Abstract
Importance
Despite 3 decades of study, there remains ongoing debate regarding whether vasectomy is associated with prostate cancer.
Objective
To determine if vasectomy is associated with prostate cancer.
Data Sources
The MEDLINE, EMBASE, Web of Science, and Scopus databases were searched for studies indexed from database inception to March 21, 2017, without language restriction.
Study Selection
Cohort, case-control, and cross-sectional studies reporting relative effect estimates for the association between vasectomy and prostate cancer were included.
Data Extraction and Synthesis
Two investigators performed study selection independently. Data were pooled separately by study design type using random-effects models. The Newcastle-Ottawa Scale was used to assess risk of bias.
Main Outcomes and Measures
The primary outcome was any diagnosis of prostate cancer. Secondary outcomes were high-grade, advanced, and fatal prostate cancer.
Results
Fifty-three studies (16 cohort studies including 2 563 519 participants, 33 case-control studies including 44 536 participants, and 4 cross-sectional studies including 12 098 221 participants) were included. Of these, 7 cohort studies (44%), 26 case-control studies (79%), and all 4 cross-sectional studies were deemed to have a moderate to high risk of bias. Among studies deemed to have a low risk of bias, a weak association was found among cohort studies (7 studies; adjusted rate ratio, 1.05; 95% CI, 1.02-1.09; P < .001; I2 = 9%) and a similar but nonsignificant association was found among case-control studies (6 studies; adjusted odds ratio, 1.06; 95% CI, 0.88-1.29; P = .54; I2 = 37%). Effect estimates were further from the null when studies with a moderate to high risk of bias were included. Associations between vasectomy and high-grade prostate cancer (6 studies; adjusted rate ratio, 1.03; 95% CI, 0.89-1.21; P = .67; I2 = 55%), advanced prostate cancer (6 studies; adjusted rate ratio, 1.08; 95% CI, 0.98-1.20; P = .11; I2 = 18%), and fatal prostate cancer (5 studies; adjusted rate ratio, 1.02; 95% CI, 0.92-1.14; P = .68; I2 = 26%) were not significant (all cohort studies). Based on these data, a 0.6% (95% CI, 0.3%-1.2%) absolute increase in lifetime risk of prostate cancer associated with vasectomy and a population-attributable fraction of 0.5% (95% CI, 0.2%-0.9%) were calculated.
Conclusions and Relevance
This review found no association between vasectomy and high-grade, advanced-stage, or fatal prostate cancer. There was a weak association between vasectomy and any prostate cancer that was closer to the null with increasingly robust study design. This association is unlikely to be causal and should not preclude the use of vasectomy as a long-term contraceptive option.
This systematic review and meta-analysis investigates whether vasectomy is associated with prostate cancer.
Key Points
Question
Is there an association between vasectomy and prostate cancer, high-grade prostate cancer, advanced prostate cancer, and/or fatal prostate cancer?
Findings
In this systematic review and meta-analysis including 53 studies, there was a weak, clinically insignificant association between vasectomy and prostate cancer. No association was found between vasectomy and risk of high-grade, advanced, or fatal prostate cancer.
Meaning
At most, there is a trivial association between vasectomy and prostate cancer that is unlikely to be causal; therefore, concerns about prostate cancer should not preclude the use of vasectomy as an option for long-term contraception.
Introduction
Vasectomy is a highly efficacious long-term contraceptive method that involves a simple outpatient procedure under local anesthetic. It is less expensive and has a lower risk of complications compared with tubal ligation, the analogous female surgical sterilization procedure. Although 43 million women worldwide rely on their partner’s vasectomy for contraception, vasectomy is still considered to be underused in the United States, with only 8% to 12% of couples using vasectomy for birth control.
In the late 1980s and early 1990s, several reports began to emerge of an epidemiologic association between vasectomy and the risk of prostate cancer. This finding ignited a controversy captured in numerous editorials, reviews, and original research articles supporting or refuting the association. Several meta-analyses of this association have been performed, but they did not bring closure to the debate. One older meta-analysis pooling 5 cohort studies and 17 case-control studies found a significant association between vasectomy and prostate cancer. Meanwhile, 2 more recent meta-analyses of cohort studies found no statistically significant association, although the CIs of pooled effect estimates precluded definitive conclusions. Moreover, none of these meta-analyses included a sufficient number of studies with a low risk of bias to analyze them as a separate subset, nor were the meta-analyses able to evaluate the association between vasectomy and risk of high-grade, advanced-stage, and fatal prostate cancer.
Recently, several large, high-quality analyses demonstrating either an association or no association between vasectomy and prostate cancer have reignited the controversy. With the aim of shedding some light onto a debate that is 3 decades old, we conducted a systematic review of the literature and performed a meta-analysis, with particular attention to study quality, to determine if there is an association between vasectomy and any prostate cancer, high-risk prostate cancer, advanced prostate cancer, and lethal prostate cancer.
Methods
Research Question
Is there an association between vasectomy and a subsequent diagnosis of prostate cancer? More specifically, is vasectomy associated with a diagnosis of any prostate cancer, high-risk prostate cancer, advanced prostate cancer, and/or fatal prostate cancer? The Mayo Clinic Institutional Review Board waived the need for review of this study.
Types of Studies
We included cohort, case-control, and cross-sectional studies. Case series lacking comparator groups were excluded. Other publications, including editorials, commentaries, review articles, and those not subject to peer review (ie, reports of data from Vital Statistics and dissertations or theses), were excluded. When there was more than 1 publication resulting from the same patient cohort, we selected a single representative study, with a preference for more contemporary publications and publications with a larger number of patients and more reliable methods of exposure and outcome ascertainment.
Types of Participants and Exposure
We reviewed studies reporting on men of any age who underwent vasectomy compared with those who did not undergo vasectomy. Vasectomy exposure was determined by administrative and clinical health records, survey results, and/or patient recall.
Outcome Measures
The primary outcome was any subsequent diagnosis of prostate cancer. Secondary outcomes included the diagnoses of high-grade prostate cancer (based on individual study definition, typically Gleason score ≥8), advanced prostate cancer (based on individual study definition, typically T3/4, N+, or M+), and fatal prostate cancer.
Methods of Systematic Review
We used Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines for reporting of this systematic review and meta-analysis.
Search Strategy
The MEDLINE, EMBASE, Web of Science, and Scopus databases were searched by a professional librarian using the OvidSP platform for studies indexed from database inception to March 21, 2017. We used both subject headings and text word terms for vasectomy, ductus deferens, prostatic neoplasms, and related and exploded terms including MeSH terms in combination with key word searching. A full search strategy is presented in eAppendix 1 in the Supplement. No limitations were placed with respect to publication language or publication year. Following the literature search, all duplicates were excluded. References from review articles, commentaries, editorials, included studies, and conference publications of relevant medical societies were hand searched and cross-referenced to ensure completeness. Conference abstracts were included when sufficient information could be obtained from the corresponding authors.
Study Review Methods
Two of us performed study selection independently (B.B. and C.J.D.W.). Disagreements were resolved by consensus. Titles and abstracts were used to screen for initial study inclusion. Full-text review was used when abstracts were insufficient to determine if the study met inclusion or exclusion criteria. Studies were considered relevant if they reported an effect estimate for an association between vasectomy and any prostate cancer outcome, or provided sufficient data for this estimate to be calculated. One of us (B.B.) performed all data abstraction, including evaluation of study characteristics, risk of bias, and outcome measures, with independent verification performed by another one of us (C.J.D.W.).
Assessment of Risk of Bias
We used the Newcastle-Ottawa Scale for assessment of risk of bias. This scale assesses risk of bias in the following 3 domains: selection of the study groups, comparability of groups, and ascertainment of exposure and outcome. Studies with scores of less than 4 were considered to have a high risk of bias, those with scores of 4 to 6 an intermediate risk of bias, and those with scores of 7 or more a low risk of bias.
Measures of Treatment Effect
Measures of treatment effect varied among study designs. Among cross-sectional and case-control studies, we pooled odds ratios (ORs). In each case, we examined unadjusted and adjusted measures of effect separately. Among cohort studies, we first pooled ratio measures of effect (including hazard ratios [HRs], rate ratios [RRs], and ORs). Subsequently, we performed subgroup analysis using only studies that reported time-to-event data (HRs).
Assessment of Heterogeneity
We identified heterogeneity using the Q test, estimated it using the DerSimonian-Laird method, and quantified it using I2 values. Furthermore, we used random-effects models for each of our analyses, given the identified clinical heterogeneity.
Assessment of Reporting Bias
We assessed publication bias for outcomes with more than 10 included studies using funnel plots.
Data Synthesis
Meta-analysis was performed using Review Manager, version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration). We used the inverse variance technique for pooling of measures of effect. Owing to the clinical heterogeneity inherent in our data, random-effects models were used for all meta-analyses. A sensitivity analysis was performed to determine if excluding any individual study altered our results.
Subgroup and Exploratory Analyses
We performed a priori subgroup analyses. First, as already mentioned, we examined cohort studies that used a time-to-event analysis separately. Second, we examined studies identified as having a low risk of bias. Third, we evaluated the studies that provided effect estimates stratified by time since vasectomy and age at which vasectomy was performed.
We also performed post hoc subgroup analyses. First, we examined cohort studies in which all patients were following a prostate-specific antigen (PSA) screening protocol or in which PSA screening was accounted for in multivariable models. Second, we examined cohort studies in which PSA screening was not performed or was very uncommon.
Finally, to facilitate interpretation of relative effect estimates for clinicians and policymakers, the pooled effect estimate from the meta-analysis was used to estimate the absolute risk increase, the number needed to harm, and the population attributable fraction. For these calculations, the lifetime risk of prostate cancer was considered to be 12.9% and the probability of undergoing vasectomy was 10%.
Results and Evidence Synthesis
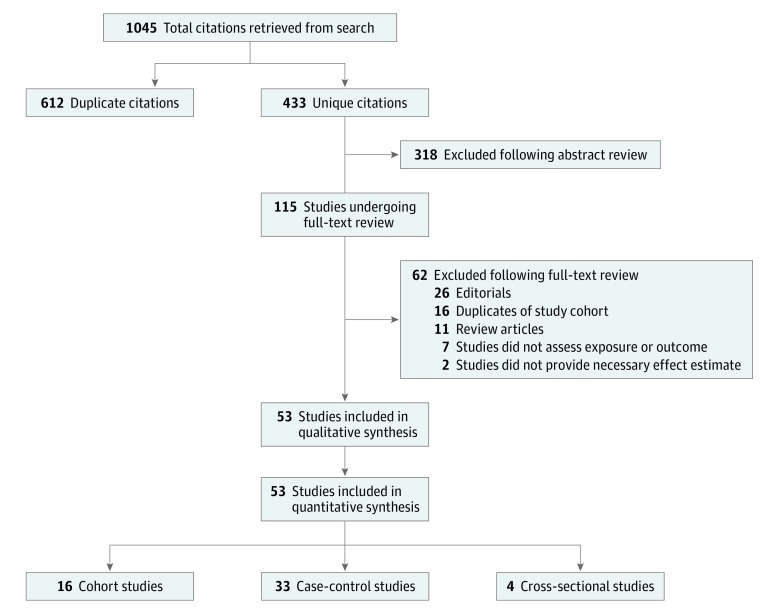
Our literature search identified 433 unique references (Figure 1). After a full-text review of 115 manuscripts, 53 were selected for inclusion. The reasons for exclusion are summarized in Figure 1 and eAppendix 2 in the Supplement.
Figure 1. PRISMA Flow Diagram Outlining Search Strategy and Final Included and Excluded Studies.
Description of Studies
Among the 53 included studies, 16 were cohort studies (2 563 519 participants), 33 were case-control studies (44 536 participants), and 4 were cross-sectional studies (12 098 221 participants). Although most studies were population- or registry-based analyses, several articles presented single- or multiple-institution analyses (Table). Duration of follow-up varied widely among cohort studies, with a minimum of 1.8 years and a maximum of 24 years. In general, the patients with and the patients without a history of vasectomy were similar in age among cohort studies. Among the case-control studies, the patients with and the patients without a diagnosis of cancer were generally similar in age, although, in some analyses, patients who received a diagnosis of prostate cancer were older. The risk-adjustment approach varied across studies (eTable 1 in the Supplement).
Table. Characteristics of 53 Included Studies.
| Source | Data Source | Study Interval | Follow-up Duration, y | Sample Size, No. | Patients With Vasectomy, No. (%) | Age | Outcomes | Patients Diagnosed With PCa, No. | |
|---|---|---|---|---|---|---|---|---|---|
| Vasectomy | No Vasectomy | ||||||||
| Cohort studies | |||||||||
| Coulson et al, 1993 | Minnesota and California, US | NA | Vasectomy, 8.3; no vasectomy, 8.8 | 21 180 | 10 590 (50.0) | Mean, y: 36 | NA | PCa | 13 |
| Davenport et al, 2016 | NIH-AARP Diet and Health Study, US | NA | 18 | 100 134 | 30 803 (30.8) | NA | NA | PCa | 14 127 |
| Eisenberg et al, 2015 | Truven Health Marketscan claims database, US | 2001-2009 | Summary value, NA | 873 485 | 112 655 (12.9) | No. (%): 18-19 y, 14 (0.01); 20-29 y, 10324 (9.2); 30-39 y, 64372 (57.1); 40-50 y, 37945 (33.7) | No. (%): 18-19 y, 1110 (0.2); 20-29 y, 133310 (17.52); 30-39 y, 452740 (59.5); 40-50 y, 173670 (22.83) | PCa | 4905 |
| Giovannucci et al, 1993 | Nurses’ Health Study, US | 1976-1989 | 11 | 25 340 | 13 034 (51.4) | Mean (SD), y: 42.0 (7.0) | Mean (SD), y: 42.2 (7.1) | PCa | 96 |
| Goldacre et al, 2005 | National Health System, Oxford, England | 1963-1999 | Vasectomy, 12.7; no vasectomy, NA | 184 253 | 24 773 (13.4) | No. (%): 20-24 y, 190 (0.8); 25-29 y, 2916 (11.8); 30-34 y, 8088 (32.6); 35-39 y, 7628 (30.8); 40-44 y, 3894 (15.7); 45-49 y, 1463 (5.9); 50-54 y, 435 (1.8); 55-59 y, 159 (0.6) | No.: 20-24 y, 33 308; 25-29 y, 25 865; 30-34 y, 20 195; 35-39 y, 16 621; 40-44 y, 15 576; 45-49 y, 15 496; 50-54 y, 16 032; 55-59 y, 16 387 | PCa | 656 |
| Hiatt et al, 1994 | California, US | 1979-1985 | 4.6 | 43 432 | NR | Mean (SD), y: 47.4 (13.0) | PCa | 238 | |
| Jacobs et al, 2016 | CPS-II + CPS-II Nutrition, US | 1982-2012 | CPS-II, 21.4; CPS-II Nutrition, 12.9 | CPS-II, 363 726; CPS-II Nutrition, 66 542 | CPS-II, 42 015 (11.6); CPS-II Nutrition, 10 589 (15.9) | %: 40-49 y, 16.6; 50-59 y, 39.2; 60-69 y, 32.1; 70-79 y, 10.9; ≥80 y, 1.2 | %: 40-49 y, 37.1; 50-59 y, 48.5; 60-69 y, 12.8; 70-79 y, 1.4; ≥80 y, 0.1 | PCa, HG-PCa, advanced PCa, and fatal PCa | PCa, 9133; HG-PCa, 1250; advanced PCa, 939; and fatal PCa, 7451 |
| Lynge, 2002 | Danish population-based registry | 1977-1995 | 12.7 | 57 931 (cohort of men who had vasectomy compared with Danish population using standardized incidence ratio) | 57 931 (100) | No. (%): ≤24 y, 76 (<1); 25-29 y, 5629 (10); 30-34 y, 16628 (29); 35-39 y, 18843 (33); 40-44 y, 11198 (19); 45-49 y, 4050 (7); 50-54 y, 1081 (2); 55-59 y, 292 (<1); ≥60 y, 134 (<1) | NA | PCa | 46 |
| Nayan et al, 2016 | Ontario, Canada, population-based registry | 1994-2012 | 10.9 | 653 214 | 326 607 (50.0) | Mean (SD), y: 37.3 (6.2) | Mean (SD), y: 37.3 (6.2) | PCa, HG-PCa, advanced PCa, and fatal PCa | PCa, 3462; and fatal PCa, 50 |
| Rohrmann et al, 2005 | CLUE II Cohort, Maryland, US | 1989-2004 | 8.3 | 3373 | 918 (27.2) | Mean (SD), y: 49.2 (9.0) | Mean (SD), y: 54.8 (11.3) | PCa, HG-PCa, and advanced PCa | PCa, 78; HG-PCa, 24; and advanced PCa, 15 |
| Romero et al, 2012 | Curitiba, Brazil | 2006-2011 | 1.8 | 2121 | 259 (12.2) | No. (%): 40-49 y, 805 (38.0); 50-59 y, 859 (40.5); 60-69 y, 350 (16.5); ≥70, 107 (5.0) | NA | PCa | 58 |
| Shoag et al, 2016 | PLCO trial, US | 1993-2009 | 13 | 73 180 | 19 965 (27.3) | NA | NA | PCa | NA |
| Smith et al, 2017 | European Prospective Investigation into Cancer and Nutrition | 1992-2012 | 15.4 | 84 743 | 12 712 (15.0) | Median (IQR), y: 52.0 (47.0-57.0) | Median (IQR), y: 54.0 (48.0-60.0) | PCa, HG-PCa, advanced PCa, and fatal PCa | PCa, 4377; HG-PCa, 544; advanced PCa, 633; and fatal PCa, 632 |
| Siddiqui et al, 2014 | Health Professionals Follow-up Study, US | 1986-2010 | 24 | 49 405 | 12 321 (24.9) | Mean, y: 51.8 | Mean, y: 55.5 | PCa, HG-PCa, advanced PCa, and fatal PCa | PCa, 6023; HG-PCa, 732; advanced PCa, 1052; and fatal PCa, 811 |
| Tangen et al, 2016 | PCPT trial, US | 1994-2003 | 7 | 8052 | 2644 (32.8) | No. (%): ≤59 y, 2497 (31.0); 60-64 y, 2429 (30.2); 65-69 y, 1901 (23.6); and ≥70, 1225 (15.2) | PCa | 574 | |
| van Leeuwen et al, 2011 | ERSPC-Rotterdam screening arm, the Netherlands | 1993-2008 | 11.1 | 19 950 | 5141 (25.8) | Median, y: 63 | Advanced PCa; PCa mets; and fatal PCa |
Advanced PCa: 2420; and fatal PCa: 104 |
|
| Case-control studies | Cases | Controls | |||||||
| Andersson et al, 1996 | Orebro County, Sweden | 1989-1991 | NA | 508 | 9 (1.8) | Mean (SD), y: 70.0 (6.1) | Mean (SD), y: 69.8 (6.2) | PCa | 256 |
| Cossack et al, 2014 | Creighton University, Northeast, US | NA | NA | 74 | 17 (23.0) | NA | NA | PCa | 24 |
| Cox et al, 2002 | New Zealand Cancer Registry | 1996-1998 | NA | 2147 | 549 (25.6) | Mean, y: 66.3 | Mean, y: 65.1 | PCa and advanced PCa | 923 |
| Emard et al, 2001 | Quebec, Canada, population-based registry | 1984-1993 | NA | 6349 | 110 (1.7) | Mean (SD), y: 68.2 (2.8) | Mean (SD), y: 67.9 (2.9) | PCa | 2962 |
| Ewings et al, 1996 | Taunton, Yeovil, Exeter Hospitals, England | 1989-1991 | NA | 484 | 8 (1.7) | No. (%): <70 y, 33 (21); 70-79 y, 90 (57); ≥80 y, 36 (23) | No. (%): <70 y, 86 (26); 70-79 y, 172 (53); ≥80 y, 67 (21) | PCa | 159 |
| Ganesh et al, 2011 | Tata Memorial Hospital, India | 1999-2001 | NA | 275 | 39 (14.2) | Mean, y: 64 | Mean, y: 45 | PCa | 123 |
| Hayes et al, 1993 | Population-based cancer registries in Michigan, Georgia, and New Jersey, US | 1986-1989 | NA | 2257 | 139 (6.2) | No. (%): 40-59 y, 279 (29); 50-69 y, 338 (35); ≥70 y, 348 (36) | No. (%): 40-59 y, 537 (42); 50-69 y, 395 (31); ≥70 y, 360 (28) | PCa | 965 |
| Hennis et al, 2013 | Barbados nationwide cohort | 2002-2011 | NA | 1904 | 1.5% of Cases; 0.7% of controls (exact number not given) | Mean (SD), y: 67.2 (9.0) | Mean (SD), y: 67.0 (9.2) | PCa | 963 |
| Holt et al, 2008 | Seattle-Puget Sound Tumor Registry, Washington, US | 2002-2005 | NA | 1943 | 36% (exact number not given) | %: 40-49 y, 7.9; 50-54 y, 9.4; 55-59 y, 19.1; 60-64 y, 21.2; 65-69 y, 23.8; 70-74 y, 18.5 | %: 40-49 y, 11.5; 50-54 y, 13.5; 55-59 y, 18.1; 60-64 y, 19.1; 65-69 y, 20.1; 70-74 y, 17.8 | PCa | 1001 |
| Honda et al, 1988 | Los Angeles, California, US, population-based registry | 1979-1982 | NA | 392 | 103 (26.3) | No. (%): <53 y, 32 (15); 53-57 y, 67 (31); 58-60 y, 117 (54) | NA | PCa | 1988 |
| Hsing et al, 1994 | Multi-institutional Chinese cohort | 1989-1992 | NA | 776 | 33 (4.3) | No. (%): 40-59 y, 13 (9.6); 60-69 y, 54 (39.7); ≥70 y, 69 (50.7) | No. (%): 40-59 y, 69 (10.8); 60-69 y, 252 (39.5); ≥70 y, 317 (49.7) | PCa | 138 |
| John et al, 1995 | Population-based registries in US and Canada | 1987-1991 | NA | 3278 | 336 (10.3) | Mean, y: 70.5 | Mean, y: 70.0 | PCa | 1642 |
| Kobayashi et al, 2012 | Kingston General Hospital, Ontario, Canada | 1997-1999 | NA | 414 | 116 (28.0) | Mean (SD), y: 65.1 (6.0) | Mean (SD), y: 63.6 (6.9) | PCa | 80 |
| Lesko et al, 1999 | Massachusetts, US, Cancer Registry | 1992-1996 | NA | 2616 | 414 (15.8) | Vasectomy (%): <60 y, 46; 60-64 y, 28; ≥65 y, 26. No vasectomy (%): <60 y, 20; 60-64 y, 29; ≥65 y, 50 | Vasectomy (%): <60 y, 40; 60-64 y, 33; ≥65 y, 27. No vasectomy (%): <60 y, 22; 60-64 y, 29; ≥65 y, 49 | PCa and advanced PCa | 1216 |
| Liang et al, 2007 | Multi-institutional Chinese cohort | 2005-2006 | NA | 186 | 16 (8.6) | Median, y: 69.5 | Median, y: 69.0 | PCa | 62 |
| Lightfoot et al, 2004 | Northeastern Ontario, Canada | 1995-1999 | NA | 2354 | 449 (19.1) | No. (%): 45-49 y, 8 (1.1); 50-54 y, 25 (3.3); 55-59 y, 50 (6.6); 60-64 y, 134 (17.6); 65-69 y, 222 (29.2); 70-74 y, 181 (23.8); 75-79 y, 109 (14.3); 80-84 y, 31 (4.1) | No. (%): 45-49 y, 19 (1.2); 50-54 y, 69 (4.2); 55-59 y, 138 (8.5); 60-64 y, 271 (16.6); 65-69 y, 445 (27.3); 70-74 y, 389 (23.8); 75-79 y, 205 (12.6); 80-84 y, 96 (5.9) | PCa | 744 |
| Mazdak et al, 2012 | Isfahan, Iran | 2005-2009 | NA | 190 | 22 (11.6) | Mean (SD), y: 73.1 (7.5) | Mean (SD), y: 67.9 (8.3) | PCa | 95 |
| Mettlin et al, 1990 | Roswell Park Memorial Institute, New York, US | 1982-1988 | NA | 3202 | 154 (4.8) | Mean (SD), y: 68.4 (7.5) | Mean (SD), y: 64.9 (8.5) | PCa | 614 |
| Nair-Shalliker et al, 2017 | New South Wales, Australia | 2006-2014 | NA | 2056 | NR | Median, y: 65.6 | Median, y: 59.0 | PCa | 1181 |
| Patel et al, 2005 | Wayne County, Michigan, US | 1996-1998 | NA | 1304 | 164 (12.6) | No. (%): 50-54 y, 59 (8.4); 55-59 y, 93 (13.3); 60-64 y, 135 (19.3); 65-69 y, 205 (29.3); 70-74 y, 208 (29.7) | No. (%): 50-54 y, 73 (12.1); 55-59 y, 97 (16.1); 60-64 y, 93 (15.4); 65-69 y, 179 (29.6); 70-74 y, 162 (26.8) | PCa | 700 |
| Platz et al, 1997 | Bombay Cancer Registry, India | 1993-1994 | NA | 1153 | 100 (8.7) | Mean, y: 67.3 | Mean, y: 59.1 | PCa | 175 |
| Pourmand et al, 2007 | Multi-institutional Iranian cohort | 2005-2007 | NA | 205 | 19 (9.3) | Mean (SD), y: 70.5 (8.3) | Mean (SD), y: 65.7 (9.9) | PCa | 130 |
| Rosenberg et al, 1994 | Multi-institutional US cohort: Boston, New York, Philadelphia, and Baltimore | 1977-1992 | NA | 7580 | 468 (6.2) | No. (%): 30-49 y, 19 (34.3); 50-59 y, 119 (21.5); 60-69 y, 415 (75.0) | No. (%): 30-49 y, 3769 (53.6); 50-59 y, 1817 (25.9); 60-69 y, 1441 (20.5) | PCa and advanced PCa |
553 |
| Ross et al, 1983 | Los Angeles, California, US, population-based registry | 1972-1980 | NA | 220 | 15 (6.8) | NA | NA | PCa | 110 |
| Schwingl et al, 2009 | Multi-institutional cohort from China, Nepal, and Korea | 1994-1997 | NA | 1173 | 120 (10.2) | Mean (SD), y: 66.6 (6.1) | Mean (SD), y: 66.4 (6.1) | PCa and advanced PCa | 294 |
| Spitz et al, 1991 | MD Anderson Cancer Center, Texas, US | NA | NA | 703 | NR | NA | NA | PCa | 343 |
| Sridhar et al, 2010 | Virginia, US, private urology practice | 2000-2005 | NA | 3710 | 348 (9.4) | No. (%): ≤60 y, 70 (13.2); 61-70 y, 183 (34.4); >70 y, 279 (52.4) | No. (%): ≤60 y, 375 (59.7); 61-70 y, 175 (27.9); >70 y, 78 (12.4) | PCa | 1237 |
| Stanford et al, 1999 | Seattle-Puget Sound Cancer Registry, Washington, US | 1993-1996 | NA | 1456 | 562 (38.6) | NA | Vasectomy (%): 40-49 y, 7.9; 50-54 y, 21.9; 55-59 y, 40.8; 60-64 y, 29.4. No vasectomy (%): 40-49 y, 8.4; 50-54 y, 18.3; 55-59 y, 35.6; 60-64 y, 37.7 | PCa, HG-PCa, and advanced PCa | 753 |
| Sunny, 2005 | Bombay Cancer Registry, India | 1998-2000 | NA | 1170 | 136 (11.6) | Mean, y: 71.2 | Mean, 64.4 | PCa | 390 |
| Tyagi et al, 2010 | Delhi Cancer Registry, India | 1998-2000 | NA | 909 | 119 (13.1) | Mean, y: 69.7 | Mean, y: 65.6 | PCa | 303 |
| Wei et al, 1994 | First Affiliated Hospital of West China University of Medical Sciences | NA | NA | 81 | 4 (4.9) | NA | NA | PCa | 27 |
| Weinmann et al, 2010 | Kaiser Permanente California and Northwest Region and Henry Ford Health System, US | 1974-2000 | NA | 1697 | 101 (6.0) | No. (%): <60 y, 91 (11.8); 60-69 y, 326 (42.4); 70-79 y, 326 (42.4); 80-84 y, 25 (3.3) | NA | Fatal PCa | 768 |
| Zhu et al, 1996 | Group Health Cooperative of Puget Sound, Washington, US | 1989-1991 | NA | 433 | 154 (35.6) | NA | NA | PCa | 175 |
| Cross-sectional studies | Participants With or Without Vasectomy | ||||||||
| Alqahtani et al, 2015 | Nationwide Inpatient Sample, US | 2007-2011 | NA | 12 000 718 | 0.03% of Cohort (exact number not reported) | Mean (SD), y: 64.2 (14.7) | PCa | 642 383 | |
| Chacko et al, 2002 | Stanford Medical Center, California, US | 1998-2001 | NA | 303 | 101 (33.3) | Mean (range), y: 65 (39-86) | PCa and HG-PCa | 144 | |
| DeAntoni et al, 1997 | Multi-institutional PCa screening cohort, US | 1993-1995 | NA | 95 961 | 26 632 (27.8) | Mean (SD), y: 61.7 (8.0) | PCa | 766 | |
| Garzotto et al, 2003 | Portland Veterans Affairs Hospital, Oregon, US | 1993-2000 | NA | 1239 | NR | Median, y: 66 | PCa | 300 | |
Abbreviations: CLUE II, Cohort Study–CLUE II [see Cancer Epidemiology Descriptive Cohort Study Database website, Johns Hopkins Bloomberg School of Public Health]; CPS-II, Cancer Prevention Study II; ERSPC, European Randomized Study of Screening for Prostate Cancer; HG-PCa, high-grade prostate cancer; IQR, interquartile range; NA, not available; NIH-AARP, National Institutes of Health–American Association of Retired Persons; NR, not reported; PCa, prostate cancer; PLCO, Prostate, Lung, Colorectal and Ovarian Cancer screening trial; PCPT, Prostate Cancer Prevention Trial; US United States.
Assessment of Risk of Bias
Assessment of risk of bias was performed according to study methods (eTable 2 in the Supplement). Nine of the cohort studies (56%) were assessed as having a low risk of bias. Exposure was ascertained by self-report or questionnaire results in several of these studies. Eight of 16 cohort studies (50%) accounted for PSA testing and/or health-seeking behavior. Most case-control studies (26 [79%]) were considered to have a moderate to high risk of bias. Although cases were typically well defined, the use of hospital-based instead of community-based controls was considered a potential source of bias among many case-control studies. Ascertainment of exposure was also a common source of bias among case-control studies. All 4 cross-sectional studies were thought to have a moderate to high risk of bias.
All Prostate Cancer
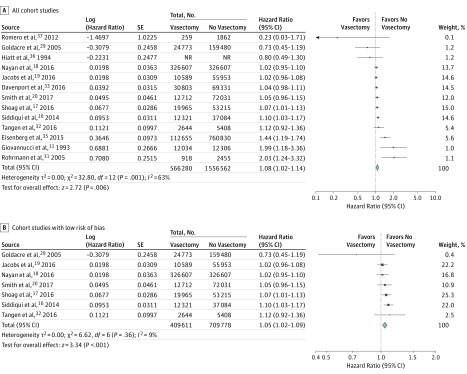
To assess the association between vasectomy and any diagnosis of prostate cancer, we first pooled results from cohort studies (Figure 2). Among the 13 of 16 cohort studies reporting on 2 563 519 patients that used adjusted measures of effect, we found a small but statistically significant increased risk of any diagnosis of prostate cancer among patients with a history of vasectomy (adjusted RR, 1.08; 95% CI, 1.02-1.14; P = .006; I2 = 63%) (Figure 2A). A similar outcome was observed among cohort studies using a time-to-event analysis (9 studies; adjusted HR, 1.09; 95% CI, 1.03-1.15; P = .004; I2 = 70%) (eFigure 1 in the Supplement). The effect estimate among studies deemed at low risk of bias remained statistically significant but was closer to the null (7 studies; adjusted RR, 1.05; 95% CI, 1.02-1.09; P < .001; I2 = 9%) (Figure 2B). Results were not meaningfully different when abstracts were excluded (eTable 3 in the Supplement).
Figure 2. Forest Plots for Meta-analyses of the Adjusted Estimates for the Association Between Vasectomy and Any Prostate Cancer by Study Design and Risk of Bias (Cohort Studies).
Data were pooled separately by study design type using random-effects models; the inverse variance technique was used for pooling of measures of effect. NR indicates not reported.
Among cohort studies in which all patients explicitly underwent PSA screening (as in the screening arm of the PLCO [Prostate, Lung, Colorectal and Ovarian] Cancer Screening trial) or in which PSA screening was accounted for in multivariable models, the association between vasectomy and prostate cancer was consistent with our overall findings (6 studies; adjusted RR, 1.06; 95% CI, 1.02-1.09; P < .001; I2 = 16%) (eFigure 2 in the Supplement). Furthermore, among studies that reported on populations in which PSA screening was not performed or very uncommonly performed, we observed no association between vasectomy and prostate cancer, although this finding was limited by few studies and significant heterogeneity (2 studies; adjusted RR, 1.26; 95% CI, 0.51-3.07; P = .62; I2 = 84%).
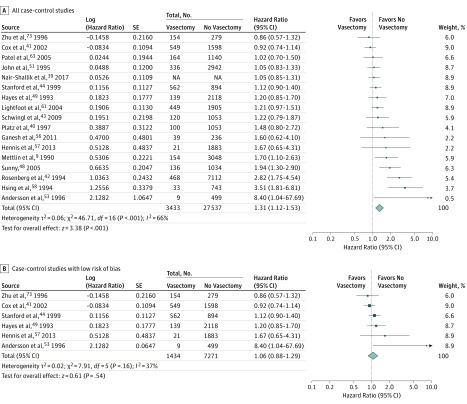
We then separately assessed the association between vasectomy and any diagnosis of prostate cancer among case-control studies (Figure 3). Meta-analysis of studies reporting adjusted ORs demonstrated a statistically significant association between vasectomy and prostate cancer (17 studies; adjusted OR, 1.31; 95% CI, 1.12-1.53; P < .001; I2 = 66%) (Figure 3A). When we restricted analysis to 6 studies deemed to have low risk of bias, there was no significant association (adjusted OR, 1.06; 95% CI, 0.88-1.29; P = .54; I2 = 37%) (Figure 3B).
Figure 3. Forest Plots for Meta-analyses of the Adjusted Estimates for the Association Between Vasectomy and Any Prostate Cancer by Study Design and Risk of Bias (Case-Control Studies).
Data were pooled separately by study design type using random-effects models; the inverse variance technique was used for pooling of measures of effect. NA indicates not available.
There were no cross-sectional studies reporting adjusted measures of effect. Analyses of cohort, case-control, and cross-sectional studies reporting unadjusted measures of effect were performed for completeness and are summarized in eAppendix 3 and eFigure 3 in the Supplement. These results did not meaningfully alter conclusions. In the sensitivity analysis, excluding any individual study did not alter the results in each of the respective meta-analyses.
Secondary Outcomes
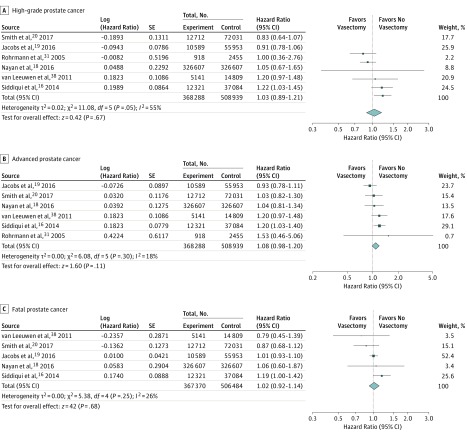
Among cohort studies, there was no statistically significant association noted between vasectomy and the diagnosis of high-grade prostate cancer (6 studies; adjusted HR, 1.03; 95% CI, 0.89-1.21; P = .67; I2 = 55%), advanced prostate cancer (6 studies; adjusted HR, 1.08; 95% CI, 0.98-1.20; P = .11; I2 = 18%), or fatal prostate cancer (5 studies; adjusted HR, 1.02; 95% CI, 0.92-1.14; P = .68; I2 = 26%) (Figure 4).
Figure 4. Forest Plots for Meta-analyses of the Associations Between Vasectomy and High-Grade, Advanced, and Fatal Prostate Cancers.
Data were pooled separately by study design type using random-effects models; the inverse variance technique was used for pooling of measures of effect.
Five case-control studies assessed the association between vasectomy and prostate cancer, stratified by stage. The varied definitions of advanced prostate cancer in these studies precluded meta-analysis of these results. Qualitatively, there was a greater association between vasectomy and low-risk prostate cancer than between vasectomy and advanced disease among these studies. Meanwhile, a single case-control study assessed the association between vasectomy and fatal prostate cancer and found no association (unadjusted OR, 1.3; 95% CI, 0.85-1.9). Similarly, a single cross-sectional study examined the association between vasectomy and high-grade prostate cancer and found no association (unadjusted OR, 0.69; 95% CI, 0.37-1.27).
Duration of Exposure and Age at Vasectomy
Six cohort studies, 15 case-control studies, and 1 cross-sectional study reported on the association of time since vasectomy with the development of prostate cancer (eTable 4 in the Supplement). Of these, 1 cohort study and 6 case-control studies suggested a stronger association between vasectomy and prostate cancer with increasing time since vasectomy, although in several instances the CIs of the individual strata included unity and formal tests for trend were only performed in 3 studies. The remainder of these studies did not find any such biological gradient associated with time since vasectomy.
Four cohort studies and 11 case-control studies reported effect estimates stratified by age at vasectomy (eTable 5 in the Supplement). Of these, 1 cohort study and 4 case-control studies found that the association between vasectomy and prostate cancer was stronger among men who had their vasectomy performed at a younger age. Conversely, 1 cohort study and 1 case-control study found that men who were older when they had their vasectomy were at greater risk for incident prostate cancer. The remainder of these studies did not find any association of age at vasectomy with development of prostate cancer. Effect estimates for time since vasectomy and age at vasectomy could not be pooled owing to the varying types of effect estimates and time intervals used by the individual studies.
Publication Bias
We assessed publication bias using funnel plots comparing effect size and measure of precision of the effect size among case-control studies reporting unadjusted and adjusted measures of effect and cohort studies reporting adjusted measures of effect (eFigure 4 in the Supplement). There was potential publication bias among cohort studies, with a relative paucity of small studies demonstrating a large increased risk, and among case-control studies reporting adjusted measures of effect, with a relative paucity of small studies demonstrating no increased risk.
Exploratory Estimations
Using the pooled effect estimate from the meta-analysis of cohort studies with a low risk of bias, we found that the absolute increase in lifetime risk of prostate cancer is estimated to be 0.6% (95% CI, 0.3%-1.2%), that the number needed to harm (assuming a causal association, the number of men who would need to undergo vasectomy to result in 1 incident case of prostate cancer) is estimated to be 156, and that the population attributable fraction (assuming a causal association, the proportion the lifetime risk of prostate cancer would be reduced if no vasectomies were performed) is estimated to be 0.5% (95% CI, 0.2%-0.9%) (eAppendix 4 in the Supplement).
Discussion
To date, the potential for bias in the studies on the association between vasectomy and prostate cancer has been a major focus of criticism of this body of literature. Accordingly, we found that the effect estimates of the association between vasectomy and prostate cancer were increasingly closer to the null when examining studies with increasingly robust study design and study quality. In our meta-analysis of cohort studies with a low risk of bias, we found a 5% increase in the risk of incident prostate cancer with vasectomy. This result was not driven by any single study alone. Meanwhile, the associations between vasectomy and high-grade, advanced, and fatal prostate cancer were not statistically significant, although the point estimates were similar to those for total diagnoses of prostate cancer. If assuming causality, for the individual patient, the effect estimate for overall prostate cancer corresponds to a 0.6% absolute increase in lifetime risk of incident prostate cancer, or a number needed to harm of 156. At the population level, only 0.5% of prostate cancers are estimated to be associated with vasectomy. It is questionable whether such a small increased risk is important to the public.
Although meta-analyses have been previously performed, an analysis of cohort studies with a low risk of bias has only recently been possible, with 6 of 7 such studies reported within the last 3 years. One meta-analysis published in 2002 pooled data from 5 cohort and 17 case-control studies and found a significant association between vasectomy and prostate cancer. Meanwhile, 2 more recent meta-analyses combined data from 9 cohort studies and found no significant association but had wide CIs. Another meta-analysis combined data from 10 cohort studies and found no association, but the lower limit of its CI barely crossed 1 (RR, 1.11; 95% CI, 0.98-1.27). Our meta-analysis, which included 53 studies, was able to separately analyze case-control, cross-sectional, and cohort studies and was well powered and achieved effect estimates with narrow CIs.
In our assessment, most of the 33 case-control studies, all 4 of the cross-sectional studies, and almost half of the 16 cohort studies had a moderate to high risk of bias. Accordingly, the point estimates for association between vasectomy and prostate cancer were furthest from the null among pooled analyses of case-control and cross-sectional studies. Publication bias might also have contributed, in part, based on funnel plots. In contrast, the observed association was smaller in magnitude in the pooled analysis of cohort studies, and even smaller when restricted to studies (cohort and case-control designs) deemed as having a low risk of bias. In addition, despite methodological rigor, failure to account for differential use of PSA screening may bias results because patients who have undergone vasectomy are more likely to undergo PSA screening and thus receive a diagnosis of prostate cancer. In our study, the association between vasectomy and prostate cancer held in a subset analysis restricted to studies that accounted for PSA screening. Our analysis illustrates the susceptibility of observational studies to bias and highlights the importance of meticulous study design.
More important, simply because a statistically significant association was detected, one cannot confirm with certainty that a causal association exists. Owing to the observational nature of pooled studies, residual unmeasured bias is still possible. Residual detection bias remains an ongoing concern, even though several studies accounted for serum PSA screening and/or contact with the health care system. A recent article by Tangen et al illustrated that detection bias influences the evaluation of several risk factors that have been described as associated with decreased or increased prostate cancer risk. The only way to address this source of bias would be a trial randomizing men to undergo vasectomy vs no vasectomy. However, a sufficiently powered trial with long enough follow-up would be neither practical nor ethical. Therefore, the present meta-analysis likely approaches the highest level of clinical evidence reasonably attainable in evaluating the association of vasectomy with risk of prostate cancer.
If applying the criteria of Hill, which have been widely accepted as aiding in making causal inferences, one cannot make a strong argument for a causal association between vasectomy and prostate cancer (eAppendix 5 in the Supplement). Moreover, the case for biological plausibility is tenuous. Although hormonal imbalances, immunologic effects, and cell proliferative changes have been suggested to play a role, the exact mechanisms remain to be described in animal models. Although 1 study found that serum testosterone levels are elevated in men who underwent a vasectomy more than 20 years ago relative to men who did not undergo a vasectomy, most studies have shown no changes in testosterone levels following vasectomy. Moreover, there is no established association between elevated serum testosterone level and risk of prostate cancer. Although vasectomy may lead to the development of antisperm antibodies, there is no evidence that these antibodies or the subsequent formation of immune complexes leads to prostate cancer. One study found increased cell proliferation 7 days after vasectomy in the ductal system of the rat ventral prostate, although the mechanism remains unclear.
On the other hand, the benefits of vasectomy as a method of contraception must be considered. More than 99% of women who have ever been sexually active have used a form of contraception at some point. However, according to the most recent data, 45% of pregnancies in 2011 in the United States were unintended; 41% of these pregnancies occurred among women who used contraception inconsistently. Two-thirds of women using contraception rely on nonpermanent methods, many of which have a higher failure rate with typical use vs perfect use. Meanwhile, 25% of women rely on surgical sterilization for contraception and 8% rely on male surgical sterilization. Given the lower costs and lower risk of complications for vasectomy compared with tubal ligation, it is clear that vasectomy is underused and should be offered more routinely to couples seeking a long-term method of contraception.
Strengths and Limitations
There are several strengths to this study, including its size, its comprehensive search strategy in all languages, its careful review for study inclusion, its thorough assessment of study quality, and its use of a priori secondary analyses. This study is the first, to our knowledge, to separately evaluate studies with a low risk of bias and to evaluate high-grade, advanced, and fatal prostate cancers as secondary outcomes. Furthermore, we used relative effect estimates to calculate absolute effect estimates, which are more useful and more readily interpretable for clinicians, policymakers, and patients.
There are also limitations to our study. First, this meta-analysis is based on observational data because randomized trials are neither presently available nor likely to be performed in the future. As such, the unmeasured biases present in the individual studies must be taken into account. Our analytic approach addressed this issue in part by separately evaluating cross-sectional, case-control, and cohort studies and, additionally, separately evaluating the cohort and case-control studies with a low risk of bias. Second, publication bias cannot be ruled out. On the other hand, publication bias would have had outcomes in opposite directions in case-control and cohort meta-analyses, yet these analyses were consistent with each other. Finally, our analysis cannot definitively prove or disprove causality. However, as already outlined, a strong argument for a causal association between vasectomy and prostate cancer does not exist based on our data and on other existing literature.
Although it is tempting to consider potential avenues of further research on the link between vasectomy and prostate cancer, there are many other research topics that warrant greater priority in a system with finite health care research resources. We have demonstrated that any risk, if present, is sufficiently small that it is unlikely to be of clinical importance. We believe that this meta-analysis, drawing on 3 decades of epidemiologic literature, provides sufficiently robust data to inform clinical care and supports the current guidelines of the American Urological Association.
Conclusions
Our meta-analysis found a weak association between vasectomy and the risk of prostate cancer among cohort studies with a low risk of bias and a similar but nonsignificant association among case-control studies with a low risk of bias. There was a similar nonsignificant association between vasectomy and high-grade, advanced-stage, or fatal prostate cancer. The association between vasectomy and prostate cancer was stronger when studies with moderate to high risk of bias were included. If assuming a causal association, which is unlikely based on our data and other existing literature, vasectomy would confer only a 5% relative increase or a 0.6% absolute increase in lifetime risk of prostate cancer, and would be responsible for only 0.5% of cases of prostate cancer in the population. Therefore, although patients should be appropriately counseled, concerns about the risk of prostate cancer should not preclude clinicians from offering vasectomy to couples seeking long-term contraception.
eAppendix 1. Literature Search Strategy
eAppendix 2. Exclusion Following Full Text Review
eAppendix 3. Pooled Estimates of Studies Reporting Unadjusted Effect Estimates
eAppendix 4. Calculation of Estimates for Absolute Risk Increase, Number Needed to Harm, and Population-Attributable Fraction
eAppendix 5. Discussion of Hill’s Criteria of Causation
eFigure 1. Forest Plots for Meta-Analyses of the Adjusted Estimates for the Association Between Vasectomy and Any Prostate Cancer for Cohort Studies Reporting on Time-to-Event Analyses
eFigure 2. Meta-Analysis of Cohort Studies That Accounted for PSA Testing
eFigure 3. Forest Plots for Meta-Analyses of Unadjusted Estimates for the Association Between Vasectomy and Any Prostate Cancer by Study Design and Risk of Bias
eFigure 4. Funnel Plots
eTable 1. Risk Adjustment for Each Included Study
eTable 2. Newcastle-Ottawa Scale for Risk of Bias Assessment of Studies Included in the Meta-Analysis
eTable 3. Pooled Adjusted Estimates for Association Between Vasectomy and Prostate Cancer, Excluding Abstracts
eTable 4. Studies Reporting on the Impact Time Since Vasectomy on the Association Between Vasectomy and Prostate Cancer
eTable 5. Studies Reporting on the Impact of Age at Vasectomy on the Association Between Vasectomy and Prostate Cancer
eReferences.
References
- 1.Sharlip ID, Belker AM, Honig S, et al. ; American Urological Association . Vasectomy: AUA guideline. J Urol. 2012;188(6)(suppl):2482-2491. [DOI] [PubMed] [Google Scholar]
- 2.Shih G, Turok DK, Parker WJ. Vasectomy: the other (better) form of sterilization. Contraception. 2011;83(4):310-315. [DOI] [PubMed] [Google Scholar]
- 3.Eisenberg ML, Henderson JT, Amory JK, Smith JF, Walsh TJ. Racial differences in vasectomy utilization in the United States: data from the national survey of family growth. Urology. 2009;74(5):1020-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pile JM, Barone MA. Demographics of vasectomy—USA and international. Urol Clin North Am. 2009;36(3):295-305. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Daniels K, Daugherty J, Jones J Current contraceptive Status Among Women Aged 15-44: United States, 2011-2013. https://www.cdc.gov/nchs/data/databriefs/db173.pdf. Published December 2014. Accessed February 3, 2017.
- 6.Guttmacher Institute. Contraceptive use in the United States. https://www.guttmacher.org/fact-sheet/contraceptive-use-united-states. Published September 2016. Accessed February 3, 2017.
- 7.Rosenberg L, Palmer JR, Zauber AG, Warshauer ME, Stolley PD, Shapiro S. Vasectomy and the risk of prostate cancer. Am J Epidemiol. 1990;132(6):1051-1055. [DOI] [PubMed] [Google Scholar]
- 8.Honda GD, Bernstein L, Ross RK, Greenland S, Gerkins V, Henderson BE. Vasectomy, cigarette smoking, and age at first sexual intercourse as risk factors for prostate cancer in middle-aged men. Br J Cancer. 1988;57(3):326-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mettlin C, Natarajan N, Huben R. Vasectomy and prostate cancer risk. Am J Epidemiol. 1990;132(6):1056-1061. [DOI] [PubMed] [Google Scholar]
- 10.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. A prospective cohort study of vasectomy and prostate cancer in US men. JAMA. 1993;269(7):873-877. [PubMed] [Google Scholar]
- 11.Giovannucci E, Tosteson TD, Speizer FE, Ascherio A, Vessey MP, Colditz GA. A retrospective cohort study of vasectomy and prostate cancer in US men. JAMA. 1993;269(7):878-882. [PubMed] [Google Scholar]
- 12.Dennis LK, Dawson DV, Resnick MI. Vasectomy and the risk of prostate cancer: a meta-analysis examining vasectomy status, age at vasectomy, and time since vasectomy. Prostate Cancer Prostatic Dis. 2002;5(3):193-203. [DOI] [PubMed] [Google Scholar]
- 13.Shang Y, Han G, Li J, et al. . Vasectomy and prostate cancer risk: a meta-analysis of cohort studies. Sci Rep. 2015;5:9920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang XL, Yan JJ, Pan SH, Pan JG, Ying XR, Zhang GF. Vasectomy and the risk of prostate cancer: a meta-analysis of cohort studies. Int J Clin Exp Med. 2015;8(10):17977-17985. [PMC free article] [PubMed] [Google Scholar]
- 15.Liu LH, Kang R, He J, et al. . Vasectomy and risk of prostate cancer: a systematic review and meta-analysis of cohort studies. Andrology. 2015;3(4):643-649. [DOI] [PubMed] [Google Scholar]
- 16.Siddiqui MM, Wilson KM, Epstein MM, et al. . Vasectomy and risk of aggressive prostate cancer: a 24-year follow-up study. J Clin Oncol. 2014;32(27):3033-3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shoag J, Mittal S, Halpern J, et al. . Vasectomy and risk of prostate cancer in a screening trial. J Urol. 2016;195(4 suppl):e1157-e1158. doi: 10.1016/j.juro.2016.02.2599 [DOI] [Google Scholar]
- 18.Nayan M, Hamilton RJ, Macdonald EM, et al. ; Canadian Drug Safety and Effectiveness Research Network (CDSERN) . Vasectomy and risk of prostate cancer: population based matched cohort study. BMJ. 2016;355:i5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobs EJ, Anderson RL, Stevens VL, Newton CC, Gansler T, Gapstur SM. Vasectomy and prostate cancer incidence and mortality in a large US cohort. J Clin Oncol. 2016;19:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith K, Byrne, Castaño JM, et al. . Vasectomy and prostate cancer risk in the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Clin Oncol. 2017;35(12):1297-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012. [DOI] [PubMed] [Google Scholar]
- 22.Stroup DF, Berlin JA, Morton SC, et al. . Meta-analysis of observational studies in epidemiology: a proposal for reporting: Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. [DOI] [PubMed] [Google Scholar]
- 23.Deeks JJ, Dinnes J, D’Amico R, et al. ; International Stroke Trial Collaborative Group; European Carotid Surgery Trial Collaborative Group . Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7(27):iii-x, 1-173. [DOI] [PubMed] [Google Scholar]
- 24.The Ottawa Hospital. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed Sept 14, 2014.
- 25.World Cancer Research Fund Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington, DC: American Institute for Cancer Research; 2007. [Google Scholar]
- 26.Roerecke M, Rehm J. Chronic heavy drinking and ischaemic heart disease: a systematic review and meta-analysis. Open Heart. 2014;1(1):e000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Surveillance, Epidemiology, and End Results Program, National Cancer Institute. SEER cancer statistics review, 1975-2013. https://seer.cancer.gov/csr/1975_2013/. Updated September 12, 2016. Accessed February 3, 2017.
- 29.Goldacre MJ, Wotton CJ, Seagroatt V, Yeates D. Cancer and cardiovascular disease after vasectomy: an epidemiological database study. Fertil Steril. 2005;84(5):1438-1443. [DOI] [PubMed] [Google Scholar]
- 30.Lynge E. Prostate cancer is not increased in men with vasectomy in Denmark. J Urol. 2002;168(2):488-490. [PubMed] [Google Scholar]
- 31.Rohrmann S, Paltoo DN, Platz EA, Hoffman SC, Comstock GW, Helzlsouer KJ. Association of vasectomy and prostate cancer among men in a Maryland cohort. Cancer Causes Control. 2005;16(10):1189-1194. [DOI] [PubMed] [Google Scholar]
- 32.Tangen CM, Goodman PJ, Till C, Schenk JM, Lucia MS, Thompson IM Jr. Biases in recommendations for and acceptance of prostate biopsy significantly affect assessment of prostate cancer risk factors: results from two large randomized clinical trials. J Clin Oncol. 2016;34(36):4338-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davenport M, Li S, Brooks J, Cullen M, Eisenberg M. Vasectomy and the risk of prostate cancer in a prospective us cohort: analysis in the presence of selection bias. J Urol. 2016;195(4):e34. doi: 10.1016/j.juro.2016.02.1950 [DOI] [Google Scholar]
- 34.Coulson AH, Crozier R, Massey FJ Jr, O’Fallon WM, Schuman LM, Spivey GH. Health status of American men—a study of post-vasectomy sequelae: results. J Clin Epidemiol. 1993;46(8):857-920. doi: 10.1016/0895-4356(93)90192-4 [DOI] [PubMed] [Google Scholar]
- 35.Eisenberg ML, Li S, Brooks JD, Cullen MR, Baker LC. Increased risk of cancer in infertile men: analysis of U.S. claims data. J Urol. 2015;193(5):1596-1601. [DOI] [PubMed] [Google Scholar]
- 36.Hiatt RA, Armstrong MA, Klatsky AL, Sidney S. Alcohol consumption, smoking, and other risk factors and prostate cancer in a large health plan cohort in California (United States). Cancer Causes Control. 1994;5(1):66-72. [DOI] [PubMed] [Google Scholar]
- 37.Romero FR, Romero AW, Almeida RM, Oliveira FC Jr, Tambara Filho R. The significance of biological, environmental, and social risk factors for prostate cancer in a cohort study in Brazil. Int Braz J Urol. 2012;38(6):769-778. [DOI] [PubMed] [Google Scholar]
- 38.van Leeuwen PJ, van den Bergh RC, Wolters T, et al. . Critical assessment of prebiopsy parameters for predicting prostate cancer metastasis and mortality. Can J Urol. 2011;18(6):6018-6024. [PubMed] [Google Scholar]
- 39.Nair-Shalliker V, Yap S, Nunez C, et al. . Adult body size, sexual history and adolescent sexual development, may predict risk of developing prostate cancer: results from the New South Wales Lifestyle and Evaluation of Risk Study (CLEAR). Int J Cancer. 2017;140(3):565-574. [DOI] [PubMed] [Google Scholar]
- 40.Platz EA, Yeole BB, Cho E, Jussawalla DJ, Giovannucci E, Ascherio A. Vasectomy and prostate cancer: a case-control study in India. Int J Epidemiol. 1997;26(5):933-938. [DOI] [PubMed] [Google Scholar]
- 41.Cox B, Sneyd MJ, Paul C, Delahunt B, Skegg DC. Vasectomy and risk of prostate cancer. JAMA. 2002;287(23):3110-3115. [DOI] [PubMed] [Google Scholar]
- 42.Rosenberg L, Palmer JR, Zauber AG, et al. . The relation of vasectomy to the risk of cancer. Am J Epidemiol. 1994;140(5):431-438. [DOI] [PubMed] [Google Scholar]
- 43.Schwingl PJ, Meirik O, Kapp N, Farley TM; HRP Multicenter Study of Prostate Cancer and Vasectomy . Prostate cancer and vasectomy: a hospital-based case-control study in China, Nepal and the Republic of Korea. Contraception. 2009;79(5):363-368. [DOI] [PubMed] [Google Scholar]
- 44.Stanford JL, Wicklund KG, McKnight B, Daling JR, Brawer MK. Vasectomy and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1999;8(10):881-886. [PubMed] [Google Scholar]
- 45.Weinmann S, Shapiro JA, Rybicki BA, et al. . Medical history, body size, and cigarette smoking in relation to fatal prostate cancer. Cancer Causes Control. 2010;21(1):117-125. [DOI] [PubMed] [Google Scholar]
- 46.Lesko SM, Louik C, Vezina R, Rosenberg L, Shapiro S. Vasectomy and prostate cancer. [published correction appears in J Urol. 1999;162(3, pt 1):809]. J Urol. 1999;161(6):1848-1852. [PubMed] [Google Scholar]
- 47.Emard JF, Drouin G, Thouez JP, Ghadirian P. Vasectomy and prostate cancer in Québec, Canada. Health Place. 2001;7(2):131-139. [DOI] [PubMed] [Google Scholar]
- 48.Sunny L. Is it reporting bias doubled the risk of prostate cancer in vasectomised men in Mumbai, India? Asian Pac J Cancer Prev. 2005;6(3):320-325. [PubMed] [Google Scholar]
- 49.Hayes RB, Pottern LM, Greenberg R, et al. . Vasectomy and prostate cancer in US blacks and whites. Am J Epidemiol. 1993;137(3):263-269. [DOI] [PubMed] [Google Scholar]
- 50.Holt SK, Salinas CA, Stanford JL. Vasectomy and the risk of prostate cancer. J Urol. 2008;180(6):2565-2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.John EM, Whittemore AS, Wu AH, et al. . Vasectomy and prostate cancer: results from a multiethnic case-control study. J Natl Cancer Inst. 1995;87(9):662-669. [DOI] [PubMed] [Google Scholar]
- 52.Spitz MR, Fueger JJ, Babaian RJ, Newell GR, Rosenberg L. Re: ‘Vasectomy and the risk of prostate cancer’. Am J Epidemiol. 1991;134(1):107-109. [PubMed] [Google Scholar]
- 53.Andersson SO, Baron J, Bergström R, Lindgren C, Wolk A, Adami HO. Lifestyle factors and prostate cancer risk: a case-control study in Sweden. Cancer Epidemiol Biomarkers Prev. 1996;5(7):509-513. [PubMed] [Google Scholar]
- 54.Cossack M, Ghaffary C, Watson P, Snyder C, Lynch H. Aspirin use is associated with lower prostate cancer risk in male carriers of BRCA mutations. J Genet Couns. 2014;23(2):187-191. [DOI] [PubMed] [Google Scholar]
- 55.Ewings P, Bowie C. A case-control study of cancer of the prostate in Somerset and east Devon. Br J Cancer. 1996;74(4):661-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ganesh B, Saoba SL, Sarade MN, Pinjari SV. Risk factors for prostate cancer: an hospital-based case-control study from Mumbai, India. Indian J Urol. 2011;27(3):345-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hennis AJM, Wu SY, Nemesure B, Leske MC. Urologic characteristics and sexual behaviors associated with prostate cancer in an African-Caribbean population in Barbados, west indies. Prostate Cancer. 2013;2013:682750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hsing AW, Wang RT, Gu FL, et al. . Vasectomy and prostate cancer risk in China. Cancer Epidemiol Biomarkers Prev. 1994;3(4):285-288. [PubMed] [Google Scholar]
- 59.Kobayashi LC, Limburg H, Miao Q, et al. . Folate intake, alcohol consumption, and the methylenetetrahydrofolate reductase (MTHFR) C677T gene polymorphism: influence on prostate cancer risk and interactions. Front Oncol. 2012;2:100. doi: 10.3389/fonc.2012.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liang CH, Liu Q, Zhou FJ, Gao X, Chen LW. [Etiologic correlations of prostate cancer in Guangdong, China to family history of cancers, and sexual and marital factors-a case-control study] [in Chinese]. Ai Zheng. 2007;26(5):484-488. [PubMed] [Google Scholar]
- 61.Lightfoot N, Conlon M, Kreiger N, Sass-Kortsak A, Purdham J, Darlington G. Medical history, sexual, and maturational factors and prostate cancer risk. Ann Epidemiol. 2004;14(9):655-662. [DOI] [PubMed] [Google Scholar]
- 62.Mazdak H, Mazdak M, Jamali L, Keshteli AH. Determination of prostate cancer risk factors in Isfahan, Iran: a case-control study. Med Arh. 2012;66(1):45-48. [DOI] [PubMed] [Google Scholar]
- 63.Patel DA, Bock CH, Schwartz K, Wenzlaff AS, Demers RY, Severson RK. Sexually transmitted diseases and other urogenital conditions as risk factors for prostate cancer: a case-control study in Wayne County, Michigan. Cancer Causes Control. 2005;16(3):263-273. [DOI] [PubMed] [Google Scholar]
- 64.Pourmand G, Salem S, Mehrsai A, et al. . The risk factors of prostate cancer: a multicentric case-control study in Iran. Asian Pac J Cancer Prev. 2007;8(3):422-428. [PubMed] [Google Scholar]
- 65.Ross RK, Paganini-Hill A, Henderson BE. The etiology of prostate cancer: what does the epidemiology suggest? Prostate. 1983;4(4):333-344. [DOI] [PubMed] [Google Scholar]
- 66.Sridhar G, Masho SW, Adera T, Ramakrishnan V, Roberts JD. Association between family history of cancers and risk of prostate cancer. J Mens Health. 2010;7(1):45-54. doi: 10.1016/j.jomh.2009.10.006 [DOI] [Google Scholar]
- 67.Tyagi B, Manoharan N, Raina V. A case control study on prostate cancer in Delhi. Asian Pac J Cancer Prev. 2010;11(2):397-401. [PubMed] [Google Scholar]
- 68.Wei Q, Tang X, Yang Y, Zhan Y, Yin H. Risk factors of prostate cancer—a matched case-control study [in Chinese]. Hua Xi Yi Ke Da Xue Xue Bao. 1994;25(1):87-90. [PubMed] [Google Scholar]
- 69.Chacko JA, Zafar MB, McCallum SW, Terris MK. Vasectomy and prostate cancer characteristics of patients referred for prostate biopsy. J Urol. 2002;168(4, pt 1):1408-1411. [DOI] [PubMed] [Google Scholar]
- 70.DeAntoni EP, Göktaş S, Stenner J, O’Donnell C, Crawford ED. A cross-sectional study of vasectomy, time since vasectomy and prostate cancer. Prostate Cancer Prostatic Dis. 1997;1(2):73-78. [DOI] [PubMed] [Google Scholar]
- 71.Alqahtani KS, Srinivasan S, Mital DP, Haque S. Analysis of risk factors for prostate cancer patients. Int J Med Eng Inform. 2015;7(4):365-380. doi: 10.1504/IJMEI.2015.072322 [DOI] [Google Scholar]
- 72.Garzotto M, Hudson RG, Peters L, et al. . Predictive modeling for the presence of prostate carcinoma using clinical, laboratory, and ultrasound parameters in patients with prostate specific antigen levels ≤10 ng/mL. Cancer. 2003;98(7):1417-1422. [DOI] [PubMed] [Google Scholar]
- 73.Zhu K, Stanford JL, Daling JR, et al. . Vasectomy and prostate cancer: a case-control study in a health maintenance organization. Am J Epidemiol. 1996;144(8):717-722. [DOI] [PubMed] [Google Scholar]
- 74.Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295-300. [PMC free article] [PubMed] [Google Scholar]
- 75.Mo ZN, Huang X, Zhang SC, Yang JR. Early and late long-term effects of vasectomy on serum testosterone, dihydrotestosterone, luteinizing hormone and follicle-stimulating hormone levels. J Urol. 1995;154(6):2065-2069. [PubMed] [Google Scholar]
- 76.Howards SS. Possible biological mechanisms for a relationship between vasectomy and prostatic cancer. Eur J Cancer. 1993;29A(7):1060-1062. [DOI] [PubMed] [Google Scholar]
- 77.Flickinger CJ, Bush LA, Williams MV, Naaby-Hansen S, Howards SS, Herr JC. Post-obstruction rat sperm autoantigens identified by two-dimensional gel electrophoresis and western blotting. J Reprod Immunol. 1999;43(1):35-53. [DOI] [PubMed] [Google Scholar]
- 78.Pereira S, Martinez M, Martinez FE, Júnior WM. Repercussions of castration and vasectomy on the ductal system of the rat ventral prostate. Cell Biol Int. 2006;30(2):169-174. [DOI] [PubMed] [Google Scholar]
- 79.Nutt M, Reed Z, Köhler TS. Vasectomy and prostate cancer risk: a historical synopsis of undulating false causality. Res Rep Urol. 2016;8:85-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khera M, Crawford D, Morales A, Salonia A, Morgentaler A. A new era of testosterone and prostate cancer: from physiology to clinical implications. Eur Urol. 2014;65(1):115-123. [DOI] [PubMed] [Google Scholar]
- 81.Jarow JP, Goluboff ET, Chang TS, Marshall FF. Relationship between antisperm antibodies and testicular histologic changes in humans after vasectomy. Urology. 1994;43(4):521-524. [DOI] [PubMed] [Google Scholar]
- 82.Sonfield A, Hasstedt K, Gold RB. Moving Forward: Family Planning in the Era of Health Reform. New York, NY: Guttmacher Institute; 2014. [Google Scholar]
- 83.Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374(9):843-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trussell J. Contraceptive failure in the United States. Contraception. 2011;83(5):397-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. N Engl J Med. 1988;318(26):1728-1733. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Literature Search Strategy
eAppendix 2. Exclusion Following Full Text Review
eAppendix 3. Pooled Estimates of Studies Reporting Unadjusted Effect Estimates
eAppendix 4. Calculation of Estimates for Absolute Risk Increase, Number Needed to Harm, and Population-Attributable Fraction
eAppendix 5. Discussion of Hill’s Criteria of Causation
eFigure 1. Forest Plots for Meta-Analyses of the Adjusted Estimates for the Association Between Vasectomy and Any Prostate Cancer for Cohort Studies Reporting on Time-to-Event Analyses
eFigure 2. Meta-Analysis of Cohort Studies That Accounted for PSA Testing
eFigure 3. Forest Plots for Meta-Analyses of Unadjusted Estimates for the Association Between Vasectomy and Any Prostate Cancer by Study Design and Risk of Bias
eFigure 4. Funnel Plots
eTable 1. Risk Adjustment for Each Included Study
eTable 2. Newcastle-Ottawa Scale for Risk of Bias Assessment of Studies Included in the Meta-Analysis
eTable 3. Pooled Adjusted Estimates for Association Between Vasectomy and Prostate Cancer, Excluding Abstracts
eTable 4. Studies Reporting on the Impact Time Since Vasectomy on the Association Between Vasectomy and Prostate Cancer
eTable 5. Studies Reporting on the Impact of Age at Vasectomy on the Association Between Vasectomy and Prostate Cancer
eReferences.