This case-control study investigates the association of gel-forming mucins and aquaporin 5 gene expression with inflammation, effusion viscosity, and hearing loss in pediatric otitis media with effusion.
Key Points
Question
Is there an association of mucins and aquaporin 5 gene expression with effusion viscosity and hearing loss in pediatric otitis media with effusion?
Findings
In this case-control study of 24 patients with otitis media with effusion and 7 control individuals, mucin 5B and aquaporin 5 gene expression were associated with effusion viscosity and mucin 5B with middle ear epithelial thickness and hearing loss.
Meaning
Further study is warranted to investigate whether mucin 5B–targeted therapy may reduce effusion viscosity and ameliorate hearing loss, a serious consequence of otitis media with effusion.
Abstract
Importance
Persistent, viscous middle ear effusion in pediatric otitis media (OM) contributes to increased likelihood of anesthesia and surgery, conductive hearing loss, and subsequent developmental delays. Biomarkers of effusion viscosity and hearing loss have not yet been identified despite the potential that such markers hold for targeted therapy and screening.
Objective
To investigate the association of gel-forming mucins and aquaporin 5 (AQP5) gene expression with inflammation, effusion viscosity, and hearing loss in pediatric OM with effusion (OME).
Design, Setting, and Participants
Case-control study of 31 pediatric patients (aged 6 months to 12 years) with OME undergoing tympanostomy tube placement and control individuals (aged 1 to 10 years) undergoing surgery for cochlear implantation from February 1, 2013, through November 30, 2014. Those with 1 or more episodes of OM in the previous 12 months, immunologic abnormality, anatomical or physiologic ear defect, OM-associated syndrome (ie, Down syndrome, cleft palate), chronic mastoiditis, or history of cholesteatoma were excluded from the study. All patients with OME and 1 control were recruited from Children’s Hospital of Wisconsin, Milwaukee. The remainder of the controls were recruited from Sick Kids Hospital in Toronto, Ontario, Canada.
Main Outcomes and Measures
Two to 3 middle ear biopsy specimens, effusions, and preoperative audiometric data (obtained <3 weeks before surgery) were collected from patients; only biopsy specimens were collected from controls. Expression of the mucin 2 (MUC2), mucin 5AC (MUC5AC), mucin 5B (MUC5B), and AQP5 genes were assayed in middle ear biopsy specimens by quantitative polymerase chain reaction. One middle ear biopsy specimen was sectioned for histopathologic analysis. Reduced specific viscosity of effusions was assayed using rheometry.
Results
Of the 31 study participants, 24 patients had OME (mean [SD] age, 50.4 [31.9] months; 15 [62.5%] male; 16 [66.7%] white) and 7 acted as controls (mean [SD] age, 32.6 [24.4] months; 2 [26.6%] male; 6 [85.7%] white). Mucins and AQP5 gene expression were significantly higher in patients with OME relative to controls (MUC2: ratio, 127.6 [95% CI, 33.7-482.7]; MUC5AC: ratio, 3748.8 [95% CI, 558.1-25 178.4]; MUC5B: ratio, 471.1 [95% CI, 130.7-1697.4]; AQP5: ratio, 2.4 [95% CI, 1.1-5.6]). A 2-fold increase in MUC5B correlated with increased hearing loss (air-bone gap: 7.45 dB [95% CI, 2.65-12.24 dB]; sound field: 6.66 dB [95% CI, 6.63-6.69 dB]), effusion viscosity (2.75 mL/mg; 95% CI, 0.89-4.62 mL/mg), middle ear epithelial thickness (3.5 μm; 95% CI, 1.96-5.13 μm), and neutrophil infiltration (odds ratio, 1.7; 95% CI, 1.07-2.72). A 2-fold increase in AQP5 correlated with increased effusion viscosity (1.94 mL/mg; 95% CI, 0.08-3.80 mL/mg).
Conclusions and Relevance
Further exploration of the role of MUC5B in the pathophysiology of OME holds promise for development of novel, targeted therapies to reduce effusion viscosity, facilitation of effusion clearance, and prevention of disease chronicity and hearing loss in patients with OME.
Introduction
Otitis media (OM) is the most common diagnosis in pediatric patients who visit physicians for illness in the United States and is responsible for approximately $5 billion in US health care expenditures annually. Although the development of otitis media with effusion (OME) represents only a subset of patients affected by OM, it can lead to conductive hearing loss as a result of persisting OME and subsequent developmental delays. Such effusions are characterized by persistent, often viscous middle ear effusions and an increased likelihood of anesthesia and surgery. For individuals who represent First Peoples (such as Native Americans, Australian Aboriginals, North American Eskimos, and Greenlanders), chronic OME and associated complications represent an even greater difficulty because of its incidence and severity in this population. Therefore, OM represents an important pediatric health care problem around the world for which additional understanding of its underlying molecular mechanisms and novel solutions are needed, particularly for specific patient populations, such as those with chronic disease, developmental delays, economic or societal disadvantages, or other health care disparities. To address the potential solutions for subpopulations, it is imperative that targeted solutions using a personalized genomic approach are explored. This article. to our knowledge, represents the first of its kind to examine the association of genetic expression to OM disease metrics in a group of patients chronically affected by OME and requiring surgery.
Secreted, oligomeric, or gel-forming mucins (GFMs) are the primary constituent of middle ear effusion and are largely responsible for its viscoelastic properties. These high-molecular-weight proteins contain multiple serine- and threonine-rich mucin-like domains that are extensively glycosylated. Glycosylation contributes as much as 80% of the weight of the molecule and causes it to become stiff and voluminous, resulting in characteristic space-filling or gel-making properties. Entanglement of long mucin oligomers is considered to be the primary mechanism for mucous gel formation. Therefore, the size, concentration, and chemical nature of the mucins are important factors for determining the properties of effusion and its propensity for clearance. The GFMs expressed in the inflamed middle ear include mucin 2 (MUC2), mucin 5AC (MUC5AC), and mucin 5B (MUC5B). Expression of these mucins during OME is triggered by bacterial infection and proinflammatory cytokines. Although mucins play a key role in the innate immune response by entrapping foreign particles and pathogens and facilitating their clearance, mucin hypersecretion alters mucous gel properties, impedes effusion clearance, and is a pathologic feature of airway diseases, including OM.
In addition to mucin expression, availability of water on the epithelial surface also significantly affects secreted mucin hydration and rheologic properties of the mucous gel. Aquaporins are integral membrane proteins that play a significant role in local water homeostasis by serving as selective pores for the rapid movement of water across diverse cell membranes. Aquaporin subtypes are expressed with region-specific localization in the middle ear, where they may be responsible for maintaining the fluid-free middle ear cavity or accumulation of effusion. Levels of aquaporins were elevated in an animal model of secretory OM and found to be 1 of the 2 major groups of homeostasis genes affected and in a mouse model of acute OM. Recent research indicates that a specific aquaporin, aquaporin 5 (AQP5), also regulates GFM expression in airway cells via epidermal growth factor receptor signaling, presenting a novel mechanism whereby aquaporin expression may influence middle ear effusion viscosity by altering its mucin composition.
The association among GFM and AQP5 (OMIM 600442) expression, inflammation, middle ear effusion viscosity, and hearing loss has not, to our knowledge, been previously investigated. In this study, we assessed these associations in a pediatric population with OME undergoing tympanostomy tube (TT) placement relative to an OM-free control population.
Methods
Study Participants
Study design was reviewed by epidemiologic and biostatistical consultants before initiation of the study, with determination of group sizes informed by previous observational studies of patients with OM and control individuals. The number of controls was limited to that deemed necessary by power analysis in keeping with the best interest of patients; analysis methods accounted for any imbalance in population size, and resultant effects were reflected in the CIs. The study followed a clinical course design wherein the OME population was composed of referrals treated in a routine practice setting to most accurately reflect and inform clinical practice. Approval was obtained from the Children's Hospital of Wisconsin Institutional Review Board for collection of clinical data and middle ear biopsy specimens from pediatric patients aged 6 months to 12 years with diagnosis of OME requiring surgical intervention with TT from February 1, 2013, through November 30, 2014. Approval was obtained under this protocol for collection of clinical data and middle ear biopsy specimens from similarly aged children undergoing cochlear implantation during the same period. Further approval was obtained from the Sick Kids Hospital in Toronto Research Ethics Board for collection of clinical data and middle ear biopsy specimens from patients aged 1 to 10 years undergoing cochlear implantation from February 1 through October 31, 2014, as a control population; the difference in age bracket was attributable to an expectation of mostly very young patient enrollees with cochlear implants.
During the study period, consecutive patients who met the inclusion criteria were offered enrollment in the study. Patients considered in this study presented with OME, defined as 3 or more months of persisting middle ear effusion, to the pediatric otolaryngology clinic after referral from their primary physician with a history of persisting middle ear infections and middle ear fluid. Strict criteria were used in considering patients with OME for study inclusion: effusion that persisted for more than 3 months and substantive hearing or developmental concerns for more than 6 months with less severe hearing or developmental concerns. Specifically, all patients with OME considered in this study had unilateral or bilateral middle ear fluid at the time of their clinic evaluation and met surgical criteria for TT placement, including persisting middle ear fluid with meaningful hearing loss (>20 dB bilaterally), persisting recurrent infections despite hearing thresholds that were better than 20 dB, or associated speech delay and persisting OME despite hearing levels better than 20 dB. Exclusion criteria included 1 or more episodes of OM in the previous 12 months; history of immunologic, intrinsic, or pharmacologic abnormality; anatomical or physiologic defect of the ear; syndrome associated with OM (ie, Down syndrome, cleft palate); chronic mastoiditis; and history of cholesteatoma. Exclusion criteria for cochlear implantation controls matched the aforementioned criteria. Written informed consent was obtained before study enrollment and surgery. All data were deidentified.
For patients with OME, after performance of the myringotomy incision, fluid in the middle ear space was collected using Juhn Tym Tap middle ear fluid aspirator or collectors; for extremely thick, mucoid effusions, nonbacteriostatic saline was used to assist suction; if no effusion was present, no sample was collected. A surgeon (R.H.C., M.E.M., J.E.K.) noted the effusion viscosity at the time of collection using a ranking system (0, absent; 1, thin serous; 2, thick serous; 3, mucoid; 4, thick mucoid; 5, extremely thick mucoid resistant to 7F suctioning). Two to 3 small (<1-mm) biopsy specimens were taken from the middle ear with a cup forceps near the eustachian tube orifice (anterior-inferior promontory), and a TT was placed according to routine care. From each patient, one specimen was placed in RNAlater (Thermo Fisher Scientific) and the other in Hank’s buffered saline solution (HBSS; Thermo Fisher Scientific) before immediate transport to the research laboratory. Samples in RNALater were stored at −80°C before RNA extraction. Samples in HBSS were immediately embedded for cryosectioning as described in the Analysis of Middle Ear Epithelial Thickness and Neutrophil Infiltration subsection of Methods. Mucosal biopsy specimens were obtained from a similar location of the middle ear of patients with cochlear implants during cochlear implantation. From each patient, one specimen was placed in RNAlater and the other in HBSS (specimens from Children’s Hospital of Wisconsin, Milwaukee) or 10% formalin (specimens from Sick Kids Hospital in Toronto, Ontario, Canada). Samples in RNALater were stored at −80°C before RNA extraction. Samples in HBSS were immediately embedded for cryosectioning, and samples in formalin were stored at 4°C before embedding for cryosectioning as described in the Analysis of Middle Ear Epithelial Thickness and Neutrophil Infiltration subsection of Methods.
Audiometry tests with an auditory threshold assessment (expressed in decibels) were performed for all patients with OME in the study according to routine care by an experienced audiologist within 21 days before TT insertion. Pure-tone audiometry was performed for patients older than 3 years: air conduction and bone conduction thresholds were measured at frequencies of 500, 1000, 2000, and 4000 Hz, and the mean and air-bone gap were noted. In younger children unable to be assessed by pure-tone audiometry, sound field audiometry was used in concert with behavioral testing (ie, conditioned play, behavioral observation, and visual reinforcement) to measure response thresholds at frequencies of 500, 1000, 2000, and 4000 Hz and the mean sound field threshold noted.
RNA Isolation and Quantitative Polymerase Chain Reaction
RNA was isolated from middle ear biopsy specimens using TRIzol according to the manufacturer’s instructions (Thermo Fisher Scientific). Spectrophotometry was performed to assess RNA purity and concentration, and RNA was stored at −80°C until use. A total of 150 ng of RNA extracted from middle ear biopsy specimens and commercially obtained trachea RNA (reference sample; Clontech/Takara) were reverse transcribed using Superscript VILO (Thermo Fisher Scientific) per the manufacturer’s instructions. Expression of mucins, AQP5, and hypoxanthine phosphoribosyltransferase 1 (HPRT1 [OMIM 308000]; internal control/housekeeping gene) was examined using TaqMan assays. Amplification was performed on the Applied Biosystems ViiA7 (Thermo Fisher Scientific) at 52°C for 2 minutes, 95°C for 10 minutes, and 50 cycles of 95°C for 15 seconds and 60°C for 1 minute. Reactions were performed in triplicate. Gene expression across multiple plates was normalized to the reference sample, then to the internal control/housekeeping gene. Relative expression differences were determined using the 2-∆∆CT method.
Analysis of Middle Ear Epithelial Thickness and Neutrophil Infiltration
After transport to the laboratory in HBSS, one middle ear biopsy specimen was embedded in Tissue-Tek OCT Compound (Sakura Finetek), placed on dry ice, and held at −80°C until use or cryosectioned to 6 μm. Sections were mounted on glass slides and stained with hematoxylin-eosin with use of an automated stainer. Slides were scanned at a magnification of ×400 (Nanozoomer; Hamamatsu Photonics). Images were analyzed by a board-certified pathologist (A.M.) using NDP.view2 software (Hamamatsu Photonics). Mean values from up to 13 distinct regions of tissue per patient were used as a representative sample of the middle ear. Areas in which the epithelium was detached or disrupted or areas with underlying specialized structures were not analyzed. Epithelial thickness in the number of cells was measured and recorded as a range (0-1, 2-5, and ≥5 cells) and/or assessed by the software (in micrometers) and verified by a micrometer. Percentage of neutrophil infiltrate was measured similarly across the tissue and recorded as present (1%-10%) or absent (0%).
Viscometry
Middle ear effusion samples (0.4-1.5 mL) were analyzed using a Kinexus Pro rheometer (Malvern). For mucoid effusions that required saline for suction, rheometry was performed exclusively on the mucoid portion of the specimen. All samples were prepared and tested in triplicate, and the reduced specific viscosity (ie, viscosity divided by specimen weight per volume) was calculated.
Statistical Analysis
The primary analytic approach was generalized linear regression modeling. Specifically, a gaussian linear regression was used to examine the effect of gene expression on continuous measures, such as hearing thresholds, epithelial thickness, and viscosity, as well as for comparing the gene expression between patient groups. A logistic regression model was used for the presence of neutrophil infiltration and a proportional odds logistic regression model for the categorized epithelial thickness measurement. We adjusted for correlation attributable to inclusion of measurements from both ears in a person by using generalized estimating equations with an independence working covariance structure and used robust sandwich estimators for the SE. All results are presented with 95% CIs. All analyses were conducted using SAS statistical software, version 9.4 (SAS Institute Inc).
Results
Patient Demographic Characteristics and Association of Gene Expression With Diagnosis
No significant difference in age, race, or sex representation was observed in OME relative to controls (Table 1). Although all patients with OME were recruited from the Children’s Hospital of Wisconsin, only one control with cochlear implants was recruited in Wisconsin. The remainder were recruited from Sick Kids Hospital in Toronto because of higher patient volume. Although strict criteria were used to identify patients with OME, the overlap of OME and recurrent acute OM is an unalterable confounding issue in all studies of OM; 6 patients with OME also had recurrent acute OM (≥3 acute ear infections in 6 months). Expression of GFMs and AQP5 was elevated in patients with OME relative to controls (Table 2).
Table 1. Patient Demographic Characteristics.
| Characteristic | Control Individuals (n = 7) |
Patients With OME (n = 24) |
Difference (95% CI)a |
|---|---|---|---|
| Age, mo | |||
| Mean (SD) | 32.6 (24.4) | 50.4 (31.9) | −17.8 (−44.7 to 9.0) |
| Mean (95% CI) | 32.6 (14.5 to 50.7) | 50.4 (37.6 to 63.2) | |
| Sex, No. (%) | |||
| Female | 5 (71.4) | 9 (37.5) | 0.34 (−0.05 to 0.73) |
| Male | 2 (28.6) | 15 (62.5) | |
| Race/ethnicity, No. (%) | |||
| White | 6 (85.7) | 16 (66.7) | 0.19 (−0.13 to 0.51) |
| Nonwhite | |||
| African American | 0 | 4 (16.7) | |
| Asian | 1 (14.3) | 0 | |
| Hispanic | 0 | 2 (8.3) | |
| Unknown | 0 | 2 (8.3) | |
| Total | 1 (14.3) | 8 (33.3) |
Abbreviation: OME, otitis media with effusion.
Difference in mean (age) or risk ratio (sex, race) between controls and patients with OME.
Table 2. Gel-Forming Mucin and AQP5 Expression in Patients With OME Relative to Control Individuals.
| Gene | Gene Expression Ratio in OME Relative to Control (95% CI) |
|---|---|
| MUC2 | 127.6 (33.7-482.7) |
| MUC5AC | 3748.8 (558.1-25 178.4) |
| MUC5B | 471.1 (130.7-1697.4) |
| AQP5 | 2.4 (1.1-5.6) |
Abbreviations: AQP5, aquaporin 5; MUC2, mucin 2; MUC5AC, mucin 5AC; MUC5B, mucin 5B; OME, otitis media with effusion.
Hearing Loss Association With Gene Expression
Of the 24 patients with OME, 15 underwent pure-tone audiometry; data were obtained from both ears of all patients but one. Eight patients with OME underwent sound field audiometry. Audiometry data could not be obtained from one patient; this patient was not excluded from the study (gene expression, effusion viscosity, and middle ear epithelial thickness data were used from this patient). Correlation of mucin and AQP5 gene expression with hearing loss was evaluated. Strong correlation of only middle ear MUC5B (OMIM 600770) gene expression was observed with hearing loss (air-bone gap and sound field threshold) (Table 3). Measures reported in Table 3 are the estimated effects of a 2-fold increase in the gene expression on the outcome variable. As reported in Table 3, if MUC5B expression doubled, the mean air-bone gap increased by a mean of 7.45 dB (95% CI, 2.65-12.24 dB) and sound field threshold increased by a mean of 6.66 dB (95% CI, 6.63-6.69 dB).
Table 3. Association of a 2-Fold Increase in Gel-Forming Mucin and AQP5 With Hearing Loss, Effusion Viscosity, and Inflammation.
| Variable | Estimate (95% CI) |
|---|---|
| Hearing Loss, dB | |
| Mean air-bone gap | |
| MUC2 | 0.94 (−0.11 to 1.98) |
| MUC5AC | 0.51 (−0.04 to 1.06) |
| MUC5B | 7.45 (2.65 to 12.24) |
| AQP5 | −0.84 (−5.13 to 3.45) |
| Mean sound field threshold | |
| MUC2 | 0.1 (−0.25 to 0.46) |
| MUC5AC | −0.47 (−0.96 to 0.03) |
| MUC5B | 6.66 (6.63 to 6.69) |
| AQP5 | −0.82 (−1.88 to 0.24) |
| Effusion Viscosity | |
| Clinical scorea | |
| MUC2 | −0.02 (−0.19 to 0.16) |
| MUC5AC | 0.02 (−0.09 to 0.13) |
| MUC5B | 0.46 (0.11 to 0.81) |
| AQP5 | −0.23 (−0.58 to 0.11) |
| Viscometry, mL/mg | |
| MUC2 | −0.68 (−1.4 to 0.04) |
| MUC5AC | −0.14 (−0.36 to 0.08) |
| MUC5B | 2.75 (0.89 to 4.62) |
| AQP5 | 1.94 (0.08 to 3.80) |
| Middle ear epithelial thickness, μm | |
| MUC2 | 0.47 (−0.68 to 1.63) |
| MUC5AC | −0.15 (−0.7 to 0.41) |
| MUC5B | 3.54 (1.96 to 5.13) |
| AQP5 | 0.51 (−2.88 to 3.89) |
Abbreviations: AQP5, aquaporin 5; MUC2, mucin 2; MUC5AC, mucin 5AC; MUC5B, mucin 5B.
Ranging from 0 to 5, with 0 indicating absent; 1, thin serous; 2, thick serous; 3, mucoid; 4, thick mucoid; and 5, extremely thick mucoid. Score was determined by the surgeon as described in the Study Participants subsection of Methods.
Middle Ear Epithelial Thickness and Neutrophil Infiltrate Association With Gene Expression
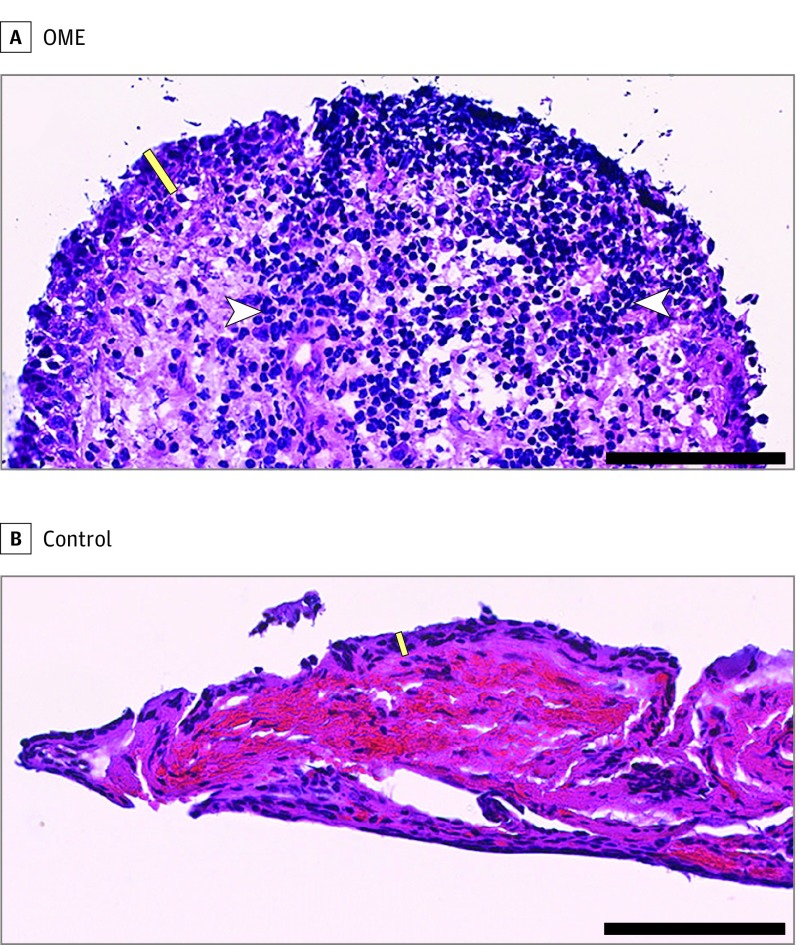
Mucin and AQP5 gene expression were also evaluated with respect to middle ear epithelial thickness in micrometers and neutrophil infiltration as a measure of inflammation and middle ear epithelial thickness in cell number as a measure of dysplasia. Middle ear MUC5B expression correlated with middle ear thickness in micrometers; as reported in Table 3, when MUC5B expression doubled, the middle ear epithelial thickness increased by 3.54 µm (95% CI, 1.96-5.13 μm). Representative histopathologic images of OME and control specimens are displayed in the Figure. In the OME specimen (Figure, A), nuclei are enlarged and rounded, partially overlap, and contain coarse, granular chromatin. In many places, the epithelium is detached from the underlying stroma, and the epithelial cells are discohesive in places. The stroma is looser and less dense than the stroma of controls (Figure, B). A dense, widespread inflammatory infiltrate composed of plasma cells, lymphocytes, and polymorphonuclear cells is present, consistent with the clinical diagnosis of acute OM. In contrast to the OME specimen, the control specimen demonstrates compact, cohesive epithelium approximately a mean of 2 cells thick. The epithelial cells are flat to oval with abundant cytoplasm and nonoverlapping, elongated, small, flattened nuclei arranged in a parallel, organized pattern. The subepithelial stroma is dense and paucicellular. Most of the vascular spaces in the stroma are full of normal-appearing red blood cells. Inflammatory cells are inconspicuous throughout the specimen.
Figure. Association of Mucin 5B (MUC5B) Expression With Middle Ear Epithelial Thickness.
A, A middle ear epithelial specimen from a patient with otitis media with effusion reveals greater MUC5B gene expression and thicker epithelium (MUC5B ΔCt, 0.20; epithelial thickness, 45 μm). B, Corresponding specimen from a control individual reveals lower MUC5B gene expression and thinner epithelium (MUC5B ΔCt, 9.32; epithelial thickness, 13 μm). Sections were stained with hematoxylin-eosin. Yellow bar indicates epithelial layer; white arrowheads, inflammatory cells. Scale bar indicates 100 μm.
Middle ear MUC5B expression also correlated with middle ear epithelial thickness in cell number and neutrophil infiltration of the mucosa; as reported in Table 4, doubled MUC5B expression was associated with a 1.79-fold greater odds (95% CI, 1.04-3.07) of being in a higher category of middle ear epithelial thickness in cell number and 1.70-fold greater odds (95% CI, 1.07-2.72) of neutrophil infiltration.
Table 4. Association of a 2-Fold Increase in Gel-Forming Mucins and AQP5 With the Likelihood of Neutrophil Infiltration and Increased Thickness.
| Variable | Odds Ratio (95% CI) |
|---|---|
| Neutrophil Infiltration (Presence vs Absence) | |
| MUC2 | 1.12 (0.9-1.4) |
| MUC5AC | 1.10 (0.95-1.28) |
| MUC5B | 1.70 (1.07-2.72) |
| AQP5 | 1.45 (0.86-2.45) |
| Middle Ear Epithelial Thickness (0-1, 2-5, or >5 Cells)a | |
| MUC2 | 0.86 (0.62-1.19) |
| MUC5AC | 0.97 (0.84-1.12) |
| MUC5B | 1.79 (1.04-3.07) |
| AQP5 | 1.27 (0.66-2.46) |
Abbreviations: AQP5, aquaporin 5; MUC2, mucin 2; MUC5AC, mucin 5AC; MUC5B, mucin 5B.
The odds ratio quantifies the effect of a 2-fold increase in gene expression on the odds of having a higher value for the outcome (ie, higher category of cell number).
Effusion Viscosity Association With Gene Expression
An association of MUC5B gene expression was observed with effusion viscosity as evaluated by rheometry (Table 3). Similarly, an association was observed between AQP5 gene expression and effusion viscosity (Table 3).
Discussion
Many potential health difficulties are associated with OME, including infectious complications, the development of cholesteatoma, and the need for surgical interventions, which are sometimes complex and have their own attendant risks. However, one of the most important deleterious effects of OME is the potential for OME and associated hearing loss. Otitis media with effusion is the most common cause of hearing loss in children and has important developmental consequences in children if left untreated. Little research has been conducted to investigate the specific molecular and pathologic events in the middle ear that lead to hearing loss despite the potential that manipulation of such events holds for ameliorating the consequences of OME. Furthermore, a clear need has arisen for biomarkers to identify patients who may be particularly susceptible to OME-attributed hearing loss and may benefit from early intervention. To our knowledge, this study is the first to link phenotypic characteristics, such as patient hearing levels, middle ear inflammation, and middle ear fluid viscosity, to expression of mucin genes, which have been demonstrated in our and other laboratories to be hypersecreted during OME and a cause of OME pathophysiologic findings. Although the results of our study warrant validation in a larger population, this data set provides a number of important correlative observations that may provide avenues of future investigation into individualized assessment of patient characteristics for a personalized approach to OM.
Consistent with previous reports, this data set demonstrates elevated expression of MUC2 (OMIM 158370), MUC5AC (OMIM 158373), and MUC5B genes in the middle ears of patients with OME relative to a control population without OM. The analysis of mucin correlation with OME disease metrics reported herein is the first, to our knowledge, to demonstrate that, of these mucins, only MUC5B expression is significantly associated with effusion viscosity, neutrophil infiltration, middle ear mucosal inflammation and hyperplasia, and hearing loss. The association of MUC5B with OME pathophysiologic findings is in accordance with its expression during chronic airway disease. Although MUC5AC predominates in the healthy lung, MUC5B is the predominant mucin in cystic fibrosis and chronic obstructive pulmonary disease. Similarly, MUC5B is the predominant mucin in middle ear effusion and mucosa during OME. Prolonged stimulation is required for MUC5B expression relative to other mucins in in vitro models of OM, further supporting its role as a chronic response gene in the middle ear. The association of MUC5B expression with viscous, difficult-to-clear effusion, chronic disease, and concomitant hearing loss is additionally supported by its biochemical properties. MUC5B is the largest and potentially the most viscous mucin: it hosts the longest repeat or glycosylation region of any mucin in the largest exon found in any vertebrate gene and, given its numerous cysteine residues, bears the capacity to oligomerize at both ends of the molecule via degradation-resistant disulfide bonds. Effusion viscosity in OM has previously been associated with 2 primary determinants: glycoproteins and DNA. MUC5B is not only the predominant glycoprotein in middle ear effusion but also has previously been found to be associated with neutrophil extracellular traps, the predominant innate immune response during OME. Accordingly, in our study, MUC5B was associated with increased neutrophil infiltration. Neutrophil extracellular traps are incorporated into bacterial biofilms, and neutrophil extracellular trap mediators, such as neutrophil elastase and metabolites from the biofilm, perpetuate inflammation and sustain epithelial mucin hypersecretions, thereby generating a feedback loop that reduces likelihood of effusion clearance and results in chronic illness.
These data are also the first, to our knowledge, to demonstrate elevation of the level of AQP5 in a clinical population with OME and its correlation with effusion viscosity. Similar to GFMs in the middle ear during OM, AQP5 expression in the lung is responsive to tumor necrosis factor and lipopolysaccharide. Posttranscriptional mechanisms that control gating and subcellular localization are important regulators of AQP function, and lipopolysaccharide also stimulates translocation of intracellular AQP5 to the plasma membrane in airway cells. On expression in the lung, AQP5 promotes airway MUC5AC and MUC5B expression. In this study, levels of MUC5B and AQP5 expression were elevated in patients with OME relative to controls, suggesting that similar regulatory mechanisms for AQP5 and GFM expression may exist in the middle ear during OM. Aside from its role in mucin regulation, AQP5 serves other key functions in innate immunity and maintenance of epithelial barrier integrity. AQP5 promotes airway epithelial expression of an adhesion molecule (intercellular adhesion molecule) and chemokine (lipopolysaccharide-induced CXC) secretion essential for neutrophil recruitment; accordingly, Aqp5−/− mice exposed to cigarette smoke have reduced alveolar neutrophil recruitment and protection from lung damage relative to wild-type controls. AQP5 also directly interacts with microtubules in epithelial cells to influence paracellular permeability independently of water transport. Additional work in in vitro and in vivo models will be required to determine the requirement of AQP5 for GFM expression and accumulation of effusion in the middle ear and the overall contribution of AQP5 to the disease process of OME.
Given the findings from our study, a logical next step will be to follow up patients in a prospective manner to assess the potential correlation of MUC5B expression with the likelihood of ongoing difficulties, such as disease recurrence and additional operations to determine whether these patients represent a group who may benefit from closer follow-up or earlier intervention. To this end, our laboratory has already initiated work to bring the findings of the current study to the threshold of a personalized approach to patients with OME through analysis of genetic responses, inflammation, and associated morbidity with hearing loss. An additional related avenue for investigation is identification of specific genetic characteristics, such as MUC5B polymorphisms, that may influence patient susceptibility to OME or efficacy of treatment, similar to the association that our laboratory identified between a long MUC5AC allele and recurrent OM.
Limitations
A limitation of this study is the difference in demographic characteristics and geographic origin of patients with OME and controls. Genes that control the immune response to infection are highly polymorphic and subject to confounding by population stratification, presenting a limitation that should be considered when interpreting candidate gene associations without family member controls. As noted in the Methods section, the current study had a natural history and natural care pattern design; patients were referrals treated in a routine practice setting rather than screened and followed up from initial enrollment. Strict criteria were used when considering patients for surgery as noted; however, given the natural care pattern design, there were inherent limitations, for example, in knowing the exact pattern or length of effusion. However, inasmuch as the study participants met a consistent definition for OME, the design provides the best ability to eventually generalize the findings more broadly in the future to clinical practice; rather than generating an artificial construct irreproducible in clinical practice, the design facilitates investigation of significant differences in a setting that mirrors that of clinical practice. The statistically significant results observed with such design may be considered to be highly substantive for future work and consideration.
Additional limitations include differences in the method of fixation of specimens for histopathologic analysis that were obtained from Canada relative to the United States. The potential for such differences to influence outcomes was mitigated by assessing the epithelial thickness in micrometers and cell count. The latter is not affected by potential tissue shrinkage during fixation and corroborated the difference between groups that was observed in thickness in micrometers. Last, specimens were collected from patients with unilateral and bilateral effusion. Although we were able to detect phenotypic differences in OME relative to controls, the OME phenotype may have been stronger in a population with exclusively bilateral effusion. This is an important question directed at the interface of genotypic characteristics, phenotypic characteristics, and environmental characteristics that drive disease expression, for which we have proposed future work in a larger trial.
Conclusions
To our knowledge, these data are the first to show association of expression of a specific mucin with inflammation, effusion viscosity, and hearing loss during OM and the first to demonstrate elevation of AQP5 levels during OM and its association with effusion viscosity. Although additional work is required to validate causation, our findings implicate MUC5B in OME-associated hearing loss and suggest that MUC5B-targeted treatment strategies may be beneficial for treatment of OME.
References
- 1.Bondy J, Berman S, Glazner J, Lezotte D. Direct expenditures related to otitis media diagnoses: extrapolations from a pediatric medicaid cohort. Pediatrics. 2000;105(6):E72. [DOI] [PubMed] [Google Scholar]
- 2.Klein JO. The burden of otitis media. Vaccine. 2000;19(suppl 1):S2-S8. [DOI] [PubMed] [Google Scholar]
- 3.Teele DW, Klein JO, Rosner BA. Otitis media with effusion during the first three years of life and development of speech and language. Pediatrics. 1984;74(2):282-287. [PubMed] [Google Scholar]
- 4.Dodson KM, Cohen RS, Rubin BK. Middle ear fluid characteristics in pediatric otitis media with effusion. Int J Pediatr Otorhinolaryngol. 2012;76(12):1806-1809. [DOI] [PubMed] [Google Scholar]
- 5.Daly KA, Hoffman HJ, Kvaerner KJ, et al. . Epidemiology, natural history, and risk factors: panel report from the Ninth International Research Conference on Otitis Media. Int J Pediatr Otorhinolaryngol. 2010;74(3):231-240. [DOI] [PubMed] [Google Scholar]
- 6.Smith DF, Boss EF. Racial/ethnic and socioeconomic disparities in the prevalence and treatment of otitis media in children in the United States. Laryngoscope. 2010;120(11):2306-2312. [DOI] [PubMed] [Google Scholar]
- 7.Carrie S, Hutton DA, Birchall JP, Green GG, Pearson JP. Otitis media with effusion: components which contribute to the viscous properties. Acta Otolaryngol. 1992;112(3):504-511. [DOI] [PubMed] [Google Scholar]
- 8.Lin J, Tsuprun V, Kawano H, et al. . Characterization of mucins in human middle ear and Eustachian tube. Am J Physiol Lung Cell Mol Physiol. 2001;280(6):L1157-L1167. [DOI] [PubMed] [Google Scholar]
- 9.Kerschner JE, Hong W, Khampang P, Johnston N. Differential response of gel-forming mucins to pathogenic middle ear bacteria. Int J Pediatr Otorhinolaryngol. 2014;78(8):1368-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Val S, Kwon HJ, Rose MC, Preciado D. Middle ear response of Muc5ac and Muc5b mucins to nontypeable Haemophilus influenzae. JAMA Otolaryngol Head Neck Surg. 2015;141(11):997-1005. [DOI] [PubMed] [Google Scholar]
- 11.Kerschner JE, Meyer TK, Wohlfeill E. Middle ear epithelial mucin production in response to interleukin 1β exposure in vitro. Otolaryngol Head Neck Surg. 2003;129(1):128-135. [DOI] [PubMed] [Google Scholar]
- 12.Kerschner JE, Meyer TK, Yang C, Burrows A. Middle ear epithelial mucin production in response to interleukin-6 exposure in vitro. Cytokine. 2004;26(1):30-36. [DOI] [PubMed] [Google Scholar]
- 13.Kerschner JE, Meyer TK, Burrows A. Chinchilla middle ear epithelial mucin gene expression in response to inflammatory cytokines. Arch Otolaryngol Head Neck Surg. 2004;130(10):1163-1167. [DOI] [PubMed] [Google Scholar]
- 14.Morris LM, DeGagne JM, Kempton JB, Hausman F, Trune DR. Mouse middle ear ion homeostasis channels and intercellular junctions. PLoS One. 2012;7(6):e39004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Q, Liu C, Gao X, et al. . Expression pattern of aquaporin 1 in the middle ear of the guinea pig with secretory otitis media. ORL J Otorhinolaryngol Relat Spec. 2009;71(2):70-77. [DOI] [PubMed] [Google Scholar]
- 16.MacArthur CJ, Hausman F, Kempton JB, Choi D, Trune DR. Otitis media impacts hundreds of mouse middle and inner ear genes. PLoS One. 2013;8(10):e75213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z, Wang X, Gao L, Bai L, Zhu R, Bai C. Regulation of MUC5AC mucin secretion by depletion of AQP5 in SPC-A1 cells. Biochem Biophys Res Commun. 2006;342(3):775-781. [DOI] [PubMed] [Google Scholar]
- 18.Zhang ZQ, Zhu ZX, Bai CX, Chen ZH. Aquaporin 5 expression increases mucin production in lung adenocarcinoma. Oncol Rep. 2011;25(6):1645-1650. [DOI] [PubMed] [Google Scholar]
- 19.Kerschner JE, Tripathi S, Khampang P, Papsin BC. MUC5AC expression in human middle ear epithelium of otitis media patients. Arch Otolaryngol Head Neck Surg. 2010;136(8):819-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ubell ML, Kerschner JE, Wackym PA, Burrows A. MUC2 expression in human middle ear epithelium of patients with otitis media. Arch Otolaryngol Head Neck Surg. 2008;134(1):39-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402-408. [DOI] [PubMed] [Google Scholar]
- 22.Rosenfeld R. Current guidelines for otitis media. Paper presented at: International Symposium on Recent Advances in Otitis Media; June 9, 2015; National Harbor, Maryland. [Google Scholar]
- 23.Kirkham S, Sheehan JK, Knight D, Richardson PS, Thornton DJ. Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem J. 2002;361(Pt 3):537-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirkham S, Kolsum U, Rousseau K, Singh D, Vestbo J, Thornton DJ. MUC5B is the major mucin in the gel phase of sputum in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178(10):1033-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preciado D, Goyal S, Rahimi M, et al. . MUC5B Is the predominant mucin glycoprotein in chronic otitis media fluid. Pediatr Res. 2010;68(3):231-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin J, Tsuboi Y, Rimell F, et al. . Expression of mucins in mucoid otitis media. J Assoc Res Otolaryngol. 2003;4(3):384-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desseyn JL, Guyonnet-Dupérat V, Porchet N, Aubert JP, Laine A. Human mucin gene MUC5B, the 10.7-kb large central exon encodes various alternate subdomains resulting in a super-repeat: structural evidence for a 11p15.5 gene family. J Biol Chem. 1997;272(6):3168-3178. [DOI] [PubMed] [Google Scholar]
- 28.Val S, Poley M, Brown K, et al. . Proteomic characterization of middle ear fluid confirms neutrophil extracellular traps as a predominant innate immune response in chronic otitis media. PLoS One. 2016;11(4):e0152865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thornton RB, Wiertsema SP, Kirkham LA, et al. . Neutrophil extracellular traps and bacterial biofilms in middle ear effusion of children with recurrent acute otitis media–a potential treatment target. PLoS One. 2013;8(2):e53837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juneau RA, Pang B, Weimer KE, Armbruster CE, Swords WE. Nontypeable Haemophilus influenzae initiates formation of neutrophil extracellular traps. Infect Immun. 2011;79(1):431-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou J, Perelman JM, Kolosov VP, Zhou X. Neutrophil elastase induces MUC5AC secretion via protease-activated receptor 2. Mol Cell Biochem. 2013;377(1-2):75-85. [DOI] [PubMed] [Google Scholar]
- 32.Towne JE, Krane CM, Bachurski CJ, Menon AG. Tumor necrosis factor-alpha inhibits aquaporin 5 expression in mouse lung epithelial cells. J Biol Chem. 2001;276(22):18657-18664. [DOI] [PubMed] [Google Scholar]
- 33.Shen Y, Wang X, Wang Y, et al. . Lipopolysaccharide decreases aquaporin 5, but not aquaporin 3 or aquaporin 4, expression in human primary bronchial epithelial cells. Respirology. 2012;17(7):1144-1149. [DOI] [PubMed] [Google Scholar]
- 34.Ohinata A, Nagai K, Nomura J, et al. . Lipopolysaccharide changes the subcellular distribution of aquaporin 5 and increases plasma membrane water permeability in mouse lung epithelial cells. Biochem Biophys Res Commun. 2005;326(3):521-526. [DOI] [PubMed] [Google Scholar]
- 35.Shen Y, Wang Y, Chen Z, et al. . Role of aquaporin 5 in antigen-induced airway inflammation and mucous hyperproduction in mice. J Cell Mol Med. 2011;15(6):1355-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aggarwal NR, Chau E, Garibaldi BT, et al. . Aquaporin 5 regulates cigarette smoke induced emphysema by modulating barrier and immune properties of the epithelium. Tissue Barriers. 2013;1(4):e25248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sidhaye VK, Chau E, Srivastava V, et al. . A novel role for aquaporin-5 in enhancing microtubule organization and stability. PLoS One. 2012;7(6):e38717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ubell ML, Khampang P, Kerschner JE. Mucin gene polymorphisms in otitis media patients. Laryngoscope. 2010;120(1):132-138. doi: 10.1002/lary.20688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas DC, Witte JS. Point: population stratification: a problem for case-control studies of candidate-gene associations? Cancer Epidemiol Biomarkers Prev. 2002;11(6):505-512. [PubMed] [Google Scholar]
- 40.Tian C, Gregersen PK, Seldin MF. Accounting for ancestry: population substructure and genome-wide association studies. Hum Mol Genet. 2008;17(R2):R143-R150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chokkalingam AP, Aldrich MC, Bartley K, et al. . Matching on race and ethnicity in case-control studies as a means of control for population stratification. Epidemiology (Sunnyvale). 2011;1:101. [DOI] [PMC free article] [PubMed] [Google Scholar]