Key Points
Question
Can intravenous acetaminophen given at the time of sinus surgery control postoperative pain better than placebo?
Findings
In this prospective, randomized clinical trial including 62 patients undergoing endoscopic sinus surgery for chronic rhinosinusitis, intravenous acetaminophen given within the first hour after surgery was associated with a reduction in pain and this difference may be clinically meaningful.
Meaning
Given our inconclusive results and the high cost of intravenous acetaminophen we cannot recommend it as a pain control regimen after sinus surgery.
This randomized clinical trial examines the use of intravenous acetaminophen vs placebo in patients undergoing endoscopic sinus surgery for chronic rhinosinusitis.
Abstract
Importance
Intravenous acetaminophen is a commonly prescribed analgesic for the prevention and treatment of postsurgical pain. Its efficacy in the context of endoscopic sinus surgery (ESS) has yielded mixed results.
Objective
To compare the efficacy of perioperative intravenous acetaminophen (IVAPAP) with that of placebo in improving early postoperative pain after endoscopic sinus surgery (ESS).
Design, Setting, and Participants
A prospective, randomized clinical trial including 62 patients undergoing ESS for chronic rhinosinusitis in a single tertiary referral hospital.
Interventions
Participants were randomized to receive 1 g of IVAPAP or 100 mL of placebo consisting of saline infusions immediately before the start of surgery and 4 hours after the initial dose.
Main Outcomes and Measures
The primary outcome was postoperative pain measured by visual analog scale (VAS) scores up to 24 hours after surgery by blinded observers. Secondary endpoints included postoperative opioid (intravenous and oral) use and adverse events in the 24-hour postoperative period.
Results
Of the 62 enrolled adult participants, 60 were randomized (31 to IVAPAP intervention and 29 to placebo). The mean (SD) age of participants was 53.7 (14.7) years and 35 (58%) of the participants were men and 25 (42%) were women. Within the first hour, mean pain scores were reduced in the IVAPAP group compared with the control group, reaching a maximum difference of 7.7 mm on a VAS scale favoring the treatment group with a true difference possibly as high as 22 mm, and the data are compatible with a clinically meaningful difference. At 12- and 24-hours, average pain scores were less in the placebo group and the data are compatible with a clinically meaningful difference of 5.8 (−5.2 to 16.8) and 8.2 (−1.9 to 18.4), respectively, favoring the placebo group. However, at all time points the CIs included the null value and were wide, thus preventing definitive conclusions. Inspection of the secondary outcomes favored IVAPAP, but the wide range of the CIs and inclusion of the null value prevent definitive conclusions.
Conclusions and Relevance
The results of this study are inconclusive. The data suggest that perioperative intravenous acetaminophen may reduce immediate postoperative pain and opioid requirements compared with placebo and these differences could be clinically meaningful. Unfortunately, the imprecision of the estimates prevents definitive conclusion. Use of IVAPAP does not seem to increase adverse events.
Trial Registration
clinicaltrials.gov Identifier: NCT01608308
Introduction
Chronic rhinosinusitis (CRS) is a common inflammatory condition of the nasal cavity and paranasal sinuses that affects an estimated 30 million individuals in the United States. Endoscopic sinus surgery (ESS) is an increasingly prevalent treatment option for patients with CRS whose symptoms are refractory to standard medical treatment. Prior studies have found that ESS provides an effective method to improve disease-specific quality-of-life outcomes by widening the natural sinonasal outflow tracts, improving mucociliary clearance, and optimizing delivery of medical therapy to diseased sites. An estimated 200 000 to 300 000 outpatient ESS procedures are performed annually in the United States, making it among the most common ambulatory surgeries performed.
Postoperative pain control has garnered the attention of many major medical societies. Pain assessment and follow-up is recognized by the American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS), for example, as an individual quality measure reportable to the Physician Quality Reporting System (PQRS). The American Pain Society has also identified several research gaps in postoperative pain management, including studies that examine the efficacy of various analgesics according to type of surgery performed.
There currently is no consensus regarding optimal pain control after functional endoscopic sinus surgery (FESS) for CRS. Available research indicates that, on average, the pain experienced in the acute postoperative period after FESS is mild; however, some studies cite up to a 70% incidence of pain requiring opioid analgesics in the absence of a nonopioid alternative. Furthermore, opioid analgesic use is still common after FESS. Despite its associated mild pain, unplanned admissions after ESS occur in 2% to 9% of cases, and acute pain remains among the most common causes for readmission. Taken together, the available evidence underscores an apparent mismatch between perceived pain after FESS and its management. While pain associated with FESS is mild, it is not trivial and should be treated; however, employing opioid analgesics in the setting of FESS may be excessive and lead to undue adverse effects. High-quality studies that evaluate the efficacy of alternative, nonopioid regimens are necessary to promote a new standard of care in the management of pain after FESS. Currently, there are limited double-blinded, placebo-controlled studies that evaluate nonopioid analgesics in the postoperative period, and the findings from available studies are mixed.
We compared the efficacy of perioperative intravenous acetaminophen (IVAPAP) with placebo in controlling postoperative pain following ESS. A prospective and double-blinded study was conducted to determine whether IVAPAP improved postoperative pain as measured by a patient-reported rating system. We also sought to determine whether perioperative IVAPAP use could reduce postoperative opioid requirements and opioid-related adverse events.
Methods
This was a prospective, double-blinded, single-center, randomized clinical trial conducted as a joint collaboration between the Departments of Anesthesiology and Otorhinolaryngology–Head and Neck Surgery at McGovern Medical School at the University of Texas Health Science Center (UTHSC). The study was registered with the US National Institutes of Health and received approval from the UTHSC at Houston institutional review board. Written informed consent was obtained from all participants prior to their participation in the study and they were not compensated. The study duration was between July 2012 and September 2014. The trial protocol is included in the Supplement.
Patient Selection and Randomized Enrollment
This clinical trial enrolled individuals aged at least 18 years and undergoing planned ESS for medically refractory CRS. Participants underwent a preoperative computed tomography scan of the paranasal sinuses with a navigation protocol and demonstrated objective evidence of CRS. Exclusion criteria included history of hypersensitivity to acetaminophen; known or suspected history of opioid intolerance; a history of chronic pain or use of opioid or any other pain modulator in the previous 2 weeks; end-stage renal disease; end-stage liver disease; severe depression or anxiety; seizure disorder; and known or suspected history of alcohol or drug abuse.
Prior to their surgical procedure, patients were randomly assigned to either the experimental group or placebo group by an investigational pharmacist. In the experimental group, participants received 1000 mg IVAPAP in 100 mL of normal saline during induction of general anesthesia, or about 15 minutes before the start of surgery. In the control group, 100 mL of normal saline was administered. Patients received a second dose 4 hours after the initial dose. The total dose of IVAPAP did not exceed 2000 mg for any patient on the day of surgery. Patients, surgeons, anesthesiologists, and research staff were blinded to the test medications administered at all stages of data acquisition.
Anesthesia and Surgical Protocols
The delivery of anesthesia was standardized for all participants undergoing ESS. All participants were medicated in the preoperative period with dexamethasone sodium phosphate (10 mg), midazolam (1 or 2 mg based on body weight), and either cefazolin or clindamycin (1 g and 600 mg, respectively). Standard intravenous induction of general anesthesia was provided by the attending anesthesiologists using propofol (2 mg/kg), lidocaine (0.5 mg/kg), and rocuronium (0.5 g/kg). Intraoperatively, patients were monitored by the anesthesiologists using electrocardiography, noninvasive blood pressure monitoring, pulse oximetry, and a temperature probe. Maintenance anesthesia was achieved with 2% sevoflurane and fentanyl citrate (2 µg/kg). Additional doses of fentanyl at 1 µg/kg were administered intraoperatively to patients if their heart rates and blood pressures were greater than 15% to 20% of their expected baseline.
Following induction of general anesthesia, ESS was performed by 1 of 3 fellowship-trained rhinologists. Prior to the surgical start, patients received topical decongestion through the placement of pledgets soaked in oxymetazoline hydrochloride under the middle turbinates bilaterally. Local anesthesia was also provided through the submucosal injection of approximately 3 mL of lidocaine, 1% , with 1:100 000 epinephrine into the medial infundibular wall and sphenopalatine region. Surgical procedures were performed with standard ESS technique, using a combination of both nonpowered instruments and a microdebrider to dissect the sinuses with objective evidence of CRS. Hemostasis was maintained at the end of procedures through the placement of bioresorbable nasal dressings (Nasopore, Stryker Corporation) in the middle meatus. Patients were extubated when they were fully awake and were transferred to the postanesthesia care unit (PACU) for monitoring of vital signs, assessment of pain, and adverse events.
Postoperative Outcome Measurements
Pain measurement was performed using a validated VAS on a 100-mm horizontal line in the manner similar to that used by Church et al. For each VAS measurement, patients were presented with a VAS card and asked to indicate their level of pain from 0 (no pain) to 100 (most severe pain imaginable) by placing a mark on a vertical line corresponding to a numerical value on the VAS scale. A minimum clinically significant change in pain on VAS scale is defined as the minimum difference in millimeters resulting in patients noting "a little more" or "a little less pain" from preceding VAS score. Using patients reporting acute pain from trauma and subsequently validating in a heterogenous group of adult patients presenting to an urban emergency department, a minimum clinically significant change in pain was defined as a change in 13 mm on the VAS scale.
After ESS, patients were asked to report their pain at 7 different postoperative time points: 15 minutes, 30 minutes, 45 minutes, 60 minutes, 2 hours, 12 hours, and 24 hours. The VAS pain assessments at 15 minutes, 30 minutes, 45 minutes, 60 minutes, and 2 hours were completed in the PACU, whereas at 12 hours and 24 hours, participants recorded their assessments in a diary that was collected and recorded at their next clinical visit. All adverse events in the immediate postoperative period were also recorded. Adverse events were recorded at each time point as noted above and included, but were not limited to, VAS over 40, hypertension, hypotension, self-reported nausea, emesis, tachycardia, bradycardia, and allergic reaction.
In an effort to evaluate the effectiveness of IVAPAP for postoperative pain control in an intention-to-treat approach, postoperative oral and intravenous opioid requirements were also recorded during the first 24 hours after surgery. In the PACU, morphine was administered intravenously as a rescue analgesic to patients who reported considerable pain; doses of intravenous morphine were repeated at 30-minute intervals until patients indicated that their pain was consistent at only mild intensity. No other analgesic medication was administered during the immediate postoperative period. The total number of doses and total dosage of intravenous morphine were both recorded for each participant, allowing for a comparison of immediate needs for rescue analgesic medication between IVAPAP and control groups. At the time of discharge from the PACU, all participants were provided prescriptions for 500 mg acetaminophen tablets and an oral narcotic (325/5 mg acetaminophen-hydrocodone) tablets. The participants were instructed to take an oral acetaminophen tablet every 6 hours for the first 12 hours after discharge if their VAS pain score exceeded 40. Twelve hours after discharge, participants were instructed to take an oral narcotic every 6 hours if their VAS pain score exceeded 40. Participants were contacted 24 to 48 hours after surgery by research staff and pain as well as the number of acetaminophen-hydrocodone doses taken was recorded.
Statistical Analysis
Power analysis was conducted using the following hypotheses: (1) at the time of arrival in PACU recovery, the incidence of postoperative pain in the control group would be 70%, and (2) the incidence of postoperative pain would be less than 35% in the experimental group. We determined that at least 25 patients in each group should be enrolled to achieve a power of 80%, allowing for a type I error of .05.
For data analysis, continuous variables were summarized by means and standard deviations. Categorical variables were summarized by frequencies and percentages. The differences between 2 treatment groups in terms of demographics, preoperative variables, and intraoperative data were compared using 2 sample t test (or Wilcoxon rank sum test as appropriate for continuous variables) and χ2 test (or Fisher exact test as appropriate) for categorical variables. Generalized estimating equations was applied to evaluate the treatment effect on VAS collected at 7 different postoperative time points to account for the correlation within patients. Differences in the mean VAS scores with 95% CIs were reported for each time point. All statistical analyses were performed using SAS 9.4 statistical software (SAS Institute, Inc).
Results
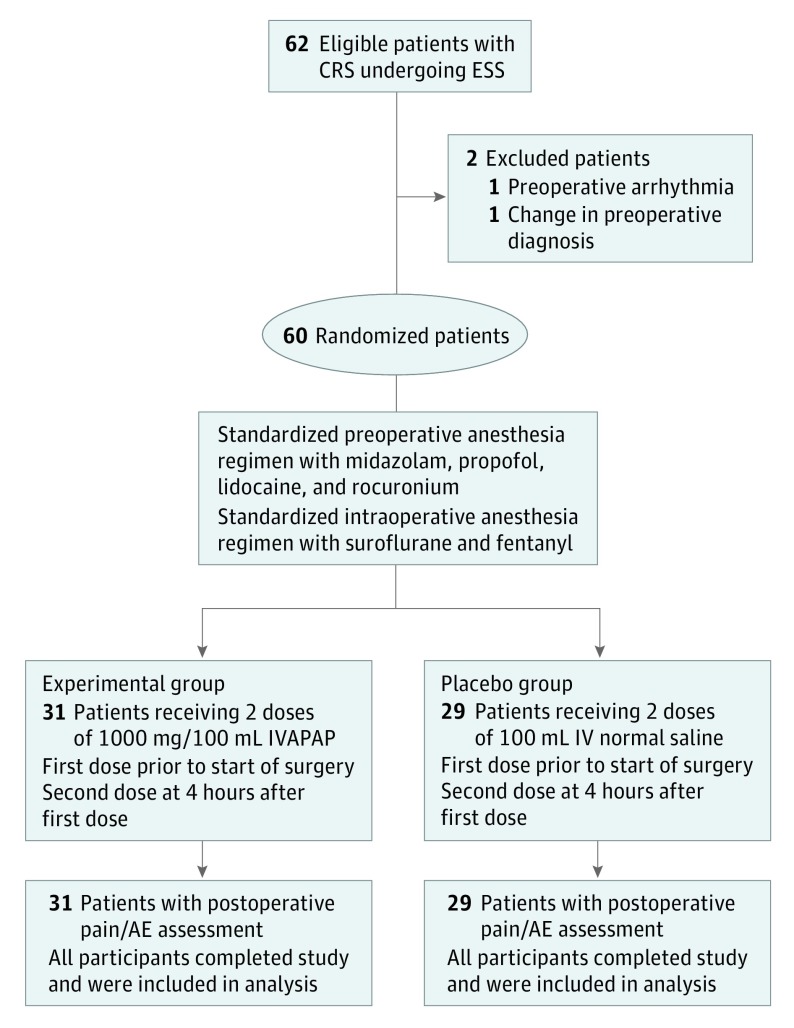
A diagram summarizing the study’s flow is included (Figure). A total of 62 adult participants were enrolled in this prospective study from July 2012 to September 2014. Two patients were excluded owing to a change in the preoperative diagnosis from CRS to a sinonasal mass in 1, and the development of an induction arrhythmia following general anesthesia, not related to the administration of the investigational medication, that prompted the surgery to be cancelled. Thirty-one patients were randomized into the IVAPAP group and 29 patients were randomized into the placebo group. Evaluation of the demographics (Table 1) demonstrated that the 2 groups were similar in terms of age, disease phenotype, severity of disease, and intraoperative variables. All 60 randomized patients provided a minimum of 3 VAS pain assessment scores in the immediate postoperative period. No patient experienced significant postoperative complications related to the administration of IVAPAP.
Figure. Study Flow Diagram.
AE Indicates adverse events; CRS, chronic rhinosinusitis; ESS, endoscopic sinus surgery; IVAPAP, intravenous acetaminophen.
Table 1. Preoperative and Intraoperative Parameters for Patients Receiving Intravenous Acetaminophen (IVAPAP) and Placebo.
| Parameter | IVAPAP Group (n=31) | Placebo Group (n=29) |
|---|---|---|
| Preoperative variables | ||
| Age, mean (SD), y | 53.7 (14.6) | 53.7 (14.7) |
| Sex, No. (%)a | ||
| Men | 22 (71) | 13 (45) |
| Women | 9 (29) | 16 (55) |
| Ethnicity, No. (%)a of patients | ||
| Asian | 2 (7) | 1 (3) |
| Black | 4 (13) | 3 (10) |
| Hispanic | 2 (7) | 1 (3) |
| Other | 0 | 1 (3) |
| White | 23 (74) | 23 (79) |
| Disease phenotypes, No. (%)a of patients | ||
| CRSwNP | 19 (61) | 20 (69) |
| CRSsNP | 12 (38) | 9 (31) |
| Lund Mackay score, mean (SD) | 13.8 (6.7) | 15.3 (6.1) |
| Preoperative VAS score, mean (SD), | 6.8 (16.3) | 3.4 (13.2) |
| Intraoperative variables | ||
| Surgical time, mean (SD), minutes | 168.8 (63.8) | 164.7 (69.9) |
| Estimated blood loss, mean (SD), mL | 206.8 (162.0) | 202.7 (164.0) |
| Supplemental fentanyl, No. (%)a | 9 (29.0) | 4 (13.8) |
| Supplemental fentanyl doses, No. (%)a | 9 | 4 |
| 1 | 6 (66.7) | 3 (75.0) |
| 2 | 1 (11.1) | 0 |
| 3 | 1 (11.1) | 0 |
| 4 | 1 (11.1) | 1 (25.0) |
Abbreviations: CRSsNP, chronic rhinosinusitis without nasal polyps; CRSwNP, chronic rhinosinusitis with nasal polyps.
Percentages have been rounded to the nearest percent and may not add up to 100%.
At each of the 3 first time points (15, 30, and 45 minutes), there were large differences in VAS score between the IVAPAP and placebo groups favoring the IVAPAP group. Furthermore, the upper bound of the CIs around the difference in VAS between the IVAPAP and placebo groups exceeded the clinically meaningful difference of 13, suggesting that the data are compatible with important clinical effects (Table 2). For instance, the upper bound of the CI at 45 minutes suggests that the true difference could be as great as 22 mm on the VAS scale. However, at the 1-hour assessment after surgery, the absolute VAS difference (95% CI) was −1.7 (−13.0 to 9.6). Whereas the upper bound of the CI of 13 suggests a clinically significant effect, the point estimate of −1.7 suggests little effect. The width of the CIs at all time points indicates imprecision in the estimate of the effect of IVAPAP, thus preventing any solid conclusion about the effect of IVAPAP compared with placebo.
Table 2. Comparison of Postoperative Pain Control After Endoscopic Sinus Surgery for Patients Receiving Intravenous Acetaminophen (IVAPAP) and Placebo.
| Variable | IVAPAP Group | Placebo Group | Difference (95% CI) | ||
|---|---|---|---|---|---|
| No. | Mean (SD) | No. | Mean (SD) | ||
| Postoperative Visual Analogue Scale (VAS) Pain Scores | |||||
| VAS Score | |||||
| 15 minutes | 26 | 20.0 (28.1) | 28 | 26.4 (29.5) | −6.4 (−22.2 to 9.4)a |
| 30 minutes | 29 | 21.4 (28.4) | 29 | 26.5 (28.6) | −5.1 (−20.1 to 9.8)a |
| 45 minutes | 30 | 19.3 (24.9) | 29 | 27.0 (29.4) | −7.7 (−21.9 to 6.5)a |
| 60 minutes | 29 | 16.0 (18.1) | 27 | 17.7 (23.7) | −1.7 (−13.0 to 9.6)a |
| 2 hours | 10 | 20.7 (19.6) | 10 | 20.4 (19.7) | 0.3 (−18.2 to 18.8)a |
| 12 hours | 31 | 20.8 (21.8) | 24 | 15.0 (17.6) | 5.8 (−5.2 to 16.8)a |
| 24 hours | 31 | 15.9 (21.0) | 24 | 7.7 (14.7) | 8.2 (−1.9 to 18.4)a |
| Postoperative Opioid Requirements | |||||
| Outcomes | |||||
| Patients receiving supplemental intravenous morphine doses, No. (%) | 31 | 8 (25.8) | 29 | 10 (34.5) | −8.7 (−31.8 to 14.5)b |
| Total supplemental morphine doses given, No. | 31 | 0.5 (0.9) | 29 | 1.0 (1.7) | −0.5 (−1.2 to 0.2)a |
| Total dosage of supplemental morphine given, mL | 31 | 0.9 (2.0) | 29 | 1.7 (2.9) | −0.8 (−2.0 to 0.5)a |
| Patients receiving supplemental oral narcotic doses, No. (%) | 31 | 7 (22.6) | 26 | 7 (26.9) | −4.3 (−26.9 to 18.2)b |
| Total supplemental oral narcotic doses given, No. | 31 | 0.5 (1.2) | 26 | 0.5 (1.0) | 0.1 (−0.5 to 0.7)a |
Indicates the difference in mean between 2 groups as well as its 95% CI.
Indicates the difference in percentage between 2 groups as well as its 95% CI.
Importantly, at the 12- and 24-hour time points the absolute differences in VAS score favored the placebo group and inspection of the CIs suggest that a clinically meaningful reduction in pain perception in the placebo group relative to the IVAPAP group is possible. This change in pain at these later time points favoring the placebo group reflects the difference in VAS trend over the 24-hour postoperative time period. The IVAPAP group was characterized by a stable mean VAS score while the placebo group was associated with a decrease in mean VAS score with time, with the most significant drops noted at 12 and 24 hours after the procedure.
There was no significant difference in the number of patients requiring intravenous morphine and oral narcotics. In the PACU, 8 of 31 patients (26%) in the IVAPAP group required supplemental intravenous morphine, whereas 10 of 29 patients (34%) in the placebo group required supplemental intravenous morphine for an absolute reduction in the percentage of participants requiring morphine of 8.7% (95% CI, −14.5% to 31.8%). No participants required hospital admission for observation secondary to any adverse event or other cause. There was no difference in incidence of adverse events between the IVAPAP and placebo group (IVAPAP, 55%; placebo, 55%), yet there was a 7.5% absolute increase (95% CI, −17.1% to 32.0%) in the proportion of patients in the IVAPAP group self-reporting postoperative nausea compared with placebo (Table 3).
Table 3. Comparison of Adverse Events in Patients Receiving IVAPAP and Placebo.
| Outcomes | IVAPAP Group (n = 31) | Placebo Group (n = 29) | Difference % (95% CI) |
|---|---|---|---|
| Recovery speed, median (Q1, Q3), minutes | 45 (15, 60) | 45 (30, 60) | 0 (−23.7 to 23.7)a |
| Incidence of postoperation nausea, No. (%) | 13 (41.9) | 10 (34.5) | 7.5 (−17.1 to 32.0)b |
| Incidence of postoperation adverse events, No. (%) | 17 (54.8) | 16 (55.2) | −0.3 (−25.5 to 24.9)b |
| No. (%) of adverse events | NR | ||
| 0 | 14 (45.2) | 13 (44.8) | |
| 1 | 11 (35.5) | 11 (37.9) | |
| 2 | 6 (19.4) | 3 (10.3) | |
| 3 | 0 | 2 (6.9) |
Abbreviations: NR, not reported; Q1, 1st quartile; Q3, 3rd quartile.
Indicates the difference in median between 2 groups as well as its 95% CI.
Indicates the difference in percentage between 2 groups as well as its 95% CI.
Discussion
In this study, we found differences in pain perception between IVAPAP and placebo favoring IVAPAP during the first 45 minutes. None of these differences were large enough to be clinically meaningful, although inspection of the 95% CIs around the differences suggest that the true effect of IVAPAP could be clinically meaningful. Importantly, at 12 and 24 hours, pain perception was actually less among the patients in the placebo group than patients in the IVAPAP group and the data are compatible with a clinically meaningful reduction in pain among the placebo group. The wide CI around the difference, the inclusion of the null value for the difference (0) in the CI, and the inclusion of clinically meaningful values for the difference in the CI range makes this an inconclusive study with regards to the primary study question.
A recent Cochrane meta-analysis found that intravenous acetaminophen formulations (paracetamol, proparacetamol) were superior to placebo in reducing postoperative pain and reducing opioid consumption, while demonstrating a similar safety profile to that of placebo. The intravenous formulation of acetaminophen has emerged in the United States as an attractive therapeutic agent given its predictable bioavailability and its relatively low adverse effect profile. To date, there have been few studies which investigate the efficacy of IVAPAP in controlling postoperative pain after ESS, the results of which have been mixed.
Kemppainen et al conducted a randomized, double-blind study including 74 patients (36 IVAPAP, 38 normal saline placebo) and compared the efficacy of IVAPAP vs placebo given immediately after surgery in reducing postoperative pain in patients undergoing FESS. The authors found that a single 1 g dose of IVAPAP given postoperatively was effective at reducing maximum pain experienced for the first 3 hours after surgery, with a significant difference in mean numeric rating score for pain at 2 hours. Total rescue opioid consumption use was reduced in the experimental group, but this did not result in reduction in opioid-related adverse events. In another study, Koteswara et al designed a randomized, double-blind study of 39 patients undergoing ESS to compare the preoperative and postoperative pain control related to the administration of 1 g of paracetamol. This study found a significant reduction in numeric pain score rating and amount of rescue opioid use in patients receiving preemptive doses of paracetamol. Similar to other studies, no differences in adverse events were noted. This study included only a limited description of the clinical characteristics of patients enrolled in the study.
Observation of the changes of the mean VAS score over the first 24 postoperative hours shows a relatively stable report of pain with intravenous acetaminophen. However, the placebo group reported a higher mean VAS score immediately after surgery, which decreased over the 24 postoperative hours resulting ultimately in a clinically meaningful difference in VAS score noted at both 12 and 24 hours after surgery. Similar differences in the trends in the mean VAS score over time were noted by Kemppainen et al when study medication was given immediately after FESS. Given the subjectivity of pain assessment, this trend in VAS score over time may suggest that IVAPAP stabilizes pain and minimizes spikes in postoperative pain, but it also may suggest that IVAPAP can prevent expected reduction in postoperative pain over time. This study is unable to differentiate between these 2 possibilities.
Limitations
There are several limitations of this study, including low precision and incomplete and missing data from patients after discharge. The low precision, as demonstrated by the wide CIs, associated with the primary outcome measure VAS score and the secondary outcome measures, undermines the ability to make definitive conclusions from the data. In addition, the incomplete and missing data from patients after discharge limits conclusion at these later time points. Despite the study’s limitations and its inconclusive results, inspection of the CIs highlight salient points regarding the value of intravenous acetaminophen in FESS. Acetaminophen may be effective in treating early postoperative pain, thus avoiding opioids and other narcotics. Although this study evaluated the effects of intravenous acetaminophen, the results warrant a study with oral acetaminophen. At the time of preparing this manuscript, intravenous acetaminophen was protected by patent. At approximately US $12 per unit, the associated costs of intravenous acetaminophen make it considerably more expensive than alternative analgesics. Although we did not conduct a cost analysis as part of our study, the value of intravenous acetaminophen in managing postoperative pain after FESS seems limited and alternative strategies, especially those with reduced associated cost, should be considered.
Conclusions
In this randomized clinical trial, we observed reduction in pain in the IVAPAP group relative to the placebo control in the first postoperative hour and the upper bound of the CI around this difference suggests a clinically meaningful difference is possible. Yet, the wide CI around the observed pain difference and the inclusion of the null value prevents definitive conclusions. The use of IVAPAP was associated with a reduction in postoperative opioid requirements but the inclusion of the null value in the CI and the wide CI also prevents definitive conclusions. Given the high cost of IVAPAP and inconclusive results, we cannot recommend its use for the control of perioperative pain reduction after ESS.
Trial Protocol
References
- 1.Pleis JR, Ward BW, Lucas JW. Summary health statistics for U.S. adults: National Health Interview Survey, 2009. Vital Health Stat 10. 2010;10(249):1-207. [PubMed] [Google Scholar]
- 2.Fokkens WJ, Lund VJ, Mullol J, et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl. 2012;23(23):3–, 1-298.. [PubMed] [Google Scholar]
- 3.Smith KA, Smith TL, Mace JC, Rudmik L. Endoscopic sinus surgery compared to continued medical therapy for patients with refractory chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014;4(10):823-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith TL, Kern R, Palmer JN, et al. Medical therapy vs surgery for chronic rhinosinusitis: a prospective, multi-institutional study with 1-year follow-up. Int Forum Allergy Rhinol. 2013;3(1):4-9. [DOI] [PubMed] [Google Scholar]
- 5.Poetker DM, Smith TL. Adult chronic rhinosinusitis: surgical outcomes and the role of endoscopic sinus surgery. Curr Opin Otolaryngol Head Neck Surg. 2007;15(1):6-9. [DOI] [PubMed] [Google Scholar]
- 6.Martin TJ, Yauck JS, Smith TL. Patients undergoing sinus surgery: constructing a demographic profile. Laryngoscope. 2006;116(7):1185-1191. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharyya N. Ambulatory sinus and nasal surgery in the United States: demographics and perioperative outcomes. Laryngoscope. 2010;120(3):635-638. [DOI] [PubMed] [Google Scholar]
- 8.2016. Physician Quality Reporting System (PQRS) Measure Specifications Manual for Claims and Registry Reporting of Individual Measures. Centers for Medicare & Medicaid Services. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS/MeasuresCodes.html. Accessed February 1, 2017.
- 9.Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. [DOI] [PubMed] [Google Scholar]
- 10.Kemppainen T, Kokki H, Tuomilehto H, Seppä J, Nuutinen J. Acetaminophen is highly effective in pain treatment after endoscopic sinus surgery. Laryngoscope. 2006;116(12):2125-2128. [DOI] [PubMed] [Google Scholar]
- 11.Friedman M, Venkatesan TK, Lang D, Caldarelli DD. Bupivacaine for postoperative analgesia following endoscopic sinus surgery. Laryngoscope. 1996;106(11):1382-1385. [DOI] [PubMed] [Google Scholar]
- 12.Wise SK, Wise JC, DelGaudio JM. Evaluation of postoperative pain after sinonasal surgery. Am J Rhinol. 2005;19(5):471-477. [PubMed] [Google Scholar]
- 13.Wong A, Kacker A. Incidence of unplanned admissions after sinonasal surgery: a 6-year review. Int Forum Allergy Rhinol. 2014;4(2):143-146. [DOI] [PubMed] [Google Scholar]
- 14.Georgalas C, Obholzer R, Martinez-Devesa P, Sandhu G. Day-case septoplasty and unexpected re-admissions at a dedicated day-case unit: a 4-year audit. Ann R Coll Surg Engl. 2006;88(2):202-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhattacharyya N. Unplanned revisits and readmissions after ambulatory sinonasal surgery. Laryngoscope. 2014;124(9):1983-1987. [DOI] [PubMed] [Google Scholar]
- 16.Leykin Y, Casati A, Rapotec A, et al. Comparison of parecoxib and proparacetamol in endoscopic nasal surgery patients. Yonsei Med J. 2008;49(3):383-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koteswara CM, D S. A study on pre-emptive analgesic effect of intravenous paracetamol in functional endoscopic sinus surgeries (FESSs): a randomized, double-blinded clinical study. J Clin Diagn Res. 2014;8(1):108-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Church CA, Stewart C IV, O-Lee TJ, Wallace D. Rofecoxib versus hydrocodone/acetaminophen for postoperative analgesia in functional endoscopic sinus surgery. Laryngoscope. 2006;116(4):602-606. [DOI] [PubMed] [Google Scholar]
- 19.Goodman SN, Berlin JA. The use of predicted confidence intervals when planning experiments and the misuse of power when interpreting results. Ann Intern Med. 1994;121(3):200-206. [DOI] [PubMed] [Google Scholar]
- 20.Guyatt GH, Juniper EF, Walter SD, Griffith LE, Goldstein RS. Interpreting treatment effects in randomised trials. BMJ. 1998;316(7132):690-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallagher EJ, Liebman M, Bijur PE. Prospective validation of clinically important changes in pain severity measured on a visual analog scale. Ann Emerg Med. 2001;38(6):633-638. [DOI] [PubMed] [Google Scholar]
- 22.Todd KH. Clinical versus statistical significance in the assessment of pain relief. Ann Emerg Med. 1996;27(4):439-441. [DOI] [PubMed] [Google Scholar]
- 23.Todd KH, Funk KG, Funk JP, Bonacci R. Clinical significance of reported changes in pain severity. Ann Emerg Med. 1996;27(4):485-489. [DOI] [PubMed] [Google Scholar]
- 24.Tzortzopoulou A, McNicol ED, Cepeda MS, Francia MB, Farhat T, Schumann R. Single dose intravenous propacetamol or intravenous paracetamol for postoperative pain. Cochrane Database Syst Rev. 2011;(10):CD007126. [DOI] [PubMed] [Google Scholar]
- 25.Gupta A, Lee LK, Rao S, Aancha S, Dadachanji C, Voralu K. Retrospective pharmacoeconomic analysis of perioperative use of intravenous acetaminophen. Austin J Anesthesia and Analgesia. 2014;2(3):1020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol