Key Points
Question
What was the impact of a 2014 consensus statement endorsing a minimal negative margin for invasive breast cancer on postlumpectomy surgery and final surgical treatment?
Findings
In a population-based sample of 3729 women undergoing initial lumpectomy between 2013 and 2015, reexcision and conversion to mastectomy declined significantly among patients with negative margins, and final rates of breast-conserving surgery increased from 52% to 65% with a decrease in both unilateral and bilateral mastectomy.
Meaning
The decrease in additional surgery after initial lumpectomy increased rates of breast-conserving surgery, consistent with a benefit of evidence-based guidelines in accelerating practice change to reduce overtreatment.
Abstract
Importance
Surgery after initial lumpectomy to obtain more widely clear margins is common and may lead to mastectomy.
Objective
To describe surgeons’ approach to surgical margins for invasive breast cancer, and changes in postlumpectomy surgery rates, and final surgical treatment following a 2014 consensus statement endorsing a margin of “no ink on tumor.”
Design, Setting, and Participants
This was a population-based cohort survey study of 7303 eligible women ages 20 to 79 years with stage I and II breast cancer diagnosed in 2013 to 2015 and identified from the Georgia and Los Angeles County, California, Surveillance, Epidemiology, and End Results registries. A total of 5080 (70%) returned a survey. Those with bilateral disease, missing stage or treatment data, and with ductal carcinoma in situ were excluded, leaving 3729 patients in the analytic sample; 98% of these identified their attending surgeon. Between April 2015 and May 2016, 488 surgeons were surveyed regarding lumpectomy margins; 342 (70%) responded completely. Pathology reports of all patients having a second surgery and a 30% sample of those with 1 surgery were reviewed. Time trends were analyzed with multinomial regression models.
Main Outcomes and Measures
Rates of final surgical procedure (lumpectomy, unilateral mastectomy, bilateral mastectomy) and rates of additional surgery after initial lumpectomy over time, and surgeon attitudes toward an adequate lumpectomy margin.
Results
The 67% rate of initial lumpectomy in the 3729 patient analytic sample was unchanged during the study. The rate of final lumpectomy increased by 13% from 2013 to 2015, accompanied by a decrease in unilateral and bilateral mastectomy (P = .002). Surgery after initial lumpectomy declined by 16% (P < .001). Pathology review documented no significant association between date of treatment and positive margins. Of 342 responding surgeons, 69% endorsed a margin of no ink on tumor to avoid reexcision in estrogen receptor–positive progesterone receptor–positive cancer and 63% for estrogen receptor–negative progesterone- receptor–negative cancer. Surgeons treating more than 50 breast cancers annually were significantly more likely to report this margin as adequate (85%; n = 105) compared with those treating 20 cases or fewer (55%; n = 131) (P < .001).
Conclusions and Relevance
Additional surgery after initial lumpectomy decreased markedly from 2013 to 2015 concomitant with dissemination of clinical guidelines endorsing a minimal negative margin. These findings suggest that surgeon-led initiatives to address potential overtreatment can reduce the burden of surgical management in patients with cancer.
This cohort study describes surgeons’ approach to surgical margins for invasive breast cancer, changes in postlumpectomy surgery rates, and final surgical treatment following a 2014 consensus statement endorsing a margin of “no ink on tumor.”
Introduction
Physicians are increasingly aware of the need to address overtreatment in cancer care. Breast cancer exemplifies these concerns because most newly diagnosed patients with a favorable prognosis are treated with multiple modalities for which the benefit of each treatment may be small, but the burden is cumulative and substantial. Surgeons increasingly recognize that with multimodality treatment, “bigger” surgery is not necessarily better, making surgery a particular focus of initiatives to reduce the burden of treatment. However, the use of breast-conserving surgery (BCS) has recently declined after years of steadily increasing rates, accompanied by increased use of bilateral mastectomy. Although BCS is a less morbid surgical approach, an important downside to its use is the historically high rate of additional operations (reexcision lumpectomy and/or mastectomy) after initial lumpectomy, ranging from 23% to 38% in published reports.
Reoperation after lumpectomy is required when tumor is present at the margin surface. In patients without tumor at the inked margin, the surgeon’s assessment of what constitutes an adequate tumor-free margin largely determines whether a patient undergoes additional operations to remove more breast tissue. Over time, as surgeons and radiation oncologists sought to minimize rates of local recurrence, wide variation arose in attitudes toward what was considered an appropriate negative margin width for lumpectomy.
Reoperation after initial lumpectomy has major implications for treatment burden on patients. The procedures require a return to the operating room, prolong recovery, and are traumatic to patients and families. In addition, reoperation after lumpectomy has been associated with increased rates of bilateral mastectomy, potentially increasing the burden of surgical treatment, because many women with small, localized unilateral breast cancers opt for treatment with bilateral mastectomy. Thus, a major dichotomy has emerged in breast cancer surgery: lumpectomy, a brief outpatient procedure, is selected by some women, while others with the same clinical characteristics undergo bilateral mastectomies with microvascular tissue flap reconstructions—major surgery requiring inpatient hospitalization and a prolonged recovery period.
The observation that rates of local recurrence have decreased substantially since the performance of the initial trials of BCS and radiotherapy, coupled with reports of high rates of reexcision for patients without tumor at the inked margin, motivated an initiative to reduce the use of unnecessary additional surgery in patients undergoing BCS. The Society of Surgical Oncology (SSO) and the American Society of Radiation Oncology (ASTRO) developed evidence-based consensus recommendations supporting the use of “no ink on tumor” as the definition of a clear margin in patients being treated with BCS and radiotherapy that were presented at national meetings in late 2013 and published electronically in February 2014.
In this study, we examined time trends in the use of additional surgery after lumpectomy in the time period immediately before and after the dissemination of the guidelines, using a population-based sample of women diagnosed between 2013 and 2015, and determined the impact of these changes on rates of BCS.
Methods
Study Sample and Data Collection
After University of Michigan institutional review board (IRB) approval, we selected women 20 to 79 years of age diagnosed with stage I and II breast cancer who were reported to the Surveillance Epidemiology and End Results (SEER) registries of Georgia and Los Angeles County. Eligible patients were identified on a monthly basis approximately 2 months after surgery via surgical pathology report. Surveys were mailed shortly afterward (mean [SD] diagnosis to survey completion time, 7 [3] months). Patients with stage III and IV disease, tumors greater than 5 cm, or more than 3 involved lymph nodes were excluded. Black, Asian, and Hispanic women were oversampled in Los Angeles using an approach we previously described. Patients were selected between July 2013 and August 2015. Written informed consent was waived by the IRB because survey completion was considered consent. To encourage response, we provided a $20 cash incentive and used a modified Dillman recruitment method, including reminders to nonrespondents. All materials were sent in English. We included Spanish-translated materials to women with surnames suggesting Hispanic ethnicity. Responses to the survey were merged with clinical data from SEER.
We selected 7810 women diagnosed as having early-stage breast cancer based on rapid reporting systems from the SEER registries; 507 were deemed ineligible owing to a prior breast cancer diagnosis, stage III and IV disease, residing outside the SEER registry area, being deceased, too ill and/or incompetent, or unable to complete a survey in Spanish or English. Of 7303 eligible women, 2223 did not return a survey or refused participation. Of 5080 respondents (70%), we excluded 279 with bilateral disease, 68 missing stage or treatment data, and 1004 with ductal carcinoma in situ (DCIS), leaving 3729 patients with invasive disease in the analytic sample (eFigure 1 in the Supplement). Patients were asked to identify their attending surgeon for the purpose of collecting information on attitudes regarding margins and nearly all patients (98%) did so: all 488 identified surgeons were sent surveys between April 2015 and May 2016, and 376 (77%) responded. Of these, 342 provided complete information and were used in this analysis. Based on a clinical scenario of a 60-year-old with a 0.8 cm, grade III, ERBB2 (HER2)-negative breast cancer, respondents were asked what margin width precluded the need for reexcision for an estrogen receptor–positive and progesterone receptor–positive and an estrogen receptor–negative and progesterone receptor–negative tumor (eAppendix 1 in the Supplement). Response options included no ink on tumor and margin widths 1 to 2 mm, 5 mm, and 1 cm. Pathology reports were reviewed (with reviewers blinded to the treatment outcomes) for the initial lumpectomy for all patients undergoing further surgery after initial lumpectomy (n = 509) and a 30% sample of those without further procedures (n = 507).
Statistical Analysis
Trends were analyzed using SAS statistical software (version 9.4; SAS Institute Inc). Two separate multinomial logistic models were created regressing treatment on date of diagnosis (treated as a continuous variable), and clinical and demographic covariates. The first model looked at final treatment across all patients with the outcomes of BCS, unilateral mastectomy, and bilateral mastectomy. The second model was restricted to patients with an initial lumpectomy, with the outcomes of lumpectomy only, lumpectomy with reexcision, and lumpectomy with subsequent mastectomy. The covariates included were age (measured continuously in years), race, geographic site, tumor grade, tumor size (categorized as T stage), number of positive nodes (categorized as N stage), and surgeon. Both patient-level models incorporated survey weights to adjust for oversampling in our design, and nonresponse weights to correct for differing responses based on age, race, stage, and SEER site.
Results
Of the 3729 patients, the median patient age was 61 years, 2016 (54%) self-identified as white, 657 (18%) as black, 675 (18%) as Latina, and 293 (8%) as Asian, and 2784 (75%) had T1 tumors. (eTable in the Supplement). Overall, 2509 patients had an initial lumpectomy (67%), and the rate of initial lumpectomy did not differ significantly over the study period after adjusting for other covariates (odds ratio [OR] for 1 quarter change in lumpectomy rate, 1.03 (95% CI, 0.99-1.06; P = .10). The final surgical treatment was lumpectomy in 63% (n = 2345), unilateral mastectomy in 21% (n = 763), and bilateral mastectomy in 17% (n = 621).
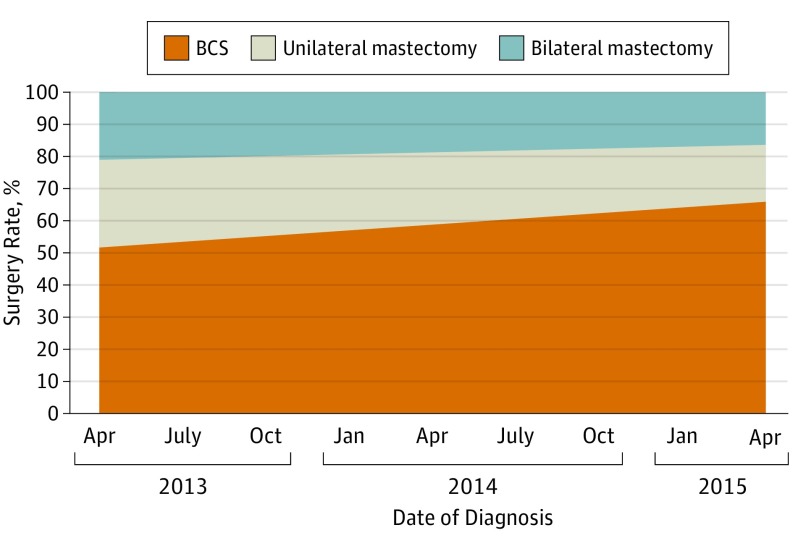
The date of diagnosis for the analytic cohort ranged from April 2013 to April 2015. Significant trends were observed during this diagnosis period in final treatment and the use of postlumpectomy surgery: the use of BCS increased; while the use of unilateral and bilateral mastectomy decreased over time (Figure 1). The predicted marginal rates of BCS, unilateral mastectomy, and bilateral mastectomy (based on multinomial logistic regression) were 52%, 27%, and 21% for patients diagnosed in April 2013; the respective marginal rates were 65%, 18%, and 16% for patients diagnosed in April 2015. The test for time trends in the rates of the 3 procedures shown in Figure 1 was significant (P < .002).
Figure 1. Adjusted Rates of Final Breast Surgery in a Sample of 3729 Patients, April 2013 to April 2015.
These are marginal rates of ultimate treatment, based on a multivariate logistic model adjusting for age, race, site, behavior, tumor size (T code), grade, and nodes (N code) and surgeon, and weighted to reflect sampling and response rates.
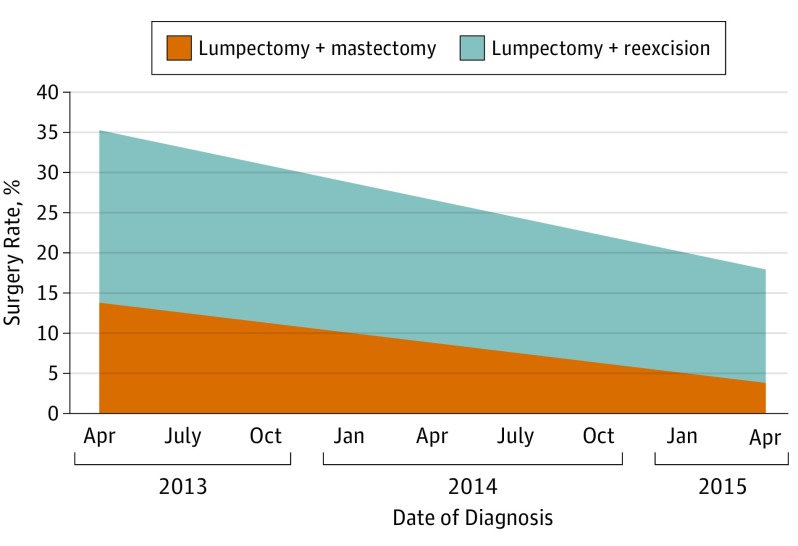
A total of 543 of 2509 patients (22%) reported an additional procedure after initial lumpectomy; reexcision in 378 (15%), and mastectomy with or without reexcision in 165 (7%). Postlumpectomy reexcision and mastectomy markedly decreased over the study time period (Figure 2). The marginal rates (based on multinomial logistic regression) of lumpectomy with reexcision and lumpectomy with subsequent mastectomy were 21% and 13% in April 2013. Two years later, the respective rates were 14% and 4%. The test for time trends of these 2 procedures was significant (P < .001).
Figure 2. Adjusted Postlumpectomy Surgery Rates in a Sample of 2509 Patients Having an Initial Lumpectomy, April 2013 to April 2015.
Marginal rates of postlumpectomy treatment, based on multivariable logistic model adjusting for age, race, site, behavior, tumor size (T code), grade, nodes (N code), and surgeon, and weighted to reflect sampling and response rates.
Review of initial lumpectomy pathology demonstrated positive margins in 330 and unknown margins in 5 cases (33% and 0.5%, respectively). A bivariate analysis of variance test found no significant association date of treatment and rate of positive margins (OR for 1 quarter change in positive margin rate, 0.91 (95% CI, 0.82-1.02) (P = .11). Of the 509 patients who underwent surgery after lumpectomy, 299 had positive margins (59%) and 210 had negative margins (41%). Of those with negative margins undergoing additional surgery, the margin was not defined numerically in 26 (12%). Of the 184 with margin measurements, 135 had a margin of 1 mm or less (73%) and 49 had a margin greater than 1 mm (27%).
Of 342 attending surgeons completing the clinician survey, 69% endorsed a margin of no ink on tumor as adequate to avoid reexcision in a 60-year-old with a grade 3, T1b ERBB2-negative carcinoma that was estrogen receptor–positive progesterone receptor–positive. For the same patient with an estrogen receptor–negative progesterone receptor–negative carcinoma, 63% endorsed no ink on tumor. A significant relationship was seen between the volume of breast surgery performed and what was considered an acceptable margin width (eFigure 2 in the Supplement). For estrogen receptor–positive progesterone receptor–positive cancer, 85% of surgeons treating more than 50 breast cancers annually (n = 105) accepted no ink on tumor as an adequate margin compared with 55% of those treating 20 cases or fewer (n = 131) (P < .001). A similar, statistically significant relationship was seen for the estrogen receptor–negative progesterone receptor–negative scenario, with 78% of the highest-volume surgeons accepting no ink on tumor compared with 50% of the lowest-volume surgeons (P < .001).
Discussion
We observed a marked decrease in the use of additional surgery, both reexcision and mastectomy, after initial lumpectomy for patients diagnosed between mid-2013 and mid-2015 resulting in an overall absolute increase in the use of BCS of 13% during the study period. This increase was accompanied by a decline in both unilateral and bilateral mastectomy, suggesting that decreasing the need for additional surgery after initial lumpectomy has the potential to reduce the trend of women opting for bilateral mastectomy for the treatment of small, unilateral breast cancers.
The 14% rate of reexcision and the 4% rate of conversion to mastectomy at the end of this study contrasts dramatically with past studies reporting rates of additional surgery after initial lumpectomy ranging from 34% to 75%. In a study using the same methodology to sample patients from the Los Angeles and Detroit SEER regions diagnosed between June 2005 and February 2007, 23% of the 1100 patients with stage I and II cancer attempting lumpectomy had reexcision and 11% were converted to mastectomy. A 23% rate of postlumpectomy surgery was noted in a National Cancer Database of the American College of Surgeons study, including 253 052 patients with stage I and II invasive breast cancer treated between 2004 and 2010, but the rate of additional surgery decreased by only 3% during the 6-year study period. In contrast, we observed a 16% decrease in the use of additional surgery during the 2 years of this study.
We argue that the decreased use of additional surgery reflects changing surgeon approaches regarding what constitutes an adequate lumpectomy margin in invasive breast cancer rather than changes in clinical factors. The rate of initial lumpectomy did not change over time—indicating that the decrease in additional surgery and overall higher rate of BCS do not reflect a more favorable patient population or change in patient attitudes—and analyses controlled for clinical factors, including tumor size. In addition, in pathology review, rates of positive margins were stable over time. We also found that more than two-thirds of surgeons now endorse a margin of no ink on tumor as adequate to avoid reexcision for both estrogen receptor–positive progesterone receptor–positive and estrogen receptor–negative progesterone receptor–negative patients, a clear change in approach compared with that reflected in older surgeon surveys in which this margin width was felt to be adequate by only 11% to 30% of surgeons. The change in surgeon approach and decrease in postlumpectomy surgery correspond chronologically to the widespread dissemination of the joint SSO-ASTRO consensus guideline on margins in invasive breast cancer. This guideline was presented in the fall of 2013, published online in February 2014, in print in March 2014, and endorsed by the American Society of Clinical Oncology and the American Society of Breast Surgeons in addition to the sponsoring organizations. Further support for our conclusion that the observed changes, at least in part, occurred in response to the guideline comes from a secondary analysis of our sample of patients with DCIS treated with initial lumpectomy (n = 673) for whom the margins guideline did not apply and where we did not observe a significant downward trend in reexcision after lumpectomy (P = .16). However, the power to detect a difference in trends between invasive and noninvasive breast cancer was limited. Our observation that the use of additional surgery postlumpectomy did not change significantly in patients with DCIS, a group not included in the SSO-ASTRO guideline, suggests that the guideline, rather than a general change in attitude regarding breast cancer treatment, was a major factor in the increase in rates of BCS. Although there have been smaller studies in convenience samples that showed a decrease in reexcision after the margin guideline was published, to our knowledge, our study is the first to document a decrease in the use of both reexcision and mastectomy with a resulting increase in the rate of BCS using a population-based sample.
This change in practice based on greater agreement about what constitutes an adequate margin has important implications for health policy and health care costs. The high rates of reexcision previously reported have led to a variety of efforts to reduce the frequency of reexcision, including the use of intraoperative frozen sections of margins, removal of cavity shave margins, large resections with oncoplastic reconstructions, and the intraoperative use of probes to detect tumor at margin surfaces. All of these approaches increase cost, either through increased operating time or requirement for specialized equipment. Our study documents a decrease in postlumpectomy surgery corresponding to an increased acceptance of the “no tumor on ink” margin, a clear example of a decrease in overtreatment. Although these results are encouraging, there is room for further improvement. While positive margins are sometimes unavoidable, we found that 41% of reexcisions were done for patients with negative margins, and that acceptance of smaller margins was greater among high-volume surgeons than among their lower-volume counterparts, indicating the need for further educational outreach to the surgical community. These results are congruent with estimates across conditions that there is a 50% to 67% probability that physicians will follow guidelines in their practice.
Strengths and Limitations
Strengths of the study include a large, contemporary, diverse patient sample; high patient response rate; granular clinical information including pathologic margin status; and an attending surgeon survey with a very high response rate performed after the promulgation of the guidelines. However, there were some limitations. Surgical procedures were identified based on patient report, and it is likely that some reports were inaccurate. However, it seems unlikely that inaccuracies in patient report would vary across time period. We report on only surgeons’ approaches toward a negative margin, but other members of the multidisciplinary breast team, particularly radiation oncologists, may influence decisions to perform additional surgery after lumpectomy and merit investigation. In addition, the generalizability of the results is limited to 2 very large, diverse populations in the United States.
Conclusions
We have demonstrated a significant decrease in the use of additional surgery after lumpectomy between 2013 and 2015, which resulted in a significant increase in the overall rate of BCS. This change seems to be associated with a change in surgeon approach regarding what constitutes an adequate lumpectomy margin. Our findings provide support for an argument that evidence-based, multidisciplinary guidelines that address issues of clinical controversy can be an effective, relatively low-cost approach to accelerating practice change and reducing overtreatment in cancer care.
eFigure 1. Consort diagram of study sample
eFigure 2. Preferred margin width by surgeon volume and estrogen receptor/progesterone receptor status
eTable. Patient and tumor characteristics
eAppendix 1. Margin case scenarios
References
- 1.Katz SJ, Morrow M. Addressing overtreatment in breast cancer: the doctors’ dilemma. Cancer. 2013;119(20):3584-3588. [DOI] [PubMed] [Google Scholar]
- 2.Morrow M, Harris JR, Schnitt SJ. Surgical margins in lumpectomy for breast cancer: bigger is not better. N Engl J Med. 2012;367(1):79-82. [DOI] [PubMed] [Google Scholar]
- 3.Morrow M, Katz SJ. Margins in ductal carcinoma in situ: is bigger really better? J Natl Cancer Inst. 2012;104(7):494-495. [DOI] [PubMed] [Google Scholar]
- 4.Albornoz CR, Matros E, Lee CN, et al. Bilateral mastectomy versus breast-conserving surgery for early-stage breast cancer: the role of breast reconstruction. Plast Reconstr Surg. 2015;135(6):1518-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg. 2015;150(1):9-16. [DOI] [PubMed] [Google Scholar]
- 6.McCahill LE, Single RM, Aiello Bowles EJ, et al. Variability in reexcision following breast conservation surgery. JAMA. 2012;307(5):467-475. [DOI] [PubMed] [Google Scholar]
- 7.Morrow M, Jagsi R, Alderman AK, et al. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. JAMA. 2009;302(14):1551-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azu M, Abrahamse P, Katz SJ, Jagsi R, Morrow M. What is an adequate margin for breast-conserving surgery? Surgeon attitudes and correlates. Ann Surg Oncol. 2010;17(2):558-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jagsi R, Abrahamse P, Morrow M, Hamilton AS, Graff JJ, Katz SJ. Coordination of breast cancer care between radiation oncologists and surgeons: a survey study. Int J Radiat Oncol Biol Phys. 2012;82(5):2072-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King TA, Sakr R, Patil S, et al. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J Clin Oncol. 2011;29(16):2158-2164. [DOI] [PubMed] [Google Scholar]
- 11.Steiner CA, Weiss AJ, Barrett ML, Fingar KR, Davis PH. Trends in Bilateral and Unilateral Mastectomies in Hospital Inpatient and Ambulatory Settings, 2005-2013: Statistical Brief 201. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare Research and Quality; 2006. [PubMed] [Google Scholar]
- 12.Bouganim N, Tsvetkova E, Clemons M, Amir E. Evolution of sites of recurrence after early breast cancer over the last 20 years: implications for patient care and future research. Breast Cancer Res Treat. 2013;139(2):603-606. [DOI] [PubMed] [Google Scholar]
- 13.Moran MS, Schnitt SJ, Giuliano AE, et al. ; Society of Surgical Oncology; American Society for Radiation Oncology . Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J Clin Oncol. 2014;32(14):1507-1515. [DOI] [PubMed] [Google Scholar]
- 14.Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Int J Radiat Oncol Biol Phys. 2014;88(3):553-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Ann Surg Oncol. 2014;21(3):704-716. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton AS, Hofer TP, Hawley ST, et al. Latinas and breast cancer outcomes: population-based sampling, ethnic identity, and acculturation assessment. Cancer Epidemiol Biomarkers Prev. 2009;18(7):2022-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dillman DA, Smyth JD, Christian LM, eds. Internet, Phone, Mail and Mixed-Mode Surveys: The Tailored Design Method. 4th ed Hoboken, NJ: John Wiley; 2014. [Google Scholar]
- 18.Sanchez C, Brem RF, McSwain AP, Rapelyea JA, Torrente J, Teal CB. Factors associated with re-excision in patients with early-stage breast cancer treated with breast conservation therapy. Am Surg. 2010;76(3):331-334. [PubMed] [Google Scholar]
- 19.Unzeitig A, Kobbermann A, Xie XJ, et al. Influence of surgical technique on mastectomy and reexcision rates in breast-conserving therapy for cancer. Int J Surg Oncol. 2012;2012:725121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright MJ, Park J, Fey JV, et al. Perpendicular inked versus tangential shaved margins in breast-conserving surgery: does the method matter? J Am Coll Surg. 2007;204(4):541-549. [DOI] [PubMed] [Google Scholar]
- 21.Wilke LG, Czechura T, Wang C, et al. Repeat surgery after breast conservation for the treatment of stage 0 to II breast carcinoma: a report from the National Cancer Data Base, 2004-2010. JAMA Surg. 2014;149(12):1296-1305. [DOI] [PubMed] [Google Scholar]
- 22.Blair SL, Thompson K, Rococco J, Malcarne V, Beitsch PD, Ollila DW. Attaining negative margins in breast-conservation operations: is there a consensus among breast surgeons? J Am Coll Surg. 2009;209(5):608-613. [DOI] [PubMed] [Google Scholar]
- 23.Parvez E, Hodgson N, Cornacchi SD, et al. Survey of American and Canadian general surgeons’ perceptions of margin status and practice patterns for breast conserving surgery. Breast J. 2014;20(5):481-488. [DOI] [PubMed] [Google Scholar]
- 24.Chung A, Gangi A, Amersi F, Bose S, Zhang X, Giuliano A. Impact of consensus guidelines by the Society of Surgical Oncology and the American Society for Radiation Oncology on margins for breast-conserving surgery in stages 1 and 2 invasive breast cancer. Ann Surg Oncol. 2015;22(suppl 3):S422-S427. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberger LH, Mamtani A, Fuzesi S, et al. Early Adoption of the SSO-ASTRO consensus guidelines on margins for breast-conserving surgery with whole-breast irradiation in stage I and II invasive breast cancer: initial experience from Memorial Sloan Kettering Cancer Center. Ann Surg Oncol. 2016;23(10):3239-3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boughey JC, Hieken TJ, Jakub JW, et al. Impact of analysis of frozen-section margin on reoperation rates in women undergoing lumpectomy for breast cancer: evaluation of the National Surgical Quality Improvement Program data. Surgery. 2014;156(1):190-197. [DOI] [PubMed] [Google Scholar]
- 27.Chagpar AB, Killelea BK, Tsangaris TN, et al. A randomized, controlled trial of cavity shave margins in breast cancer. N Engl J Med. 2015;373(6):503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schnabel F, Boolbol SK, Gittleman M, et al. A randomized prospective study of lumpectomy margin assessment with use of MarginProbe in patients with nonpalpable breast malignancies. Ann Surg Oncol. 2014;21(5):1589-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zysk AM, Chen K, Gabrielson E, et al. Intraoperative assessment of final margins with a handheld optical imaging probe during breast-conserving surgery may reduce the reoperation rate: results of a multicenter study. Ann Surg Oncol. 2015;22(10):3356-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Timmermans S, Mauck A. The promises and pitfalls of evidence-based medicine. Health Aff (Millwood). 2005;24(1):18-28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Consort diagram of study sample
eFigure 2. Preferred margin width by surgeon volume and estrogen receptor/progesterone receptor status
eTable. Patient and tumor characteristics
eAppendix 1. Margin case scenarios