This randomized clinical trial determines the efficacy of a depression prevention strategy among mothers whose families are enrolled in Head Start.
Key Points
Question
Is a lay-delivered, problem-solving intervention embedded in Head Start efficacious in preventing clinically important depressive symptom episodes among at-risk, low-income mothers?
Findings
In this randomized clinical trial of 230 Head Start mothers, those receiving problem-solving education experienced a 60% incident rate of depressive symptom episodes compared with those not receiving it. Among the subpopulation with low symptom levels at baseline, those receiving problem-solving education experienced a 39% incident rate.
Meaning
The efficacy of problem-solving education demonstrates the promise of embedding maternal depression prevention programs in Head Start; additional effectiveness studies are necessary to develop meaningful public health programs.
Abstract
Importance
Low-income and minority mothers experience a disproportionate incidence of depression and lack access to treatment services. Development of prevention strategies in accessible community-based venues is a potentially important public health strategy.
Objective
To determine the efficacy of a depression prevention strategy embedded in Head Start.
Design, Setting, and Participants
This randomized clinical trial was performed from February 15, 2011, through May 9, 2016, at 6 Head Start agencies serving families at or below the federal poverty level. Participants included mothers with depressed mood, anhedonia, or depression history but who were not in a current major depressive episode. Participants were followed up for 12 months with masked outcome assessments. Final follow-up was completed on May 9, 2016.
Interventions
Participants were randomized to a problem-solving education (PSE) intervention (n = 111) or usual Head Start services (n = 119).
Main Outcomes and Measures
Primary outcomes were problem-solving skills and depressive symptoms. To capture the chronicity and intensity of symptoms, the Quick Inventory of Depressive Symptoms was administered bimonthly, and rates of clinically significant symptom elevations were compared across groups. Secondarily, the presence of a major depressive episode was assessed using the Structured Clinical Interview for DSM-IV Axis I Disorders.
Results
Among the 230 participants, 152 (66.1%) were Hispanic, with a mean (SD) age of 31.4 (7.3) years. An intention-to-treat analysis among 223 participants contributing follow-up data found no differences in problem-solving skills across groups. The mean (SD) number of depressive symptom elevations among the PSE participants was 0.84 (1.39) compared with 1.12 (1.47) among the usual service participants (adjusted incident rate ratio [aIRR], 0.60; 95% CI, 0.41-0.90). In analyses stratified according to baseline depressive symptoms, PSE exerted a preventive effect among those with lower-level baseline symptoms, with a mean (SD) of 0.39 (0.84) elevations among PSE participants compared with 0.88 (1.37) among usual service participants (aIRR, 0.39; 95% CI, 0.21-0.75). However, no difference was observed among those with higher-level baseline symptoms (mean [SD] elevations, 2.06 [1.92] for PSE and 2.00 [1.91] for usual service; aIRR, 1.10; 95% CI, 0.67-1.80). Analysis of symptom scores followed the same pattern, with an adjusted mean reduction of 1.33 (95% CI, 0.36-2.29) among participants with lower-level baseline symptoms.
Conclusions and Relevance
The PSE intervention is efficacious in preventing depressive symptom episodes and performs optimally among those with initial low-level symptoms. Additional effectiveness studies in Head Start are necessary to develop meaningful public health programs.
Trial Registration
clinicaltrials.gov Identifier: NCT01298804
Introduction
Maternal depression disproportionately affects low-income and minority women and negatively affects children. In 2009, the Institute of Medicine published a report, Depression in Parents, Parenting, and Children: Opportunities to Improve Identification, Treatment, and Prevention, in which it called for initiatives to take place in community-based venues capable of providing services for adults and children. One such venue in the United States is Head Start, a federally funded early learning program that provides services for approximately 1 million low-income families each year. Depression affects almost half of all Head Start mothers.
Because of disparities in access to mental health services for low-income and minority populations, embedding effective mental illness prevention strategies in accessible community-based venues such as Head Start is a potentially important public health strategy. Our particular approach used the paradigm of screening, brief intervention, and referral to treatment, in which screening identified an at-risk population, a brief intervention aimed to prevent the emergence or worsening of depressive symptoms, and individuals with persistent or escalating symptoms were referred to formal behavioral health services.
Our brief intervention was problem-solving education (PSE), a 6-session cognitive behavioral program. Although problem solving has served as a component of other depression prevention and treatment models, our study, to the best of our knowledge, is the first to embed a lay-delivered intervention in a community-based agency charged with addressing the needs of low-income families. In the case management infrastructure of Head Start, we randomized participants to receive PSE or usual Head Start case management services. During 12 months of follow-up, we measured problem-solving skills and depressive symptoms as our primary intervention outcomes.
Methods
Design
We conducted a parallel-group efficacy trial with 1:1 randomization. We worked within 6 Head Start centers in Boston, Massachusetts, serving families of children from birth to 5 years of age at or below the federal poverty level. A copy of the study protocol is found in the Supplement. We enrolled participants from February 15, 2011, through May 20, 2015. Head Start caseworkers screened mothers for depression risk; research staff assessed eligibility and obtained informed consent. The institutional review board of Boston University Medical Center approved this study. All participants provided written informed consent.
Participants
We recruited mothers whose children were expected to remain in Head Start for at least 6 months, targeting those at increased risk for depression but excluding those in a current major depressive episode (MDE). At risk was defined as experiencing depressed mood or anhedonia according to the Patient Health Questionnaire–2 or a recent history of depression according to the Composite International Diagnostic Interview. We determined the presence of an MDE using the Mini-International Neuropsychiatric Interview. We excluded mothers with high levels of suicidal ideation according to the suicide screen of the MacArthur Initiative on Depression and Primary Care and those with cognitive limitation according to the MacArthur Competence Assessment Tool. We enrolled English- and Spanish-speaking mothers.
Randomization
We used stratified, blocked randomization to allocate participants to PSE or usual Head Start services according to computer-generated lists. Randomization occurred independently at each Head Start site in strata defined by depression history and was balanced in randomly varying blocks of 2 and 4. Lists were concealed in opaque envelopes. Outcome assessors, investigators, and Head Start personnel were masked to allocation.
Study Arms
Usual Head Start services included regular family needs assessments, home visitation, parenting groups, referrals to behavioral health services, and assistance with accessing community resources for food, job training, and housing. We decided against a formal attention control group because we wanted to compare our intervention with real-world Head Start services and because such services already represent a high level of interpersonal attention.
The PSE intervention included the following 3 components: a series of 6 one-on-one workbook-based problem-solving sessions (adapted from Hegel and Arean), depressive symptom monitoring, and linkage to formal mental health services when necessary. Problem-solving sessions lasted 30 to 60 minutes and were conducted as home visits or in Head Start centers for 6 to 8 weeks. Each session consisted of the following 7 steps: defining a problem, goal setting, generating solutions, implementing decision-making guidelines, evaluating solutions, implementing solutions, and evaluating outcomes. Motivational interviewing was used to promote intervention adherence.
The PSE providers assessed depressive symptoms with the Patient Health Questionnaire–9 at every other session. Participants with moderate symptoms on 2 assessments or severe symptoms on a single assessment were referred to mental health services using motivational interviewing techniques. A crisis management plan was implemented for suicidal ideation.
Intervention Provider Training
We trained 15 lay intervention providers (not licensed mental health clinicians). Training workshops lasted 1 day and were followed by up to 5 training cases. Trainees were certified as PSE providers after completing 2 cases in which they met fidelity criteria according to standardized criteria developed in prior work. Trainees learned motivational interviewing in a separate 2-day workshop. Participants were randomly assigned to linguistically matched providers.
Supervision and Fidelity Monitoring
Provider supervision consisted of weekly group meetings, facilitated by a master’s-level social worker (Y.D.-L.). We audiotaped 1 randomly selected session for each participant and used the same fidelity criteria as in provider training. Fidelity was rated according to the proportion of core PSE components delivered appropriately on a scale ranging from poor (<60%) to excellent (≥90%).
Baseline Data
Before randomization, we collected a self-report of mothers’ age and number of children, race/ethnicity, educational level, work status, and household status (single- vs dual-parent). We assessed anxiety symptoms with the Beck Anxiety Inventory, trauma history and posttraumatic stress disorder (PTSD) symptoms with the modified PTSD Symptom Scale, and problem-solving ability with the Social Problem Solving Inventory, which includes subscales in positive and negative orientation, avoidance, impulsivity, and rationality.
We assessed depressive symptoms with the Quick Inventory of Depressive Symptoms (QIDS). We used the widely accepted QIDS score cut points of 11 or greater, the clinical threshold for moderately severe symptoms, and 14 or greater, the threshold that optimally balances sensitivity and specificity for estimating MDE.
Outcome Assessment
We followed up participants for 12 months, beginning data collection 2 months after randomization. To determine use of mental health services, we administered an adapted version of the Collaborative Psychiatric Epidemiology Studies for bimonthly assessment of use of specialty services (eg, psychiatrist, psychologist, therapist, or social worker) during the preceding 2 months. Follow-up was completed on May 9, 2016.
We assessed problem-solving skills at 6 and 12 months, examining results as change scores for the composite measure and for the positive problem orientation and rational problem-solving subscales. We assessed depressive symptoms bimonthly, operationalizing our primary outcome as elevations to the moderately severe threshold (QIDS score, ≥11). We conducted sensitivity analyses using the QIDS threshold score of at least 14. As an exploratory analysis, we assessed at 12 months of follow-up whether participants met criteria for MDE at any point during the follow-up period or during the follow-up period’s final month. We had initially planned to use the Composite International Diagnostic Interview for this purpose but, after trial enrollment began, decided to use the Structured Clinical Interview for DSM-IV Axis I Disorders. We made this change before assessing any participants for MDE.
Statistical Analysis
To compare use of mental health services across study groups, we conducted χ2 analyses of utilization data aggregated across the full follow-up period. To estimate intervention effect on our primary outcome measures, we conducted intention-to-treat analyses using a set of binary variables to model the effects of the Head Start site. To assess problem-solving skills, we used linear regression to compare change scores between baseline and 6 months and between baseline and 12 months. We used negative binomial regression to compare rates of depressive symptom elevations during follow-up, using an offset to standardize rates according to the number of assessments completed. Consistent with prior work, we adjusted all main effects models for baseline QIDS score.
We conducted stratified analyses to determine whether a differential intervention effect occurred among mothers whose baseline QIDS scores were higher or lower than either of the 2 prespecified thresholds and formally assessed effect modification by entering group-by-QIDS threshold interaction terms into the models. In our primary analysis, we stratified according to the QIDS threshold of 11 or greater; in our sensitivity analysis, we stratified according to the threshold of 14 or greater. For those with baseline scores below the threshold, we used Kaplan-Meier curves and Cox proportional hazards regression models to determine differences in time to symptom elevation. In stratified models, we removed baseline QIDS score as a covariate.
We analyzed mean QIDS scores over time using linear models to examine time-averaged scores and group-by-time interaction effects. We used logistic regression to model the incidence of MDE.
We explored provider effects by estimating regression models to determine variation in participant outcomes across PSE providers, controlling for Head Start site. We verified our main results using multiple imputation techniques for missing data. We imputed data in 20 sequential data sets using information (maternal age, educational level, work, and single-vs-dual parent status; number of children; depression and anxiety scores; and trauma history) at earlier points to impute data at later points. We conducted all analyses with SAS software (version 9.3; SAS Institute Inc). Unless otherwise indicated, data are expressed as mean (SD).
Sample Size
We estimated our sample size to provide power to test a clinically significant difference across intervention arms on the composite problem-solving measure and rate of symptom elevations. We estimated that a sample size of 100 per group would be able to detect a 1-point difference in mean composite problem-solving scores, and a Poisson regression analysis would be able to detect a 33% reduction in the rate of symptom elevations from 1.2 to 0.8 per 6 assessments. Our sample size of 230 assumed 80% power, a 2-sided α value of .05, and 15% loss to follow-up.
Results
Enrollment
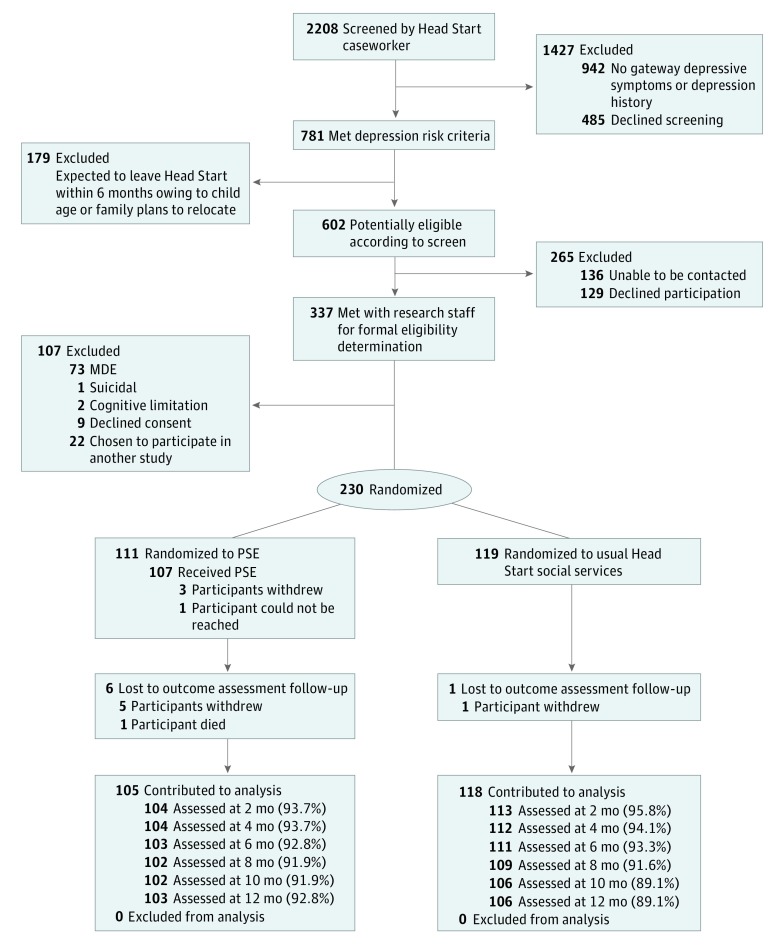
Head Start caseworkers screened 2208 mothers (Figure 1); 781 met depression risk criteria. Of these, 179 were ineligible because the child was expected to leave Head Start within 6 months. Of the remaining 602 mothers, 136 could not be contacted and 129 refused participation. Staff met with the remaining 337 mothers for eligibility determination; of these, 73 met criteria for MDE, 1 had suicidal ideation, and 2 had cognitive limitations, leaving 261 eligible participants. Nine of these declined consent, and 22 were randomly selected to participate in a separate study. We enrolled the remaining 230 mothers (mean [SD] age, 31.4 [7.3] years).
Figure 1. CONSORT Diagram .
MDE indicates major depressive episode; PSE, problem-solving education.
Baseline Characteristics
Hispanic mothers constituted most of our sample (152 [66.1%]). Seventy mothers (30.4%) had moderately severe depressive symptoms at baseline. Thirty-three women (14.3%) were taking antidepressant medication; 97 (42.2%) had a history of MDE. None of these baseline measures differed between study groups (Table 1). Although baseline mean depressive symptom scores were balanced between groups (8.11 [5.20] in PSE vs 7.59 [4.38] in usual service groups), the PSE group had a higher proportion of mothers at the QIDS threshold of 14 or greater (23 [20.7%] vs 8 [6.7%]).
Table 1. Baseline Characteristics of Participants.
| Characteristic | Study Group | |
|---|---|---|
| PSE Intervention (n = 111) |
Usual Head Start Services (n = 119) |
|
| Maternal demographic characteristics | ||
| Age, mean (SD), y | 31.4 (7.1) | 31.3 (7.5) |
| No. of children, mean (SD) | 2.5 (1.3) | 2.1 (1.2) |
| Race/ethnicity, No. (%) | ||
| Black | 37 (33.3) | 44 (37) |
| Asian | 0 | 3 (2.5) |
| White | 28 (25.2) | 33 (27.7) |
| Hispanic | 75 (67.6) | 77 (64.7) |
| Other, including multiracial | 46 (41.4) | 39 (32.8) |
| Educational level, No. (%) | ||
| Less than high school, including GEDa | 57 (51.8) | 39 (32.8) |
| High school diploma | 16 (14.5) | 47 (39.5) |
| Some college | 28 (25.4) | 25 (21.0) |
| College degree or higher | 9 (8.2) | 8 (6.7) |
| Works outside of the home, No. (%) | 45 (40.5) | 53 (44.5) |
| Single-parent household, No. (%) | 67 (60.4) | 69 (58) |
| Maternal mental health measures | ||
| Depressive symptom score on QIDS, mean (SD)b | 8.11 (5.20) | 7.59 (4.38) |
| Beck Anxiety Inventory score, mean (SD)c | 12.08 (10.61) | 12.07 (10.25) |
| QIDS score ≥11, No. (%) | 36 (32.4) | 34 (28.6) |
| QIDS score ≥14, No. (%) | 23 (20.7) | 8 (6.7) |
| Currently receiving antidepressive treatment, No. (%) | 14 (12.6) | 19 (16.0) |
| History of MDE, No. (%) | 50 (45.0) | 47 (39.5) |
| Ever seen a mental health professional, psychologist, therapist, or mental health social worker, No. (%) | 54 (48.6) | 60 (50.4) |
| Trauma history, No. (%) | 78 (70.3) | 74 (62.2) |
| Social Problem Solving Inventory scores, mean (SD) | ||
| Totald | 13.04 (2.83) | 13.02 (2.63) |
| Positive problem orientation subscalee | 2.49 (0.82) | 2.54 (0.81) |
| Rational problem-solving subscalef | 2.41 (0.85) | 2.49 (0.85) |
Abbreviations: GED, general equivalency diploma; MDE, major depressive episode; PSE, problem-solving education; QIDS, Quick Inventory of Depressive Symptoms.
Data were missing for 1 participant in the PSE group.
Scores range from 0 to 20; 11 or greater indicates moderately severe depressive symptoms, and 14 or greater indicates more severe depressive symptoms.
Scores range from 0 to 46, with higher scores indicating a greater level of anxiety symptoms.
Scores range from 5.8 to 19.0, with higher scores indicating better performance on the composite measure.
Scores range from 0.8 to 4.0, with higher scores indicating a more positive orientation to problem solving.
Scores range from 0.2 to 4.0, with higher scores indicating a more rational approach to problem solving.
Intervention Delivery and Fidelity
The PSE group included 111 mothers. Across 15 PSE providers, the mean caseload size was 7.4 (7.5) clients, with a range from 1 to 26. Of a possible 6 PSE sessions, the mean number completed was 4.64 (2.06); 65 participants (58.6%) completed a full PSE course. Of 54 audiotaped PSE sessions (57 mothers declined audiotaping), 28 met criteria for good model fidelity (≥80% of PSE components delivered), 25 met criteria for excellent fidelity (≥90%), and 1 audiofile was damaged. Problem-solving education providers referred 10 mothers to formal mental health services and responded to possible mental health crises for 13, none of which eventuated in an adverse event. Thirty-seven of 105 PSE participants (35.2%) engaged with specialty mental health services during follow-up compared with 39 of 118 (33.1%) usual service participants (P = .73).
Problem Solving
Mean composite problem-solving scores in the PSE group changed from 13.04 (2.83) at baseline to 13.55 (2.68) at 6 months and 14.12 (2.79) at 12 months. In the usual service group, they changed from 13.02 (2.63) at baseline to 13.34 (2.38) at 6 months and 13.81 (2.78) at 12 months, amounting to no statistically significant differences across groups (Table 2).
Table 2. Outcomes for Full Samplea.
| Outcome | Study Group | Adjusted Measure of Association (95% CI) | |
|---|---|---|---|
| PSE Intervention (n = 105) |
Usual Head Start Services (n = 118) |
||
| Change in Social Problem Solving Inventory scores from baseline to 6 mo, mean (SD) | |||
| Totalb | 0.58 (2.06) | 0.24 (2.17) | Adjusted difference, 0.31 (−0.25 to 0.87) |
| Positive problem orientation subscalec | 0.03 (0.83) | −0.14 (0.90) | Adjusted difference, 0.17 (−0.07 to 0.40) |
| Rational problem-solving subscaled | −0.03 (0.80) | −0.20 (0.88) | Adjusted difference, 0.18 (−0.05 to 0.40) |
| Depressive symptoms and MDE | |||
| Rate of moderately severe symptom spikese | 0.17 (0.28) | 0.28 (0.35) | aIRR, 0.60 (0.41 to 0.90) |
| Rate of more severe symptom spikesf | 0.08 (0.18) | 0.11 (0.22) | aIRR, 0.51 (0.29 to 0.90) |
| Met criteria for MDE during the 12-mo follow-up, No. (%) | 25/102 (24.5) | 32/110 (29.1) | aOR, 0.70 (0.37 to 1.32) |
| Met criteria for MDE during the final study month, No. (%) | 6/102 (5.9) | 12/110 (10.9) | aOR, 0.43 (0.15 to 1.25) |
Abbreviations: aIRR, adjusted incident rate ratio; aOR, adjusted odds ratio; MDE, major depressive episode; PSE, problem-solving education; QIDS, Quick Inventory of Depressive Symptoms.
Includes 223 participants. All models adjusted for Head Start site and baseline QIDS.
Change scores range from −5.2 to 6.2, with higher scores indicating more positive change.
Change scores range from −2.4 to 2.4, with higher scores indicating more positive change.
Change scores range from −2.6 to 2.4, with higher scores indicating more positive change.
The mean (SD) number of moderately severe depressive symptom spikes (QIDS score ≥11) in the PSE group was 0.84 (1.39) compared with 1.12 (1.47) in the usual service group.
The mean (SD) number of more severe spikes in the PSE group (QIDS score ≥14) was 0.45 (1.01) compared with 0.61 (1.17) in the usual service group.
Depressive Symptoms
The QIDS scores were missing from 102 of 1380 possible follow-up assessments (7.4%). In the full sample, the mean number of moderately severe symptom elevations in the PSE group was 0.84 (1.39) compared with 1.12 (1.47) in the usual service group; considering a possible 6 outcome assessments, rates were 0.17 (0.28) and 0.28 (0.35), respectively. This difference produced an adjusted incident rate ratio (aIRR) of 0.60 (95% CI, 0.41-0.90) in favor of PSE.
Because we found a significant interaction between study group and baseline QIDS score (group-by-QIDS interaction, P = .03), we conducted stratified analyses to determine the difference in intervention effect among mothers above or below the QIDS threshold of 11 or greater at baseline (Table 3). Among those below the threshold, PSE exerted a preventive effect on symptom elevations (aIRR, 0.39; 95% CI, 0.21-0.75); however, among those above the threshold, PSE had no effect (aIRR, 1.10; 95% CI, 0.67-1.80).
Table 3. Primary Outcomes in Strata Corresponding to Baseline Depressive Symptoms.
| Outcome | Population With Lower Depressive Symptoms at Baselinea | Population With Higher Depressive Symptoms at Baselinea | ||||
|---|---|---|---|---|---|---|
| Study Group | Adjusted Measure of Association (95% CI) | Study Group | Adjusted Measure of Association (95% CI) | |||
| PSE Intervention (n=70) |
Usual Head Start Services (n=84) |
PSE Intervention (n=35) |
Usual Head Start Services (n=34) |
|||
| Change in Social Problem Solving Inventory scores from baseline to 6 mo, mean (SD) | ||||||
| Totalb | 0.43 (2.08) | 0.05 (2.22) | Adjusted difference, 0.41 (−0.27 to 1.10) | 0.89 (2.00) | 0.71 (1.98) | Adjusted difference, 0.37 (−0.71 to 1.45) |
| Positive problem orientation subscalec | −0.03 (0.87) | −0.22 (0.88) | Adjusted difference, 0.20 (−0.09 to 0.49) | 0.17 (0.74) | 0.07 (0.91) | Adjusted difference, 0.22 (−0.22 to 0.67) |
| Rational problem-solving subscaled | −0.01 (0.80) | −0.21 (0.82) | Adjusted difference, 0.22 (−0.05 to 0.48) | −0.08 (0.80) | −0.18 (1.00) | Adjusted difference, 0.17 (−0.33 to 0.67) |
| Depressive symptoms and MDE | ||||||
| Rate of moderately severe symptom spikes (QIDS score ≥11) | 0.07 (0.15)e | 0.15 (0.29)e | aIRR, 0.39 (0.21 to 0.75)e | 0.36 (0.37)f | 0.36 (0.37)f | aIRR, 1.10 (0.67 to 1.80)f |
| Rate of more severe symptom spikes (QIDS score ≥14)g | 0.04 (0.10)e | 0.10 (0.19)e | aIRR, 0.35 (0.17 to 0.73)e | 0.22 (0.34)f | 0.23 (0.56)f | aIRR, 0.89 (0.28 to 2.86)f |
| Time to moderately severe symptom spike | NA | NA | aHR, 0.52 (0.28 to 0.95) | NA | NA | NA |
| Time to more severe symptom spike | NA | NA | aHR, 0.48 (0.25 to 0.92) | NA | NA | NA |
| Met criteria for MDE during the 12-mo follow-up period, No. (%) | 12/68 (17.6) | 18/78 (23.1) | aOR, 0.72 (0.31 to 1.69) | 13/34 (38.2) | 14/32 (43.8) | aOR, 1.10 (0.36 to 3.39) |
| Met criteria for MDE during the final study month, No. (%) | 6/68 (4.4) | 6/78 (7.7) | aOR, 0.55 (0.13 to 2.36) | 3/34 (8.8) | 6/32 (18.8) | aOR, 0.47 (0.10 to 2.19) |
Abbreviations: aHR, adjusted hazard ratio; aIRR, adjusted incident rate ratio; aOR, adjusted odds ratio; NA, not applicable; MDE, major depressive episode; PSE, problem-solving education; QIDS, Quick Inventory of Depressive Symptoms.
Sample sizes are provided for the strata defined by baseline QIDS score above or below a threshold of 11 or greater. If we apply the threshold of 14 or greater, the lower baseline symptom stratum has 83 PSE and 110 usual service participants, and the higher baseline symptoms stratum has 22 PSE and 8 usual service participants. All models are adjusted for Head Start site.
Change scores range from −5.2 to 6.2, with higher scores indicating more positive change.
Change scores range from −2.4 to 2.4, with higher scores indicating more positive change.
Change scores from −2.6 to 2.4, with higher scores indicating more positive change.
Among the subgroup below the threshold, the mean (SD) number of moderately severe depressive symptom spikes in the PSE group was 0.39 (0.84) compared with 0.88 (1.37) in the usual service group; the mean (SD) number of more severe spikes in the PSE group was 0.24 (0.73) compared with 0.56 (1.15) in the usual service group.
Among the subgroup above the threshold, the mean (SD) number of moderately severe depressive symptom spikes in the PSE group was 2.06 (1.92) compared with 2.00 (1.91) in the usual service group; the mean (SD) number of more severe spikes in the PSE group was 1.23 (1.48) compared with 1.25 (1.28) in the usual service group.
When we model symptom spikes as elevations to the QIDS threshold of 14 or greater, we also define our strata according to that threshold.
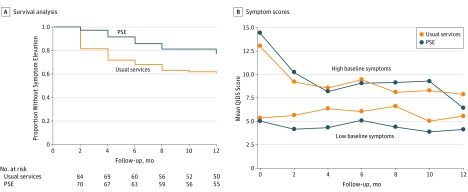
Figure 2A shows Kaplan-Meier curves for the PSE and usual service groups for those under the symptom threshold of 11 or greater at baseline. Cox proportional hazards regression models estimated an adjusted hazard ratio of 0.52 (95% CI, 0.28-0.95) in favor of PSE. We replicated all models using the threshold of 14 or greater for the outcome measure and stratification variable and obtained nearly identical results.
Figure 2. Trajectory of Depressive Symptoms Over Time.
A, One hundred fifty-four patients were included in the survival analysis. B, Two hundred twenty-three patients were included in the mean symptom scores assessed using the Quick Inventory of Depressive Symptoms (QIDS). Scores range from 0 to 20, with higher scores indicating higher levels of depressive symptoms. PSE indicates problem-solving education.
In the full sample, time-averaged depressive symptom scores were lower in the PSE group (5.84 [4.61] vs 6.70 [4.78]), for an adjusted difference in symptom scores of 0.90 (95% CI, 0.09-1.71). However, because of an interaction (P = .08) between study group and baseline QIDS score, we conducted stratified analyses to determine the difference in intervention effect among mothers with and without moderately severe symptoms at baseline (Figure 2B). Among those with scores below the threshold, PSE produced a significant reduction in time-averaged symptom scores (adjusted difference, 1.33; 95% CI, 0.36-2.29); however, among those with scores above the threshold at baseline, PSE produced no significant reduction (adjusted difference, −0.33; 95% CI, −2.10 to 1.44). In all models, the group-by-time interaction terms were nonsignificant.
Twenty-five of 102 PSE participants (24.5%) met MDE criteria during the full follow-up period compared with 32 of 110 usual service participants (29.1%), for an adjusted odds ratio of 0.70 (95% CI, 0.37-1.32). During the final month of the follow-up, 6 of 102 PSE participants (5.9%) met criteria for MDE compared with 12 of 110 usual service participants (10.9%), for an adjusted odds ratio of 0.43 (95% CI, 0.15-1.25).
We found no evidence of variation in outcomes by PSE provider. Imputing missing data did not change our results.
Discussion
The PSE intervention substantially reduced the rate of symptomatic person-time during a full calendar year, particularly for those with low symptom burdens at baseline. Among this subgroup, PSE also produced lower time-averaged depressive symptom scores, following a pattern that suggested early and sustained improvement in symptoms. These differences in depressive symptoms have clear implications for participants’ overall quality of life.
Previous studies have demonstrated the effectiveness of problem-solving interventions in treating and preventing adult depression. Participants in these trials, however, have rarely represented the US demographic groups with the poorest access to conventional treatment services. Few such prevention programs, furthermore, have harnessed the infrastructure of community-based organizations to serve as intervention delivery systems. For young, low-income mothers, embedding efficacious mental health programs into accessible, community-based structures is a potentially important public health strategy with implications for both parents and children.
One notable finding in our study was the PSE intervention’s optimal performance among mothers with lower baseline symptoms. This pattern of results is consistent with PSE’s design as a prevention intervention, and we offer 2 explanations for this finding. First, as a stand-alone intervention, PSE may not be intense enough to break through clinically significant symptoms to produce its effect. Second, because PSE was designed as a preventive model, we intentionally uncoupled the problems that participants were asked to solve from feelings of sadness, and we emphasized behavioral activation over cognitive restructuring. In theory, this approach could have worked better among a subpopulation without the intrinsic deactivation associated with a higher depressive symptom burden.
Limitations
Our study has limitations. Although PSE providers had educational levels and cultural backgrounds similar to those of Head Start caseworkers, the providers were paid study personnel. Although such an efficacy design was necessary to ensure safety and proof of principle, additional effectiveness testing is necessary. Second, whether our principal outcome (elevations in depressive symptoms, measured bimonthly) was a metric equally robust as a diagnostic measure of MDE administered at the end of the follow-up period is debatable. We believed that the former represented a more granular analysis of the intensity and chronicity of symptoms, which may be the primary mechanisms by which maternal depression affects children. We thus powered our trial on symptomatic person-time and considered MDE as an exploratory measure, differences in which our trial was not powered to detect. Last, although the positive results on depressive symptoms produced by a problem-solving intervention in the absence of differences in problem-solving abilities may appear to be curious, such a finding is not new. These findings highlight the need to conduct additional research into potential intervention mechanisms.
Conclusions
Our study contributes to the literature on the role of problem solving as a viable depression prevention strategy across diverse populations and settings. Additional effectiveness studies, specifically in the Head Start setting, are necessary to translate our results into meaningful public health programs.
Trial Protocol
References
- 1.Hammen C, Gordon D, Burge D, Adrian C, Jaenicke C, Hiroto D. Maternal affective disorders, illness, and stress: risk for children’s psychopathology. Am J Psychiatry. 1987;144(6):736-741. [DOI] [PubMed] [Google Scholar]
- 2.England MJ, Sim L. Depression in Parents, Parenting, and Children: Opportunities to Improve Identification, Treatment, and Prevention. Washington, DC: Institute of Medicine, National Academy Press; 2009. [PubMed] [Google Scholar]
- 3.Koepsell TD, Diehr PH, Cheadle A, Kristal A. Invited commentary: symposium on community intervention trials. Am J Epidemiol. 1995;142(6):594-599. [DOI] [PubMed] [Google Scholar]
- 4.Early Head Start Research and Evaluation Project. Depression in the Lives of Early Head Start Families: Research to Practice. United States Department of Health and Human Services; April 2006. https://www.acf.hhs.gov/sites/default/files/opre/research_brief_depression.pdf. Accessed May 5, 2017.
- 5.Office of the Surgeon General (US); Center for Mental Health Services (US) . National Institute of Mental Health (US). Mental Health: Culture, Race, and Ethnicity: A Supplement to Mental Health: A Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2001. [PubMed] [Google Scholar]
- 6.Wang PS, Berglund P, Kessler RC. Recent care of common mental disorders in the United States: prevalence and conformance with evidence-based recommendations. J Gen Intern Med. 2000;15(5):284-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babor TF, McRee BG, Kassebaum PA, Grimaldi PL, Ahmed K, Bray J. Screening, brief intervention, and referral to treatment (SBIRT): toward a public health approach to the management of substance abuse. Subst Abus. 2007;28(3):7-30. [DOI] [PubMed] [Google Scholar]
- 8.Buntrock C, Ebert DD, Lehr D, et al. . Effect of a web-based guided self-help intervention for prevention of major depression in adults with subthreshold depression: a randomized clinical trial. JAMA. 2016;315(17):1854-1863. [DOI] [PubMed] [Google Scholar]
- 9.Unützer J, Katon W, Callahan CM, et al. ; IMPACT Investigators. . Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288(22):2836-2845. [DOI] [PubMed] [Google Scholar]
- 10.Ciechanowski P, Wagner E, Schmaling K, et al. . Community-integrated home-based depression treatment in older adults: a randomized controlled trial. JAMA. 2004;291(13):1569-1577. [DOI] [PubMed] [Google Scholar]
- 11.Mrazek PJ, Haggerty RJ, eds. Reducing Risks for Mental Disorders: Frontiers for Preventive Intervention Research. Washington, DC: National Academies Press; 1994. [PubMed] [Google Scholar]
- 12.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire–2: validity of a two-item depression screener. Med Care. 2003;41(11):1284-1292. [DOI] [PubMed] [Google Scholar]
- 13.Kessler RC, Abelson J, Demler O, et al. . Clinical calibration of DSM-IV diagnoses in the World Mental Health (WMH) version of the World Health Organization (WHO) Composite International Diagnostic Interview (WMHCIDI). Int J Methods Psychiatr Res. 2004;13(2):122-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheehan DV, Lecrubier Y, Sheehan KH, et al. . The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22-33. [PubMed] [Google Scholar]
- 15.John D. & Catherine T. MacArthur Foundation’s Initiative on Depression and Primary Care. Depression Management Tool kit. The MacArthur Initiative on Depression and Primary Care. http://www.integration.samhsa.gov/clinical-practice/macarthur_depression_toolkit.pdf 2009. Accessed May 5, 2017.
- 16.Appelbaum PS, Grisso T, Frank E, O’Donnell S, Kupfer DJ. Competence of depressed patients for consent to research. Am J Psychiatry. 1999;156(9):1380-1384. [DOI] [PubMed] [Google Scholar]
- 17.Hegel MT, Arean PA Problem Solving Treatment for Primary Care: A Treatment Manual for Depression. 2003. http://www.public-health.uiowa.edu/icmha/outreach/documents/ProblemSolvingTreatmentforPrimaryCare.PDF. Accessed May 5, 2017.
- 18.Miller W, Rollnick S. Motivational Interviewing: Preparing People for Change. 2nd ed New York, NY: Guilford Press; 2002. [Google Scholar]
- 19.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feinberg E, Augustyn M, Fitzgerald E, et al. . Improving maternal mental health after a child’s diagnosis of autism spectrum disorder: results from a randomized clinical trial. JAMA Pediatr. 2014;168(1):40-46. [DOI] [PubMed] [Google Scholar]
- 21.Osman A, Kopper BA, Barrios FX, Osman JR, Wade T. The Beck Anxiety Inventory: reexamination of factor structure and psychometric properties. J Clin Psychol. 1997;53(1):7-14. [DOI] [PubMed] [Google Scholar]
- 22.Coffey SF, Dansky BS, Falsetti SA, Saladin ME, Brady KT. Screening for PTSD in a substance abuse sample: psychometric properties of a modified version of the PTSD Symptom Scale Self-Report: posttraumatic stress disorder. J Trauma Stress. 1998;11(2):393-399. [DOI] [PubMed] [Google Scholar]
- 23.Chang EC, D’Zurilla TJ. Relations between problem orientation and optimism, pessimism, and trait affectivity: a construct validation study. Behav Res Ther. 1996;34(2):185-194. [DOI] [PubMed] [Google Scholar]
- 24.Kessler RC, Berglund P, Demler O, et al. ; National Comorbidity Survey Replication . The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095-3105. [DOI] [PubMed] [Google Scholar]
- 25.Merikangas KR, Akiskal HS, Angst J, et al. . Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamoureux BE, Linardatos E, Fresco DM, Bartko D, Logue E, Milo L. Using the QIDS-SR16 to identify major depressive disorder in primary care medical patients. Behav Ther. 2010;41(3):423-431. [DOI] [PubMed] [Google Scholar]
- 27.Heeringa SG, Wagner J, Torres M, Duan N, Adams T, Berglund P. Sample designs and sampling methods for the Collaborative Psychiatric Epidemiology Studies (CPES). Int J Methods Psychiatr Res. 2004;13(4):221-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV). Washington, DC: American Psychiatric Association; 1996. [Google Scholar]
- 29.Localio AR, Berlin JA, Ten Have TR, Kimmel SE. Adjustments for center in multicenter studies: an overview. Ann Intern Med. 2001;135(2):112-123. [DOI] [PubMed] [Google Scholar]
- 30.Cohen RM, Greenberg JM, IsHak WW. Incorporating multidimensional patient-reported outcomes of symptom severity, functioning, and quality of life in the Individual Burden of Illness Index for Depression to measure treatment impact and recovery in MDD. JAMA Psychiatry. 2013;70(3):343-350. [DOI] [PubMed] [Google Scholar]
- 31.Ishak WW, Balayan K, Bresee C, et al. . A descriptive analysis of quality of life using patient-reported measures in major depressive disorder in a naturalistic outpatient setting. Qual Life Res. 2013;22(3):585-596. [DOI] [PubMed] [Google Scholar]
- 32.Ishak WW, Christensen S, Sayer G, et al. . Sexual satisfaction and quality of life in major depressive disorder before and after treatment with citalopram in the STAR*D study. J Clin Psychiatry. 2013;74(3):256-261. [DOI] [PubMed] [Google Scholar]
- 33.Robinson RG, Jorge RE, Moser DJ, et al. . Escitalopram and problem-solving therapy for prevention of poststroke depression: a randomized controlled trial. JAMA. 2008;299(20):2391-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rovner BW, Casten RJ, Hegel MT, Leiby BE, Tasman WS. Preventing depression in age-related macular degeneration. Arch Gen Psychiatry. 2007;64(8):886-892. [DOI] [PubMed] [Google Scholar]
- 35.Arean P, Hegel M, Vannoy S, Fan MY, Unuzter J. Effectiveness of problem-solving therapy for older, primary care patients with depression: results from the IMPACT project. Gerontologist. 2008;48(3):311-323. [DOI] [PubMed] [Google Scholar]
- 36.Brennan PA, Hammen C, Andersen MJ, Bor W, Najman JM, Williams GM. Chronicity, severity, and timing of maternal depressive symptoms: relationships with child outcomes at age 5. Dev Psychol. 2000;36(6):759-766. [DOI] [PubMed] [Google Scholar]
- 37.Mynors-Wallis L. Does problem-solving treatment work through resolving problems? Psychol Med. 2002;32(7):1315-1319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol