Key Points
Question
What is the association between maternal preeclampsia and retinopathy of prematurity (ROP) in infants?
Findings
In this cohort study, preeclampsia was associated with a 6.1-fold increased risk of infants developing ROP. In the preterm cohort with very low birth weights, preeclampsia was inversely associated with the development of all ROP.
Meaning
These results may add clarity to the association between preeclampsia and ROP and are consistent with the current understanding of the pathophysiology of these diseases.
Abstract
Importance
Studies report conflicting associations between preeclampsia and retinopathy of prematurity (ROP). This study provides explanations for the discrepancies to clarify the relationship between preeclampsia and ROP.
Objective
To evaluate the association of maternal preeclampsia and risk of ROP among infants in an unrestricted birth cohort and a restricted subcohort of preterm, very low birth weight (P-VLBW) infants.
Design, Setting, and Participants
A retrospective review of 290 992 live births within the Intermountain Healthcare System in Utah from January 1, 2001, through December 31, 2010, was performed. Generalized estimating equations for logistic regressions with covariate adjustment were applied to relate ROP to preeclampsia among the full cohort and in a subcohort of P-VLBW infants born at younger than 31 weeks’ gestation and weighing less than 1500 g.
Main Outcomes and Measures
The occurrence of ROP was related to maternal preeclampsia in the full cohort and in a subcohort of P-VLBW infants.
Results
In the full cohort, 51% of the infants were male and the mean (SD) gestational age was 38.38 (1.87) weeks. In the P-VLBW cohort, 55% were male and the mean (SD) gestational age was 26.87 (2.40) weeks. In the full cohort, preeclampsia was associated with an increased risk of all ROP (adjusted odds ratio [aOR], 6.07; 95% CI, 4.72–7.79; P < .001), severe ROP (aOR, 5.21; 95% CI, 3.44–7.91; P < .001), infant death (aOR, 1.66; 95% CI, 1.16–2.38; P = .006), and giving birth to a P-VLBW infant (aOR, 7.74; 95% CI, 6.92–8.67; P < .001). In the P-VLBW subcohort, preeclampsia was inversely associated with the development of all ROP (aOR, 0.62; 95% CI, 0.46–0.85; P = .003), severe ROP (aOR, 0.62; 95% CI, 0.36–1.06; P = .08), and infant death (aOR, 0.19; 95% CI, 0.11-0.32; P < .001).
Conclusions and Relevance
Preeclampsia was associated with an increased risk of developing ROP among an unrestricted cohort but with a reduced risk of ROP among a restricted subcohort of P-VLBW infants. Although the conflicting associations in the full and P-VLBW cohorts may reflect true differences, the association of a reduced risk of ROP among the P-VLBW subcohort also may reflect biases from restricting the cohort to prematurity, because prematurity is an outcome of preeclampsia.
This cohort study examines the association between maternal preeclampsia and the risk for infants to develop retinopathy of prematurity.
Introduction
Maternal preeclampsia is estimated to occur in 4% to 18% of live births worldwide and increases morbidity and mortality rates among mothers and infants.1 Evidence of circulating levels of placental antiangiogenic factors supports the theory that interference with angiogenesis delays fetal organ vascular development and thereby contributes to infant growth restriction.2,3 Nonetheless, clinical reports are controversial, with some linking maternal preeclampsia to increased infant survival4,5 and others to increased death.6,7,8,9
Conflicting findings concerning the effects of preeclampsia also exist for retinopathy of prematurity (ROP), a leading cause of childhood blindness. Retinopathy of prematurity manifests initially with delayed physiological retinal vascular development followed by aberrant vasoproliferation and is highly correlated with extreme prematurity and poor postnatal growth.10,11 These observations align with the premise that circulating antiangiogenic factors in preeclampsia interfere with retinal vascular development and contribute to later pathology. Some studies report that maternal preeclampsia is associated with an increased risk of infants developing ROP,12,13,14,15,16 others report a seemingly protective effect of preeclampsia on ROP,17,18,19 and a recent meta-analysis concluded that there was no association.20 Although their findings differed, all of these studies analyzed the association of preeclampsia with ROP in cohorts restricted to preterm infants. Because preeclampsia increases the risk of preterm births and preterm births are closely linked to ROP, restriction of the infant cohort to preterm infants removes a path whereby preeclampsia may affect ROP and also creates a risk of selection bias that can result when an analytic cohort is restricted based on an outcome of the exposure.
In this report, our goal was to address the conflicting literature by performing 2 parallel sets of analyses to estimate the effect of preeclampsia on the incidence and severity of ROP. The first set of analyses was performed in a complete birth cohort and addressed the overall, or “total,” effect of preeclampsia on ROP, irrespective of the role of prematurity or low birth weight. Evaluating the total effect avoids the risk of introducing selection bias and addresses the clinically relevant question of what the overall association between preeclampsia and ROP risk is. The second set of analyses was performed in a restricted subcohort that was limited to include preterm and very low-birth-weight (P-VLBW) infants, who are known to have a greater risk for developing ROP based on the literature. Because preterm births are an outcome of preeclampsia, restricting analyses to a P-VLBW subcohort, as has traditionally been done in the literature, can be viewed as an attempt to estimate the “direct” effect of preeclampsia on ROP within this subcohort that is not mediated by preterm births, but it leads to increased risk of selection bias.
Preterm infants who die before their first ROP screening examination are often excluded from analyses. Infants at risk of experiencing an early death might also have higher risk of developing ROP should they have lived, and their exclusion can introduce bias in the analyses. To address the effects of infant death, we determined the percentage of infants who died in both groups before receiving the first ROP examination and evaluated the composite outcomes of ROP or death in the complete and P-VLBW cohorts.
Methods
Institutional review board/ethics committee approval was obtained from the University of Utah, and as this was a retrospective electronic medical record review, informed consent was waived. We analyzed all live births within the Intermountain Healthcare System (IH) in Utah from 2001 to 2010. The IH is the largest health care system in the intermountain west and has an extensive electronic medical record system. The IH operates 21 hospitals where infant deliveries occur and one tertiary children’s hospital for care throughout Utah and Idaho. Administrative and clinical data from mothers and infants were linked for prenatal visits, labor and delivery encounters, and any subsequent transfer visits within IH, thus capturing data from all infants, including those who transferred among different IH hospitals to receive different levels of neonatal care. We then defined a subcohort of infants younger than 31 weeks’ gestational age and smaller than 1500 g as P-VLBW in accordance with other studies.21 These criteria identify a group of at-risk infants who are currently being screened for ROP in the United States. All previous studies assessing the risk of ROP in preeclampsia restricted their analyses to subcohorts with similar characteristics to this group.12,13,14,15,16,17,18,19 We defined the P-VLBW subcohort to simulate the effect of restricting the analytic cohort similarly to previous studies.
Infants with ROP were identified by International Classification of Diseases, Ninth Revision (ICD-9) codes for ROP. Severe ROP was defined as ROP that required laser treatment, cryotherapy, or incisional surgery. Intravitreal antiangiogenic treatments were not used for ROP during this time. Death before the first ROP examination was recorded and included in the analyses. Preeclampsia was identified by ICD-9 codes. Maternal factors recorded from the patient records included maternal age, multiple gestations, preeclampsia, the infant’s sex, and self-reported use of alcohol, illicit drugs, and/or tobacco.
Statistical Methods
We evaluated the total and direct effects of preeclampsia on the following outcomes: (1) all ROP, which included severe and nonsevere ROP and was treated as the primary ROP end point, (2) severe ROP, (3) death prior to discharge, (4) the composite of severe ROP or death before the first ROP examination, and (5) the composite of all ROP or death before the first ROP examination. The total effects of preeclampsia were estimated using generalized estimating equations (GEE) to fit logistic regression models in the full study cohort to relate each outcome to preeclampsia while controlling for the following factors as covariates: maternal age, maternal race/ethnicity, indicator variable for multiple gestations, the infant’s sex, maternal alcohol use during pregnancy, substance abuse during pregnancy, and tobacco exposure during pregnancy. The direct effects of preeclampsia on the same outcomes given preterm births were estimated first by repeating the same GEE/logistic regressions within the restricted P-VLBW subcohort. We then obtained an additional set of direct effect estimates by repeating the GEE/logistic regressions after both restricting to the P-VLBW subcohort and including birth weight and gestational age at birth as additional covariates. Our presentation of these analyses is accompanied by detailed assessments of the risk that the results are subject to selection bias resulting from the restriction to preterm births, which are an outcome of preeclampsia. A separate GEE/logistic regression model using the same covariates described the association of preeclampsia with the incidence of preterm birth or very low birth weight. We conducted sensitivity analyses to address the sensitivity of our results to an uncontrolled confounding factor using the method of VanderWeele and Arah.22
The logistic regressions were fit using GEEs with an independent working covariance model to account for clustering because of multiple births from the same mother, which occurred for 169 066 births (58.1%) in the full cohort and for 1390 births (69%) in the P-VLBW subcohort. Robust empirical standard errors were used to compute confidence intervals and P values. Statistical significance was set at P < .05. All of the statistical analyses were performed using SAS, version 9.3 (SAS Institute).
Results
The full cohort included 290 992 live births over 10 years with 2015 infants (0.69%) in the P-VLBW subcohort. Table 1 demonstrates the baseline characteristics of the cohorts, including mean age and race/ethnicity distributions. Table 2 summarizes the preeclampsia exposure, covariates, and the 5 outcome variables for the 2 cohorts. The proportion of patients with missing values was low. A total of 289 infants in the full cohort died before receiving their first retinal examination, and 99 were transferred to another facility or discharged from the neonatal intensive care unit before being screened for ROP.
Table 1. Baseline Characteristics.
| Characteristic | Full Cohort (N = 290 992) |
P-VLBW Subcohorta (N = 2015) |
|---|---|---|
| Maternal age, mean (SD), y | 27.16 (5.34) | 27.08 (5.74) |
| Birth weight, mean (SD), g | 3294.64 (539.96) | 980.89 (300.56) |
| Gestational age, mean (SD), wk | 38.38 (1.87) | 26.87 (2.40) |
| Preeclampsia, No. (%) | 8996 (3.09) | 469 (23.28) |
| Maternal race/ethnicity, No. (%)b | ||
| White | 236 443 (81.4) | 1569 (77.98) |
| Black | 1453 (0.50) | 23 (1.14) |
| Asian, Pacific Islander, and American Indian | 6824 (2.35) | 56 (2.78) |
| Hispanic | 32 265 (11.11) | 256 (12.87) |
| Other or unknown | 13 475 (4.64) | 105 (5.22) |
| Infant sex, male, No. (%)c | 148 983 (51.23) | 1099 (54.57) |
| Alcohol use, No. (%)d | 5141 (1.77) | 110 (5.46) |
| Substance abuse, No. (%)d | 801 (0.28) | 54 (2.68) |
| Tobacco exposure, No. (%)d | 18 685 (6.42) | 266 (12.20) |
| Multiple gestations, No. (%)d | 8907 (3.06) | 537 (26.65) |
Abbreviation: P-VLBW, preterm very low-birth-weight.
Preterm very low birth weight was considered younger than 31 weeks’ gestational age and weighing less than 1500 g.
Five hundred thirty-two births (0.18%) in the full cohort and 3 births (0.15%) in the P-VLBW subcohort did not have maternal race/ethnicity reported.
One hundred seventy-four births (0.15%) in the full cohort and 1 birth (0.05%) in the P-VLBW subcohort had missing infant sex.
Self-reported parameters.
Table 2. Frequencies of Eventsa.
| Characteristic | Patients in the Full Cohort,b No. (%) (N = 290 992) |
Patients in the P-VLBW Subcohort, No. (%) (N = 2015) |
|---|---|---|
| All ROP | 579 (0.20) | 548 (32.16) |
| Severe ROP | 148 (0.05) | 147 (8.63) |
| All ROP or death before the first ROP examination | 868 (0.30) | 800 (40.90) |
| Severe ROP or death before the first ROP examination | 437 (0.15) | 399 (20.40) |
| Death before discharge | 565 (0.19) | 297 (15.18) |
Abbreviations: NICU, neonatal intensive care unit; P-VLBW, preterm very low-birth-weight; ROP, retinopathy of prematurity.
Ninety-nine patients were excluded from both cohorts because they transferred from the NICU before receiving the initial ROP examination.
Two hundred eighty-nine additional patients in the full cohort who died before their first ROP examination were excluded from the analysis of ROP outcomes, which did not incorporate death. These patients were included in the analyses for composite outcomes, which included death.
Estimates of Total and Direct Effects
Table 3 displays the results of the logistic regression analyses that evaluated the total and direct effects of preeclampsia on all ROP among the full cohort and the P-VLBW subcohort. The direct effect estimates presented in Table 3 were obtained after adjusting for the extended set of covariates, including birth weight and gestational age; corresponding direct effect estimates without an adjustment for birth weight and gestational age are provided in the supplementary materials (eTable in the Supplement). The adjusted odds ratio (aOR) corresponding to the total effect of preeclampsia was 6.07 (95% CI, 4.72-7.79; P < .001), indicating that preeclampsia was associated with an increased risk of ROP among the full cohort, whereas the aOR corresponding to the estimated direct effect was 0.62 (95% CI, 0.46-0.85; P = .003), indicating that preeclampsia was associated with a reduced risk of ROP among the restricted P-VLBW subcohort. After controlling for the same covariates as the total effect model, preeclampsia was associated with an increased occurrence of preterm births and very low birth weight among the full cohort (aOR, 7.74; 95% CI, 6.92–8.67; P < .001) (data not shown). Also, in the full cohort, the birth of male infants, maternal multiple gestations, alcohol use, tobacco exposure, and substance abuse were all independently associated with the occurrence of ROP.
Table 3. Association of All Retinopathy of Prematurity With Preeclampsia and Covariates in the Full Cohort and P-VLBW Subcohort.
| Covariates | Total Effect of Preeclampsia in the Full Cohort (N = 290 992) |
Direct Effect of Preeclampsia in the P-VLBW Subcohort (N = 2015) |
||
|---|---|---|---|---|
| aOR (95% CI) | P Value | aOR (95% CI) | P Value | |
| Preeclampsia | 6.07 (4.72-7.79) | <.001 | 0.62 (0.46-0.85) | .003 |
| Birth weight (per 10 g)a | NA | NA | 0.97 (0.96-0.97) | <.001 |
| Gestational age, wka | NA | NA | 0.72 (0.67-0.78) | <.001 |
| Maternal age, y | 0.99 (0.97-1.01) | .37 | 1.01 (0.99-1.04) | .27 |
| Infant sex, male (ref = female) | 1.21 (1.02-1.44) | .030 | 1.49 (1.14-1.94) | .004 |
| Multiple gestations | 7.90 (6.04-10.35) | <.001 | 0.83 (0.59-1.15) | .26 |
| Maternal race/ethnicity (ref = white) | ||||
| Black | 1.77 (0.78-4.02) | .18 | 0.32 (0.10-1.00) | .051 |
| Asian, Pacific Islander, or American Indian | 1.02 (0.58-1.79) | .96 | 1.15 (0.60-2.21) | .67 |
| Hispanic | 1.43 (1.11-1.83) | .005 | 1.24 (0.88-1.75) | .22 |
| Other or unknown | 0.99 (0.64-1.55) | .97 | 0.46 (0.24-0.86) | .015 |
| Alcohol use | 2.27 (1.51-3.42) | <.001 | 1.24 (0.72-2.14) | .43 |
| Tobacco exposure | 1.75 (1.30-2.35) | <.001 | 0.79 (0.52-1.20) | .27 |
| Substance abuse | 4.97 (2.75-8.98) | <.001 | 2.02 (0.99-4.12) | .053 |
Abbreviations: aOR, adjusted odds ratio; NA, not applicable; P-VLBW, preterm very low-birth-weight; Ref, reference; ROP, retinopathy of prematurity.
Birth weight and gestational age were included as covariates in the regression model for the direct effect of preeclampsia in the P-VLBW cohort but not in the regression model for the total effect of preeclampsia in the full cohort.
Table 4 summarizes the aORs corresponding to the estimated total and direct effects for severe ROP, death, and composites of all ROP and severe ROP with death before the first ROP examination. The aORs corresponding to the total effects indicate that after adjusting for covariates, preeclampsia was associated with a greater risk of each of these outcomes among the full cohort, whereas the aORs corresponding to the direct effects indicate that preeclampsia was associated with a lower risk of each of these outcomes among the restricted P-VLBW cohort. The estimated direct effects were similar when the analysis was performed in the restricted P-VLBW subcohort but without inclusion of preterm births and gestational age as covariates (eTable in the Supplement).
Table 4. Association of the Retinopathy of Prematurity-Related Outcomes With Preeclampsia in the Full Cohort and in the P-VLBW Subcohort.
| Outcome | Total Effect of Preeclampsia in the Full Cohort (N = 290 992)a |
Direct Effect of Preeclampsia in the P-VLBW Subcohort (N = 2015)b |
||
|---|---|---|---|---|
| aOR (95% CI) | P Value | aOR (95% CI) | P Value | |
| Severe ROP | 5.21 (3.44-7.91) | <.001 | 0.62 (0.36-1.06) | .08 |
| All ROP or death before the first ROP examination | 4.35 (3.49-5.43) | <.001 | 0.51 (0.37-0.69) | <.001 |
| Severe ROP or death before the first ROP examination | 2.59 (1.82-3.67) | <.001 | 0.32 (0.22-0.48) | <.001 |
| Predischarge death | 1.66 (1.16-2.38) | .006 | 0.19 (0.11-0.32) | <.001 |
Abbreviations: aOR, adjusted odds ratio; P-VLBW, preterm very low-birth-weight; ROP, retinopathy of prematurity.
Adjusted odds ratios for the total effects of preeclampsia were estimated using generalized estimating equations for logistic regression in the full cohort with adjustment for maternal age, sex, history of multiple gestations, maternal race/ethnicity (classified as white, black, Asian/Pacific Islander, Hispanic, other or unknown), alcohol use, tobacco exposure, and substance abuse.
Adjusted odds ratios for the direct effects of preeclampsia were estimated using generalized estimating equations for logistic regression in the restricted cohort of P-VLBW infants with covariate adjustment for each of the covariates in the total effect models plus birth weight and gestational age.
Discussion
Circulating antiangiogenic factors in the preeclamptic maternal environment may interfere with fetal retinal vascular development and predispose preterm infants to developing ROP. In addition, preeclampsia increases the risk and severity of preterm births, which are strongly linked to ROP risk.21 Thus, it is plausible from a biological perspective that maternal preeclampsia would increase the risk and severity of ROP. A recent experimental study removed the confounder of preterm births and exposed full-term rat pups newly born to pregnant dams with uteroplacental insufficiency, a feature of preeclampsia, to oxygen stresses similar in degree to those experienced by preterm infants who are at risk of severe ROP. The severity of oxygen-induced retinopathy was then compared with that in pups born to pregnant control rats without uteroplacental insufficiency. Pups born to dams with uteroplacental insufficiency and placed in variable oxygen had less severe retinopathy than those born to control dams. This suggested that in the absence of preterm births, surviving offspring may have an advantage that is induced by variable oxygen, a condition preterm infants often experience.23
The clinical literature is mixed with some studies reporting that preeclampsia is associated with increased risk of ROP12,13,14,15,16 and others reporting that there is a reduced risk.17,18,19 These studies all restricted analyses to preterm infants and excluded full-term births during the same time periods. As a result of this restriction, the estimates of the association of preeclampsia with ROP from these studies exclude the indirect effects of preeclampsia (eg, preeclampsia increases the risk of having a preterm birth, which is a risk factor for ROP), and instead evaluate only the direct effects of preeclampsia on ROP that occur within preterm birth subcohorts through paths that are unrelated to preterm births.
To provide a more comprehensive evaluation that would be useful in clinical care, we investigated the association of preeclampsia with ROP both among an unrestricted birth cohort and among a restricted subcohort of P-VLBW infants. Our analyses in the unrestricted cohort estimate the total effects of preeclampsia and account for both direct and indirect effects, whereas our analyses in the restricted P-VLBW subcohort estimate the direct effects of preeclampsia on ROP, similar to previous studies. We believe that the total effect is more relevant clinically, for instance when counseling women with preeclampsia regarding the relative risk of their child developing ROP. In this study, we determined that preeclampsia increased the odds of ROP and severe ROP among the full cohort (total effect). The magnitudes of the estimated adverse total effects of preeclampsia on ROP are sufficiently large enough that it is unlikely that they can be fully explained by the uncontrolled confounding that would occur when analyzing direct effects. For example, if we allow for the possibility that there may be an uncontrolled confounder with an 8-fold higher prevalence among infants of mothers with preeclampsia vs those without (0.80 vs 0.10), this confounder would have to lead to a 27-fold increase in the risk of ROP to account for the adjusted odds ratio of 6.07 (95% CI, 4.72-7.79; P < .001) that we estimated for the relationship between preeclampsia and all ROP. Moreover, a presupposed uncontrolled confounder with an 8-fold higher prevalence (0.80 vs 0.10) among infants of mothers with preeclampsia would lead to a 16-fold increased ROP risk to account for the estimated aOR of 5.21 (95% CI, 3.44–7.91; P < .001) for severe ROP. Although not impossible, it is unlikely that such strong unmeasured confounders are present.22
In contrast to the total effects, preeclampsia appeared to be protective among the subcohort P-VLBW infants. Thus, one interpretation of our results is that they are consistent with the hypothesis that preeclampsia has both an adverse total effect and a protective direct effect on ROP in which the adverse effect that is mediated by prematurity is stronger than the direct effect through other mechanisms. We found a similar pattern for early mortality rates, with preeclampsia exhibiting adverse total effects but also protective direct effects, similar to a previous report,24 and for composite outcomes of ROP and death occurring before the first ROP examination. Including death before the first ROP examination did not alter our results, and the relationship between preeclampsia and ROP in both cohorts held regardless of whether deaths occurring before the first ROP examination were censored or included as events. Among the P-VLBW subcohort, we found similar estimates of a protective direct effect with and without covariate adjustment for residual variation in gestational age and birth weight.
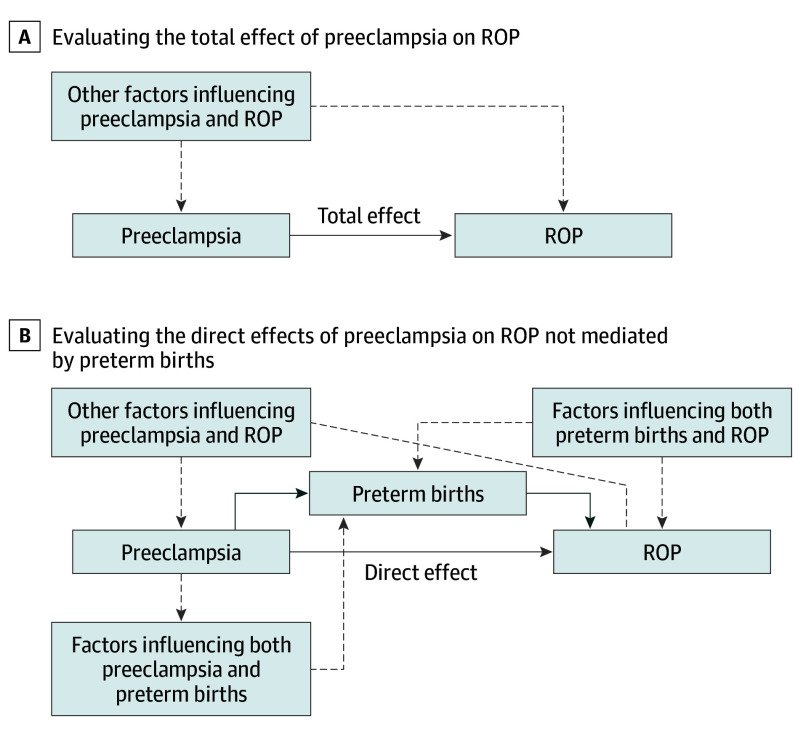
However, the analyses suggested that protective direct effects of preeclampsia on ROP are subject to a substantially greater risk of bias from uncontrolled confounding than are the analyses of the total effects. This is illustrated in the directed acyclic graphs that define the relationships of the total effect (Figure, A) and the direct effect (Figure, B) of preeclampsia on ROP. Confounding factors that must be adjusted for in the analysis are noted as dashed lines. Unbiased estimations of the total effect require adjusting for confounding factors that jointly influence preeclampsia and ROP (Figure). Unbiased estimations of the direct effect require adjusting for the same confounding factors, factors that influence preeclampsia and premature birth, and factors that influence premature birth and ROP risk among infants who were born prematurely (Figure). It is less likely that covariates known to us are sufficient to control for 3 classes of confounders than it is that they are sufficient to control for the single class of confounders. It is possible that the conflicting directions of the relationships between preeclampsia and ROP in previous studies restricted to premature birth cohorts reflect variations in the patterns of 1 or more of the 3 sources of confounding present across these studies. There is great variance in the risks associated with ROP worldwide that are believed to reflect differences in resources for prenatal and perinatal care, and this may have played a role in some of the differences in previous studies.25 The risk of uncontrolled confounding in this setting has been termed collider bias, which is a type of selection bias. Among the P-VLBW subcohort, infants whose mothers did not have preeclampsia were more likely to have been exposed to other risk factors for preterm births than infants whose mothers did have preeclampsia. If these factors pose a greater risk for ROP than does maternal preeclampsia, failing to control for them provides a false appearance of a protective effect from preeclampsia. Collider bias has been discussed in studies of cohorts that were restricted by gestational age that relate preeclampsia to infant mortality rates24 and other neonatal outcomes.26,27,28 For example, maternal smoking is associated with an increased risk of low-birth-weight infants, and a low birth weight increases mortality rates among infants. However, analyses that were restricted to low-birth-weight infants suggest that maternal smoking is protective against mortality. This has led to the conjecture that other, more significant risk factors for both low birth weight and mortality are involved, and restricting by birth weight causes smoking to appear falsely protective.27
Figure. Directed Acyclic Graphs (DAGs) Corresponding to the Estimation of the Total and Direct Effects of Preeclampsia on Retinopathy of Prematurity (ROP).
The DAGs describe the classes of confounders, which must be controlled to obtain unbiased estimates of the (total effect of preeclampsia on ROP (A) and the direct effect of preeclampsia on ROP (B), which is distinct from effect of preeclampsia on preterm births. The solid lines represent the direct and total effect between preeclampsia and ROP, and the dashed lines represent confounders that must be controlled.
Several covariates were associated with a risk of all ROP, particularly in the full unrestricted cohort. Notably, male sex was associated with an increased risk of all ROP among both the full and restricted cohorts, consistent with other studies in which males had a greater risk of experiencing perinatal adverse events than females.29
Strengths and Limitations
Strengths of this study include the large sample size and comprehensive cohort of all live births in a 10-year period, the distinction between total and direct effects in our analyses, and the inclusion of infant deaths to address the potential effect of death before the first eye examination on the risk of ROP in preeclampsia. Limitations include reliance on ICD-9 codes for the diagnoses of preeclampsia and ROP and the fact that infants in only 1 region of the country were analyzed. Also, because of the retrospective nature of the analysis, we were unable to further categorize ROP by stage or zone; however, patients with diagnosis codes for laser treatment or surgery for ROP were categorized as severe, which has been used in other large retrospective studies on ROP.30,31 Despite these study limitations, these results clarify the association between preeclampsia and ROP and are consistent with the current understanding of the pathophysiology of these diseases.
Conclusions
We interpret our results as strongly suggestive of an adverse total effect of preeclampsia on ROP. Our results also are consistent with the presence of a protective direct effect of preeclampsia among infants who were born preterm and with a very low birth weight. It is unclear, however, whether the estimates of a protective direct effect that were obtained in this study and in previous studies among restricted premature birth cohorts reflect a true protective direct effect, collider bias, or another related form of uncontrolled confounding. While the direct effect is relevant to the biological mechanism by which preeclampsia affects ROP, it is the total effect that is clinically relevant and informs prognostication and the implications of treatments to reduce the incidence of preeclampsia in the prevention of ROP.
eTable. Association of the ROP-related Outcomes With Preeclampsia in the P-VLBW Subcohort Without Adjustment for Birth Weight and Gestational Age
References
- 1.Villar J, Say L, Gulmezoglu AM, et al. Eclampsia and preeclampsia: a health problem for 2000 years. In: Critchly H, MacLean A, Poston L, Walker J, eds. Preeclampsia. London, England: RCOG Press; 2003:189-207. [Google Scholar]
- 2.Wu D, Huang L, He Z, Huang X, Fang Q, Luo Y. Preeclampsia in twin pregnancies: association with selective intrauterine growth restriction. J Matern Fetal Neonatal Med. 2016;29(12):1967-1971. [DOI] [PubMed] [Google Scholar]
- 3.Hod T, Cerdeira AS, Karumanchi SA. Molecular mechanisms of preeclampsia. Cold Spring Harb Perspect Med. 2015;5(10):a023473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen XK, Wen SW, Smith G, Yang Q, Walker M. Pregnancy-induced hypertension is associated with lower infant mortality in preterm singletons. BJOG. 2006;113(5):544-551. [DOI] [PubMed] [Google Scholar]
- 5.Piper JM, Langer O, Xenakis EM, McFarland M, Elliott BD, Berkus MD. Perinatal outcome in growth-restricted fetuses: do hypertensive and normotensive pregnancies differ? Obstet Gynecol. 1996;88(2):194-199. [DOI] [PubMed] [Google Scholar]
- 6.SocolIov D, Ilea C, Lupascu IA, et al. Maternal-fetal prognosis in HELLP syndrome in a level 3 maternal-fetal care centre. Clin Exp Obstet Gynecol. 2016;43(3):374-378. [PubMed] [Google Scholar]
- 7.Auger N, Le TU, Park AL, Luo ZC. Association between maternal comorbidity and preterm birth by severity and clinical subtype: retrospective cohort study. BMC Pregnancy Childbirth. 2011;11:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basso O, Rasmussen S, Weinberg CR, Wilcox AJ, Irgens LM, Skjaerven R. Trends in fetal and infant survival following preeclampsia. JAMA. 2006;296(11):1357-1362. [DOI] [PubMed] [Google Scholar]
- 9.Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209(6):544.e1-544.e12. [DOI] [PubMed] [Google Scholar]
- 10.Holmes JM, Duffner LA. The effect of postnatal growth retardation on abnormal neovascularization in the oxygen exposed neonatal rat. Curr Eye Res. 1996;15(4):403-409. [DOI] [PubMed] [Google Scholar]
- 11.Lundgren P, Wilde Å, Löfqvist C, Smith LE, Hård AL, Hellström A. Weight at first detection of retinopathy of prematurity predicts disease severity. Br J Ophthalmol. 2014;98(11):1565-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JW, McElrath T, Chen M, et al. Pregnancy disorders appear to modify the risk for retinopathy of prematurity associated with neonatal hyperoxemia and bacteremia. J Matern Fetal Neonatal Med. 2013;26(8):811-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gagliardi L, Rusconi F, Da Frè M, et al. Pregnancy disorders leading to very preterm birth influence neonatal outcomes: results of the population-based ACTION cohort study. Pediatr Res. 2013;73(6):794-801. [DOI] [PubMed] [Google Scholar]
- 14.Yang CY, Lien R, Yang PH, et al. Analysis of incidence and risk factors of retinopathy of prematurity among very-low-birth-weight infants in North Taiwan. Pediatr Neonatol. 2011;52(6):321-326. [DOI] [PubMed] [Google Scholar]
- 15.Ozkan H, Cetinkaya M, Koksal N, Ozmen A, Yıldız M. Maternal preeclampsia is associated with an increased risk of retinopathy of prematurity. J Perinat Med. 2011;39(5):523-527. [DOI] [PubMed] [Google Scholar]
- 16.Shah VA, Yeo CL, Ling YL, Ho LY. Incidence, risk factors of retinopathy of prematurity among very low birth weight infants in Singapore. Ann Acad Med Singapore. 2005;34(2):169-178. [PubMed] [Google Scholar]
- 17.Araz-Ersan B, Kir N, Akarcay K, et al. Epidemiological analysis of retinopathy of prematurity in a referral centre in Turkey. Br J Ophthalmol. 2013;97(1):15-17. [DOI] [PubMed] [Google Scholar]
- 18.Fortes Filho JB, Costa MC, Eckert GU, Santos PG, Silveira RC, Procianoy RS. Maternal preeclampsia protects preterm infants against severe retinopathy of prematurity. J Pediatr. 2011;158(3):372-376. [DOI] [PubMed] [Google Scholar]
- 19.Yu XD, Branch DW, Karumanchi SA, Zhang J. Preeclampsia and retinopathy of prematurity in preterm births. Pediatrics. 2012;130(1):e101-e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan PYL, Tang SM, Au SCL, et al. Association of gestational hypertensive disorders with retinopathy of prematurity: a systematic review and meta-analysis. Sci Rep. 2016;6:30732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Good WV; Early Treatment for Retinopathy of Prematurity Cooperative Group . Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc. 2004;102:233-248. [PMC free article] [PubMed] [Google Scholar]
- 22.VanderWeele TJ, Arah OA. Bias formulas for sensitivity analysis of unmeasured confounding for general outcomes, treatments, and confounders. Epidemiology. 2011;22(1):42-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becker S, Wang H, Yu B, et al. Protective effect of maternal uteroplacental insufficiency on oxygen-induced retinopathy in offspring: removing bias of premature birth. Sci Rep. 2017;7:42301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auger N, Naimi AI, Fraser WD, et al. Three alternative methods to resolve paradoxical associations of exposures before term. Eur J Epidemiol. 2016;31(10):1011-1019. [DOI] [PubMed] [Google Scholar]
- 25.Quinn GE. Retinopathy of prematurity blindness worldwide: phenotypes in the third epidemic. Eye Brain. 2016;8:31-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernández-Díaz S, Schisterman EF, Hernán MA. The birth weight “paradox” uncovered? Am J Epidemiol. 2006;164(11):1115-1120. [DOI] [PubMed] [Google Scholar]
- 27.Wilcox AJ, Weinberg CR, Basso O. On the pitfalls of adjusting for gestational age at birth. Am J Epidemiol. 2011;174(9):1062-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.VanderWeele TJ, Hernández-Diaz S. Is there a direct effect of pre-eclampsia on cerebral palsy not through preterm birth? Paediatr Perinat Epidemiol. 2011;25(2):111-115. [DOI] [PubMed] [Google Scholar]
- 29.Wallace ME, Mendola P, Kim SS, et al. Racial/ethnic differences in preterm perinatal outcomes. Am J Obstet Gynecol. 2017;216(3):306.e1-306.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sood BG, Madan A, Saha S, et al. ; NICHD neonatal research network . Perinatal systemic inflammatory response syndrome and retinopathy of prematurity. Pediatr Res. 2010;67(4):394-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hagen EW, Sadek-Badawi M, Albanese A, Palta M. A comparison of Wisconsin neonatal intensive care units with national data on outcomes and practices. WMJ. 2008;107(7):320-326. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Association of the ROP-related Outcomes With Preeclampsia in the P-VLBW Subcohort Without Adjustment for Birth Weight and Gestational Age