This cross-sectional study assesses the functional burden of diabetic retinopathy across severity levels in the United States.
Key Points
Question
What is the association between worsening diabetic retinopathy and visual function?
Findings
This cross-sectional analysis of 1004 persons in the National Health and Nutrition Examination Surveys 2005-2008 who had diabetes and valid ocular and sociodemographic outcomes found that vision-related functional burden was significantly greater among those with severe nonproliferative diabetic retinopathy or proliferative diabetic retinopathy than among those with no retinopathy.
Meaning
The data suggest the importance of preventing severe forms of diabetic retinopathy to mitigate the vision-related functional burden among US adults with diabetes.
Abstract
Importance
Among adults with diabetes in the United States, severe forms of diabetic retinopathy (DR) are significantly associated with a greater vision-related functional burden.
Objective
To assess the functional burden of DR across severity levels in the United States.
Design, Setting, and Participants
This cross-sectional study was based on 1004 participants 40 years or older with diabetes and valid ocular and sociodemographic outcomes in the National Health and Nutrition Examination Surveys (NHANES) (2005-2006 and 2007-2008). Diabetic retinopathy was based on fundus photograph grading, using the Early Treatment Diabetic Retinopathy Study severity scale. The analysis was performed from October 15, 2016, to June 15, 2017.
Main Outcomes and Measures
Functional difficulties secondary to vision were assessed during a household questionnaire in which participants self-reported difficulty with reading, visuospatial tasks (ie, close-up work or finding things on a crowded shelf), mobility (ie, walking down steps, stairs, or curbs), and driving. The main outcome measure was vision-related functional burden, which was defined as present for individuals reporting moderate or greater difficulty in any of the aforementioned tasks.
Results
Of the 1004 persons with diabetes analyzed for this study (mean age, 65.7 years [95% CI, 64.0-67.3 years]; 51.1% male [95% CI, 47.1-55.2] and 48.9% female [95% CI, 44.8-52.9]), the prevalence was 72.3% for no retinopathy, 25.4% for mild and moderate nonproliferative diabetic retinopathy (NPDR), and 2.3% for severe NPDR or proliferative diabetic retinopathy (PDR). The prevalence of vision-related functional burden was 20.2% (95% CI, 16.3%-24.1%) for those with no retinopathy, 20.4% (95% CI, 15.3%-27.8%) for those with mild and moderate NPDR, and 48.5% (95% CI, 25.6%-71.5%) for those with severe NPDR or PDR (P = .02). In multivariable analysis, the odds of vision-related functional burden were significantly greater among those with severe NPDR or PDR relative to those with no retinopathy (adjusted odds ratio [aOR], 3.59; 95% CI, 1.29-10.05; P = .02). Those with severe NPDR or PDR did not have a statistically significant greater odds of vision-related functional burden than did those with mild or moderate NPDR (aOR, 2.70; 95% CI, 0.93-7.78; P = .07).
Conclusions and Relevance
Among US adults with diabetes, approximately half of those with severe NPDR or PDR had difficulty with at least one visual function task. Moreover, vision-related functional burden was significantly greater among those with severe NPDR or PDR than among those with no retinopathy. These data suggest the importance of preventing severe forms of DR to mitigate the vision-related functional burden among US adults with diabetes. Future studies should complement our study by assessing the association of worsening retinopathy with objectively measured functional outcomes.
Introduction
Diabetic retinopathy (DR) is a leading cause of blindness among working age adults in the United States and can significantly affect an individual’s quality of life and functional capabilities. The estimated number of Americans 40 years and older with a DR diagnosis is expected to triple between 2005 and 2050, from 5.5 million to 16 million individuals. Although many of these patients will have mild DR, they are at risk for progressing to the more severe forms of DR and experiencing a significant decline in productivity, mental health, and quality of life. In particular, Mazhar et al found that worsening severity of DR was significantly associated with worse general and vision-specific quality of life in a Latino-based population. However, these studies were conducted in clinic-based populations or population-based studies focused on particular ethnic/racial groups, limiting the generalizability of the results to the US population.
The National Health and Nutrition Examination Survey (NHANES), an ongoing cross-sectional study conducted during 2-year cycles to reflect a representative sample of the US civilian, noninstitutionalized population, previously evaluated the prevalence and severity of DR through the evaluation of retinal photographs. In addition, NHANES has ascertained self-reported functional difficulties related to vision. The primary aim of this study was to evaluate the prevalence of vision-related functional burden among individuals with varying severity levels of DR in the United States, while controlling for various ocular and sociodemographic factors.
Methods
The research adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all participants. The NHANES 2005-2006 and 2007-2008 protocols were approved by the National Center for Health Statistics Research Ethics Review Board.
Study Population
Data were obtained from the 2005-2006 and 2007-2008 rounds of NHANES, a rolling cross-sectional study performed in 2-year cycles on a new cohort of participants chosen to reflect a representative sample of the US civilian, noninstitutionalized population through a complex, multistage probability design. Survey participants were invited to undergo a comprehensive health examination in a mobile examination center, including visual acuity testing and blood testing. Retinal photographs were obtained for participants 40 years or older. Home interviews provided self-reported basic sociodemographic data, medical history, and vision-related functional difficulties.
Evaluation of Diabetes
Individuals were categorized as having underlying diabetes on the basis of an algorithm developed by Varma et al. Specifically, individuals were classified as having diabetes if they (1) self-reported a history of diabetes based on the question, “Have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?”; (2) had a glycosylated hemoglobin A1c value of at least 6.5%; (3) used antidiabetic drugs according to the medication inventory file; or (4) had a positive response to the question, “Are you now taking insulin?” or “Are you now taking diabetic pills to lower your blood sugar?”
Evaluation of DR
Diagnosis of DR was based on grading of fundus photographs by masked graders at the University of Wisconsin Ocular Epidemiologic Reading Center by using a nonmydriatic image of the retina in each eye from a Canon CR6-45NM ophthalmic digital imaging system and Canon EOS 10D digital camera (Canon Inc). A senior grader and/or a senior ophthalmologist resolved intergrader discrepancies regarding the level of DR. Severity of DR was based on the Early Treatment Diabetic Retinopathy Study protocol. Participants were then categorized based on the severity of DR of the worse eye as having (1) no retinopathy, (2) mild nonproliferative diabetic retinopathy (NPDR), (3) moderate NPDR, (4) severe NPDR, or (5) proliferative diabetic retinopathy (PDR). Those with mild or moderate NPDR were categorized together because of the similarities in the distribution of vision-related functional burden. Similarly, those with severe NPDR or PDR were categorized together because of the similarities in the distribution of vision-related functional burden (eFigures 1, 2, and 3 in the Supplement).
Evaluation of Difficulty With Visual Function
Self-reported functional difficulties secondary to vision problems were assessed over multiple categories, including (1) reading; (2) close-up work; (3) finding objects on a crowded shelf; (4) walking down steps, stairs, or curbs; (5) noticing objects to the side during ambulation; and (6) driving. For each category, participants described difficulty on a Likert scale: (1) no difficulty, (2) little difficulty, (3) moderate difficulty, (4) extreme difficulty, and (5) unable to do because of eyesight. Participants were categorized as having difficulty with a specific task if they reported moderate or extreme difficulty or if they noted that they were unable to do the activity because of their vision.
Evaluation of Vision
Visual acuity was measured for each eye as previously described. Presenting acuity for each eye was assessed using an autorefractor that contained built-in visual acuity charts (Nidek ARK-760). Presenting acuity was recorded as the smallest line for which 4 or more characters were read correctly. Presenting acuity was also analyzed as a continuous variable after conversion into logMAR units.
Statistical Analysis
Group differences in the prevalence of the aforementioned specific functional difficulties across DR severity levels were evaluated using χ2 analyses. Furthermore, we created a composite dichotomous variable, defined as vision-related functional burden, which was present when individuals had moderate difficulty or greater in any of the above 6 functional tasks. Multiple logistic regression analyses were used to evaluate the association between objectively measured levels of DR and vision-related functional burden, controlling for presenting visual acuity (better eye), age, sex, educational level (ie, less than college or any college), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, or other), and self-perceived health status (poor/fair or good/excellent). Furthermore, because diabetes can lead to diabetic macular edema (DME) and DR, we wanted to assess the relative effects of these conditions on visual function. Thus, the presence of DME was also controlled for in the multiple logistic regression models, in which the presence of DME was ascertained through the analysis of fundus photographs. In addition, because DME may have been underdiagnosed given that it was detected by only fundus photography and not optical coherence tomography, we adjusted for central visual acuity, which is frequently affected by the presence of DME. Multiple logistic regression analyses were also conducted to assess the association between the various levels of retinopathy and specific functional difficulties as judged by the responses to individual questions. Wald χ2 test 2-sided P<.05 was considered statistically significant. Data were analyzed using SAS statistical software (SAS Institute Inc).
Results
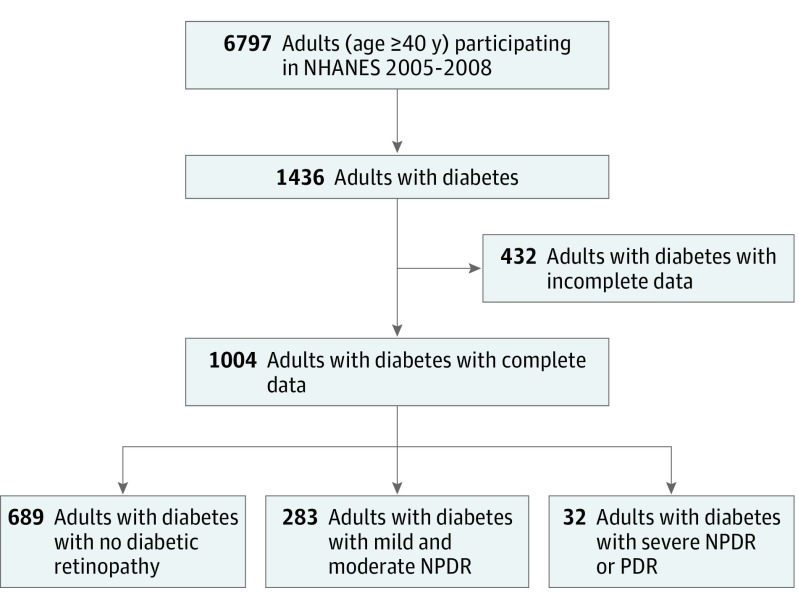
A total of 6797 persons 40 years or older participated in NHANES 2005-2006 and 2007-2008, and 1436 (21.1%) of these individuals had diabetes (mean [SD] age, 64.0 [11.2] years; 95% CI, 63.4-64.6 years; 712 [49.6%] male and 724 [50.4%] female). Among these individuals with diabetes, 1038 had complete visual function and retinal photography data, of whom 1004 had complete visual acuity and sociodemographic data. Among the individuals with complete data, the weighted prevalence was 72.3% (95% CI, 68.8%-75.9%) for no retinopathy, 25.4% (95% CI, 20.3%-30.4%) for mild and moderate NPDR, and 2.4% (95% CI, 0.9%-3.7%) for severe NPDR or PDR (Figure 1). Compared with persons with complete data, persons with incomplete data were older, had worse vision, and differed in their racial/ethnic distribution (Table 1).
Figure 1. Flowchart for Sampling of US Adults With Diabetic Retinopathy.
Diabetes was defined based on an algorithm developed by Varma et al. Specifically, individuals were classified as having diabetes if they self-reported a history of diabetes based on the question, “Have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?” Individuals were also categorized as having diabetes if they had a glycosylated hemoglobin A1c value of at least 6.5%, antidiabetic medication use according to the medication inventory file, or a positive response to the question “Are you now taking insulin?” or “Are you now taking diabetic pills to lower your blood sugar?” NHANES indicates National Health and Nutrition Examination Survey; NPDR, nonproliferative diabetic retinopathy; and PDR, proliferative diabetic retinopathy.
Table 1. Characteristics of Study Participants With Complete and Incomplete Data: NHANES 2005-2008a .
| Characteristic | Complete Data (n = 1004) |
Incomplete Data for All Adults With Diabetes (n = 432) |
P Valueb | |||
|---|---|---|---|---|---|---|
| No Retinopathy (n = 689) |
Mild and Moderate Retinopathy (n = 283) |
Severe NPDR or PDR (n = 32) |
All Adults With Diabetes (n = 1004) |
|||
| Male | 46.6 (41.6-51.5) | 56.1 (48.5-63.7) | 54.6 (32.5-76.7) | 49.2 (45.0-53.29) | 43.7 (37.5-49.9) | .15 |
| Age, y | 59.9 (58.9-61.0) | 61.4 (59.6-63.2) | 63.2 (59.1-67.4) | 60.4 (59.5-61.3) | 65.7 (64.0-67.3) | <.001 |
| Race/ethnicity | ||||||
| Non-Hispanic white | 67.6 (63.5-71.7) | 61.7 (54.9-68.5) | 48.7 (25.3-72.0) | 65.7 (62.2-69.14) | 56.8 (50.9-62.7) | .002 |
| Non-Hispanic black | 14.5 (12.1-16.9) | 22.9 (17.8-27.9) | 35.4 (15.9-55) | 17.1 (14.9-19.32) | 20.3 (16.6-24.1) | |
| Hispanic | 12.2 (9.8-14.5) | 13.5 (9.2-17.8) | 15.9 (5.1-26.7) | 12.6 (10.6-14.63) | 12.4 (9.6-15.3) | |
| Other | 5.8 (3-8.5) | 1.9 (0.1-3.8) | NA | 4.7 (2.6-6.73) | 10.4 (5.8-15.0) | |
| Health status | ||||||
| Fair or poor | 64.0 (59.4-68.6) | 53.1 (45.4-60.8) | 60.5 (38.7-82.3) | 61.2 (57.2-65.11) | 33.5 (27.5-39.5) | .001 |
| Excellent or good | 36.0 (31.4-40.6) | 46.9 (39.2-54.6) | 39.5 (17.7-61.3) | 38.8 (34.9-42.76) | 37.2 (31.3-43.0) | |
| Any college | 43.9 (38.9-48.9) | 41 (33.2-48.9) | 41.1 (18.4-63.9) | 43.1 (38.9-47.3) | 35.8 (29.6-42.0) | .06 |
| Presenting visual acuity, logMAR [Snellen equivalents] | 0.30 (0.30-0.30) [20/40 (20/40-20/40)] | 0.40 (0.30-0.40) [20/50 (20/40-20/50)] | 1.10 (0.80-1.30) [20/250 (20/125-20/400)] | 0.34 (0.30-0.37) [20/50 (20/40-20/50)] | 0.80 (0.70-0.90) [20/125 (20/100-20/160)] | <.001 |
| Diabetic macular edema | NA | 12.3 (7.9-16.7) | 30.8 (10.9-50.8) | 3.8 (2.6-5.1) | 0.2 (0-0.5) | <.001 |
Abbreviations: NA, not applicable; NHANES, National Health and Nutrition Examination Surveys; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy.
Data are presented as percentage (95% CI) of study participants unless otherwise indicated. Those with incomplete data were defined as US adults with diabetes and incomplete retinal imaging data, visual acuity data, or self-reported health status data.
Difference between adults with diabetes who have complete vs incomplete data.
DR and Vision-Related Functional Burden
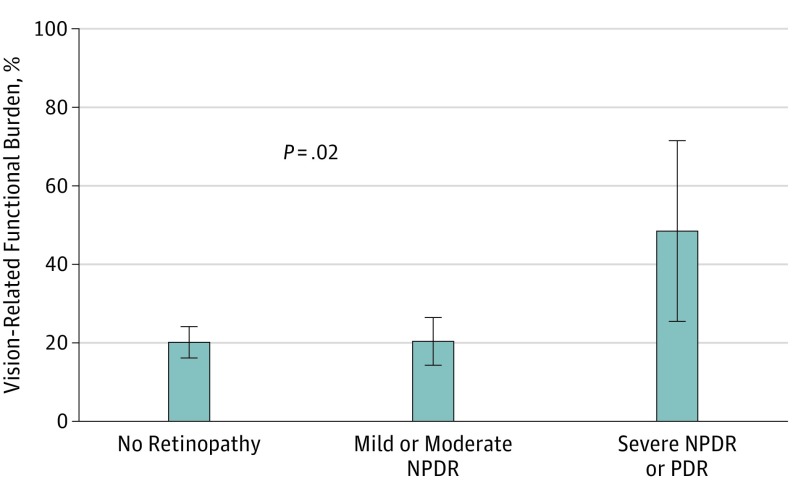
Among US adults 40 years or older with diabetes, the weighted prevalence of vision-related functional burden was 20.9% (95% CI, 17.6%-24.2%). The weighted prevalence of vision-related functional burden was 20.2% (95% CI, 16.3%-24.1%) for those with no retinopathy, 20.4% (95% CI, 14.2%-26.5%) for those with mild and moderate NPDR, and 48.5% (95% CI, 25.6%-71.5%) for those with severe NPDR or PDR (P = .02) (Figure 2). Multivariate logistic regression analysis showed that the odds of vision-related functional burden were significantly higher (adjusted odds ratio [aOR], 3.59; 95% CI, 1.29-10.05; P = .02) among those with severe NPDR or PDR than among those with no retinopathy. There was no significant difference in vision-related functional burden between those with mild or moderate DR compared with those without retinopathy (aOR, 0.99; 95% CI, 0.61-1.63; P = .98) (Table 2). These results did not change after controlling for presenting visual acuity or DME (Table 2). Male sex was associated with greater odds of vision-related functional burden compared with their female counterparts (aOR, 2.16; 95% CI, 1.40-3.34; P < .001), whereas those with better self-perceived health status had lower odds compared with those with poor self-perceived health status (aOR, 0.50; 95% CI, 0.32-0.77; P < .001) (Table 2).
Figure 2. Vision-Related Functional Burden Among US Adults With Varying Levels of Diabetic Retinopathy: National Health and Nutrition Examination Survey, 2005-2008 .
Error bars indicate 95% CIs. P value represents comparison across the 3 groups (severe NPDR or PDR vs mild and moderate NPDR vs no retinopathy). NPDR indicates nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy.
Table 2. Factors Associated With Visual Function Difficulty Among 1004 US Adults With Diabetes and Complete Retinal Imaging Data in the NHANES 2005-2006 and 2007-2008 Panels.
| Characteristic | aOR of Vision-Related Functional Burdena (95% CI) |
P Value |
|---|---|---|
| Diabetic retinopathy | ||
| None | 1 [Reference] | NA |
| Mild and moderate NPDR | 0.99 (0.61-1.63) | .98 |
| Severe NPDR or PDR | 3.59 (1.29-10.05) | .02 |
| Sex | ||
| Female | 1 [Reference] | NA |
| Male | 2.16 (1.40-3.34) | <.001 |
| Age | 0.98 (0.96-1.00) | .02 |
| Race/ethnicity | ||
| Non-Hispanic white | 1 [Reference] | NA |
| Non-Hispanic black | 0.76 (0.48-1.20) | .24 |
| Hispanic | 0.97 (0.57-1.64) | .90 |
| Other | 0.29 (0.08-1.09) | .07 |
| Self-reported health status | ||
| Fair or poor | 1 [Reference] | NA |
| Excellent or good | 0.50 (0.32-0.77) | <.001 |
| Educational level | ||
| No college | 1 [Reference] | NA |
| Any college | 0.78 (0.50 -1.23) | .28 |
| Presenting visual acuity | 1.36 (0.96-1.92) | .09 |
Abbreviations: aOR, adjusted odds ratio; NA, not applicable; NHANES, National Health and Nutrition Examination Survey; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy.
Vision-related functional burden defined as having moderate or greater difficulty with at least one of the following tasks because of vision: (1) reading; (2) close-up work; (3) finding objects on a crowded shelf; (4) walking down steps, stairs, or curbs; (5) noticing objects to the side during ambulation; and (6) driving. There were no significant changes in the model when the presence of diabetic macular edema was included. Given the collinear association between visual acuity and diabetic macular edema (ie, diabetic macular edema commonly leads to decline in visual acuity), the presence of diabetic macular edema was excluded from the above regression model.
In a separate multiple logistic regression model, in which we excluded those with no retinopathy, those with severe NPDR or PDR did not have a statistically significant greater vision-related functional burden compared with those with mild or moderate NPDR (aOR, 2.70; 95% CI, 0.93-7.78; P = .07).
DR and Specific Vision-Related Functional Difficulties
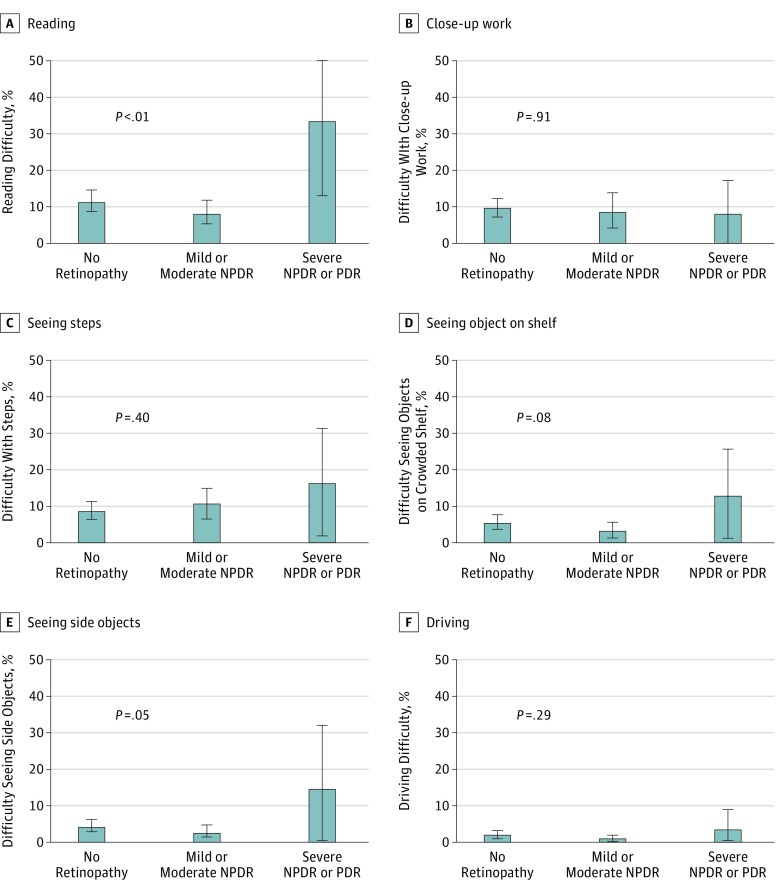
Among all US adults with diabetes, the weighted prevalence of vision-related difficulty was 11.4% (95% CI, 9.0%-13.8%) for reading, 9.7% (95% CI, 7.2%-12.1%) for close-up work, and 5.4% (95% CI, 3.7%-7.1%) for finding objects on a crowded shelf. In addition, among all US adults with diabetes, the weighted prevalence of vision-related difficulty was 9.6% (95% CI, 7.3%-11.9%) for walking down steps, stairs, or curbs; 4.3% (95% CI, 2.8%-5.7%) for noticing objects to the side during ambulation; and 1.8% (95% CI, 0.8%-2.8%) for driving.
Significant vision-related difficulty with specific tasks was more frequent in those with NPDR or PDR (33.3% for reading and 14.5% for noticing objects to the side) compared with those with mild and moderate NPDR (8.5% for reading and 2.8% for noticing objects to the side) or no retinopathy (11.7% for reading and 4.4% for noticing objects to the side) (P = .003 for reading and P = .05 for noticing objects to the side) but not with regard to close-up work; seeing objects on a crowded shelf; walking down steps, stairs, or curbs; or driving (Figure 3). However, when we controlled for potential confounders, there was no statistically significant difference in the individual vision-related functional difficulties across the 3 groups. In multivariable regression analysis, those with severe NPDR or PDR tended to have a greater reading difficulty compared with those with no retinopathy (aOR, 2.68; 95% CI, 0.87-8.3; P = .09). Those with severe NPDR or PDR also tended to have greater difficulty noticing objects to the side compared with those with no retinopathy (aOR, 2.74; 95% CI, 0.52-14.5; P = .24) (eTable in the Supplement).
Figure 3. Vision-Related Functional Difficulties by Specific Activities Among US Adults With Varying Levels of Diabetic Retinopathy: National Health and Nutrition Examination Survey, 2005-2008.
Error bars indicate 95% CIs. NPDR indicates nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy. P values represent comparison across the 3 groups (severe NPDR or PDR vs mild and moderate NPDR vs no retinopathy).
Discussion
Approximately half of US adults 40 years or older with severe NPDR or PDR reported difficulty with 1 or more visual function task, possibly affecting their daily work or activities; these patients reported significantly greater vision-related functional burden than those with no retinopathy, even after adjusting for visual acuity and other confounding variables. These results reinforce the importance of preventing severe forms of DR.
In its severe forms, DR can lead to visually devastating consequences resulting from complications such as tractional retinal detachments, vitreous hemorrhage, macular ischemia, or neovascular glaucoma. Such complications lead to a decreased quality of life and a decline in mobility. Our study found that those with severe NPDR or PDR had significantly greater levels of vision-related functional burden compared with those with no DR. Specifically, we found that those with severe NPDR or PDR had a 4-fold greater vision-related functional burden than did those with no DR, even after controlling for central visual acuity or DME. These results suggest that the greater vision-related functional burden among those with severe NPDR or PDR is likely secondary to changes in the peripheral retina, leading to visual field loss, dyschromatopsia, nyctalopia, or reduced contrast sensitivity. In addition, among those with peripheral DR, peripheral retinal changes can manifest in the forms of tractional retinal detachments, vitreous hemorrhage, or destruction of retinal tissue after panretinal photocoagulation (PRP).
The influence of worsening DR on quality of life has been reported by Mazhar et al in a Latino-based population in the United States, in which DR progression led to significant decline in driving difficulty and the composite quality-of-life measure based on the 25-item National Eye Institute Visual Function Questionnaire and the Medical Outcomes Study 12-Item Short-Form Health Survey scores. In particular, that study noted that an important threshold for the worsening of quality of life was when patients developed DR worse than moderate NPDR. Similarly, Coyne et al conducted a focus study group and found that when patients had moderate and severe NPDR or PDR, they frequently noted loss of independence and mobility. Our study adds to these findings by using a nationally representative population and by controlling for a multitude of confounding factors, including central visual acuity and DME. However, in contrast to the other 2 studies, we did not find that moderate NPDR was associated with a decline in visual function. Possible explanations for this could be secondary to the variance surrounding the grading protocol and the image quality of the retinal photographs across the various study centers; thus, those with moderate DR in other studies may actually have had severe DR. Nevertheless, given the influence of severe NPDR or PDR on visual function, there is a public health importance to slow and prevent the progression of DR, especially before conversion to the proliferative stage with the need for invasive procedures, such as PRP, intravitreal anti–vascular endothelial growth factor (VEGF) injections, and vitrectomy.
During the early stages of DR, available management options include lifestyle changes and systemic medical interventions to control hypertension, hyperglycemia, and hyperlipidemia. When DR progresses to visually threatening stages, such as very severe NPDR or PDR, PRP has been the standard of care, with vitrectomy as a surgical adjunct when PRP alone is inadequate or not feasible because of vitreous hemorrhage. The disadvantage of PRP, however, is that it is associated with nyctalopia, visual field loss, loss of color vision, and reduced contrast sensitivity. An alternative to PRP is a pharmacologic approach: intravitreal anti-VEGF injections, which may lead not only to the prevention of worsening DR severity levels but also to clinically meaningful improvements in DR severity levels. The improvements in DR severity levels may, in turn, lead to improvements in central visual acuity and contrast sensitivity. However, the disadvantage of intravitreal anti-VEGF injections is the need for adherence to monthly visits at least in the first year and not being cost-effective in the United States in the absence of simultaneous vision-impairing DME with the PDR. Taken together, these studies show the association between anatomical benefits of anti-VEGF injections and the potential influence of improving DR severity levels to maintain or improve visual function among those with diabetes in the United States. Future research should be conducted in this area to better understand how DR regression achieved by medical therapy may affect an individual’s quality of life and a variety of peripheral visual function outputs. In particular, such an approach should be systematically coordinated with recommended screening algorithms for patients with diabetes and known DR progression rates by severity levels.
Limitations
Our study has several limitations. Many individuals with diabetes did not have complete ocular and sociodemographic data. Those with incomplete data were more likely to have worse visual acuity, which may have led us to the underestimation of the true visual function burden of those with DR. In addition, fundus photographs were based on 2-field photography rather than wide-field photography, potentially contributing to a misclassification bias and an underestimation of the burden of severe DR and its associated visual function difficulty in the United States. Furthermore, critics may argue that we were unable to adequately control for other ocular pathologic conditions that may have affected visual function. For instance, because DME was not assessed by optical coherence tomography, it is possible that we inadequately controlled for the association of DME with visual function. A further limitation of the study was the small number of individuals with severe NPDR and PDR, restricting our ability to evaluate the influence of these retinopathy levels on specific functional tasks in our multivariable regression models. Finally, our outcomes were based on subjective outcomes, which may not correlate with what is seen objectively. Future studies should aim to complement our study by enrolling a greater number of patients with severe NPDR and PDR and studying the association of DR with objectively measured outcomes, such as physical balance, falls, and reading speed.
Conclusions
Half of US adults 40 years or older with severe NPDR or PDR reported difficulty with 1 or more visual function task that possibly affected their daily work and activities. These patients reported a significantly greater vision-related functional burden compared with those no DR, even after adjusting for visual acuity. Ophthalmologists should take a comprehensive approach in not only preventing severe levels of DR but also regressing severe levels of DR through the promotion of lifestyle, systemic medical, and focused retinal interventions.
eFigure 1. Vision-Related Functional Burden Among US Adults With Diabetes Across Specific Levels of Diabetic Retinopathy: NHANES 2005-2008 (P = .06)
eFigure 2. Vision-Related Functional Difficulties for Specific Activities Among US Adults With Diabetes Across Specific Levels of Diabetic Retinopathy: NHANES 2005-2008
eFigure 3. Detailed Vision-Related Functional Difficulties for Specific Activities Among US Adults With Diabetes Across Levels of Diabetic Retinopathy: NHANES 2005-2008
eTable. Factors Associated With Vision-Related Functional Difficulties for Specific Activities Among US Adults With Diabetes: NHANES 2005-2008
References
- 1.Centers for Disease Control and Prevention Vision Health Initiative (VHI) Report: Projection of Diabetic Retinopathy and Other Major Eye Diseases among People with Diabetes Mellitus United States, 2005-2050. Atlanta, GA: Centers for Disease Control and Prevention; 2015. [Google Scholar]
- 2.Coyne KS, Margolis MK, Kennedy-Martin T, et al. . The impact of diabetic retinopathy: perspectives from patient focus groups. Fam Pract. 2004;21(4):447-453. [DOI] [PubMed] [Google Scholar]
- 3.Mazhar K, Varma R, Choudhury F, McKean-Cowdin R, Shtir CJ, Azen SP; Los Angeles Latino Eye Study Group . Severity of diabetic retinopathy and health-related quality of life: the Los Angeles Latino Eye Study. Ophthalmology. 2011;118(4):649-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma S, Oliver-Fernandez A, Liu W, Buchholz P, Walt J. The impact of diabetic retinopathy on health-related quality of life. Curr Opin Ophthalmol. 2005;16(3):155-159. [DOI] [PubMed] [Google Scholar]
- 5.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 6.Varma R, Bressler NM, Doan QV, et al. . Prevalence of and risk factors for diabetic macular edema in the United States. JAMA Ophthalmol. 2014;132(11):1334-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention NHANES Digital Grading Protocol. Atlanta, GA: Centers for Disease Control and Prevention; 2005. [Google Scholar]
- 8.Willis JR, Jefferys JL, Vitale S, Ramulu PY. Visual impairment, uncorrected refractive error, and accelerometer-defined physical activity in the United States. Arch Ophthalmol. 2012;130(3):329-335. [DOI] [PubMed] [Google Scholar]
- 9.SAS [computer program]. Cary, NC; SAS Institute Inc; 2011.
- 10.American Academy of Ophthalmology Preferred Practice Patterns: Diabetic Retinopathy. San Francisco, CA: American Academy of Ophthalmology; 2014. [Google Scholar]
- 11.Henricsson M, Heijl A. Visual fields at different stages of diabetic retinopathy. Acta Ophthalmol (Copenh). 1994;72(5):560-569. [DOI] [PubMed] [Google Scholar]
- 12.Pahor D. Reduction of retinal light sensitivity in diabetic patients [in German]. Klin Monbl Augenheilkd. 2003;220(12):868-872. [DOI] [PubMed] [Google Scholar]
- 13.Chaturvedi N, Sjolie AK, Stephenson JM, et al. ; The EUCLID Study Group. . Effect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes: EURODIAB Controlled Trial of Lisinopril in Insulin-Dependent Diabetes Mellitus. Lancet. 1998;351(9095):28-31. [DOI] [PubMed] [Google Scholar]
- 14.Nathan DM, Genuth S, Lachin J, et al. ; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. [DOI] [PubMed] [Google Scholar]
- 15.Klein R, Klein BE, Moss SE. The Wisconsin epidemiological study of diabetic retinopathy: a review. Diabetes Metab Rev. 1989;5(7):559-570. [DOI] [PubMed] [Google Scholar]
- 16.Park YG, Roh YJ New diagnostic and therapeutic approaches for preventing the progression of diabetic retinopathy. J Diabetes Res 2016;2016:1753584. [DOI] [PMC free article] [PubMed]
- 17.Sjølie AK, Klein R, Porta M, et al. ; DIRECT Programme Study Group . Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): a randomised placebo-controlled trial. Lancet. 2008;372(9647):1385-1393. [DOI] [PubMed] [Google Scholar]
- 18.Fong DS, Girach A, Boney A. Visual side effects of successful scatter laser photocoagulation surgery for proliferative diabetic retinopathy: a literature review. Retina. 2007;27(7):816-824. [DOI] [PubMed] [Google Scholar]
- 19.Bressler SB, Qin H, Melia M, et al. ; Diabetic Retinopathy Clinical Research Network . Exploratory analysis of the effect of intravitreal ranibizumab or triamcinolone on worsening of diabetic retinopathy in a randomized clinical trial. JAMA Ophthalmol. 2013;131(8):1033-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ip MS, Domalpally A, Sun JK, Ehrlich JS. Long-term effects of therapy with ranibizumab on diabetic retinopathy severity and baseline risk factors for worsening retinopathy. Ophthalmology. 2015;122(2):367-374. [DOI] [PubMed] [Google Scholar]
- 21.Ip MS, Zhang J, Ehrlich JS. The clinical importance of changes in diabetic retinopathy severity score. Ophthalmology. 2017;124(5):596-603. [DOI] [PubMed] [Google Scholar]
- 22.Ehrlich JS, Zhang J. Are improvements in diabetic retinopathy severity clinically meaningful? insights from studies of intravitreal ranibizumab (RBZ) in patients with diabetic macular edema. Invest Ophthalmol Vis Sci. 2014;55:1767. [Google Scholar]
- 23.Hutton DW, Stein JD, Bressler NM, Jampol LM, Browning D, Glassman AR; Diabetic Retinopathy Clinical Research Network . Cost-effectiveness of intravitreous ranibizumab compared with panretinal photocoagulation for proliferative diabetic retinopathy: secondary analysis from a Diabetic Retinopathy Clinical Research Network randomized clinical trial. JAMA Ophthalmol. 2017;135(6):576-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gross JG, Glassman AR, Jampol LM, et al. ; Writing Committee for the Diabetic Retinopathy Clinical Research Network . Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314(20):2137-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nathan DM, Bebu I, Hainsworth D, et al. ; DCCT/EDIC Research Group . Frequency of evidence-based screening for retinopathy in type 1 diabetes. N Engl J Med. 2017;376(16):1507-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention NHANES: Anthropometry and Physical Activity Monitor Procedures Manual. Atlanta, GA: Centers for Disease Prevention and Control; 2005. [Google Scholar]
- 27.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181-188. [DOI] [PubMed] [Google Scholar]
- 28.Loprinzi PD, Brodowicz GR, Sengupta S, Solomon SD, Ramulu PY. Accelerometer-assessed physical activity and diabetic retinopathy in the United States. JAMA Ophthalmol. 2014;132(8):1017-1019. [DOI] [PubMed] [Google Scholar]
- 29.Praidou A, Harris M, Niakas D, Labiris G. Physical activity and its correlation to diabetic retinopathy. J Diabetes Complications. 2017;31(2):456-461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Vision-Related Functional Burden Among US Adults With Diabetes Across Specific Levels of Diabetic Retinopathy: NHANES 2005-2008 (P = .06)
eFigure 2. Vision-Related Functional Difficulties for Specific Activities Among US Adults With Diabetes Across Specific Levels of Diabetic Retinopathy: NHANES 2005-2008
eFigure 3. Detailed Vision-Related Functional Difficulties for Specific Activities Among US Adults With Diabetes Across Levels of Diabetic Retinopathy: NHANES 2005-2008
eTable. Factors Associated With Vision-Related Functional Difficulties for Specific Activities Among US Adults With Diabetes: NHANES 2005-2008