Abstract
Importance
Frail patients are known to have poor perioperative outcomes. There is a paucity of literature investigating how the Modified Frailty Index (mFI), a validated measure of frailty, is associated with unplanned readmission among military veterans following surgery.
Objective
To understand the association between frailty and 30-day postoperative unplanned readmission.
Design, Setting, and Participants
A retrospective cohort study was conducted among adult patients who underwent surgery and were discharged alive from Veterans Affairs hospitals for orthopedic, general, and vascular conditions between October 1, 2007, and September 30, 2014, with a postoperative length of stay between 2 and 30 days.
Exposure
Frailty, as calculated by the 11 variables on the mFI.
Main Outcomes and Measures
The primary outcome of interest is 30-day unplanned readmission. Secondary outcomes included any 30-day predischarge or postdischarge complication, 30-day postdischarge mortality, and 30-day emergency department visit.
Results
The study sample included 236 957 surgical procedures (among 223 877 men and 13 080 women; mean [SD] age, 64.0 [11.3] years) from high-volume surgical specialties: 101 348 procedures (42.8%) in orthopedic surgery, 92 808 procedures (39.2%) in general surgery, and 42 801 procedures (18.1%) in vascular surgery. The mFI was associated with readmission (odds ratio [OR], 1.11; 95% CI, 1.10-1.12; R2 = 10.3%; C statistic, 0.71). Unadjusted rates of overall 30-day readmission (26 262 [11.1%]), postdischarge emergency department visit (34 204 [14.4%]), any predischarge (13 855 [5.9%]) or postdischarge (14 836 [6.3%]) complication, and postdischarge mortality (1985 [0.8%]) varied by frailty in a dose-dependent fashion. In analysis by individual mFI components using Harrell ranking, impaired functional status, identified as nonindependent functional status (OR, 1.16; 95% CI, 1.11-1.21; P < .01) or having a residual deficit from a prior cerebrovascular accident (OR, 1.17; 95% CI, 1.11-1.22; P < .01), contributed most to the ability of the mFI to anticipate readmission compared with the other components. Acutely impaired sensorium (OR, 1.12; 95% CI, 0.99-1.27; P = .08) and history of a myocardial infarction within 6 months (OR, 0.93; 95% CI, 0.81-1.06; P = .28) were not significantly associated with readmission.
Conclusions and Relevance
The mFI is associated with poor surgical outcomes, including readmission, primarily due to impaired functional status. Targeting potentially modifiable aspects of frailty preoperatively, such as improving functional status, may improve perioperative outcomes and decrease readmissions.
This cohort study examines the association between frailty and 30-day postoperative unplanned readmission.
Key Points
Question
What is the association between frailty, as defined by the Modified Frailty Index, and 30-day unplanned readmission?
Findings
In this cohort study, increasing Modified Frailty Index scores were associated with 30-day unplanned readmission, with impaired functional status contributing most to readmisson.
Meaning
A preoperative frailty risk assessment with the Modified Frailty Index may assist clinicians in identifying potentially modifiable patient-level targets for perioperative intervention to improve outcomes.
Introduction
Frailty is defined as a state of vulnerability with reduced physiological reserves affecting the capacity to maintain or regain homeostasis when exposed to stressors, such as surgery, that place patients at increased risks of adverse health outcomes. There are various measures of frailty in the literature, with most involving a phenotypic definition and/or a compilation of deficits definition. The Modified Frailty Index (mFI) is a deficit accumulation measure of frailty validated across many surgical specialties. The mFI was derived from the Canadian Study of Health and Aging Frailty Index by matching its 70 variables to 11 comorbidity and deficit variables from the American College of Surgeons’ National Surgery Quality and Improvement Project (NSQIP). Frailty in surgical patients can exist independent of age and may be important in addressing quality of care outcomes, specifically, readmission.
According to recent data, one-fifth of all hospitalized Medicare patients are readmitted within 30 days of discharge. Specifically, 15.6% of patients undergoing surgical care are readmitted within 30 days, with 2% mortality. Overall, 67.1% of patients who undergo medical treatment and 51.5% of patients who undergo surgical treatment are readmitted or die within the first year following index discharge. The Hospital Readmission Reduction Program was enacted through the Affordable Care Act to reduce unplanned readmissions, given the excess cost to the health care system. Currently, the Centers for Medicare & Medicaid Services reimbursements are reduced for hospitals that exceed higher-than-expected 30-day readmission rates for the care of patients with congestive heart failure, myocardial infarction, pneumonia, chronic lung disease, joint replacement, or cardiac surgery, with plans to expand penalties to additional surgical cases. These financial penalties have increased efforts to anticipate and therefore potentially prevent readmissions.
The composite mFI score is associated with poor clinical outcomes overall, but there remains a paucity of literature investigating how the mFI and its 11 individual components are associated with unplanned readmission following surgery. We hypothesized that the composite mFI would be associated with 30-day unplanned readmission and that each individual mFI component would contribute differentially. In addition, we hypothesized that the mFI would be associated with poor postoperative outcomes in our population of military veterans.
Methods
This retrospective cohort study was conducted within the Veterans Health Administration. Three high-volume surgical specialties (orthopedic, general, and vascular surgery) performed at 118 Veterans Affairs hospitals between October 1, 2007, and September 30, 2014, were included in the analysis. This study was reviewed and approved by the Veterans Affairs Central Institutional Review Board with a waiver of informed consent.
Population and Data Sources
The analysis for this cohort study used data from the Veterans Affairs Surgical Quality Improvement Program (VASQIP). Veterans Affairs Surgical Quality Improvement Program variable definitions, data integrity, procedure sampling, abstraction, and follow-up procedures have been described elsewhere. General, vascular, and orthopedic surgical procedures were identified and limited to procedures in which the patient had a length of stay between 2 and 30 days. To exclude patients not at risk for readmission, only patients discharged alive were assessed.
Variables
The Veterans Affairs Corporate Data Warehouse domains were queried to enhance data captured by the VASQIP, including mental health diagnoses and sociodemographic factors such as marital and insurance status. Patient laboratory values and vital sign data closest to 30 days prior to surgery were obtained, along with data throughout the perioperative course until 30 days after hospital discharge. Any health care use through an inpatient admission or emergency department visit, within 6 months prior to surgery or 30 days after discharge, was included. Perioperative patient- and procedure-specific variables of interest included in this study have previously been described.
The main exposure of interest in this cohort study was frailty as defined by the mFI. The mFI, as a continuous measure for modeling, is scored by assigning 1 point for each frailty component present divided by the 11 possible comorbidities or deficits assessed, for an accumulation of deficits ratio indexed from 0 to 1 to account for missing variables. The 11 mFI components derived from the VASQIP include the following: (1) nonindependent functional status (partially or totally dependent activities of daily living); (2) history of no diabetes or diabetes controlled by diet alone, diabetes treated with oral antihyperglycemic therapy, or diabetes treated with insulin therapy; (3) history of chronic obstructive pulmonary disease exacerbation or pneumonia within 30 days; (4) history of congestive heart failure exacerbation within 30 days; (5) history of myocardial infarction within 6 months; (6) history of angina within 30 days or any percutaneous coronary intervention or coronary artery bypass grafting; (7) hypertension requiring medication; (8) history of peripheral vascular disease; (9) acutely impaired sensorium; (10) transient ischemic attack or cerebrovascular accident without deficits; and (11) cerebrovascular accident with deficits. Variables having multiple levels were simplified to a dichotomous variable, with any positive findings clustered together. The mFI was examined as both a categorical and continuous (ratio) variable to examine potential nonlinear associations. We present the mFI as the total number of components present, rather than as a ratio, for ease of interpretation.
The main outcome of interest for this study was the occurrence of an unplanned inpatient readmission within 30 days following discharge after an index hospitalization for surgery. When multiple operations occurred during the index hospitalization, the first procedure performed was analyzed. Readmissions were defined as any subsequent inpatient stay following surgical discharge identified using the Corporate Data Warehouse inpatient domain. Unplanned readmissions were then identified by International Classification of Disease, Ninth Revision, Clinical Modification diagnosis and procedure codes in the current Centers for Medicare & Medicaid Services planned vs unplanned readmission algorithm. Secondary outcomes include observed 30-day postoperative complications as defined by the VASQIP, 30-day postdischarge use of the emergency department, and 30-day postdischarge mortality.
Statistical Analysis
Univariate and bivariate statistics were used to describe the population, with χ2 tests for categorical variables and 2-sided t tests or Wilcoxon signed rank tests for continuous variables. Multivariable logistic regression examined the association of frailty with 30-day unplanned readmission using missing indicators for any missing data. The base risk-adjusted model for 30-day unplanned readmission using perioperative patient-level variables with 48 parameters used in this study is described in a previously published study. The mFI was entered into our base risk-adjusted model as a continuous index variable to understand its association with readmission after adjustment for known factors. Next, the individual components of the mFI were entered into the base model only as dummy variables. The Harrell ranking technique was used to rank the relative contribution of the 11 individual mFI components by calculating the difference between the χ2 for each variable and its df. Variables with higher Harrell ranking contribute more to a specified outcome than lower-ranked variables. Models were compared using adjusted R2 and C statistics. All analyses and logistic modeling were completed using SAS, version 9.4 (SAS Institute), with an a priori α = .05 considered statistically significant.
Results
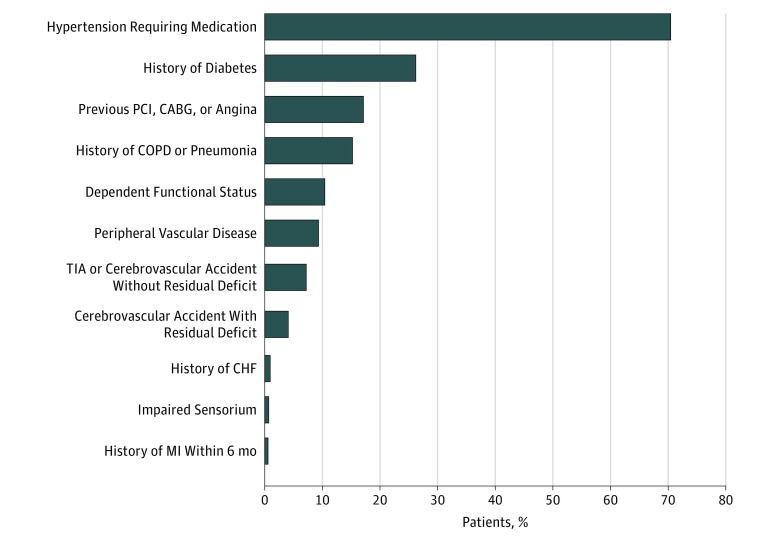
The study sample included 236 957 procedures from high-volume surgical specialties: 101 348 procedures (42.8%) in orthopedic surgery, 92 808 procedures (39.2%) in general surgery, and 42 801 procedures (18.1%) in vascular surgery. Patient demographics are those expected of a cohort of veterans and are shown in Table 1. The prevalence of each component within the mFI is shown in Figure 1; the 5 most common components were hypertension requiring medication (166 965 [70.5%]), history of diabetes (62 082 [26.2%]), previous angina or any percutaneous coronary intervention or coronary artery bypass surgery (40 572 [17.1%]), history of chronic obstructive pulmonary disease or pneumonia within 30 days (36 084 [15.2%]), and having a nonindependent functional status (24 653 [10.4%]). Patient characteristics by number of mFI components are also shown in Table 1. Compared with patients with no frailty, those with 3 or more mFI components (n = 51 780) were more likely to be older than 65 years (10 385 [22.8] vs 28 458 [57.8%]; P < .001), have an American Society of Anesthesiologists class of 4 or more (1418 [3.0%] vs 16 155 [31.2%]; P < .001), and be associated with more health care use within 6 months prior to surgery through inpatient admissions (10 286 [21.8%] vs 24 297 [46.9%]; P < .001) or emergency department visits (16 046 [34.0%] vs 24 431 [47.2%]; P < .001). In general, men were more likely than women to have a higher mFI. The frailty component burden increased with age. Patients who were less frail were admitted from community or outpatient areas, while patients who were more frail (≥3 mFI components) were more likely to be transferred from outside hospitals (1003 [1.9%]) and nursing homes (1932 [3.7%]) (P < .001). Overall, patients with higher frailty scores were more likely to be discharged to locations other than home. Furthermore, frailty varied by surgical specialty, with patients who underwent vascular surgery having more frailty components (median, 2 components; interquartile range, 2-3 components) compared with those who underwent general or orthopedic surgery (median, 1 component; interquartile range, 1-2 components; P < .001). Patients with 3 or more frailty components experienced a longer postoperative length of stay compared with patients who were less frail.
Table 1. Patient Characteristics by Frailty.
| Characteristic | Overall, Valuea | Modified Frailty Index | P Value | |||||
|---|---|---|---|---|---|---|---|---|
| Median (IQR) | P Value | No. (%)b | ||||||
| 0 | 1 | 2 | ≥3 | |||||
| Overall, No. | 236 957 | 1 (1-2) | 47 251 | 76 721 | 61 205 | 51 780 | ||
| Demographics | ||||||||
| Age, y (n = 225 960) | ||||||||
| 19-34 | 3711 (1.6) | 0 (0-0) | <.001 | 3147 (6.9) | 481 (0.7) | 71 (0.1) | 12 (0.0) | <.001 |
| 35-65 | 127 191 (56.3) | 1 (0-2) | 32 083 (70.3) | 44 428 (60.8) | 29 953 (51.6) | 20 727 (42.1) | ||
| >65 | 95 058 (42.1) | 2 (1-3) | 10 385 (22.8) | 28 149 (38.5) | 28 066 (48.3) | 28 458 (57.8) | ||
| Sex | ||||||||
| Male | 223 877 (94.5) | 1 (0-2) | <.001 | 42 436 (89.8) | 71 940 (93.8) | 58 840 (96.1) | 50 661 (97.8) | <.001 |
| Female | 13 080 (5.5) | 1 (1-2) | 4815 (10.2) | 4781 (6.2) | 2365 (3.9) | 1119 (2.2) | ||
| Race/ethnicity (n = 215 191) | ||||||||
| Black | 34 590 (16.1) | 1 (1-2) | <.001 | 6341 (13.4) | 12 273 (16.0) | 9063 (14.8) | 6913 (13.4) | <.001 |
| Hispanic | 10 185 (4.7) | 1 (1-2) | 2273 (4.8) | 3219 (4.2) | 2762 (4.5) | 1931 (3.7) | ||
| White | 155 016 (72.0) | 1 (1-2) | 30 290 (64.1) | 48 741 (63.5) | 40 009 (65.4) | 35 976 (69.5) | ||
| Other | 2306 (1.1) | 1 (0-2) | 614 (1.3) | 689 (0.9) | 582 (1.0) | 421 (0.8) | ||
| Unknown | 13 094 (6.1) | 1 (1-2) | 2380 (5.0) | 4339 (5.7) | 3519 (5.8) | 2856 (5.5) | ||
| BMI | ||||||||
| <18.5 | 7774 (3.3) | 2 (1-3) | <.001 | 1511 (3.2) | 2109 (2.8) | 1897 (3.1) | 2257 (4.4) | <.001 |
| 18.5-24.9 | 57 530 (24.3) | 1 (1-2) | 13 022 (27.6) | 17 073 (22.3) | 13 567 (22.2) | 13 868 (26.8) | ||
| >24.9 | 171 653 (72.4) | 1 (1-2) | 32 718 (69.2) | 57 539 (75.0) | 45 741 (74.7) | 35 655 (68.9) | ||
| ASA classification (n = 236 907) | ||||||||
| 1 | 1671 (0.7) | 0 (0-0) | <.001 | 1541 (3.3) | 100 (0.1) | 16 (0.0) | 14 (0.0) | <.001 |
| 2 | 41 310 (17.4) | 1 (0-1) | 19 523 (41.3) | 16 656 (21.7) | 4414 (7.2) | 717 (1.4) | ||
| 3 | 163 093 (68.8) | 2 (1-2) | 24 755 (52.4) | 54 957 (71.6) | 48 500 (79.3) | 34 881 (67.4) | ||
| 4 | 30 833 (13.0) | 3 (2-4) | 1418 (3.0) | 4996 (6.5) | 8264 (13.5) | 16 155 (31.2) | ||
| Marital status (n = 236 709) | ||||||||
| Married | 110 601 (46.7) | 1 (1-2) | <.001 | 20 362 (43.2) | 35 724 (46.6) | 29 593 (48.4) | 24 922 (48.2) | <.001 |
| Divorced | 71 893 (30.4) | 1 (1-2) | 15 295 (32.4) | 23 796 (31.0) | 18 011 (29.5) | 14 791 (28.6) | ||
| Separated | 9034 (3.8) | 1 (1-2) | 1933 (4.1) | 2887 (3.8) | 2239 (3.7) | 1975 (3.8) | ||
| Widowed | 20 563 (8.7) | 2 (1-3) | 2279 (4.8) | 6067 (7.9) | 5999 (9.8) | 6218 (12.0) | ||
| Single | 24 618 (10.4) | 1 (0-2) | 7294 (15.5) | 8179 (10.7) | 5298 (8.7) | 3847 (7.4) | ||
| Prior inpatient admission | ||||||||
| No | 166 012 (70.1) | 1 (1-2) | <.001 | 36 965 (78.2) | 58 489 (76.2) | 43 075 (70.4) | 27 483 (53.1) | <.001 |
| Yes | 70 945 (29.9) | 2 (1-3) | 10 286 (21.8) | 18 232 (23.8) | 18 130 (29.6) | 24 297 (46.9) | ||
| Prior ED use | ||||||||
| No | 148 638 (62.7) | 1 (1-2) | <.001 | 31 205 (66.0) | 51 418 (67.0) | 38 666 (63.2) | 27 349 (52.8) | <.001 |
| Yes | 88 319 (37.3) | 2 (1-3) | 16 046 (34.0) | 25 303 (33.0) | 22 539 (36.8) | 24 431 (47.2) | ||
| Index Hospital Characteristics | ||||||||
| Admission source | ||||||||
| Community | 107 968 (45.6) | 1 (1-2) | <.001 | 22 085 (46.7) | 35 096 (45.7) | 27 810 (45.4) | 22 977 (44.4) | <.001 |
| Outpatient | 121 751 (51.4) | 1 (1-2) | 24 005 (50.8) | 40 145 (52.3) | 31 893 (52.1) | 25 708 (49.7) | ||
| Transfer from other hospital | 2730 (1.2) | 2 (1-3) | 532 (1.1) | 583 (0.8) | 612 (1.0) | 1003 (1.9) | ||
| Nursing home or domiciliary | 3419 (1.4) | 3 (2-4) | 258 (0.6) | 546 (0.7) | 683 (1.1) | 1932 (3.7) | ||
| Nonveteran facility | 732 (0.3) | 1 (0-1) | 291 (0.6) | 273 (0.4) | 125 (0.2) | 43 (0.1) | ||
| Other | 357 (0.2) | 2 (1-3) | 80 (0.2) | 78 (0.1) | 82 (0.1) | 117 (0.2) | ||
| Discharge location | ||||||||
| Community | 207 401 (87.5) | 1 (1-2) | <.001 | 43 334 (91.7) | 68 606 (89.4) | 53 636 (87.6) | 41 825 (80.8) | <.001 |
| Other | 29 556 (12.5) | 2 (1-3) | 3917 (8.3) | 8115 (10.6) | 7569 (12.4) | 9955 (19.2) | ||
| Surgery classification (n = 236 937) | ||||||||
| Elective | 215 922 (91.1) | 1 (1-2) | <.001 | 42 719 (90.4) | 70 919 (92.4) | 55 995 (91.5) | 46 289 (89.4) | <.001 |
| Emergency | 21 015 (8.9) | 2 (1-3) | 4531 (9.6) | 5798 (7.6) | 5207 (8.5) | 5479 (10.6) | ||
| Operative type | ||||||||
| Abdominal general surgery | 92 808 (39.2) | 23 041 (48.8) | 30 430 (39.7) | 23 574 (38.5) | 15 763 (30.4) | |||
| Open | 64 161 (69.1) | 1 (1-2) | <.001 | 15 115 (65.7) | 20 799 (68.4) | 16 534 (70.2) | 11 648 (74.0) | <.001 |
| Laparoscopic | 28 647 (30.9) | 1 (0-2) | 7905 (34.3) | 9607 (31.6) | 7033 (29.8) | 4102 (26.0) | ||
| Vascular surgery | 42 801 (18.1) | 2027 (4.3) | 7642 (10.0) | 11 933 (19.5) | 21 199 (40.9) | |||
| Open | 35 478 (82.9) | 3 (2-4) | <.001 | 1539 (75.9) | 5809 (76.0) | 9539 (79.9) | 18 591 (87.7) | <.001 |
| Endovascular | 7323 (17.1) | 2 (1-3) | 488 (24.1) | 1833 (24.0) | 2394 (20.1) | 2608 (12.3) | ||
| Orthopedic | 101 348 (42.8) | 22 183 (47.0) | 38 649 (50.4) | 25 698 (42.0) | 14 818 (28.6) | |||
| Open | 100 025 (98.7) | 1 (1-2) | .002 | 21 858 (98.5) | 38 251 (99.0) | 25 380 (98.8) | 14 536 (98.1) | <.001 |
| Arthroscopic | 1323 (1.3) | 1 (1-2) | 325 (1.5) | 398 (1.0) | 318 (1.2) | 282 (1.9) | ||
| Operative time, mean (SD), h | 2 (1.5) | NA | NA | 2.4 (1.4) | 2.5 (1.4) | 2.5 (1.5) | 2.5 (1.7) | <.001 |
| Work relative value unit, mean (SD), U | 20 (7.4) | NA | NA | 19.4 (7.7) | 20.2 (7.2) | 19.8 (7.5) | 18.6 (7.4) | <.001 |
| Postoperative length of stay, mean (SD), d | 6 (4.5) | NA | NA | 5.1 (4.0) | 5.5 (4.3) | 5.9 (4.6) | 6.5 (5.0) | <.001 |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); ED, emergency department; IQR, interquartile range; NA, not applicable.
Data are presented as number (percentage) of patients unless otherwise indicated.
Percentages represent the column percentages of nonmissing values.
Figure 1. Population Prevalence of Frailty Components.
The most common Modified Frailty Index comorbidities include hypertension requiring medication and history of diabetes requiring treatment with oral antihyperglycemics or insulin. CABG indicates coronary artery bypass graft; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; MI, myocardial infraction; PCI, percutaneous coronary intervention; and TIA, transient ischemic attack.
We observed less than 0.05% missing data (67 of 236 957) for all 11 variables used to calculate mFI scores. There were 47 251 patients (19.9%) with 0 frailty components, 76 721 patients (32.4%) with 1 mFI component, 61 205 patients (25.8%) with 2 mFI components, and 51 780 patients (21.9%) with 3 or more mFI components, with most of this group having 3 components (31 357 [13.2%]). In our study, there were no patients at the highest end of the mFI (total of 10 or 11 frailty components).
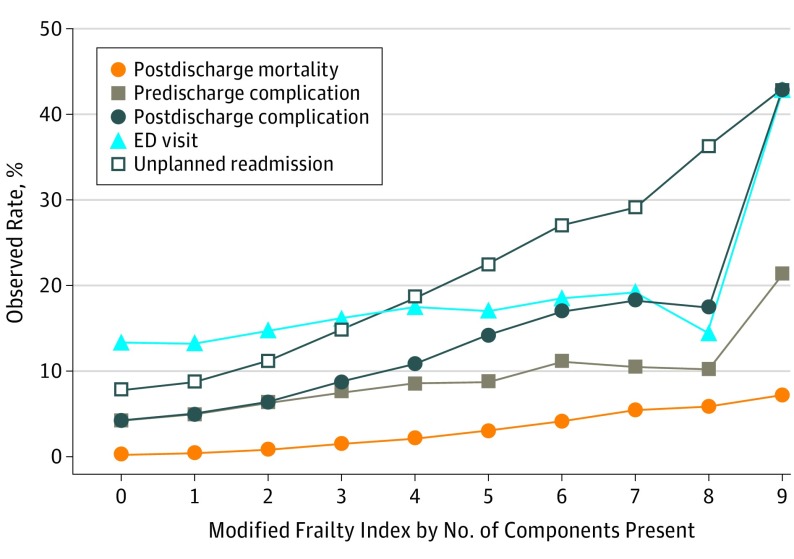
The overall readmission rate was 11.1% (n = 26 262), which significantly varied by patient frailty in a linear fashion, shown in Table 2 and Figure 2, with the lowest readmission rate (3722 [7.9%]) among patients with no frailty components and the highest readmission rate (6 [42.9%]) among patients with 9 frailty components (P < .001). All frailty components were significantly associated with unadjusted readmission rates and other surgical outcomes (eTable in the Supplement). Similarly, the unadjusted rates of emergency department use (14.4% [n = 34 204]), any predischarge complication (5.9% [n = 13 855]), postdischarge complication (6.3% [n = 14 836]), and postdischarge mortality (0.8% [n = 1985]) varied by patient frailty, with increasing rates observed in a dose-dependent fashion.
Table 2. Observed 30-Day Postoperative Outcomes by Frailty.
| Characteristic | Valuea (N = 236 957) | P Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unplanned Readmission | P Value | ED Use | P Value | In-Hospital Complication | P Value | Postdischarge Complication | P Value | Postdischarge Mortality | ||
| Overall | 26 262 (11.1) |
NA | 34 204 (14.4) |
NA | 13 855 (5.9) |
NA | 14 836 (6.3) |
NA | 1985 (0.8) |
NA |
| Specialty | ||||||||||
| Orthopedic | 7735 (7.6) |
<.001 | 11 320 (11.2) |
<.001 | 3213 (3.2) |
<.001 | 4079 (4.0) |
<.001 | 686 (0.7) |
<.001 |
| General | 11 930 (12.9) |
16 009 (17.3) |
8185 (8.8) |
7161 (7.7) |
890 (1.0) |
|||||
| Vascular | 6597 (15.4) |
6875 (16.1) |
2457 (5.7) |
3596 (8.4) |
409 (1.0) |
|||||
| Modified Frailty Indexb | ||||||||||
| Median (IQR) | 2.0 (1-3) |
<.001 | 2.0 (1-3) |
<.001 | 2.0 (1-3) |
<.001 | 2.0 (1-3) |
<.001 | 3.0 (2-4) |
<.001 |
| Mean (SD) | 2.0 (1.5) |
<.001 | 1.7 (1.3) |
<.001 | 1.9 (1.4) |
<.001 | 2.1 (1.5) |
<.001 | 2.7 (1.5) |
<.001 |
| Components present, No.b | ||||||||||
| 0 | 3722 (7.9) |
<.001 | 6343 (13.4) |
<.001 | 1998 (4.2) |
<.001 | 1970 (4.2) |
<.001 | 122 (0.3) |
<.001 |
| 1 | 6816 (8.9) |
10 188 (13.3) |
3775 (4.9) |
3738 (4.9) |
346 (0.5) |
|||||
| 2 | 6876 (11.2) |
9011 (14.7) |
3862 (6.3) |
3879 (6.3) |
501 (0.8) |
|||||
| 3 | 4673 (14.9) |
5071 (16.2) |
2419 (7.7) |
2727 (8.7) |
480 (1.5) |
|||||
| 4 | 2513 (18.6) |
2373 (17.6) |
1148 (8.5) |
1473 (10.9) |
300 (2.2) |
|||||
| 5 | 1115 (22.5) |
851 (17.2) |
434 (8.8) |
703 (14.2) |
149 (3.0) |
|||||
| 6 | 414 (27.0) |
283 (18.5) |
172 (11.2) |
263 (17.2) |
63 (4.1) |
|||||
| 7 | 102 (29.1) |
68 (19.4) |
37 (10.5) |
65 (18.5) |
19 (5.4) |
|||||
| 8 | 25 (36.2) |
10 (14.5) |
7 (10.1) |
12 (17.4) |
4 (5.8) |
|||||
| 9 | 6 (42.9) |
6 (42.9) |
3 (21.4) |
6 (42.9) |
1 (7.1) |
|||||
| 10 | 0 | 0 | 0 | 0 | 0 | |||||
| 11 | 0 | 0 | 0 | 0 | 0 | |||||
Abbreviations: ED, emergency department; IQR, interquartile range; NA, not applicable.
Data are presented as number (percentage) of patients unless otherwise indicated. Percentages represent the column percentages of nonmissing values.
The Modified Frailty Index and its components are explained in the Variables subsection of the Methods section.
Figure 2. Observed 30-Day Postoperative Outcomes by Frailty Component Burden.
No patients in this cohort experienced more than 9 frailty components (10 and 11 not pictured). Observed rates of 30-day postoperative outcomes, including unplanned readmission, emergency department (ED) use, any predischarge or postdischarge complication, and postdischarge mortality, increase as frailty components accumulate. The Modified Frailty Index and its components are explained in the Variables subsection of the Methods section.
The base risk-adjusted model of 30-day unplanned readmission used in this study, which examined contributions of 48 patient-level perioperative parameters (R2 = 10.4%; C statistic, 0.71), has been previously published. The mFI, as a continuous proportional variable, was associated with 30-day unplanned readmission after adjusting for predischarge complications, with an 11% increase in the odds of readmission for each incremental point in the mFI from 0 to 1 (odds ratio [OR], 1.11; 95% CI, 1.10-1.12; R2 = 10.3%; C statistic, 0.71). In analysis of the mFI by the individual 11 components, impaired function, whether identified as nonindependent functional status (OR, 1.16; 95% CI, 1.11-1.21; Harrell rank, 41.55; P < .001) or having any residual deficit from a prior cerebrovascular accident (OR, 1.17; 95% CI, 1.11-1.22; Harrell rank, 37.74; P < .001), contributes most to the ability of the mFI to anticipate readmission in this cohort (Table 3). Acutely impaired sensorium (OR, 1.12; 95% CI, 0.99-1.27; Harrell rank, 2.08; P = .08) and history of a myocardial infarction within 6 months (OR, 0.93; 95% CI, 0.81-1.06; Harrell rank, 0.19; P = .28) were not significantly associated with readmission.
Table 3. Risk-Adjusted Model of Unplanned Readmission.
| Characteristic | Base Model (Morris et al) |
Base Model + mFI, OR (95% CI) |
Base Model + mFI Components, OR (95% CI)a |
Harrell Ranking, χ2 (df) |
P Value |
|---|---|---|---|---|---|
| mFI (composite) | NA | 1.11 (1.10-1.12) | NA | NA | <.001 |
| Dependent functional status | NA | NA | 1.16 (1.11-1.21) | 41.55 | <.001 |
| CVA with residual deficit | NA | NA | 1.17 (1.11-1.22) | 37.74 | <.001 |
| Previous PCI, CABG, or angina | NA | NA | 1.11 (1.08-1.15) | 35.22 | <.001 |
| History of diabetes requiring oral or insulin therapy | NA | NA | 1.10 (1.06-1.13) | 32.39 | <.001 |
| History of COPD or pneumonia | NA | NA | 1.11 (1.07-1.15) | 30.76 | <.001 |
| Peripheral vascular disease | NA | NA | 1.13 (1.08-1.19) | 24.54 | <.001 |
| TIA or CVA without residual deficit | NA | NA | 1.16 (1.09-1.23) | 22.13 | <.001 |
| Hypertension requiring medication | NA | NA | 1.07 (1.04-1.11) | 16.51 | <.001 |
| History of congestive heart failure | NA | NA | 1.13 (1.01-1.25) | 3.59 | .03 |
| Impaired sensorium | NA | NA | 1.12 (0.99-1.27) | 2.08 | .08 |
| History of MI within 6 mo | NA | NA | 0.93 (0.81-1.06) | 0.19 | .28 |
| R2, % | 10.02 | 10.26 | 10.28 | NA | NA |
| C statistic | 0.703 | 0.705 | 0.705 | NA | NA |
Abbreviations: CABG, coronary artery bypass grafting surgery; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; mFI, Modified Frailty Index; MI, myocardial infarction; NA, not applicable; PCI, percutaneous coronary intervention; TIA, transient ischemic attack.
The Modified Frailty Index and its components are explained in the Variables subsection of the Methods section.
Discussion
Our national study of a large cohort of patients who underwent orthopedic, general, or vascular surgery at Veterans Affairs facilities shows that frailty measured by the mFI is independently associated with 30-day unplanned readmission and that the individual components contribute to 30-day unplanned readmission to different degrees. Increasing frailty was associated with increasing age, American Society of Anesthesiologists class of 3 and 4, widowed marital status, prior health care use within 6 months of surgery, preoperative admission source or postoperative discharge location other than home, longer postoperative length of stay, any 30-day postoperative complication, and 30-day postdischarge mortality. The association between the mFI and readmission remained after adjusting for predischarge complications.
Preoperative frailty has been associated with increased risk of postoperative complications, prolonged hospital length of stay with a need for discharge to a location other than home, and mortality. A 2006-2013 national NSQIP study of 1193 patients undergoing surgery for head and neck cancer found that the mFI was associated with postoperative complications but not readmission or mortality. Another 2011-2013 NSQIP study examined frailty in 4434 patients undergoing paraesophageal hernia repair and found the mFI associated with discharge destination locations other than home and with postoperative complications, but not with readmission or mortality. Our large multi-institutional study found an association between frailty and readmission on adjusted analyses, likely owing to the addition of more granular data on a heterogeneous, high-risk patient population with higher readmission rates compared with these prior studies. Hall and colleagues developed a Risk Analysis Index (RAI) derived from the Minimum Data Set Mortality Risk Index–Revised that can be applied both prospectively (RAI-C) and retrospectively (RAI-A), with similar outcomes as the mFI. Using 1021 Veterans Affairs patients undergoing elective surgery, the RAI-C, RAI-A, and mFI were associated with mortality (30 days, 6 months, and 1 year), having any complication, and Clavien-Dindo IV complications. However, readmissions were not assessed.
Overall, postoperative readmissions are challenging to anticipate, despite adequately powered studies with granular perioperative patient-level data. The addition of the mFI provides a small incremental improvement in our overall ability to anticipate readmission (R2 = 10.3%) despite its significant and independent association. This study builds on the literature by using administratively collected data to examine outcomes 30 days after discharge, which may occur beyond the traditional 30-day postsurgery period currently assessed by the VASQIP.
Preoperative frailty assessment provides an opportunity to improve discussions between patients and clinicians surrounding perioperative risk. Patient-level information acquired at the time of surgery contributed most in models of 30-day surgical readmission in 2 recent large national studies. Frailty assessment with the mFI can identify potentially modifiable components of frailty for “prehabilitation,” which is a “process enhancing one’s functional and mental capacity to buffer against the potential deleterious effects of a significant stressor”(p966) and aims to optimize patient comorbidities in physical, nutritional, and psychosocial domains through multidisciplinary support and education. A recent international consensus on frailty recommends exercise (resistance and aerobic), nutritional optimization (caloric, protein, and immunonutrition), and reduction of polypharmacy to improve outcomes after surgery. In addition, the American College of Surgeons adopted the “Strong for Surgery” prehabilitation strategy in recommending widespread implementation to address nutrition, smoking cessation, glycemic control, and management of polypharmacy preoperatively. The RAI by Hall et al focuses on patient factors, including general demographics, comorbidities, cognition, preoperative residence, and functional status through activities of daily living. Their index weighs significantly on functional status, with those having partially or totally dependent deficits in activities of daily living scoring higher in frailty; however, their analyses do not identify which components contribute most toward surgical outcomes. Further investigation is warranted to determine whether functional status could be modifiable and targeted to improve perioperative outcomes.
The mFI is an additional useful tool to screen frailty prospectively; however, we acknowledge that the mFI is a measure of comorbidities plus functional status, which supports the idea that frailty is a sum of cumulative deficits. It remains unclear how the mFI compares with other proven prospective assessments of frailty. The strength of the mFI is that it is inexpensive, easy to calculate, and can be calculated prospectively or retrospectively. Some prospective measures of frailty, including grip strength, Timed Up and Go, the Fried assessment, and sarcopenia assessments, require more expense, space, time, or special equipment and cannot be measured retrospectively. According to a comprehensive systematic review of 20 frailty instruments, no criterion standard exists for true comparison between measures given heterogeneous frailty definitions of varying subpopulations. Because frailty can exist apart from comorbidity, the ability of the mFI to anticipate readmission was mostly signaled by impaired functional status. A consensus is needed within the surgical community to standardize how frailty and functional status are best measured both preoperatively and retrospectively.
Limitations
Our study presents an association between readmission and poor perioperative outcomes among frail patients on a national scale. However, it is not without its limitations. This study is an observational retrospective analysis using administrative and nurse-abstracted data and cannot assign causation. Physicians poorly document functional status, and clinical nurse abstractors frequently rely on the presence of durable medical equipment, such as walkers or wheelchairs, to aid in this assessment. In addition, while we attempted to control for all clinically and statistically significant confounders, including age, in our final model, it is possible that residual confounding may remain. The associations and effect of frailty on surgical readmissions presented here are limited to a sample of mostly white, male patients undergoing elective orthopedic, general, or vascular surgical procedures within the Veterans Affairs Health Care system and may not be generalizable to other patient populations or to surgical procedures not mentioned here.
Conclusions
Frailty as identified by the mFI is associated with unplanned readmission in a large cohort of military veterans. The mFI components contribute differentially even after adjusting for predischarge complications and other information known to the clinician at the time of discharge. Future work should examine the elements of the mFI to determine whether they can be modified preoperatively to improve postoperative outcomes.
eTable. Observed 30-day Postoperative Outcomes by Frailty Component Type
References
- 1.McIsaac DI, Bryson GL, van Walraven C. Association of frailty and 1-year postoperative mortality following major elective noncardiac surgery: a population-based cohort study. JAMA Surg. 2016;151(6):538-545. [DOI] [PubMed] [Google Scholar]
- 2.Buigues C, Juarros-Folgado P, Fernández-Garrido J, Navarro-Martínez R, Cauli O. Frailty syndrome and pre-operative risk evaluation: a systematic review. Arch Gerontol Geriatr. 2015;61(3):309-321. [DOI] [PubMed] [Google Scholar]
- 3.Robinson TN, Walston JD, Brummel NE, et al. . Frailty for surgeons: review of a National Institute on Aging Conference on frailty for specialists. J Am Coll Surg. 2015;221(6):1083-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morley JE, Vellas B, van Kan GA, et al. . Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Revenig LM, Canter DJ, Taylor MD, et al. . Too frail for surgery? initial results of a large multidisciplinary prospective study examining preoperative variables predictive of poor surgical outcomes. J Am Coll Surg. 2013;217(4):665-670.e1. [DOI] [PubMed] [Google Scholar]
- 6.Fagard K, Leonard S, Deschodt M, et al. . The impact of frailty on postoperative outcomes in individuals aged 65 and over undergoing elective surgery for colorectal cancer: a systematic review. J Geriatr Oncol. 2016;7(6):479-491. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clin Interv Aging. 2014;9:433-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walston J, Hadley EC, Ferrucci L, et al. . Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54(6):991-1001. [DOI] [PubMed] [Google Scholar]
- 9.Velanovich V, Antoine H, Swartz A, Peters D, Rubinfeld I. Accumulating deficits model of frailty and postoperative mortality and morbidity: its application to a national database. J Surg Res. 2013;183(1):104-110. [DOI] [PubMed] [Google Scholar]
- 10.Saxton A, Velanovich V. Preoperative frailty and quality of life as predictors of postoperative complications. Ann Surg. 2011;253(6):1223-1229. [DOI] [PubMed] [Google Scholar]
- 11.Farhat JS, Velanovich V, Falvo AJ, et al. . Are the frail destined to fail? Frailty index as predictor of surgical morbidity and mortality in the elderly. J Trauma Acute Care Surg. 2012;72(6):1526-1530. [DOI] [PubMed] [Google Scholar]
- 12.Obeid NM, Azuh O, Reddy S, et al. . Predictors of critical care–related complications in colectomy patients using the National Surgical Quality Improvement Program: exploring frailty and aggressive laparoscopic approaches. J Trauma Acute Care Surg. 2012;72(4):878-883. [DOI] [PubMed] [Google Scholar]
- 13.Tsiouris A, Hammoud ZT, Velanovich V, Hodari A, Borgi J, Rubinfeld I. A modified frailty index to assess morbidity and mortality after lobectomy. J Surg Res. 2013;183(1):40-46. [DOI] [PubMed] [Google Scholar]
- 14.Rubinfeld I, Farooq M, Velanovich V, Syed Z. Predicting surgical risk: how much data is enough? AMIA Annu Symp Proc. 2010;2010:777-781. [PMC free article] [PubMed] [Google Scholar]
- 15.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62(7):738-743. [DOI] [PubMed] [Google Scholar]
- 16.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. [DOI] [PubMed] [Google Scholar]
- 17.Patient Protection and Affordable Care Act of 2010. Pub L No 111-148, 124 Stat 119, amended by Health Care and Education Reconciliation Act of 2010, Pub L No 111-152, 124 Stat 1029 (codified as amended in scattered sections of 42 USC) (2010).
- 18.Davis CL, Pierce JR, Henderson W, et al. . Assessment of the reliability of data collected for the Department of Veterans Affairs national surgical quality improvement program. J Am Coll Surg. 2007;204(4):550-560. [DOI] [PubMed] [Google Scholar]
- 19.Itani KM. Fifteen years of the National Surgical Quality Improvement Program in review. Am J Surg. 2009;198(5)(suppl):S9-S18. [DOI] [PubMed] [Google Scholar]
- 20.Khuri SF, Daley J, Henderson WG. The comparative assessment and improvement of quality of surgical care in the Department of Veterans Affairs. Arch Surg. 2002;137(1):20-27. [DOI] [PubMed] [Google Scholar]
- 21.Khuri SF, Daley J, Henderson W, et al. ; National VA Surgical Quality Improvement Program . The Department of Veterans Affairs’ NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. Ann Surg. 1998;228(4):491-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris MS, Graham LA, Richman JS, et al. . Postoperative 30-day readmission: time to focus on what happens outside the hospital. Ann Surg. 2016;264(4):621-631. [DOI] [PubMed] [Google Scholar]
- 23.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horwitz LI, Partovian C, Lin Z, et al. . Development and use of an administrative claims measure for profiling hospital-wide performance on 30-day unplanned readmission. Ann Intern Med. 2014;161(10)(suppl):S66-S75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrell FE., Jr Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer; 2001. [Google Scholar]
- 26.Abt NB, Richmon JD, Koch WM, Eisele DW, Agrawal N. Assessment of the predictive value of the modified frailty index for Clavien-Dindo grade IV critical care complications in major head and neck cancer operations. JAMA Otolaryngol Head Neck Surg. 2016;142(7):658-664. [DOI] [PubMed] [Google Scholar]
- 27.Chimukangara M, Frelich MJ, Bosler ME, Rein LE, Szabo A, Gould JC. The impact of frailty on outcomes of paraesophageal hernia repair. J Surg Res. 2016;202(2):259-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall DE, Arya S, Schmid KK, et al. . Development and initial validation of the risk analysis index for measuring frailty in surgical populations. JAMA Surg. 2017;152(2):175-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merkow RP, Ju MH, Chung JW, et al. . Underlying reasons associated with hospital readmission following surgery in the United States. JAMA. 2015;313(5):483-495. [DOI] [PubMed] [Google Scholar]
- 30.Santa Mina D, Scheede-Bergdahl C, Gillis C, Carli F. Optimization of surgical outcomes with prehabilitation. Appl Physiol Nutr Metab. 2015;40(9):966-969. [DOI] [PubMed] [Google Scholar]
- 31.Le Roy B, Selvy M, Slim K. The concept of prehabilitation: what the surgeon needs to know? J Visc Surg. 2016;153(2):109-112. [DOI] [PubMed] [Google Scholar]
- 32.Levett DZ, Edwards M, Grocott M, Mythen M. Preparing the patient for surgery to improve outcomes. Best Pract Res Clin Anaesthesiol. 2016;30(2):145-157. [DOI] [PubMed] [Google Scholar]
- 33.Carli F, Zavorsky GS. Optimizing functional exercise capacity in the elderly surgical population. Curr Opin Clin Nutr Metab Care. 2005;8(1):23-32. [DOI] [PubMed] [Google Scholar]
- 34.CERTAIN Strong for surgery. http://www.becertain.org/strong_for_surgery. Accessed September 12, 2016.
- 35.de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG, Nijhuis-van der Sanden MW. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10(1):104-114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Observed 30-day Postoperative Outcomes by Frailty Component Type