Key Points
Question
Can dual-task gait testing (assessing gait while performing a challenging cognitive task) identify patients with mild cognitive impairment at risk of progression to dementia?
Findings
In this cohort study of 112 older adults with mild cognitive impairment with up to 6 years of follow-up, poor performance in dual-task gait testing was significantly associated with a 2- to 3-fold risk of dementia incidence independent of age, sex, education, comorbidities, and baseline cognition.
Meaning
Dual-task gait testing may serve clinicians to detect patients with mild cognitive impairment at higher risk of progression to dementia, allowing for optimization of further biomarker testing and initiation of early interventions.
Abstract
Importance
Gait performance is affected by neurodegeneration in aging and has the potential to be used as a clinical marker for progression from mild cognitive impairment (MCI) to dementia. A dual-task gait test evaluating the cognitive-motor interface may predict dementia progression in older adults with MCI.
Objective
To determine whether a dual-task gait test is associated with incident dementia in MCI.
Design, Setting, and Participants
The Gait and Brain Study is an ongoing prospective cohort study of community-dwelling older adults that enrolled 112 older adults with MCI. Participants were followed up for 6 years, with biannual visits including neurologic, cognitive, and gait assessments. Data were collected from July 2007 to March 2016.
Main Outcomes and Measures
Incident all-cause dementia was the main outcome measure, and single- and dual-task gait velocity and dual-task gait costs were the independent variables. A neuropsychological test battery was used to assess cognition. Gait velocity was recorded under single-task and 3 separate dual-task conditions using an electronic walkway. Dual-task gait cost was defined as the percentage change between single- and dual-task gait velocities: ([single-task gait velocity – dual-task gait velocity]/ single-task gait velocity) × 100. Cox proportional hazard models were used to estimate the association between risk of progression to dementia and the independent variables, adjusted for age, sex, education, comorbidities, and cognition.
Results
Among 112 study participants with MCI, mean (SD) age was 76.6 (6.9) years, 55 were women (49.1%), and 27 progressed to dementia (24.1%), with an incidence rate of 121 per 1000 person-years. Slow single-task gait velocity (<0.8 m/second) was not associated with progression to dementia (hazard ratio [HR], 3.41; 95% CI, 0.99-11.71; P = .05)while high dual-task gait cost while counting backward (HR, 3.79; 95% CI, 1.57-9.15; P = .003) and naming animals (HR, 2.41; 95% CI, 1.04-5.59; P = .04) were associated with dementia progression (incidence rate, 155 per 1000 person-years). The models remained robust after adjusting by baseline cognition except for dual-task gait cost when dichotomized.
Conclusions and Relevance
Dual-task gait is associated with progression to dementia in patients with MCI. Dual-task gait testing is easy to administer and may be used by clinicians to decide further biomarker testing, preventive strategies, and follow-up planning in patients with MCI.
Trial Registration
clinicaltrials.gov: NCT03020381.
This cohort study determines whether a dual-task gait test is associated with incident dementia in mild cognitive impairment.
Introduction
Mild cognitive impairment (MCI) is considered a predementia state associated with a 10-fold increased risk of progression to dementia.1 However, almost one-third of individuals with MCI remain clinically stable after the initial diagnosis or even revert to normal cognitive functioning,2 which highlights the hazard of considering patients with MCI as a homogeneous group.3 This heterogeneity of outcomes challenges clinical treatment once MCI is identified because it is problematic to accurately predict progression to dementia including Alzheimer disease (AD). To overcome this challenge, the identification of clinically useful and readily available biomarkers of progression to dementia, including motor markers, is highly needed in MCI.4,5,6,7
During the past decade, large cohort studies have shown that motor impairment, in particular, slowing of gait, is not only evident early in Alzheimer and non-Alzheimer dementias but also predicts progression to dementia in the general population.8,9,10 Although the main clinical hallmark of MCI is memory impairment,11 motor dysfunction and gait impairment have been previously described.8,11,12 Few studies have focused on dual-task gait testing (walking while simultaneously performing a cognitive challenge) as a means to determine the association between cognitive-motor interaction and risk of progression to dementia in patients with MCI.13,14 Dual-task gait testing challenges the cognitive component of locomotion and can provide insight into the mechanisms of brain motor control and cognitive performance.13 Mechanistically, structural and functional brain imaging studies have shown that cognition and motor control share common brain networks, particularly in the prefrontal and temporal areas.13,15,16,17 Based on the limited capacity model, these networks can become overloaded when a motor task is concurrently performed with a cognitive task, more so in individuals with cognitive impairment who have less cognitive reserve.13,18
The dual-task gait test is unique in that it reflects the motor-cognitive interface.19,20,21 There is a linear association between the magnitude of gait slowing while dual tasking and deficits in executive, attention, and memory processes in MCI.4,20,22,23,24 The magnitude of changes in gait during dual-task performance owing to a concurrent cognitive challenge can be expressed as a dual-task gait cost, which adjusts for an individual’s baseline gait characteristics.25 To our knowledge, the capacity of dual-task gait testing to predict progression to dementia in patients with MCI has not been investigated.
In this study, we examined the longitudinal association of dual-task gait performance and the incidence of dementia in a well-characterized MCI cohort with 6 years of follow-up. We hypothesized that participants who progressed to dementia would have a higher dual-task cost in gait velocity than participants who did not progress to dementia.
Methods
Study Participants
Participants were part of the Gait and Brain Study, an ongoing prospective cohort study designed to determine whether quantitative gait deficits can predict incident cognitive and mobility decline and progression to dementia among community-dwelling older adults. Design and logistics have been described in detail elsewhere.26,27,28 After written consent was obtained, participants underwent a comprehensive baseline evaluation as well as biannual assessments during 6 years of follow-up. For this analysis, participants were required to have at least 2 assessments, including the baseline visit, and to fulfill MCI diagnostic criteria.3,29 All participants were community-living adults meeting the following inclusion criteria: age 65 years and older, able to walk 10 m independently without a gait aid, having MCI as ascertained by scoring 0.5 on the global rating of the Clinical Dementia Rating scale and satisfying the following 4 criteria1: (1) subjective cognitive challenges; (2) objective cognitive impairment in at least 1 of the following cognitive domains: memory, executive function, attention, and language3,29; (3) preserved activities of daily living30 confirmed by a clinician’s interviews; and (4) absence of dementia using criteria from the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision).31 Exclusion criteria included lack of English proficiency, parkinsonism or any neurologic disorder with residual motor deficits (eg, stroke), musculoskeletal disorders of lower limbs (eg, severe osteoarthritis or history of knee/hip replacement) affecting gait performance at clinical examination, use of neuroleptics or benzodiazepines, and major depression. Ethics approval was obtained from the University of Western Ontario Health Sciences Research ethics board, and written informed consent was obtained from participants at enrollment. Data collection occurred between July 2007 and March 2016.
Medical and Cognitive Assessments
Sociodemographic characteristics, comorbidities, chronic medications, physical activity level, history of falls, and basic and instrumental activities of daily living were collected using standardized questionnaires during face-to-face interviews (Table 1). Study clinicians performed a physical examination including a neurological examination on all participants.
Table 1. Baseline Characteristics of Participants Stratified by Progression to Dementia (N = 112).
| Characteristics | Full
Sample (n = 112) |
Progressed
MCI (n = 27) |
Stable
MCI (n = 85) |
P Valuea |
|---|---|---|---|---|
| Age, mean (SD), y | 75.97 (6.88) | 78.75 (6.35) | 75.05 (6.84) | .01b |
| Women, No. (%) | 55 (49.1) | 12 (42.9) | 43 (51.2) | .45 |
| No. of medications, mean (SD) | 7.14 (3.98) | 7.43 (4.17) | 7.04 (3.94) | .66 |
| No. of comorbidities, mean (SD) | 5.56 (3.06) | 4.57 (2.85) | 5.90 (3.08) | .05b |
| BP systolic, mean (SD), mm Hg | 128.66 (10.13) | 129.78 (12.40) | 128.48 (9.84) | .72 |
| BP diastolic, mean (SD), mm Hg | 71.52 (8.04) | 71.78 (7.34) | 71.48 (8.20) | .92 |
| Comorbidities, No. (%) | ||||
| Hypertension | 67 (60.4) | 19 (70.4) | 48 (57.1) | .23 |
| Diabetes | 22 (19.8) | 4 (14.8) | 18 (21.4) | .46 |
| Atrial fibrillation | 9 (8.0) | 2 (7.4) | 7 (8.2) | .89 |
| CHF | 2 (1.8) | 1 (3.7) | 1 (1.2) | .40 |
| Myocardial infarction | 11 (10.0) | 4 (14.8) | 7 (8.4) | .34 |
| Stroke/TIA | 13 (11.7) | 1 (3.7) | 12 (14.3) | .14 |
| Smoking | 37 (56.1) | 3 (37.5) | 34 (58.6) | .27 |
| Osteoarthritis | 37 (33) | 6 (5) | 31 (28) | .17 |
| MMSE score, mean (SD) | 27.46 (2.45) | 25.71 (3.15) | 28.05 (1.86) | <.001b |
| MMSE score, median (range) | 28 (18-30) | 26 (18-30) | 28 (20-30) | .002b |
| MoCA, mean (SD) | 23.14 (3.39) | 21.25 (3.05) | 23.77 (3.28) | .001b |
| APOE ε4 carrier, No. (%)c | 23 (39.0) | 5 (55.6) | 18 (36) | .28 |
| a-MCI, No. (%) | 77 (86.2) | 15 (57.4) | 62 (73.0) | <.001b |
| Multiple domain MCI, No. (%) | 30 (33) | 12 (43.4) | 18 (22.6) | .02b |
| Non a-MCI, No. (%) | 3 (3.336) | 0 | 3 (73.6) | NA |
| Cognitive tests, mean (SD) | ||||
| RAVLT, delayed recall | 4.54 (3.04) | 2.50 (1.52) | 4.77 (3.09) | .08 |
| Digit span forward | 10.97 (2.083) | 11.38 (2.72) | 10.91 (2.00) | .56 |
| Digit span backward | 6.82 (2.35) | 5.25 (1.98) | 7.03 (2.32) | .04b |
| TMT A, s | 48.74 (17.35) | 57.54 (25.08) | 47.53 (15.93) | .30 |
| TMT B, s | 151.63 (136.06) | 320.65 (294.80) | 128.31 (76.65) | .11 |
| BNT | 13.27 (1.57) | 12.13 (1.36) | 13.45 (1.54) | .03b |
| LNS | 7.62 (2.58) | 6.14 (4.14) | 7.79 (2.32) | .34 |
| Gait velocity, mean (SD), cm/s | ||||
| Single-task | 107.43(21.26) | 105.21 (21.91) | 108.13 (21.13) | .54 |
| Counting | 98.49 (26.38) | 88.42 (25.62) | 101.84 (25.92) | .03b |
| Serial sevens | 80.59 (29.21) | 70.02 (24.26) | 83.86 (29.96) | .03b |
| Naming animals | 87.46 (27.48) | 78.04 (26.90) | 90.45 (27.13) | .04b |
| Gait cost, mean (SD), No. (%) | ||||
| DTC counting | 8.82 (14.09) | 15.95 (16.01) | 6.56 (12.70) | .002b |
| DTC serials sevens | 24.54 (19.49) | 33.69 (18.67) | 21.67 (18.95) | .006b |
| DTC naming animals | 19.18 (17.27) | 26.57 (16.52) | 16.84 (16.83) | .01b |
Abbreviations: APOE, apolipoprotein E; BNT, Boston Naming Test; CHF, congestive heart failure; DTC, dual-task gait cost, calculated as ([single-task gait value – dual-task gait value]/ single-task gait value) × 100; LNS, Letter-Number Sequencing; MCI, mild cognitive impairment; NA, not applicable; RAVLT, Rey Auditory Verbal Learning Test; TIA, transient ischemic attack; TMT A, Trail Making Test A; TMT B, Trail Making Test B.
P value determined using χ2or t tests, as deemed appropriate.
Statistically significant value.
Data available for 59 participants.
Global cognition was assessed by using the Mini-Mental State Examination32 and the Montreal Cognitive Assessment,33 with alternative test versions used in consecutive assessments to avoid potential learning effects. The Clinical Dementia Rating scale was also performed at all visits.34 A neuropsychological test battery was administered to characterize MCI subtype and to help ascertain progression to dementia. Executive function was assessed using Trail Making Tests A and B35; verbal episodic memory using the Rey Auditory Verbal Learning Test36; naming using the Boston Naming Test37; attention using the Digit Span Test (forward and backward), and working memory using the Letter-Number Sequencing test.38 Impaired cognitive domains were identified using a cutoff of 1.5 SD below the age-adjusted norms.14,39 Participants were classified as pure amnestic MCI (impairment only in verbal episodic memory), multidomain amnestic MCI (impairment in more than 1 domain including memory), and nonamnestic MCI (solely impairment in 1 or more nonmemory domain).14,40
Gait Assessments
Gait velocity within single and dual tasks was assessed using an electronic walkway (GAITRite System, 600 cm long and 64 cm wide; CIR Systems Inc) that provides data to assess both spatial and temporal gait parameters.26 Start and end points were marked on the floor 1 meter from either walkway end to avoid recording acceleration and deceleration phases. Each participant performed 1 practice trial walking on the walkway. For the single-gait test, participants were asked to walk at their usual pace in a quiet, well-lit room wearing comfortable footwear and without the use of any mobility aids. For the dual-task tests, participants walked at their usual pace while doing the following cognitive tasks aloud: (1) counting backward from 100 by ones, (2) subtracting serial sevens from 100, and (3) naming animals. Rationale for dual-task condition selection has been described elsewhere.26 To balance and minimize the effects of learning and fatigue, only 1 trial was performed in each condition, and the order of single and dual tasks was randomized. Reliability has been previously established for this protocol in participants with MCI.26 The magnitude of the effect of the cognitive challenge on gait performance was assessed by calculating the dual-task gait cost (percent) as19:
| ([single-task gait velocity – dual-task gait velocity] / single-task gait velocity) × 100 |
Outcome Variable
Incident dementia was the main end point as determined by a clinician investigator during follow-up visits per Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision) criteria31 and when Clinical Dementia Rating increased to a score of 1 or higher. At the time of diagnosis, clinicians were blinded to baseline gait or baseline neuro-psychological test scores. The type of dementia was established using standardized clinical criteria for AD dementia,41 frontotemporal dementia,42 Lewy body dementia,43 and vascular dementia.44 Participants were reassessed after 6 months to confirm dementia status and subtype.
Predictor Variables
Gait velocity and dual-task gait cost were our main predictor variables because they have been previously associated with cognitive performance in MCI studies.14,27 These variables were modeled as continuous variables to ascertain the strength of associations and as dichotomous variables to identify potential thresholds to be used in clinics. Slow gait velocity in the single-task condition was defined as less than 0.8 m/second, a threshold previously established for predicting adverse events, including cognitive decline, in ambulatory patients.45,46,47 Because there is not an established cutoff for dual-task gait testing, receiver operating characteristic curves were produced to determine the optimal threshold value for high dual-task gait cost as a predictor of dementia for the 3 dual-tasks conditions.
Covariates
Analyses were adjusted for covariates that included demographics (age, sex, and educational level), number of comorbidities, and baseline cognition.
Statistical Analysis
Demographics and clinical characteristics were summarized using either means and standard deviations or frequencies and percentages as appropriate. Comparisons of baseline characteristics between patients with MCI who progressed to dementia and those who did not were made using χ2 or t tests as deemed appropriate. Multivariable Cox proportional hazards regression analyses were completed to assess the risk, measured as hazard ratios (HRs), for progression to dementia for gait velocity as continuous variables (single- and dual-task gait velocity and dual-task gait cost) and as dichotomous variables (slow gait velocity and high dual-task gait cost in each condition), unadjusted and adjusted for the previously mentioned covariates. Dual-task gait cost cutoffs were determined using receiver operating characteristic analysis. Time to event was calculated from enrollment to the assessment visit at which dementia was diagnosed. Proportional hazards assumption was tested using methods based on scaled Schoenfeld residuals. To account for different follow-up times, incident dementia is also presented as incident rate expressed as “total person-years at risk” for all the models. Associations were also explored by stratifying dual-task gait velocities into quartiles. Statistical significance was set at P < .05 (2-sided). Statistical analyses were conducted using SPSS, version 23 (IBM Corporation) and occurred from June 2016 to March 2017.
Results
Participant Characteristics
One hundred twelve participants (mean [SD] age, 76 [6.88] years; 55 women) were assessed with a mean follow-up of 24 months (range, 12-76 months). Characteristics of the study sample, stratified by progression to dementia (patients with MCI who progressed to dementia vs those who did not) are presented in Table 1. Ninety-two participants with MCI fulfilled criteria for pure amnestic MCI while 30 fulfilled criteria for nonamnestic MCI. A total of 24% of the sample (n = 27; 11 women [43%]) progressed to dementia, with an overall incidence rate of 121 per 1000 person-years. From the 27 participants who progressed to dementia, 23 progressed to AD (85%), 2 to Lewy body dementia (7%), 1 to frontotemporal dementia (4%), and 1 to vascular dementia (4%).
Table 1 shows that participants with MCI who progressed to dementia were older and had fewer comorbidities. They had higher hypertension prevalence (70.4% vs 57.1%) and were more likely to carry at least 1 apolipoprotein E ε4 allele, although these differences were not significant. Both groups had a mean baseline single-gait velocity greater than the normality cutoff of 0.8 m/second; however, participants who progressed to dementia had a significantly lower dual-task gait velocity and higher dual-task gait cost in the 3 test conditions.
Associations Between Gait Performance and Incident Dementia
Table 2 reports the Cox proportional hazard models for our main outcome, incident dementia, for our 2 predictor variables, gait velocity and dual-task gait cost, in 1 unadjusted and 2 adjusted models. When modeling our predictors as a continuous variable, single-gait velocity test failed to predict dementia (HR, 1.02; 95% CI, 0.99-1.04; P = .10), while all dual-task gait velocity tests (velocities and costs) predicted progression to dementia except for dual-task gait cost in serial sevens subtractions when adjusted for covariates.
Table 2. Cox Proportional Hazard Regression of the Association of Gait Velocity and Dual-Task Gait Cost With Incident Dementia Modeled as Continuous and Dichotomous Variablesa.
| Variable | Model 1 (Unadjusted) | Model 2 (Adjusted) | Model 3 (Adjusted + MMSE) | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Gait variable (continuous) | ||||||
| Gait velocity | ||||||
| Single-task | 1.01 (0.99-1.03) | .26 | 1.02 (0.99-1.04) | .10 | 1.02 (0.99-1.04) | .20 |
| Counting | 1.02 (1.00-1.03) | .01b | 1.03 (1.01-1.04) | .001b | 1.03 (1.01-1.04) | .01b |
| Serial sevens | 1.01 (1.00-1.02) | .04b | 1.01 (1.00-1.03) | .05b | 1.02 (0.99-1.03) | .11 |
| Naming animals | 1.02 (1.00-1.03) | .01b | 1.03 (1.01-1.04) | .002b | 1.02 (1.01-1.04) | .02b |
| Gait cost | ||||||
| DTC counting | 1.04 (1.01-1.06) | .003b | 1.06 (1.03-1.09) | <.001b | 1.04 (1.00-1.07) | .01b |
| DTC serial sevens | 1.03 (1.01-1.05) | .008b | 1.02 (0.99-1.04) | .08 | 1.01 (0.99-1.03) | .20 |
| DTC naming animals | 1.03 (1.01-1.06) | .004b | 1.04 (1.02-1.07) | .001b | 1.03 (1.00-1.05) | .04b |
| Gait variable (dichotomous) | ||||||
| Gait velocity | ||||||
| Slow single-task (<0.8m/s) | 2.31 (0.80-6.69) | .12 | 3.41 (0.99-11.71) | .05 | 3.17 (0.92-10.88) | .07 |
| Gait cost | ||||||
| DTC counting | 2.27 (1.04-4.97) | .04b | 3.79 (1.57-9.15) | .003b | 2.39 (0.91-6.27) | .08 |
| DTC serial sevens | 2.50 (1.00-6.24) | .05b | 1.89 (0.73-4.93) | .19 | 1.30 (0.48-3.54) | .61 |
| DTC naming animals | 2.25 (1.01-5.01) | .05b | 2.41 (1.04-5.59) | .04b | 1.96 (0.81-4.72) | .13 |
Abbreviations: DTC, dual-task gait cost, calculated as ([single-task gait velocity – dual-task gait velocity]/ single-task gait velocity) × 100; MMSE, Mini-Mental State Examination.
Model 1: unadjusted. Model 2: adjusted for age, sex, years of education, and number of comorbidities Model 3: adjusted for age, sex, years of education, number of comorbidities and MMSE.
Statistically significant values.
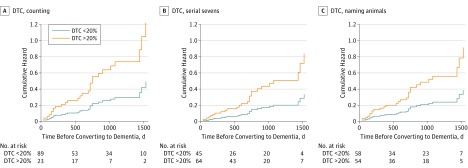
Modeling gait velocity as dichotomous variables showed that slow single-task velocity (<0.8 m/second) was not associated with dementia progression (HR, 3.41; 95% CI, 0.99-11.71; P = .05). Alternative slow single-task gait velocity cutoffs, determined by receiver operating characteristic analysis (<1.08 m/second) or using 1.5 SD below the cohort mean gait velocity (<0.76 m/second) were also not associated with dementia progression (eTable 1 in the Supplement). However, high dual-task cost in gait velocity while counting backward (HR, 3.79; 95% CI, 1.57-9.15; P = .003) and naming animals (HR, 2.41; 95% CI, 1.04-5.59; P = .04) were both associated with dementia progression (Table 2 and Figure 1A and C). After adjusting for baseline cognition (Mini-Mental State Examination scores, model 3) the associations with progression to dementia were only attenuated for dual-task gait as a dichotomous variable.
Figure 1. Cumulative Hazard Ratio for Progression to Dementia for Low and High Dual-Task Cost in Gait Velocity (n = 112) .
A, Dual-task gait cost (DTC) while counting backward. B, While performing serial sevens subtractions. C, While naming animals.
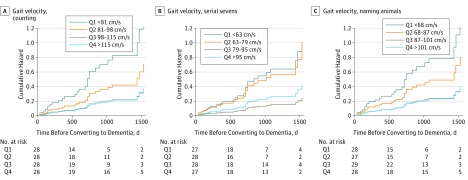
Stratification of the sample into quartiles of dual-task gait velocity (eTable 2 in the Supplement) showed that participants in the lowest quartile had the highest risk of progression to dementia while counting backward (HR, 13.39; 95% CI, 3.76-47.75; P < .001; Figure 2A) and while naming animals (HR, 9.89; 95% CI, 2.91-33.62; P < .001; Figure 2C).
Figure 2. Risk of Dementia Stratified by Gait Velocity (Centimeters per Second) Quartiles in 3 Dual-Tasks Conditions.
The 3 dual-tasks conditions are counting backward (A), serial sevens subtractions (B), and naming animals (C).
Sensitivity Analyses
Associations with apolipoprotein E ε4 carrier status and single- and dual-tasking gait performance were explored, and differences were small and in both directions (eTable 3 in the Supplement). Baseline global cognition (Mini-Mental State Examination and Montreal Cognitive Assessment) was associated with incident dementia, with comparable HRs to dual-task gait (eTable 4 in the Supplement).
Discussion
Our results suggest that dual-task gait testing is associated with the progression to dementia in a well-characterized cohort of older adults with MCI. Specifically, a high dual-task gait cost while counting backward and naming animals was associated with an increased risk of progression to dementia by 3.8 and 2.4 times, respectively. The predictive ability of single-task gait performance appeared high although not statistically significant, revealing the unique utility of dual-task gait testing in the clinical encounter when assessing patients with normal gait velocity. To the best of our knowledge, this is the first study establishing the ability of dual-task gait testing to detect incident dementia in patients with MCI.4,14,19,26,48,49,50,51,52
Assessing gait velocity is easy to perform and provides an excellent general measure of overall function. However, dual-task gait testing can uncover valuable subtleties regarding the role of cognitive control on a participant’s gait.45,53,54,55,56 It is also important to note that the single-task gait velocity in our MCI sample was greater than 0.8 m/second, the threshold recommended to identify slow gait indicative of adverse events, and consistent with the high functional abilities seen in the early stages of cognitive decline and with previous studies assessing gait in MCI.14,25,27 Thus, among these participants, using solely a slow velocity threshold would have been insufficient to identify individuals at a high risk of progression to dementia.
Our results complement the Motoric Cognitive Risk syndrome, where participants with cognitive challenges and slower gait had a higher risk of developing dementia,7 by showing that dual-task gait may predict dementia in participants with MCI while single-task gait velocity does not. Whether dual-task changes in gait in patients with MCI is associated with progression to vascular dementia or AD is unknown. Previous studies in general populations have suggested that gait slowing during dual tasking is most likely associated with incident vascular dementia.7,9 In this study, dual-task gait change was mostly associated with AD, which can be explained by the fact that our study focused only in individuals with MCI. Interestingly, emerging evidence is linking gait performance with AD neurodegenerative changes, as in a 2017 study that showed in older adults free of dementia that gait performance, including dual-task gait, was cross-sectionally associated with amyloid β brain deposition,57,58 independent of the burden of vascular changes. Taken together, these data suggest that both vascular and neurodegenerative changes may contribute to dementia progression in patients with MCI with impaired dual-task gait.59
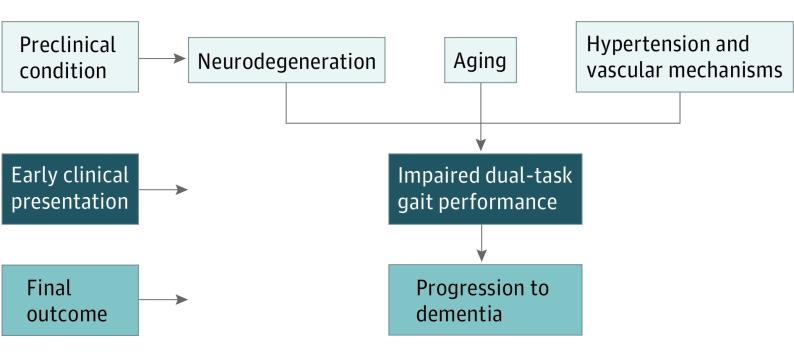
The underlying mechanisms affecting dual-task gait performance remain not completely understood. What does seem clear is that executive demands used for gait and for the selected cognitive tasks may share a similar pathogenic mechanism at the brain level.27,60,61 Episodic memory, a cognitive domain that was affected in all of our participants who progressed to dementia, relies on frontal-hippocampal circuits that are also central for gait control. In addition, gait control also relies on the prefrontal-striatal networks that are involved in executive function, which was similarly impaired in all of our participants who progressed to dementia.62 Prior imaging brain studies in MCI revealed that higher dual-task gait cost is associated with altered neurochemistry and low volume of the primary motor cortex, which is part of the executive network circuit of normal locomotion.17,63,64 In the same manner, stride time correlated negatively with hippocampal neurochemistry in MCI,65 which could reflect the role of the hippocampus in the retrieval of complex foot movement sequences necessary for regular gait patterns.66 Ultimately, these brain circuits shared by both cognition and motor-gait performance can be affected by aging, neurodegenerative, and microvascular mechanisms, providing a rationale to propose that dual-task gait testing may serve as a “brain stress test” to detect impending cognitive decline in patients with subclinical damage (Figure 3).67
Figure 3. Proposal That Dual-Task Gait Could Be an Early Clinical Marker of Progression to Dementia Syndromes.
Dementia biomarkers, including amyloid β and tau protein–related markers, are promising; however, the correlation between pathologic biomarker load and actual dementia status lessens with age.21,68 In other words, older patients with MCI with similar degrees of neuropathology burden may present different functional and clinical states as they age. This warrants expanding the prediction of dementia progression by adding “functional markers” such as the motor-cognitive interface.21
Our findings could be easily translated to the clinical setting owing to the simplicity, noninvasive nature, and low cost of dual-task gait assessment. Our sensitivity analysis showed that dual-task gait was comparable with cognitive testing to predict incident dementia, and adjustments for baseline cognition only partially attenuated the associations when modeled as a dichotomous variable, suggesting that dual-task gait test is providing extra information not captured by cognitive testing. Performances in the 3 dual tasks used were all associated with a high risk of progression to dementia, providing flexibility to clinicians to choose the most appropriate dual-task test to cognitively stress a given patient. Clinicians may use dual-task gait testing in screening patients with MCI who could benefit the most from additional testing, optimizing recommendations for imaging, spinal fluid examinations, and genetic testing. Similarly, our findings can assist in identifying high-risk individuals with MCI to plan the frequency of follow-up visits to monitor function. Finally, dual-task gait testing could help researchers plan primary prevention or intervention studies in MCI through the selection of participants at greater risk of decline and progression to dementia.
Limitations
Our sample was limited to 112 individuals with MCI and with a relatively low frequency of outcome events (27 progressed to dementia); however, the event per independent variable ratio for our Cox regression model (27/5 = 5.4) is considered robust for models with binary end point outcomes.69 Our annual conversion rate to AD was 7%, which is in line with clinic-based studies, including amnestic MCI, but higher than population studies; thus, our results are generalizable to clinic-based settings only. Quantitative techniques were used to measure velocity, which can be a limitation to wide clinical applicability. However, dual-task gait velocity can be simply measured using a stopwatch, and dual-task gait cost can be easily calculated. Although predictive validity might be improved by considering cognitive errors on dual tasking, that would make it more difficult to apply in clinics. Finally, our results need cross-validation in other MCI cohorts. The strengths of our study include a well-characterized MCI cohort with a long period of follow-up and with biannual assessments to adequately monitor time to progression to dementia. We used a validated dual-task protocol on quantitative gait analysis and standardized assignment of dementia diagnoses blinded to gait categories, with robust analyses adjusting for a number of important covariates.
Conclusions
Dual-task gait testing can detect individuals with MCI at increased risk of progression to dementia. Our results support the hypothesis that cognitive and motor dysfunction in MCI may reflect a shared pathogenic mechanism at the brain level and that gait is a candidate motor biomarker of progression to dementia.13,16,27,70,71,72 Future studies should confirm whether adding dual-task gait testing to the clinical and cognitive evaluation of patients with MCI can improve dementia prediction. Cross-validation of this approach in other MCI cohorts would further support the clinical applicability.
eTable 1. Cox Proportional Hazard Regression of the Association of Gait Velocity and Incident Dementia Modeled as a Dichotomous Variable, Using Cutoffs Based on 1.5 SD Below Cohort Mean and ROC Analysis.
eTable 2. Cox Proportional Hazard Regression of the Association of Gait Velocity Stratified By Quartiles, Under Single and Dual-Task Conditions.
eTable 3. Gait Performance in the Complete Cohort and Stratified By APOE4 Carrier Status (at Least One e4 Allele).
eTable 4. Cox Proportional Hazard Regression of the Association of MMSE and MoCA Performance With Incident Dementia Modeled As Continuous and Dichotomous Variables.
References
- 1.Petersen RC. Clinical practice: mild cognitive impairment. N Engl J Med. 2011;364(23):2227-2234. [DOI] [PubMed] [Google Scholar]
- 2.Storandt M, Grant EA, Miller JP, Morris JC. Rates of progression in mild cognitive impairment and early Alzheimer’s disease. Neurology. 2002;59(7):1034-1041. [DOI] [PubMed] [Google Scholar]
- 3.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment: beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240-246. [DOI] [PubMed] [Google Scholar]
- 4.Montero-Odasso M, Bergman H, Phillips NA, Wong CH, Sourial N, Chertkow H. Dual-tasking and gait in people with mild cognitive impairment: the effect of working memory. BMC Geriatr. 2009;9:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal NT, Wilson RS, Beck TL, Bienias JL, Bennett DA. Motor dysfunction in mild cognitive impairment and the risk of incident Alzheimer disease. Arch Neurol. 2006;63(12):1763-1769. [DOI] [PubMed] [Google Scholar]
- 6.Pettersson AF, Olsson E, Wahlund LO. Motor function in subjects with mild cognitive impairment and early Alzheimer’s disease. Dement Geriatr Cogn Disord. 2005;19(5-6):299-304. [DOI] [PubMed] [Google Scholar]
- 7.Verghese J, Wang C, Lipton RB, Holtzer R. Motoric cognitive risk syndrome and the risk of dementia. J Gerontol A Biol Sci Med Sci. 2013;68(4):412-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camicioli R, Howieson D, Oken B, Sexton G, Kaye J. Motor slowing precedes cognitive impairment in the oldest old. Neurology. 1998;50(5):1496-1498. [DOI] [PubMed] [Google Scholar]
- 9.Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H. Abnormality of gait as a predictor of non-Alzheimer’s dementia. N Engl J Med. 2002;347(22):1761-1768. [DOI] [PubMed] [Google Scholar]
- 10.Waite LM, Grayson DA, Piguet O, Creasey H, Bennett HP, Broe GA. Gait slowing as a predictor of incident dementia: 6-year longitudinal data from the Sydney Older Persons Study. J Neurol Sci. 2005;229-230:89-93. [DOI] [PubMed] [Google Scholar]
- 11.Waldemar G, Phung KT, Burns A, et al. Access to diagnostic evaluation and treatment for dementia in Europe. Int J Geriatr Psychiatry. 2007;22(1):47-54. [DOI] [PubMed] [Google Scholar]
- 12.Marquis S, Moore MM, Howieson DB, et al. Independent predictors of cognitive decline in healthy elderly persons. Arch Neurol. 2002;59(4):601-606. [DOI] [PubMed] [Google Scholar]
- 13.Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc. 2012;60(11):2127-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verghese J, Robbins M, Holtzer R, et al. Gait dysfunction in mild cognitive impairment syndromes. J Am Geriatr Soc. 2008;56(7):1244-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosano C, Studenski SA, Aizenstein HJ, Boudreau RM, Longstreth WT Jr, Newman AB. Slower gait, slower information processing and smaller prefrontal area in older adults. Age Ageing. 2012;41(1):58-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosso AL, Studenski SA, Chen WG, et al. Aging, the central nervous system, and mobility. J Gerontol A Biol Sci Med Sci. 2013;68(11):1379-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Annweiler C, Beauchet O, Celle S, et al. ; WALK Team (Working group Angers-London for Knowledge) . Contribution of brain imaging to the understanding of gait disorders in Alzheimer’s disease: a systematic review. Am J Alzheimers Dis Other Demen. 2012;27(6):371-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montero-Odasso M, Bherer L, Studenski S, et al. Mobility and cognition in seniors: report from the 2008 Institute of Aging (CIHR) mobility and cognition workshop. Can Geriatr J. 2015;18(3):159-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montero-Odasso M, Muir SW, Speechley M. Dual-task complexity affects gait in people with mild cognitive impairment: the interplay between gait variability, dual tasking, and risk of falls. Arch Phys Med Rehabil. 2012;93(2):293-299. [DOI] [PubMed] [Google Scholar]
- 20.Camicioli R, Howieson D, Lehman S, Kaye J. Talking while walking: the effect of a dual task in aging and Alzheimer’s disease. Neurology. 1997;48(4):955-958. [DOI] [PubMed] [Google Scholar]
- 21.Albers MW, Gilmore GC, Kaye J, et al. . At the interface of sensory and motor dysfunctions and Alzheimer’s disease. Alzheimers Dement. 2015;11(1):70-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bootsma-van der Wiel A, Gussekloo J, de Craen AJ, van Exel E, Bloem BR, Westendorp RG. Walking and talking as predictors of falls in the general population: the Leiden 85-Plus Study. J Am Geriatr Soc. 2003;51(10):1466-1471. [DOI] [PubMed] [Google Scholar]
- 23.Camicioli R, Bouchard T, Licis L. Dual-tasks and walking fast: relationship to extra-pyramidal signs in advanced Alzheimer disease. J Neurol Sci. 2006;248(1-2):205-209. [DOI] [PubMed] [Google Scholar]
- 24.Hausdorff JM, Yogev G, Springer S, Simon ES, Giladi N. Walking is more like catching than tapping: gait in the elderly as a complex cognitive task. Exp Brain Res. 2005;164(4):541-548. [DOI] [PubMed] [Google Scholar]
- 25.Hausdorff JM, Schweiger A, Herman T, Yogev-Seligmann G, Giladi N. Dual-task decrements in gait: contributing factors among healthy older adults. J Gerontol A Biol Sci Med Sci. 2008;63(12):1335-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montero-Odasso M, Casas A, Hansen KT, et al. Quantitative gait analysis under dual-task in older people with mild cognitive impairment: a reliability study. J Neuroeng Rehabil. 2009;6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montero-Odasso M, Oteng-Amoako A, Speechley M, et al. . The motor signature of mild cognitive impairment: results from the gait and brain study. J Gerontol A Biol Sci Med Sci. 2014;69(11):1415-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montero-Odasso MM, Barnes B, Speechley M, et al. Disentangling cognitive-frailty: results from the gait and brain study. J Gerontol A Biol Sci Med Sci. 2016;71(11):1476-1482. [DOI] [PubMed] [Google Scholar]
- 29.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985-1992. [DOI] [PubMed] [Google Scholar]
- 30.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179-186. [PubMed] [Google Scholar]
- 31.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text revision Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 32.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. [DOI] [PubMed] [Google Scholar]
- 33.Nasreddine ZS, Phillips NA, Bédirian V, et al. . The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. [DOI] [PubMed] [Google Scholar]
- 34.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412-2414. [DOI] [PubMed] [Google Scholar]
- 35.Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- 36.Estévez-González A, Kulisevsky J, Boltes A, Otermín P, García-Sánchez C. Rey verbal learning test is a useful tool for differential diagnosis in the preclinical phase of Alzheimer’s disease: comparison with mild cognitive impairment and normal aging. Int J Geriatr Psychiatry. 2003;18(11):1021-1028. [DOI] [PubMed] [Google Scholar]
- 37.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. 3rd ed Philadelphia, PA: Lea and Febiger; 1983. [Google Scholar]
- 38.Weschler D. The Wechsler Adult Intelligence Scale. 4th ed San Antonio, TX: Pearson Assessments; 2008. [Google Scholar]
- 39.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183-194. [DOI] [PubMed] [Google Scholar]
- 40.Pedersen MM, Holt NE, Grande L, et al. Mild cognitive impairment status and mobility performance: an analysis from the Boston RISE study. J Gerontol A Biol Sci Med Sci. 2014;69(12):1511-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944. [DOI] [PubMed] [Google Scholar]
- 42.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(pt 9):2456-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006;9(3)(suppl):417-423. [DOI] [PubMed] [Google Scholar]
- 44.Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s Disease Diagnostic and Treatment Centers. Neurology. 1992;42(3, pt 1):473-480. [DOI] [PubMed] [Google Scholar]
- 45.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peel NM, Kuys SS, Klein K. Gait speed as a measure in geriatric assessment in clinical settings: a systematic review. J Gerontol A Biol Sci Med Sci. 2013;68(1):39-46. [DOI] [PubMed] [Google Scholar]
- 47.Verghese J, Annweiler C, Ayers E, et al. Motoric cognitive risk syndrome: multicountry prevalence and dementia risk. Neurology. 2014;83(8):718-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gillain S, Warzee E, Lekeu F, et al. . The value of instrumental gait analysis in elderly healthy, MCI or Alzheimer’s disease subjects and a comparison with other clinical tests used in single and dual-task conditions. Ann Phys Rehabil Med. 2009;52(6):453-474. [DOI] [PubMed] [Google Scholar]
- 49.Maquet D, Lekeu F, Warzee E, et al. Gait analysis in elderly adult patients with mild cognitive impairment and patients with mild Alzheimer’s disease: simple versus dual task: a preliminary report. Clin Physiol Funct Imaging. 2010;30(1):51-56. [DOI] [PubMed] [Google Scholar]
- 50.Muir SW, Speechley M, Wells J, Borrie M, Gopaul K, Montero-Odasso M. Gait assessment in mild cognitive impairment and Alzheimer’s disease: the effect of dual-task challenges across the cognitive spectrum. Gait Posture. 2012;35(1):96-100. [DOI] [PubMed] [Google Scholar]
- 51.Persad CC, Jones JL, Ashton-Miller JA, Alexander NB, Giordani B. Executive function and gait in older adults with cognitive impairment. J Gerontol A Biol Sci Med Sci. 2008;63(12):1350-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beauchet O, Allali G, Launay C, Herrmann FR, Annweiler C. Gait variability at fast-pace walking speed: a biomarker of mild cognitive impairment? J Nutr Health Aging. 2013;17(3):235-239. [DOI] [PubMed] [Google Scholar]
- 53.Montero-Odasso M, Schapira M, Duque G, Soriano ER, Kaplan R, Camera LA. Gait disorders are associated with non-cardiovascular falls in elderly people: a preliminary study. BMC Geriatr. 2005;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51(3):314-322. [DOI] [PubMed] [Google Scholar]
- 55.Montero-Odasso M, Schapira M, Varela C, et al. Gait velocity in senior people: an easy test for detecting mobility impairment in community elderly. J Nutr Health Aging. 2004;8(5):340-343. [PubMed] [Google Scholar]
- 56.Montero-Odasso M, Schapira M, Soriano ER, et al. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. J Gerontol A Biol Sci Med Sci. 2005;60(10):1304-1309. [DOI] [PubMed] [Google Scholar]
- 57.Nadkarni NK, Lopez OL, Perera S, et al. Cerebral amyloid deposition and dual-tasking in cognitively normal, mobility unimpaired older adults. J Gerontol A Biol Sci Med Sci. 2016;glw211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nadkarni NK, Perera S, Snitz BE, et al. Association of brain amyloid-β with slow gait in elderly individuals without dementia: influence of cognition and apolipoprotein e ε4 genotype. JAMA Neurol. 2017;74(1):82-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toledo JB, Arnold SE, Raible K, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain. 2013;136(pt 9):2697-2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buchman AS, Yu L, Boyle PA, et al. Microvascular brain pathology and late-life motor impairment. Neurology. 2013;80(8):712-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buchman AS, Yu L, Wilson RS, Boyle PA, Schneider JA, Bennett DA. Brain pathology contributes to simultaneous change in physical frailty and cognition in old age. J Gerontol A Biol Sci Med Sci. 2014;69(12):1536-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watson NL, Rosano C, Boudreau RM, et al. ; Health ABC Study . Executive function, memory, and gait speed decline in well-functioning older adults. J Gerontol A Biol Sci Med Sci. 2010;65(10):1093-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Annweiler C, Beauchet O, Bartha R, et al. Motor cortex and gait in mild cognitive impairment: a magnetic resonance spectroscopy and volumetric imaging study. Brain. 2013;136(pt 3):859-871. [DOI] [PubMed] [Google Scholar]
- 64.Annweiler C, Beauchet O, Bartha R, Montero-Odasso M; WALK Team-Working group Angers-London for Knowledge . Slow gait in MCI is associated with ventricular enlargement: results from the Gait and Brain Study. J Neural Transm (Vienna). 2013;120(7):1083-1092. [DOI] [PubMed] [Google Scholar]
- 65.Zimmerman ME, Lipton RB, Pan JW, Hetherington HP, Verghese J. MRI- and MRS-derived hippocampal correlates of quantitative locomotor function in older adults. Brain Res. 2009;1291:73-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lafleur MF, Jackson PL, Malouin F, Richards CL, Evans AC, Doyon J. Motor learning produces parallel dynamic functional changes during the execution and imagination of sequential foot movements. Neuroimage. 2002;16(1):142-157. [DOI] [PubMed] [Google Scholar]
- 67.Montero-Odasso M, Hachinski V. Preludes to brain failure: executive dysfunction and gait disturbances. Neurol Sci. 2014;35(4):601-604. [DOI] [PubMed] [Google Scholar]
- 68.Perez-Nievas BG, Stein TD, Tai HC, et al. Dissecting phenotypic traits linked to human resilience to Alzheimer’s pathology. Brain. 2013;136(pt 8):2510-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165(6):710-718. [DOI] [PubMed] [Google Scholar]
- 70.Hausdorff JM, Buchman AS. What links gait speed and MCI with dementia? a fresh look at the association between motor and cognitive function. J Gerontol A Biol Sci Med Sci. 2013;68(4):409-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Montero-Odasso M. Gait as a biomarker of cognitive impairment and dementia syndromes: quo vadis? Eur J Neurol. 2016;23(3):437-438. [DOI] [PubMed] [Google Scholar]
- 72.Montero-Odasso M, Bergman H, Phillips NA, Wong C, Sourial N, Chertkow H. The effect of executive and memory dysfunction in gait performance in a cognitive impairment population. J Am Geriatr Soc. 2006;54:S154. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Cox Proportional Hazard Regression of the Association of Gait Velocity and Incident Dementia Modeled as a Dichotomous Variable, Using Cutoffs Based on 1.5 SD Below Cohort Mean and ROC Analysis.
eTable 2. Cox Proportional Hazard Regression of the Association of Gait Velocity Stratified By Quartiles, Under Single and Dual-Task Conditions.
eTable 3. Gait Performance in the Complete Cohort and Stratified By APOE4 Carrier Status (at Least One e4 Allele).
eTable 4. Cox Proportional Hazard Regression of the Association of MMSE and MoCA Performance With Incident Dementia Modeled As Continuous and Dichotomous Variables.