retina for high acuity vision: cones, the fovea and the avascular zone. Prog Retin Eye Res. 2013;35:63-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koenekoop RK, Wang H, Majewski J, et al. ; Finding of Rare Disease Genes (FORGE) Canada Consortium . Mutations in NMNAT1 cause Leber congenital amaurosis and identify a new disease pathway for retinal degeneration. Nat Genet. 2012;44(9):1035-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ostergaard P, Simpson MA, Mendola A, et al. . Mutations in KIF11 cause autosomal-dominant microcephaly variably associated with congenital lymphedema and chorioretinopathy. Am J Hum Genet. 2012;90(2):356-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwartz DA. The origins and emergence of Zika virus, the newest TORCH infection: what’s old is new again. Arch Pathol Lab Med. 2017;141(1):18-25. [DOI] [PubMed] [Google Scholar]
- 40.Hendrickson A, Djajadi H, Erickson A, Possin D. Development of the human retina in the absence of ganglion cells. Exp Eye Res. 2006;83(4):920-931. [DOI] [PubMed] [Google Scholar]
- 41.Jampol LM, Goldstein DA. Zika virus, microcephaly, and ocular findings—reply. JAMA Ophthalmol. 2016;134(8):946. [DOI] [PubMed] [Google Scholar]
- 42.Henry CR, Al-Attar L, Cruz-Chacón AM, Davis JL. Chorioretinal lesions presumed secondary to Zika virus infection in an immunocompromised adult. JAMA Ophthalmol. 2017;135(4):386-389. [DOI] [PubMed] [Google Scholar]
- 43.Kodati S, Palmore TN, Spellman FA, Cunningham D, Weistrop B, Sen HN. Bilateral posterior uveitis associated with Zika virus infection. Lancet. 2017;389(10064):125-126. [DOI] [PubMed] [Google Scholar]
- 44.Wong CW, Ng SR, Cheung CMG, Wong TY, Mathur R. Zika-related maculopathy [published online February 17, 2017]. Retin Cases Brief Rep. doi: 10.1097/ICB.0000000000000552 [DOI] [PubMed] [Google Scholar]
- 45.Parke DW III, Almeida DRP, Albini TA, Ventura CV, Berrocal AM, Mittra RA. Serologically confirmed Zika-related unilateral acute maculopathy in an adult. Ophthalmology. 2016;123(11):2432-2433. [DOI] [PubMed] [Google Scholar]
- 46.Lucchese G, Kanduc D. Zika virus and autoimmunity: from microcephaly to Guillain-Barré syndrome, and beyond. Autoimmun Rev. 2016;15(8):801-808. [DOI] [PubMed] [Google Scholar]
- 47.Zhang F, Hammack C, Ogden SC, et al. . Molecular signatures associated with ZIKV exposure in human cortical neural progenitors. Nucleic Acids Res. 2016;44(18):8610-8620. [DOI] [PMC free article] [PubMed] [Google Scholar]