Key Points
Question
What are the in vivo cortical distributions and the relationships between tau and amyloid-β deposits in elderly, cognitively normal individuals?
Findings
In this cross-sectional study of 88 elderly persons, tau and amyloid-β deposits in the elderly brain displayed well-defined hierarchical cortical relationships as well as overlaps between the principal accumulations of both abnormalities in the heteromodal association regions.
Meaning
These findings represent systematic, large-scale mechanisms of early pathologic changes of Alzheimer disease in the elderly human brain.
Abstract
Importance
Abnormal accumulation of tau and amyloid-β (Aβ) proteins in the human brain are 2 pathologic hallmarks of Alzheimer disease (AD). Because pathologic processes begin decades before the onset of the clinical manifestations, the study of the cortical distribution of early-stage pathologic alterations is critical in understanding the underpinnings of the disease.
Objectives
To identify the in vivo brain spatial distributions of tau and Aβ deposits in a sample of cognitively normal participants in the Harvard Aging Brain Study, determine spatial patterns of pathologic alterations, and provide means for improved individual in vivo staging.
Design, Setting, and Participants
Eighty-eight individuals from the general community underwent flortaucipir 18 T807 (18F-T807) and carbon 11–labeled Pittsburgh Compound B (11C-PiB) positron emission tomographic (PET) imaging. A voxel-level hierarchical clustering approach was used to obtain the main clustering partitions corresponding to the cortical distribution maps of 18F-T807 and 11C-PiB. Hierarchical relationships between areas of distinctive pathologic deposits were then studied. Using cerebellar gray reference, 18F-T807 data were expressed as standardized uptake value ratio, and 11C-PiB were given as distribution volume ratio.
Main Outcomes and Measures
Main in vivo and hierarchically organized tau and Aβ deposits in the elderly brain.
Results
Of the 88 study participants, 39 (44%) were men, with a mean (SD) age of 76.2 (6.2) years. The tau and Aβ maps both displayed optimal cortical partitions at 4 clusters. The tau deposits were grouped in the temporal lobe, distributed in heteromodal areas, medial and visual regions, and primary somatomotor cortex; the Aβ deposits were clustered in the heteromodal areas and rather patchy in distributed regions involving the primary cortices, medial structures, and temporal areas. Moreover, tau deposits in the temporal lobe and distributed heteromodal areas were tightly nested.
Conclusions and Relevance
Tau and Aβ deposits in the elderly brain generally display well-defined hierarchical cortical relationships as well as overlaps between the principal clusters of both pathologic alterations in the heteromodal association regions. These findings represent systematic, large-scale mechanisms of early AD pathology.
This cross-sectional study examines the spatial distributions of tau and Aβ deposits in the brains of elderly, cognitively normal individuals.
Introduction
The appearance and interplay of abnormal tau and amyloid-β (Aβ) proteins in the human brain have been extensively implicated in the future development of neuronal degeneration and Alzheimer disease (AD). For several decades, it has been observed that misfolded tau and Aβ proteins begin to accumulate in the cerebral tissue decades before any cognitive manifestations appear and with distinctive histologic and spatial affinities. Pathologic forms of tau protein, particularly as intraneuronal neurofibrillary tangles, preferentially concentrate in medial temporal structures, and misfolded extracellular Aβ is primarily located in the association cortex of the temporal, parietal, and frontal lobes. However, it is still not well understood why these rather stereotyped spatial patterns occur and how they may interact to create a final synergic product of large-scale neurodegeneration. Currently, the advent of high-affinity, high-selectivity tau radioligands for positron emission tomographic (PET) imaging in combination with existing Aβ tracers have opened new means to study such potential interactions in the preclinical and clinical stages of the disease.
Alzheimer disease is frequently conceptualized as an amyloid-facilitated tauopathy. The neuron-to-neuron transmission of abnormal intracellular tau via prion-like mechanisms, as well as synaptic glutamatergic excitotoxicity of amyloid abnormalities, have been postulated as major factors for tissue progression. Although AD pathologic alterations seem to spread along interconnected neuronal systems beyond spatial proximity in the brain, there are limited neuroimaging approaches that are able to characterize the putative spreading pathways of preclinical and clinical AD. For instance, if we postulate that AD pathologic alterations progress from the medial temporal to association cortex, a sensitive method should be able to detect spatiotemporal relationship or dependency between pathologic deposits of those systems. In this study, we used a cross-sectional sample of cognitively normal elderly participants in the Harvard Aging Brain Study (HABS) in which group- and individual-level fingerprints of spreading are investigated using a hierarchical clustering approach. We postulated that the AD-related abnormality is sequentially nested in interconnected systems and, as such, is optimally detected with hierarchical clustering approaches. Moreover, by studying the hierarchical nature of tau and Aβ deposits, we can address a long-standing question about their distinctive cortical distribution as well as their large-scale spatial overlaps and interactions. In summary, we aimed to characterize the spatial distribution maps and hierarchical clustering organization of tau and Aβ deposits using multitracer PET data from the same study sample and provide group-level maps for individual staging of AD-related abnormalities.
Methods
Participants
We included 88 cognitively normal participants from the HABS (mean [SD] age, 76.2 [6.2] years; 39 [44%] men; mean education, 15.5 [2.8]) years. There were 27 amyloid-positive participants (distribution volume ratio [DVR] in frontal, temporoparietal, and retrosplenial regions >1.2). All individuals had a normal neurologic examination, a mean (SD) Mini-Mental State Examination score of 28.5 (normal, ≥27), a Clinical Dementia Rating scale score of 0, and a performance within 1.5 SD on age- and education-adjusted norms on a cognitive testing battery. None of the participants in the HABS had any notable medical or neuropsychiatric illnesses, history of drug or alcohol abuse or head trauma, or a family history of autosomal dominant AD. Participants previously diagnosed with a neurologic or psychiatric condition or with a score higher than 11 on the Geriatric Depression Scale were excluded. All participants in the HABS took part in the study using protocols and written informed consent procedures approved by the Partners Human Research Committee. Participants received financial compensation.
Magnetic Resonance Imaging Acquisition and Spatial Normalization
All participants underwent magnetic resonance imaging (MRI) of the whole head on a system (3-T Tim Trio; Siemens) that included a T1-weighted magnetization-prepared rapid gradient-echo scan using the following parameters: repetition time, 6400 milliseconds; echo time, 2.8 milliseconds; flip angle, 8°; inversion time, 900 milliseconds; and voxel size, 1.0 × 1.0 × 1.2 mm. We used SPM12 (Wellcome Department of Cognitive Neurology, University College London) running under Matlab, version 8.0 (Mathworks Inc) for imaging preprocessing and normalization of the anatomical T1-weighted MRI images.
PET Acquisition and Preprocessing Procedures
All participants had 2 PET imaging acquisitions: (1) a flortaucipir 18 T807 (18F-T807) or flortaucipir-PET that binds tau neurofibrillary tangles and neurites, and (2) a carbon 11 (11C)-labeled Pittsburgh Compound B, N-methyl 11C-2-(4-methylaminophenyl)-6-hydroxybenzothiazole (11C-PiB) PET that binds fibrillar Aβ plaques. The 18F-T807 PET was acquired from 80 to 100 minutes after a 9.0- to 11.0-mCi bolus injection in 4 × 5-minute frames in 3-dimensional mode (63 image planes, 15.2-cm axial field of view, 5.6-mm transaxial resolution, and 2.4-mm slice interval). The final intensity 18F-T807 maps were calculated using the standardized uptake value ratio (SUVR) (gray cerebellar reference). The 11C-PiB PET parameters were as follows: following a transmission scan, 10 to 15 mCi of 11C-PiB was injected intravenously as a bolus and followed immediately by a 60-minute dynamic PET scan (HR+ scanner; Siemens) in a 3-dimensional mode (63 image planes, 15.2-cm axial field of view, 5.6-mm transaxial resolution, and 2.4-mm slice interval; 69 frames: 12 frames of 15 seconds and 57 frames of 60 seconds). The PiB retention intensity was expressed as the DVR at each voxel and was calculated using the Logan graphical method (gray cerebellar reference). Corrections for normalization, dead time, random coincidences, scattered radiation, and attenuation were performed. The mean time that elapsed between the 2 PET scan sessions was 5.3 (range, 0-20) months. Finally, using SPM12, all PET data in native space were spatially normalized into Montreal Neurological Institute space using the normalized T1-weighted images.
Hierarchical Clustering
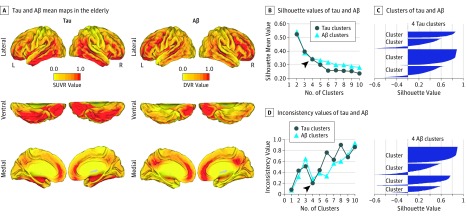
We used the 18F-T807 and 11C-PiB PET scans of 88 participants in HABS for the neuroimaging analysis. The 18F-T807 and 11C-PiB PET images of this sample have previously been studied in association with gray matter atrophy. Mean intensity maps of the 18F-T807 and 11C-PiB PET images are presented in Figure 1A for reference purposes regarding the raw spatial characteristics of the data with adaptation. We used these SUVR and DVR PET maps as inputs for the hierarchical clustering approach (Matlab linkage function with Euclidean distance and complete linkage). For computational efficiency, we first down-sampled all of the data from the PET images to 6-mm isotropic voxels. Second, we vectorized the individual images and concatenated them into a single voxel-by-participant matrix in each PET imaging modality. Before running the final hierarchical clustering approach, we randomly split the initial sample into a discovery or training data set (n = 30) and a final or testing data set (n = 58) with the holdout method. The training data set was used to identify the optimal number of clusters (k) in our data via silhouette and consistency analyses (Figure 1B-D). The final data set was used to determinate the spatial patterns of AD-related abnormalities as well as the individual features of intramodal and intermodal imaging (tau and Aβ). Silhouette analysis is a method that provides interpretation and graphical visualization of cluster partitions based on the dissimilarities between them. In our framework, the silhouette analysis helped to classify the number of voxels that lay inside and outside specific hierarchical clusters and also examine stability with increasing number of partitions within the same sample (Figure 1B and C). In addition, we used a clustering consistency method based on a sampling approach of the data described elsewhere. This analytical method provides an estimation of the consistency of the hierarchical clustering solution across multiple samples of the training data (leave-1-out strategy, 30 times) since the number of clusters is varied.
Figure 1. Tau and Amyloid-β (Aβ) Mean Maps and Hierarchical Clustering Approach.
A, The mean Aβ and tau intensity maps of the sample (adapted from Sepulcre et al). Color scales represent standardized uptake value ratio (SUVR) or distribution volume ratio (DVR) values from 0 to 1. B, Line graph between the mean silhouette values and the number of hierarchical clusters in the Aβ and tau data. C, Silhouette graph of a hierarchical clustering partition in k = 4 for tau and Aβ (positive values, good voxel classification; negative values, bad voxel classification). D, Line graph between the inconsistency values of the clustering consistency analysis and the number of hierarchical clusters in the tau and Aβ data. L indicates left; R, right. Arrowheads in parts B and D show the selection of the optimal number of clusters.
These complementary methods gave us converging results. After an initial drop, we found that the silhouette values greatly stabilized in the Aβ curve at k = 4 (light blue line in Figure 1B), and the tau curve reached that point between k = 4 to 6 (dark blue line in Figure 1B). In both cases, a k = 4 classified the majority of voxels in assigned clusters, leaving only a small proportion with bad classification values (negative silhouette values in Figure 1C). In Figure 1D, results of the clustering consistency method show that both tau (dark blue line) and Aβ (light blue line) display consistent and optimal cortical partitions at k = 4. Taking into account the outputs of both the silhouette and consistency analyses, we used k = 4 as a selection criterion for the number of clusters in our final hierarchical analysis. Based on the k = 4 partitions, we calculated the mean and total sum intensity signal of tau and Aβ cortical maps at the individual level. We used the mean intensity signal of tau and Aβ clusters of individuals to study associations within (intra) and between (inter) PET modalities. We used single and mean Pearson correlation coefficients, as well as scatter graphs and an association matrix, to study and visualize the interactions within and between PET modalities. We used Caret, version 5, software (PALS surface [PALS-B12], interpolated algorithm, and multifiducial mapping) for the visualization of the tau and Aβ clusters in cortical space.
Results
Tau and Amyloid-β Proteins Distribution
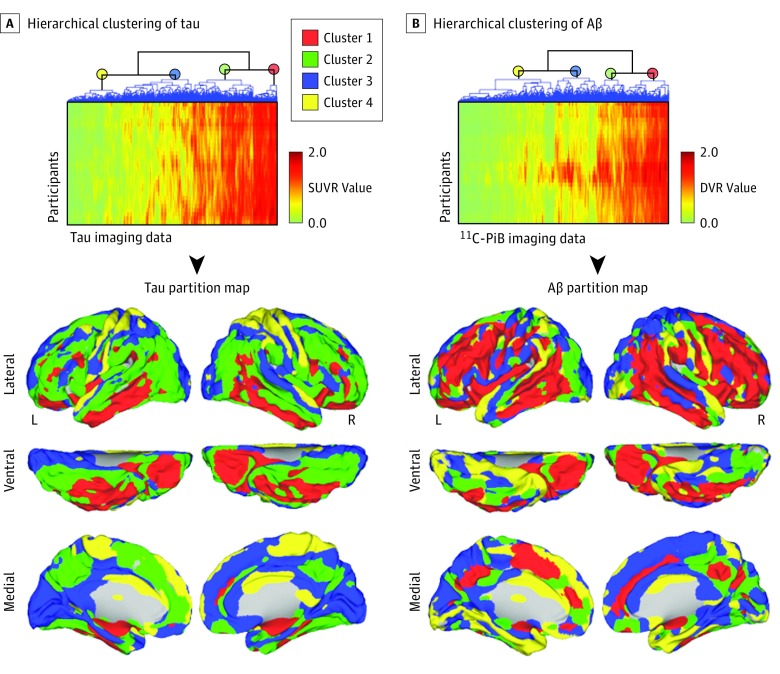
Two specific clusters of high tau intensity that nest together were identified: 1 including the medial temporal lobe, basolateral temporal cortex, and orbitofrontal cortex (Figure 2A, red), and the other involving large portions of the frontoparietal and lateral occipital areas (Figure 2A, green). In addition, we found 2 other clusters displaying less tau intensity and largely involving medial and visual regions (Figure 2A, blue) as well as primary somatomotor cortex (Figure 2A, yellow). For Aβ deposits, we identified 2 clusters located in distributed heteromodal areas, such as the lateral temporoparietal and frontal regions, in which 1 of the clusters (Figure 2B, red cluster) is surrounded by or in close proximity to the other (Figure 2B, green cluster). Two remaining Aβ clusters were found in an irregular distribution involving primary cortices, medial structures, and parahippocampal areas (Figure 2B, blue and yellow).
Figure 2. Tau and Amyloid-β (Aβ) Distribution and Assembly in Specific Brain Clusters.
A and B, Hierarchical clustering partitions in tau and Aβ data. Association matrices show participants’ voxel values of tau and Aβ positron emission tomography imaging data. Each row corresponds to an individual image in vectorized form. Color scales represent standardized uptake value ratio (SUVR) or distribution volume ratio (DVR) values from 0 to 2. Dendrograms of the hierarchical clustering partitions are displayed in the upper horizontal axes of the association matrices. 11C-PiB indicates carbon 11–labeled Pittsburgh Compound B; L, left; and R, right.
Spatial Features of Tau and Amyloid-β Clusters
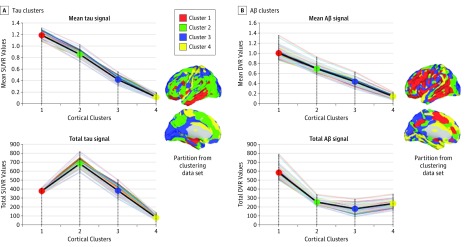
Tau and Aβ hierarchical clusters differed in intensity and size. The tau and Aβ clusters displayed characteristic patterns of mean and total signal within the topologic clusters. The 2 imaging modalities exhibited consecutive reductions of mean intensity from clusters 1 to 4 (Figure 3) but different patterns of the total amount of tau and Aβ. For instance, tau cluster 1 had the highest mean intensity, while tau cluster 2 showed the highest amount of total signal compared with the rest of the clusters. In other words, tau cluster 1 showed a relatively high density of signal due to its elevated intensity and smaller number of voxels compared with tau cluster 2. Tau cluster 2 showed less density but more total signal widely distributed across the cortical mantle. However, the spatial distribution in the Aβ modality demonstrated a more concordant pattern between the mean and total signal. Amyloid-β cluster 1 displayed both the highest intensity and the highest total signal (Figure 3). The total Aβ signal abruptly decreased after Aβ cluster 1 and plateaued in the rest of the clusters.
Figure 3. Spatial Features of Tau and Amyloid-β (Aβ) Clusters.
A and B, Average intensity and total signal of tau and Aβ from clusters 1 to 4 in the final data set of the study. Individual lines represent each participant of the sample, and the dark line indicates tau mean values in each cluster. Cortical maps were adapted from those in Figure 2. DVR indicates distribution volume ratio; SUVR, standardized uptake value ratio.
Tau and Amyloid-β Relationships in Association Cortex
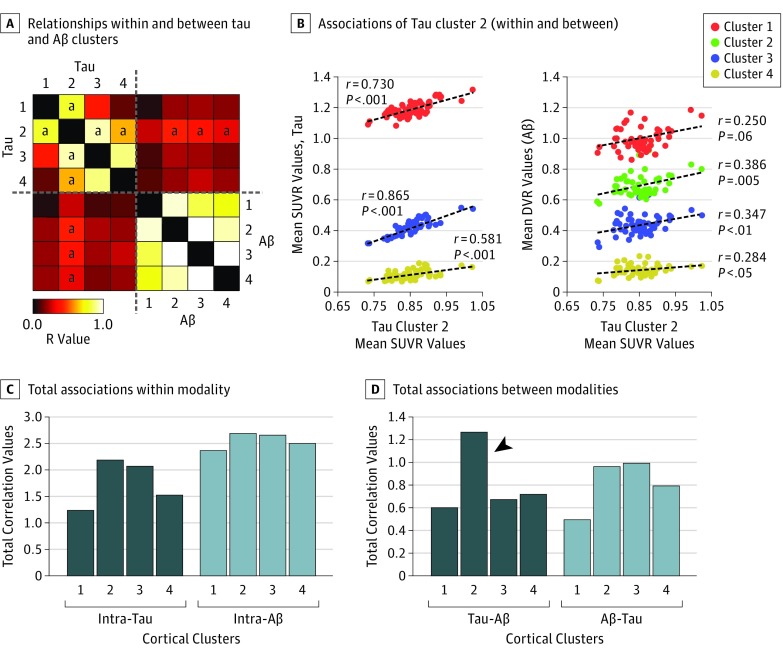
Analysis of relationships within and between tau and Aβ clusters revealed distinct intramodal and intermodal associations. First, we confirmed that each separate modality showed high associations between intramodal clusters, particularly among intra-Aβ clusters (hot colors in the association matrix of Figure 4A and light blue columns in Figure 4C). Second, we observed that only tau cluster 2 revealed significant associations both within and between modalities. Tau cluster 2 displayed significant correlations with all tau clusters and with Aβ clusters 2, 3, and 4 (Figure 4B).
Figure 4. Tau Pathology Relates to Amyloid-β (Aβ) Pathology .
A, Cross-correlation matrix containing the r values of correlations between the average intensities of all tau and Aβ cortical clusters across the individuals of the study sample. Color scale represents positive Pearson correlation coefficient values. B, Correlations of tau cluster 2 within and between modalities. C and D, Total sum of correlation values within and between modalities. The total sum of correlation values from the sample does not provide error bars. The arrowhead in panel D shows that tau cluster 2 reveals the highest degree of associations with all AB clusters. DVR indicates distribution volume ratio; SUVR, standardized uptake value ratio.
aStatistically significant correlations of tau cluster 2 at the P < .05 level.
Discussion
Several years before AD dementia manifests, abnormal accumulations of tau and Aβ insoluble proteins are visible in the temporal lobe and association cortex. Tau and Aβ deposits show some degree of spatial specificity as well as some overlap in convergent zones. However, we presently have little information about the spatial relationships between these 2 pathologic accumulations, particularly with respect to in vivo human brain data. In this study, we proposed a novel approach to comprehend the theoretically hierarchical spatial organization of tau and Aβ pathologic alterations. Using a hierarchical clustering analysis of multitracer PET data, including those obtained with a novel tau PET tracer, in a sample of elderly individuals, we identified high-resolution associations across neuronal systems, specifically, tau deposits in the medial temporal lobe, lateral temporal cortex, and orbitofrontal cortex that transversely expand to areas in the parietal and frontal lobes. Our in vivo findings show some overlap with the proposed Braak tau stages, although with a specific reorganization of spatial stages. We observed that Braak stages I and II (entorhinal cortex and hippocampus) and stages III to IV (amygdala, temporal lobe, and orbitofrontal cortex) converged into 1 cluster in our data, leaving the other main tau cluster of association cortex as the equivalent to Braak stages V to VI (isocortical areas).
The investigation of AD-related abnormalities at early stages is important in understanding the large-scale spreading mechanisms of the disease. In the past, it has been suggested that abnormal tau proteins accumulate along the cerebral tissue via transsynaptic or transneuronal connections, not only by proximity but also by distant synaptic connections. Our hierarchical approach describes this potential advance of tau accumulation. The medial temporal, basolateral temporal, and orbitofrontal cortex establish functional connections with areas of the so-called default mode network. The spatially sequential distribution of tau follows that long-distance connectivity pattern. The difference between the intensity and size of the tau cortical signal, in which tau cluster 2 has a lower mean but higher total signal than tau cluster 1, may also indicate a fingerprint of spatial progression between temporal areas and the association cortex. In contrast, Aβ deposits are already dispersed in widespread heteromodal cortex, and subsequent spatial projections primarily affect surrounding cortical areas—not a substantially different brain system. Therefore, the progression from local to distributed areas in the cortex seems more pronounced in tau than Aβ deposits, at least at the large-scale system level. Nevertheless, at very early stages of the Aβ deposits, in which individuals are classified as amyloid negative by current conventional neuroimaging cutoffs, local spatial associations between temporal, orbitofrontal, and amygdala areas have been found. These regions are part of a much larger cluster, namely, Aβ cluster 1, in the present study. Consequently, it is possible that Aβ cluster 1 is capturing more advanced stages of those detected by our previous graph theory–based approach, making the local to distributed transition of Aβ less noticeable.
An enigmatic topic in AD pathophysiology is whether tau and Aβ accumulation deposits are related or even dependent phenomena. Although the predominant deposition of each type of abnormal protein displays distinguishable locations in the cortex, their spatial distributions also show some degree of overlap in specific areas of the human brain, particularly in the association cortex. In fact, both characteristics—distinct spatial predominance and overlapping pathology—may advocate for plausible interactions between tau and Aβ deposits via concurrent local production or distant connectivity effects, respectively. We believe that the study of the spatial associations between tau and Aβ deposits can offer some important clues about the dependency of these potential relationships and predictive value for longitudinal progression. For instance, in our study, we found that tau deposits in a cluster encompassing large parts of the association cortex (tau cluster 2) showed significant correlations with many Aβ clusters. We did not find a single Aβ cluster that correlated with more than 1 tau cluster or any tau cluster other than tau cluster 2. Moreover, tau cluster 2 displayed a high degree of significant correlation with other tau clusters. In this sense, the combination of both of these findings—high associations within tau modality and between tau and Aβ—supports a prominent role of regionally specific tau deposits in the pathologic interactions and interdigitations with Aβ. These critical tau deposits largely correspond to the association cortex rather than to the initial medial temporal deposits represented in tau cluster 1 in our study. This finding may reinforce the idea that tau in the association cortex is essential to bond the appearance of focal tau in the temporal lobe and widespread Aβ elsewhere and, in some sense, that AD is a tau-centered abnormality with amyloid effects. However, although we found that tau in a specific association cortex cluster better explains widespread Aβ than the opposite, it does not imply any directionality assumption about the cerebral progression of the pathology. It could be possible that Aβ in different clusters induces tau deposits in 1 single cluster (corresponding to the association cortex) as a final product. Thus, in our opinion, the directionality question remains open and should be addressed using longitudinal study designs as soon as these data become available. Finally, we observed an interesting point about the correlations between tau cluster 2 and Aβ cluster 1. Although we expected a high correlation between tau cluster 2 and Aβ cluster 1 due to their manifest spatial overlap in the association cortex, our findings demonstrate the contrary. Tau cluster 2 and Aβ cluster 1 displayed the lowest correlation value among all of the tau cluster 2 interactions. We believe that this result may suggest a fingerprint of how AD-related pathologic changes spread in the brain. If tau and Aβ productions are spatially dependent, it is likely that they occur across axonally connected, but not colocalized, brain systems. A low correlation between tau and Aβ in the association cortex (tau cluster 2 and Aβ cluster 1) and a high correlation between tau cluster 2 and Aβ cluster 2 support this assumption. Future voxel-level approaches should focus on similar phenomena about asymmetric interactions between tau and Aβ pathology in the human cortex.
Limitations
Our study was a cross-sectional investigation of early stages of tau and Aβ in vivo cortical distributions. Thus, future longitudinal studies are needed to fully understand how these 2 hallmarks of AD dynamically relate and evolve.
Conclusions
In this study, we investigated the cortical distribution of early AD-related pathologic alterations by taking a particular neuroimaging perspective. We interpreted tau and Aβ deposits as patterns of abnormalities that follow hierarchical organized systems. As such, we used a hierarchical clustering approach and multitracer PET imaging to reveal potential spatial mechanisms of AD pathologic changes in the brains of elderly individuals. Our findings support the idea that tau is a major factor in the pathologic spreading of the disease. Moreover, our characterization of hierarchically organized maps of tau and Aβ may help in the near future to improve early individual staging and monitor the cognitive functional effect of tau accumulation on memory decline in the preclinical and clinical stages of AD.
References
- 1.Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb Cortex. 1991;1(1):103-116. [DOI] [PubMed] [Google Scholar]
- 2.Braak H, Braak E. Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol. 1991;1(3):213-216. [DOI] [PubMed] [Google Scholar]
- 3.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239-259. [DOI] [PubMed] [Google Scholar]
- 4.Hyman BT, Phelps CH, Beach TG, et al. . National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1(1):a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikonomovic MD, Klunk WE, Abrahamson EE, et al. . Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008;131(pt 6):1630-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430(7000):631-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogomori K, Kitamoto T, Tateishi J, Sato Y, Suetsugu M, Abe M. β-Protein amyloid is widely distributed in the central nervous system of patients with Alzheimer’s disease. Am J Pathol. 1989;134(2):243-251. [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh DM, Selkoe DJ. Oligomers on the brain: the emerging role of soluble protein aggregates in neurodegeneration. Protein Pept Lett. 2004;11(3):213-228. [DOI] [PubMed] [Google Scholar]
- 10.Chien DT, Bahri S, Szardenings AK, et al. . Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J Alzheimers Dis. 2013;34(2):457-468. [DOI] [PubMed] [Google Scholar]
- 11.Maruyama M, Shimada H, Suhara T, et al. . Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron. 2013;79(6):1094-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okamura N, Furumoto S, Harada R, et al. . Novel 18F-labeled arylquinoline derivatives for noninvasive imaging of tau pathology in Alzheimer disease. J Nucl Med. 2013;54(8):1420-1427. [DOI] [PubMed] [Google Scholar]
- 13.Villemagne VL, Furumoto S, Fodero-Tavoletti MT, et al. . In vivo evaluation of a novel tau imaging tracer for Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2014;41(5):816-826. [DOI] [PubMed] [Google Scholar]
- 14.Johnson K, Becker AJ, Sepulcre J, et al. . Tau PET imaging in aging and early Alzheimer’s disease. Ann Neurol. 2016;79(1):110-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho H, Choi JY, Hwang MS, et al. . In vivo cortical spreading pattern of tau and amyloid in the Alzheimer disease spectrum. Ann Neurol. 2016;80(2):247-258. [DOI] [PubMed] [Google Scholar]
- 16.Braak H, Del Tredici K, Schultz C, Braak E. Vulnerability of select neuronal types to Alzheimer’s disease. Ann N Y Acad Sci. 2000;924:53-61. [DOI] [PubMed] [Google Scholar]
- 17.Raj A, Kuceyeski A, Weiner M. A network diffusion model of disease progression in dementia. Neuron. 2012;73(6):1204-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villain N, Fouquet M, Baron JC, et al. . Sequential relationships between grey matter and white matter atrophy and brain metabolic abnormalities in early Alzheimer’s disease. Brain. 2010;133(11):3301-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Englund E, Brun A, Alling C. White matter changes in dementia of Alzheimer’s type: biochemical and neuropathological correlates. Brain. 1988;111(pt 6):1425-1439. [DOI] [PubMed] [Google Scholar]
- 20.Kuczynski B, Targan E, Madison C, et al. . White matter integrity and cortical metabolic associations in aging and dementia. Alzheimers Dement. 2010;6(1):54-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palop JJ, Chin J, Roberson ED, et al. . Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55(5):697-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palop JJ, Mucke L. Amyloid-β–induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat Neurosci. 2010;13(7):812-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sepulcre J, Sabuncu MR, Becker A, Sperling R, Johnson KA. In vivo characterization of the early states of the amyloid-beta network. Brain. 2013;136(pt 7):2239-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou J, Gennatas ED, Kramer JH, Miller BL, Seeley WW. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron. 2012;73(6):1216-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tosun D, Schuff N, Mathis CA, Jagust W, Weiner MW; Alzheimer’s Disease NeuroImaging Initiative . Spatial patterns of brain amyloid-β burden and atrophy rate associations in mild cognitive impairment. Brain. 2011;134(pt 4):1077-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teipel S, Grothe MJ, Zhou J, et al. . Measuring cortical connectivity in Alzheimer’s disease as a brain neural network pathology: toward clinical applications. J Int Neuropsychol Soc. 2016;22(2):138-163. [DOI] [PubMed] [Google Scholar]
- 28.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566-572. [DOI] [PubMed] [Google Scholar]
- 29.Morris JC, Edland S, Clark C, et al. . The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD)—part IV: rates of cognitive change in the longitudinal assessment of probable Alzheimer’s disease. Neurology. 1993;43(12):2457-2465. [DOI] [PubMed] [Google Scholar]
- 30.Becker JA, Hedden T, Carmasin J, et al. . Amyloid-β associated cortical thinning in clinically normal elderly. Ann Neurol. 2011;69(6):1032-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version In: Brink TL, ed. Clinical Gerontology: A Guide to Assessment and Intervention. New York: The Haworth Press, Inc; 1986:165-173. [Google Scholar]
- 32.Mathis CA, Wang Y, Holt DP, Huang GF, Debnath ML, Klunk WE. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem. 2003;46(13):2740-2754. [DOI] [PubMed] [Google Scholar]
- 33.Lopresti BJ, Klunk WE, Mathis CA, et al. . Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: a comparative analysis. J Nucl Med. 2005;46(12):1959-1972. [PubMed] [Google Scholar]
- 34.Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIBin a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67(3):446-452. [DOI] [PubMed] [Google Scholar]
- 35.Sepulcre J, Schultz AP, Sabuncu M, et al. . In vivo tau, amyloid, and gray matter profiles in the aging brain. J Neurosci. 2016;36(28):7364-7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rousseeuw PJ. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. J Comput Appl Math. 1987;20:53-65. [Google Scholar]
- 37.Yeo BT, Krienen FM, Sepulcre J, et al. . The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Essen DCA. A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28(3):635-662. [DOI] [PubMed] [Google Scholar]
- 39.Jack CR Jr, Knopman DS, Jagust WJ, et al. . Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson KA, Fox NC, Sperling RA, Klunk WE. Brain imaging in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(4):a006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sojkova J, Driscoll I, Iacono D, et al. . In vivo fibrillar β-amyloid detected using [11C]PiB positron emission tomography and neuropathologic assessment in older adults. Arch Neurol. 2011;68(2):232-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vlassenko AG, Mintun MA, Xiong C, et al. . Amyloid-Beta plaque growth in cognitively normal adults: longitudinal [11C]Pittsburgh compound B data. Ann Neurol. 2011;70(5):857-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schöll M, Lockhart SN, Schonhaut DR, et al. . PET imaging of tau deposition in the aging human brain. Neuron. 2016;89(5):971-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braak H, Braak E. Evolution of the neuropathology of Alzheimer’s disease. Acta Neurol Scand Suppl. 1996;165:3-12. [DOI] [PubMed] [Google Scholar]
- 45.Medina M, Avila J. The role of extracellular tau in the spreading of neurofibrillary pathology. Front Cell Neurosci. 2014;8:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jucker M, Walker LC. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501(7465):45-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frost B, Diamond MI. Prion-like mechanisms in neurodegenerative diseases. Nat Rev Neurosci. 2010;11(3):155-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100(1):253-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433-447. [DOI] [PubMed] [Google Scholar]
- 50.Sheline YI, Raichle ME. Resting state functional connectivity in preclinical Alzheimer’s disease. Biol Psychiatry. 2013;74(5):340-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thal DR, Rüb U, Orantes M, Braak H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12):1791-1800. [DOI] [PubMed] [Google Scholar]