This study aims to quantify the incidence of atrial fibrillation in patients at high risk for but without previously known atrial fibrillation using an insertable cardiac monitor.
Key Points
Question
Will insertable cardiac monitors identify a high incidence of previously undiagnosed atrial fibrillation (AF) in patients at high risk for AF and stroke?
Findings
In this study of 446 patients, the rate of AF detection was 29.3% and 40.0% at 18 and 30 months, respectively, and often resulted in prescription of oral anticoagulation.
Meaning
The incidence of previously undiagnosed AF identified by insertable cardiac monitors may be substantial in patients at high risk for AF and stroke.
Abstract
Importance
In approximately 20% of atrial fibrillation (AF)–related ischemic strokes, stroke is the first clinical manifestation of AF. Strategies are needed to identify and therapeutically address previously undetected AF.
Objective
To quantify the incidence of AF in patients at high risk for but without previously known AF using an insertable cardiac monitor.
Design, Setting, and Participants
This prospective, single-arm, multicenter study was conducted from November 2012 to January 2017. Visits took place at 57 centers in the United States and Europe. Patients with a CHADS2 score of 3 or greater (or 2 with at least 1 additional risk factor) were enrolled. Approximately 90% had nonspecific symptoms potentially compatible with AF, such as fatigue, dyspnea, and/or palpitations.
Exposures
Patients underwent monitoring with an insertable cardiac monitor for 18 to 30 months.
Main Outcomes and Measures
The primary end point was adjudicated AF lasting 6 or more minutes and was assessed at 18 months. Other analyses included detection rates at points from 30 days to 30 months and among CHADS2 score subgroups. Median time from insertion to detection and the percentage of patients subsequently prescribed oral anticoagulation therapy was also determined.
Results
A total of 446 patients were enrolled; 233 (52.2%) were male, and the mean (SD) age was 71.5 (9.9) years. A total of 385 patients (86.3%) received an insertable cardiac monitor, met the primary analysis cohort definition, and were observed for a mean (SD) period of 22.5 (7.7) months. The detection rate of AF lasting 6 or more minutes at 18 months was 29.3%. Detection rates at 30 days and 6, 12, 24, and 30 months were 6.2%, 20.4%, 27.1%, 33.6%, and 40.0%, respectively. At 18 months, AF incidence was similar among patients with CHADS2 scores of 2 (24.7%; 95% CI, 17.3-31.4), 3 (32.7%; 95% CI, 23.8-40.7), and 4 or greater (31.7%; 95% CI, 22.0-40.3) (P = .23). Median (interquartile) time from device insertion to first AF episode detection was 123 (41-330) days. Of patients meeting the primary end point, 13 (10.2%) had 1 or more episodes lasting 24 hours or longer, and oral anticoagulation therapy was prescribed for 72 patients (56.3%).
Conclusions and Relevance
The incidence of previously undiagnosed AF may be substantial in patients with risk factors for AF and stroke. Atrial fibrillation would have gone undetected in most patients had monitoring been limited to 30 days. Further trials regarding the value of detecting subclinical AF and of prophylactic therapies are warranted.
Trial Registration
clinicaltrials.gov Identifier: NCT01727297
Introduction
Atrial fibrillation (AF) affects millions of people worldwide, is associated with increased morbidity and mortality, and increases with older age, hypertension, diabetes, heart failure, and more. Importantly, these same conditions are independently associated with increased stroke risk. Atrial fibrillation combined with these comorbidities is particularly concerning.
Atrial fibrillation episodes may be symptomatic, asymptomatic (ie, silent AF), or both. Symptoms commonly associated with AF (all nonspecific) include palpitations, chest discomfort, dizziness or syncope, dyspnea, heart failure, and/or fatigue, but correlation between AF and symptoms is poor. Heart failure or stroke can be the first clinical manifestation of AF.
Other than in patients with cryptogenic stroke or implanted pacemakers/defibrillators (the latter having associated cardiac electrical disorders), silent AF incidence has not been well defined, to our knowledge. Importantly, in patients with implanted pacemakers/defibrillators, silent (sometimes also termed subclinical) AF episodes as brief as 5 minutes to 24 hours have been associated with increased stroke risk and are more common than symptomatic episodes. Recognition of previously undiagnosed AF and initiation of appropriate/indicated therapies, including oral anticoagulation (OAC) therapy, is essential for stroke prevention.
Minimally invasive prolonged electrocardiographic monitoring with insertable cardiac monitors (ICMs) could facilitate early AF diagnosis and earlier treatment. However, the diagnostic yield in high-risk patients is not well known. The REVEAL AF study was therefore performed to determine the incidence of previously undiagnosed AF using ICMs in a high-risk population.
Methods
Trial Design
The REVEAL AF study is a prospective, single-arm, open-label, multicenter clinical study designed to establish the incidence of AF using an ICM (Reveal XT or Reveal LINQ; Medtronic). Detailed methods have been published previously.
The study was supported by Medtronic and was conducted in compliance with applicable local laws and regulations of each participating country. The study protocol can be found in Supplement 1. Ethics committee or institutional review board approval was obtained at each institution (eAppendix in Supplement 2). All patients provided written informed consent prior to participation in the study. A steering committee was responsible for the study design, conduct, and reporting. Data monitoring, collection, and analysis were performed by the sponsor and steering committee in partnership.
Study Participants
Recruitment occurred at 57 clinical centers in the United States and Europe from November 2012 to June 2015. Patients with no AF history but deemed to be at risk based on demographic characteristics with or without symptoms were enrolled. All patients had either a CHADS2 score of 3 or greater or a score of 2 with at least 1 of the following AF risk factors: coronary artery disease, renal impairment, sleep apnea, or chronic obstructive pulmonary disease. All patients underwent 24 hours or more of external monitoring within 90 days prior to enrollment or before ICM insertion. Any AF was exclusionary.
Enrolled patients who received an ICM were observed for 18 or more months, until either their 30-month visit or until the last patient completed the 18-month visit. Patients had in-office visits every 6 months (plus unscheduled pro re nata) and transmitted device data monthly via remote monitoring (CareLink; Medtronic). The REVEAL AF study used the components of the Reveal ICM device. Devices were used in accordance with approved indications and were not paid for by the sponsor.
Study Outcomes
The primary outcome was the incidence of adjudicated AF 6 or more minutes in duration at 18 months. Secondary outcomes included predictors of AF and physician actions in response to AF detection. Additional exploratory objectives encompassed safety, AF incidence at additional time points, and comparison of AF incidence among CHADS2 subgroups and patients with vs without baseline symptoms.
Statistical Analysis
Sample size was chosen to (1) generate a 2-sided 95% CI for the 18-month incidence rate of AF that would be approximately 10 percentage points in width for an estimated event rate of 16% to 20% and (2) adequately power the secondary objectives. Standard statistical methods were used for summarization and analysis. To ensure a robust data set for subgroup analysis, a minimum of 70 patients with CHADS2 scores of 2, 3, and 4 or greater were included in each of these subgroups. Confidence intervals and any statistical significance testing used an α level of .05, unless otherwise stated. Tests of hypotheses were 2-tailed. Only adjudicated episodes, based on stored electrogram data, were included for analysis of the primary end point. Patients without an end point during follow-up were censored at the date of last device interrogation. The date of device implant was time 0 for survival analyses. A Cox proportional hazards model was used to identify predictors of AF, while log-rank tests were used to compare AF incidence among CHADS2 score subgroups and subgroups defined by presence of baseline symptoms, such as palpitations. Atrial fibrillation lasting 6 or more minutes was chosen as the primary end point because at the time this study was being designed, current evidence suggested that subclinical AF lasting 5 to 6 minutes or longer was associated with a significantly increased risk of stroke or systemic embolism in patients with a pacemaker and because the Reveal ICM has high sensitivity, specificity, and positive predictive value for detecting AF episodes of this duration.
Results
Patient Characteristics
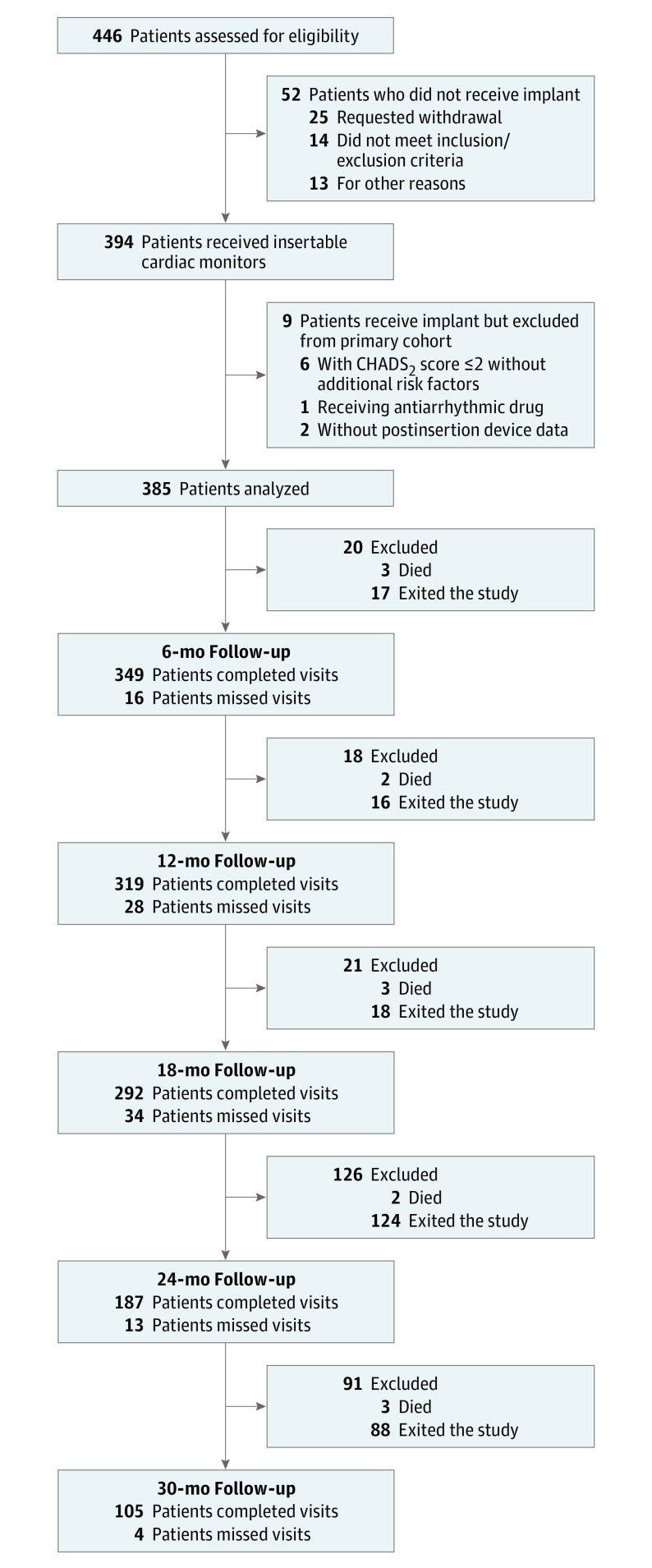
Of 446 patients screened, 394 (88.3%) underwent device insertion. Table 1 and eTable 1 in Supplement 2 summarize the devices used and baseline demographic characteristics in those who underwent device insertion. Procedure-related and/or device-related serious adverse events were infrequent (total, 13 [3.3%]; Reveal LINQ ICM, 6 [2.2%]; Reveal XT ICM, 7 [5.7%]; P = .12) and have been reported previously. There were 385 patients included in the primary end point analysis; of these, 326 (84.7%) reached the target 18-month follow-up (Figure 1). The mean (SD) age was 71.5 (9.9) years, and 297 (77.1%) were 65 years or older. See eTables 2 and 3 in Supplement 2 for details regarding study exits.
Table 1. Devices and Baseline Demographic Characteristics.
| Characteristic | Patients With Device Insertion, No. (%) (n = 394) |
|---|---|
| Device inserted/attempted | |
| Reveal LINQa | 272 (69.0) |
| Reveal XTa | 122 (31.0) |
| Demographic characteristics | |
| Male | 206 (52.3) |
| Age, y | |
| Mean (SD) | 71.6 (9.8) |
| <65 | 88 (22.3) |
| 65-75 | 131 (33.3) |
| >75 | 175 (44.4) |
| Left ventricular ejection fraction | 58.9 (8.1) |
| CHADS2 score | |
| Mean (SD) | 2.9 (0.8) |
| 1 | 1 (0.3) |
| 2 | 158 (40.0) |
| 3 | 130 (33.2) |
| ≥4 | 105 (26.6) |
| CHA2DS2-VASc score | |
| Mean (SD) | 4.4 (1.3) |
| 2 | 25 (6.3) |
| 3 | 79 (20.1) |
| 4 | 112 (28.4) |
| 5 | 100 (25.4) |
| 6 | 53 (13.5) |
| ≥7 | 25 (6.3) |
| Reason for considering possibility of atrial fibrillation | |
| Both symptoms and demographic characteristics | 332 (84.3) |
| Demographic characteristics alone | 62 (15.7) |
| Symptoms within 3 mo of consent | |
| None | 38 (9.6) |
| Chest pain | 79 (20.1) |
| Dizziness/lightheadedness/presyncope | 142 (36.0) |
| Rapid heart beat | 81 (20.6) |
| Shortness of breath | 141 (35.8) |
| Syncope | 77 (19.5) |
| Fatigue/weakness | 119 (30.1) |
| Palpitations | 201 (51.0) |
| Other | 20 (5.1) |
| Family history of atrial fibrillation | 8 (2.0) |
| Personal history of premature atrial complexes | 79 (20.1) |
| Personal history of sinus node arrhythmias or atrioventricular/bundle branch conduction disorders | 9 (2.3) |
| Medical history | |
| Renal dysfunction | 64 (16.2) |
| Congestive heart failure | 81 (20.6) |
| Coronary artery disease | 233 (59.1) |
| Prior coronary artery bypass grafting or percutaneous coronary intervention | 165 (41.9) |
| Hypertension | 369 (93.7) |
| Chronic obstructive pulmonary disease | 76 (19.3) |
| Sleep apnea | 104 (26.4) |
| Diabetes | 248 (62.9) |
| Valve disease (any, clinical or laboratory) | 222 (56.3) |
| Prior valve surgery | 12 (3.0) |
| Vascular disease | |
| Remote stroke | 80 (20.3) |
| Remote transient ischemic attack | 76 (19.3) |
Medtronic.
Figure 1. STROBE Diagram Showing Flow of the Study and Status at Each Point.
Primary Outcome
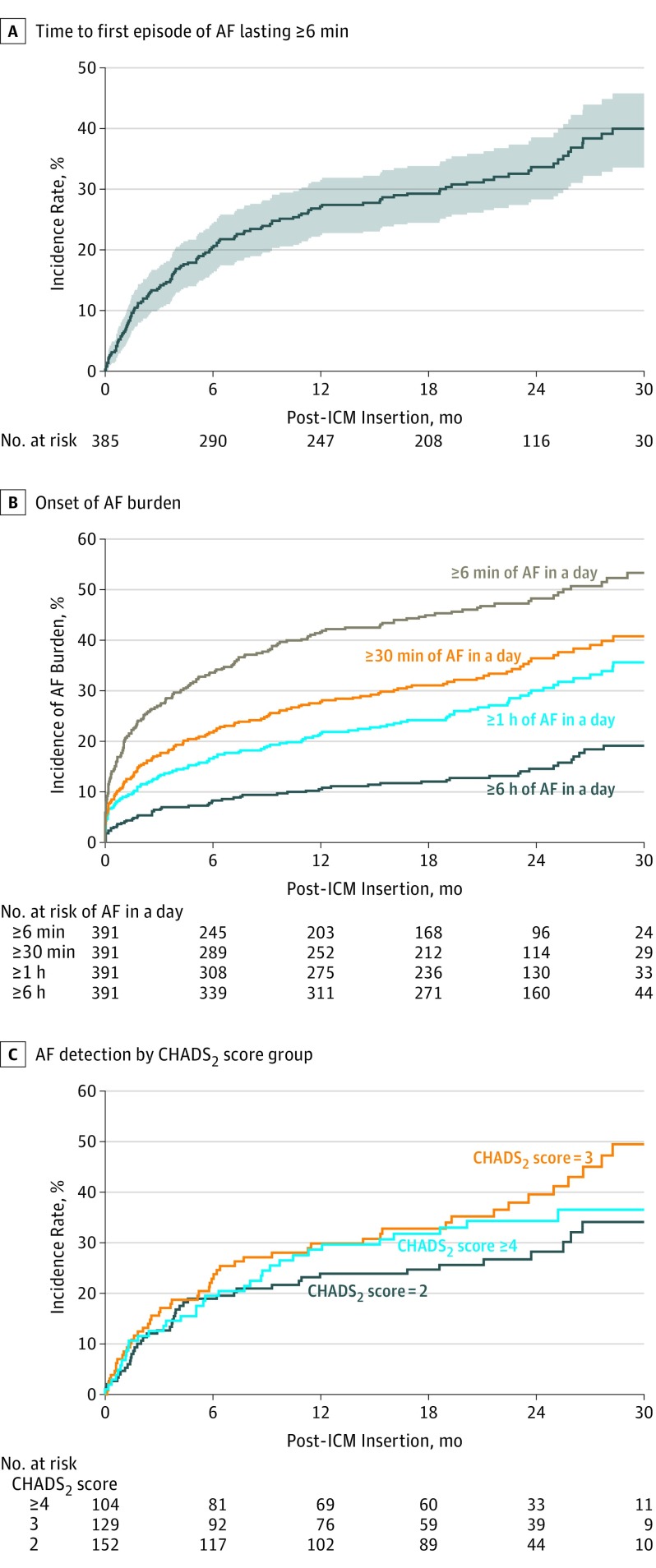
Final episode adjudication of the 10 850 device recordings revealed 6 or more minutes of AF in 128 participants, with an AF detection rate of 29.3% (95% CI, 24.4-33.8) at 18 months (Figure 2A). Atrial fibrillation detection rates at 30 days and 6, 12, 24, and 30 months were 6.2% (95% CI, 3.8-8.6), 20.4% (95% CI, 16.2-24.3), 27.1% (95% CI, 22.5-31.5), 33.6% (95% CI, 28.3-38.6), and 40.0% (95% CI, 33.6-45.8), respectively. Two sensitivity analyses were performed including all patients who exited the study prematurely; one assumed all patients had AF while the other assumed none had AF following their date of exit (the 2 extreme conditions). In the first, the 18-month incidence rate would have been 41.1%; in the second, 28.2%. Given our observed rate of 29.3%, it is possible the true incidence would have been higher had no patients exited early. Of the 128 patients with AF lasting 6 or more minutes, 113 (88.3%) had 30 or more minutes of AF, 97 (75.8%) had 1 or more hours of AF, and 53 (41.4%) had 6 or more hours of AF in a day at some point. Thirteen patients (10.2%) had at least 1 episode lasting 24 hours or longer. The time to onset of daily AF burden for the entire cohort, excluding events adjudicated to not be AF, is shown in Figure 2B. By 30 months, 35.6% (95% CI, 29.4-41.3) had at least 1 hour of AF in a day. Overall, the median (interquartile range) time from device insertion to first AF detection was 123 (41-330) days. There was no difference in AF detection rates between patients with or without any symptoms at enrollment. However, the AF detection rate at 18 months was higher in patients specifically with palpitations at baseline vs those without (35.3%; 95% CI, 29.0-42.6 vs 23.0%; 95% CI, 17.5-29.8; P = .02).
Figure 2. Detection of Atrial Fibrillation (AF).
A, Time to first episode of AF lasting 6 or more minutes. At 30 days, the incidence rate was 6.2%; at 6 months, 20.4%; at 12 months, 27.1%; at 18 months, 29.3%; at 24 months, 33.6%; and at 30 months, 40.0%. B, Onset of AF burden. At 18 months, the incidence of AF burden was 44.9%, 31.1%, 24.2%, and 12.0% for 6 or more minutes, 30 or more minutes, 1 or more hours, and 6 or more hours of AF in a day, respectively; at 30 months, 53.3%, 40.8%, 35.6%, and 19.1%. C, AF detection by CHADS2 score group. At 18 months, the incidence rate was 31.7%, 32.7% and 24.7% for those with a CHADS2 score of 4 or greater, 3, and 2, respectively. ICM indicates insertable cardiac monitor.
Other Outcomes
At 18 months, there was no significant difference in AF incidence between patients with CHADS2 scores of 2 (24.7%; 95% CI, 17.3-31.4), 3 (32.7%; 95% CI, 23.8- 40.7), or 4 or more (31.7%; 95% CI, 22.0-40.3) (Figure 2C). When individual baseline characteristics were assessed, only age (the strongest) and body mass index were significant independent predictors of AF, with AF incidence higher in older and more obese patients (Table 2). Biomarkers (brain natriuretic peptide, C-reactive protein, troponin-I, and thyrotropin) were also examined as predictors in a subset of patients, but the analysis was underpowered. No circulating biomarkers were predictive of AF when controlling for other prespecified clinical characteristics.
Table 2. Predictive Value of Baseline Characteristics for Atrial Fibrillation Onset.
| Characteristic | Hazard Ratio (95% CI)a | P Value |
|---|---|---|
| Age, y | 1.08 (1.05-1.11) | <.001 |
| Body mass index | 1.04 (1.01-1.08) | .02 |
| Male sex | 1.11 (0.77-1.61) | .56 |
| Diabetes | 1.09 (0.74-1.59) | .66 |
| Heart failure | 1.08 (0.69-1.69) | .73 |
| Hypertension | 1.23 (0.58-2.60) | .58 |
| Renal impairment | 0.92 (0.64-1.32) | .65 |
| Chronic obstructive pulmonary disease | 0.73 (0.45-1.20) | .22 |
| Stroke | 0.86 (0.54-1.38) | .53 |
| Coronary artery disease | 0.78 (0.53-1.15) | .21 |
| Sleep apnea | 0.72 (0.45-1.17) | .19 |
| Family history of atrial fibrillation | 1.97 (0.76-5.14) | .16 |
| Vascular disease | 0.89 (0.56-1.43) | .63 |
Obtained from the Cox proportional hazards model.
Among patients who met the primary outcome of 6 or more minutes of AF, 72 (56.3%) were prescribed OAC therapy and 19 (14.8%) were prescribed rhythm control at some point during follow-up. Treatment was not required per protocol. During the total 732.8 patient-years of follow-up among patients in the primary end point analysis, 132 patients (34.1%) experienced 1 or more health care uses, including 94 hospitalizations (12 associated with AF); 12 patients (3.1%) developed persistent AF (with 4 undergoing cardioversion).
There were 6 strokes in 6 patients (3 met the primary end point before the stroke event; 1 met the primary end point after having a stroke), and 12 transient ischemic attacks in 11 patients (all nonadjudicated). Their significance cannot be assessed owing to frequent initiation of OAC therapy on detection of AF.
Discussion
The at-risk population chosen for this study represents a common group of patients encountered clinically. Stroke is a high concern in patients with AF and does not require AF to be symptomatic. Results of the REVEAL AF study demonstrated a substantial incidence of previously undiagnosed AF (nearly 30%) at 18 months of follow-up in patients demographically at high risk of both AF and stroke. By 30 months, the detection rate increased to 40%. With a median time to AF detection of 123 days, most patients would not have been identified with shorter duration monitoring typical of external devices. Age and body mass index were found to be significant predictors of AF detection. In separate univariate assessments, palpitations at baseline were associated with a higher incidence of AF, but CHADS2 score was not. However, notably, in most large monitoring databases, palpitations are not specific for AF and often occur in association with sinus rhythm. Of patients who met the primary end point, 72 (56%) were prescribed OAC therapy. While the benefit of OAC in this patient population is unknown, ongoing anticoagulation trials for subclinical AF will provide insight (ie, Apixaban for the Reduction of Thromboembolism in Patients With Device-Detected Subclinical Atrial Fibrillation [ARTESiA; clinicaltrials.gov identifier: NCT01938248], Non–Vitamin K Antagonist Oral Anticoagulants in Patients With Atrial High Rate Episodes [NOAH; NCT02618577], and Atrial Fibrillation Detected by Continuous ECG Monitoring [LOOP; NCT02036450]). Additionally, results from the FIND-AF randomized trial suggest OAC therapy is beneficial for detected subclinical AF in patients with acute ischemic stroke.
Two smaller trials have also reported on the incidence of AF in high-risk populations using ICMs. The Prevalence of Sub-Clinical Atrial Fibrillation Using an Implantable Cardiac Monitor (ASSERT-II) study (NCT01694394) was a multicenter trial that enrolled 273 at-risk patients without known AF; 252 (92.3%) received an ICM and completed follow-up. The incidence rate of AF 5 or more minutes was 34.4% per person-year. The Predicting Atrial Fibrillation or Flutter (PREDATE-AF) study (NCT01851902) was a single-center trial of patients without known AF but with a CHA2DS2-VASc score of 2 or greater. In 245 patients, the incidence of AF or atrial flutter was 22% during an average follow-up of approximately 15 months. The mean time to detection was similar to the REVEAL AF study at 141 days. There was no difference in the AF detection rates between patients with CHA2DS2-VASc scores of 4 or less vs 5 or greater. Male sex was the only significant predictor of AF. Oral anticoagulation therapy was initiated in 76%. In contrast to both the PREDATE-AF study and the ASSERT-II study, the REVEAL AF study required a minimum of 24 hours of external monitoring without AF detected prior to device insertion. This, in addition to broader inclusion criteria, may explain the lower AF detection rate in the REVEAL AF study vs the ASSERT-II study.
Other studies have also reported on the use of external monitors or smart phones to detect subclinical AF. However, the AF incidence reported in such studies is low, as external monitors have a limited surveillance duration with lower diagnostic yield than ICMs. Furthermore, external devices cannot accurately determine AF burden, a parameter which may be related to the magnitude of stroke risk.
While the primary end point in the REVEAL AF study was an AF episode lasting 6 or more minutes, many patients had prolonged cumulative periods of AF; the incidence of AF lasting 30 minutes or more in a day was greater than 40% at 30 months, and the corresponding incidence of AF lasting 6 or more hours in a day was nearly 20% at 30 months. Additionally, 10.2% of patients with 6 or more consecutive minutes of AF had at least 1 episode lasting 24 hours or longer. Both the Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial (ASSERT) and the study by Israel et al demonstrated that even prolonged episodes of AF can be asymptomatic.
A recent subanalysis from ASSERT suggested that a 24-hour duration of subclinical AF may be critical for increased stroke risk. However, the mean CHADS2 score in ASSERT was only 2.2, and other reports suggest that risk is tied to synergism between AF duration and the severity of associated comorbidities rather than a specific AF duration. Importantly, pathophysiological changes in atrial tissue because of age, disease, or genetics may promote stasis and/or alter atrial endothelial function with resultant thromboembolic risk. Similar dysfunction may also be consequent to AF itself and can be additive to dysfunction produced by underlying comorbidities. The abnormal atrial milieu that underlies thrombogenesis may persist when an episode of AF ends. Thus, the duration of AF that is critical is likely not a single specific value. Moreover, the 24-hour duration has not been confirmed in other trials. Accordingly, we cannot yet determine in a given patient what degree subclinical AF is causative of embolization or whether it is just a risk marker.
Does the thromboembolic risk associated with relatively brief durations of AF justify their detection with prolonged monitoring? In the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial, nonsustained, asymptomatic episodes of AF carried the same risk for stroke as symptomatic AF. Data gathered by device interrogation from patients with implanted pacemakers and defibrillators suggest that as little as 6 minutes to 6 hours of AF may double incident stroke risk, yet such observations may not be relevant in patients without electrical disorders requiring a cardiac rhythm management device. It is also unknown whether treating such brief AF episodes with OAC therapy would significantly reduce stroke risk. Importantly, 3 trials (ARTESIA, NOAH, and LOOP) are underway to assess the potential role of OAC therapy in patients with device-detected AF.
It is noteworthy that based on the information available from the ICM, 56% of patients in the REVEAL AF study who met the primary end point received a new prescription for OACs, as did 76% of patients in the PREDATE AF study. Interestingly, this is lower than rates observed with ICM monitoring in patients with cryptogenic stroke and in response to AF detection clinically. For patients with cryptogenic stroke, the threshold to treat is likely lower owing to previous stroke history. Currently, to our knowledge, there are no data to prove the efficacy of OAC therapy in the REVEAL AF population, but this is currently under investigation in the LOOP trial. Nonetheless, REVEAL AF results support further studies to assess whether ICM-based screening for AF may be beneficial and cost-effective for stroke prevention in specific high-risk populations and demonstrate that high-risk patients were willing to undergo ICM monitoring for AF screening
Limitations
Although the REVEAL AF study is, to our knowledge, the largest trial assessing the use of ICMs for AF detection in high-risk patients, it remains modest in size. Nonetheless, the PREDATE AF, ASSERT-II, and REVEAL AF studies are concordant in finding that previously undetected AF is common in high-risk patients. Second, we were unable to assess the embolic risk posed by device-detected AF because of the small number of strokes during follow-up and potential confounding by anticoagulation use. Third, the positive predictive value of ICMs for AF depends on episode duration, device settings, and AF incidence in the population monitored. In the REVEAL AF study, devices were programmed to favor sensitivity, as this was an exploratory study in a new patient population. Importantly, all episodes that met the primary end point definition were adjudicated. Fourth, given the size of our study, the low enrollment rate, and premature dropouts, it is difficult to determine the external validity of this study cohort to the general population. However, the relative consistency of findings with the PREDATE AF study, ASSERT-II, and the pacemaker/ICD population suggests that the REVEAL AF cohort likely is representative of the general population.
Conclusions
Atrial fibrillation 6 or more minutes is frequently detected with ICM monitoring in patients with previously unknown AF but with demographic factors (with or without symptoms) that place them at risk of both AF and stroke. As the AF incidence was still rising at 30 months, the ideal monitoring duration is unclear. Our findings have important implications for AF screening and stroke prevention in this population.
Study Protocol.
eAppendix. Institutional review board and ethics committee approval.
eTable 1. Baseline echocardiogram testing results.
eTable 2. Reasons for exit amongst patients with atrial fibrillation detected during monitoring with insertable cardiac monitors.
eTable 3. Reasons for exit amongst patients with no atrial fibrillation detected during monitoring with insertable cardiac monitors.
References
- 1.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129(8):837-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18(11):1609-1678. [DOI] [PubMed] [Google Scholar]
- 3.January CT, Wann LS, Alpert JS, et al. ; ACC/AHA Task Force Members . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):2071-2104. [DOI] [PubMed] [Google Scholar]
- 4.Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69(7):841-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mozaffarian D, Benjamin EJ, Go AS, et al. ; Writing Group Members; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Executive summary: heart disease and stroke statistics–2016 update: a report from the American Heart Association. Circulation. 2016;133(4):447-454. [DOI] [PubMed] [Google Scholar]
- 6.Barbarossa A, Guerra F, Capucci A. Silent atrial fibrillation: a critical review. J Atr Fibrillation. 2014;7(3):1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirchhof P, Breithardt G, Aliot E, et al. Personalized management of atrial fibrillation: proceedings from the fourth Atrial Fibrillation competence NETwork/European Heart Rhythm Association consensus conference. Europace. 2013;15(11):1540-1556. [DOI] [PubMed] [Google Scholar]
- 8.Garimella RS, Chung EH, Mounsey JP, Schwartz JD, Pursell I, Gehi AK. Accuracy of patient perception of their prevailing rhythm: a comparative analysis of monitor data and questionnaire responses in patients with atrial fibrillation. Heart Rhythm. 2015;12(4):658-665. [DOI] [PubMed] [Google Scholar]
- 9.Belkin M, Hayes DL, Upadhyay GA Device-detected atrial tachycardia and risk of thromboembolism. https://www.acc.org/latest-in-cardiology/articles/2017/02/03/09/44/device-detected-atrial-tachycardia-and-risk-of-thromboembolism. Accessed March 8, 2017.
- 10.Boriani G, Glotzer TV, Santini M, et al. Device-detected atrial fibrillation and risk for stroke: an analysis of >10,000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). Eur Heart J. 2014;35(8):508-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Botto GL, Padeletti L, Santini M, et al. Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol. 2009;20(3):241-248. [DOI] [PubMed] [Google Scholar]
- 12.Capucci A, Santini M, Padeletti L, et al. ; Italian AT500 Registry Investigators . Monitored atrial fibrillation duration predicts arterial embolic events in patients suffering from bradycardia and atrial fibrillation implanted with antitachycardia pacemakers. J Am Coll Cardiol. 2005;46(10):1913-1920. [DOI] [PubMed] [Google Scholar]
- 13.Glotzer TV, Daoud EG, Wyse DG, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009;2(5):474-480. [DOI] [PubMed] [Google Scholar]
- 14.Glotzer TV, Hellkamp AS, Zimmerman J, et al. ; MOST Investigators . Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation. 2003;107(12):1614-1619. [DOI] [PubMed] [Google Scholar]
- 15.Healey JS, Connolly SJ, Gold MR, et al. ; ASSERT Investigators . Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366(2):120-129. [DOI] [PubMed] [Google Scholar]
- 16.Shanmugam N, Boerdlein A, Proff J, et al. Detection of atrial high-rate events by continuous home monitoring: clinical significance in the heart failure-cardiac resynchronization therapy population. Europace. 2012;14(2):230-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orlov MV, Ghali JK, Araghi-Niknam M, Sherfesee L, Sahr D, Hettrick DA; Atrial High Rate Trial Investigators . Asymptomatic atrial fibrillation in pacemaker recipients: incidence, progression, and determinants based on the atrial high rate trial. Pacing Clin Electrophysiol. 2007;30(3):404-411. [DOI] [PubMed] [Google Scholar]
- 18.Strickberger SA, Ip J, Saksena S, Curry K, Bahnson TD, Ziegler PD. Relationship between atrial tachyarrhythmias and symptoms. Heart Rhythm. 2005;2(2):125-131. [DOI] [PubMed] [Google Scholar]
- 19.Conti S, Reiffel JA, Gersh BJ, et al. Baseline demographics, safety, and patient acceptance of an insertable cardiac monitor for atrial fibrillation screening: the REVEAL AF study. J Atr Fibrillation. 2017;9(5):9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reiffel J, Verma A, Halperin JL, et al. Rationale and design of REVEAL AF: a prospective study of previously undiagnosed atrial fibrillation as documented by an insertable cardiac monitor in high-risk patients. Am Heart J. 2014;167(1):22-27. [DOI] [PubMed] [Google Scholar]
- 21.Hindricks G, Pokushalov E, Urban L, et al. ; XPECT Trial Investigators . Performance of a new leadless implantable cardiac monitor in detecting and quantifying atrial fibrillation: results of the XPECT trial. Circ Arrhythm Electrophysiol. 2010;3(2):141-147. [DOI] [PubMed] [Google Scholar]
- 22.Reiffel JA, Verma A, Kowey PR, et al. Do atrial fibrillation detection rates differ based on presenting symptomatology in patients at risk of atrial fibrillation and stroke? results from the REVEAL AF study. Poster presented at: European Society of Cardiology Annual Scientific Sessions; August 26–30, 2017; Barcelona, Spain. [Google Scholar]
- 23.Wachter R, Gröschel K, Gelbrich G, et al. ; Find-AF(randomised) Investigators and Coordinators . Holter-electrocardiogram-monitoring in patients with acute ischaemic stroke (Find-AFRANDOMISED): an open-label randomised controlled trial. Lancet Neurol. 2017;16(4):282-290. [DOI] [PubMed] [Google Scholar]
- 24.Healey JS, Alings M, Ha AC, et al. ; ASSERT-2 Investigators . Subclinical atrial fibrillation in older patients [published online August 4, 2017]. Circulation. [DOI] [PubMed] [Google Scholar]
- 25.Nasir JM, Pomeroy W, Marler A, et al. Predicting Determinants of Atrial Fibrillation or Flutter for Therapy Elucidation in Patients at Risk for Thromboembolic Events (PREDATE AF) Study. Heart Rhythm. 2017;14(7):955-961. [DOI] [PubMed] [Google Scholar]
- 26.Engdahl J, Andersson L, Mirskaya M, Rosenqvist M. Stepwise screening of atrial fibrillation in a 75-year-old population: implications for stroke prevention. Circulation. 2013;127(8):930-937. [DOI] [PubMed] [Google Scholar]
- 27.Lowres N, Neubeck L, Redfern J, Freedman SB. Screening to identify unknown atrial fibrillation: a systematic review. Thromb Haemost. 2013;110(2):213-222. [DOI] [PubMed] [Google Scholar]
- 28.Lowres N, Neubeck L, Salkeld G, et al. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies: the SEARCH-AF study. Thromb Haemost. 2014;111(6):1167-1176. [DOI] [PubMed] [Google Scholar]
- 29.Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V, Rosenqvist M. Mass screening for untreated atrial fibrillation: the STROKESTOP Study. Circulation. 2015;131(25):2176-2184. [DOI] [PubMed] [Google Scholar]
- 30.Turakhia MP, Ullal AJ, Hoang DD, et al. Feasibility of extended ambulatory electrocardiogram monitoring to identify silent atrial fibrillation in high-risk patients: the Screening Study for Undiagnosed Atrial Fibrillation (STUDY-AF). Clin Cardiol. 2015;38(5):285-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charitos EI, Ziegler PD, Stierle U, et al. Atrial fibrillation burden estimates derived from intermittent rhythm monitoring are unreliable estimates of the true atrial fibrillation burden. Pacing Clin Electrophysiol. 2014;37(9):1210-1218. [DOI] [PubMed] [Google Scholar]
- 32.Van Gelder IC, Healey JS, Crijns HJGM, et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J. 2017;38(17):1339-1344. [DOI] [PubMed] [Google Scholar]
- 33.Israel CW, Grönefeld G, Ehrlich JR, Li YG, Hohnloser SH. Long-term risk of recurrent atrial fibrillation as documented by an implantable monitoring device: implications for optimal patient care. J Am Coll Cardiol. 2004;43(1):47-52. [DOI] [PubMed] [Google Scholar]
- 34.Reiffel JA. If it were only that simple. Eur Heart J. 2016;37(20):1603-1605. [DOI] [PubMed] [Google Scholar]
- 35.Flaker GC, Belew K, Beckman K, et al. ; AFFIRM Investigators . Asymptomatic atrial fibrillation: demographic features and prognostic information from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am Heart J. 2005;149(4):657-663. [DOI] [PubMed] [Google Scholar]
- 36.Witt CT, Kronborg MB, Nohr EA, Mortensen PT, Gerdes C, Nielsen JC. Early detection of atrial high rate episodes predicts atrial fibrillation and thromboembolic events in patients with cardiac resynchronization therapy. Heart Rhythm. 2015;12(12):2368-2375. [DOI] [PubMed] [Google Scholar]
- 37.Sanna T, Diener HC, Passman RS, et al. ; CRYSTAL AF Investigators . Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370(26):2478-2486. [DOI] [PubMed] [Google Scholar]
- 38.Mazurek M, Huisman MV, Lip GY. Registries in atrial fibrillation: from trials to real-life clinical practice. Am J Med. 2017;130(2):135-145. [DOI] [PubMed] [Google Scholar]
- 39.Mittal S, Rogers J, Sarkar S, et al. Real-world performance of an enhanced atrial fibrillation detection algorithm in an insertable cardiac monitor. Heart Rhythm. 2016;13(8):1624-1630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol.
eAppendix. Institutional review board and ethics committee approval.
eTable 1. Baseline echocardiogram testing results.
eTable 2. Reasons for exit amongst patients with atrial fibrillation detected during monitoring with insertable cardiac monitors.
eTable 3. Reasons for exit amongst patients with no atrial fibrillation detected during monitoring with insertable cardiac monitors.