This cohort study identifies prognostic factors for immunosuppressive treatment in patients with neurosarcoidosis and assesses the association of such treatment with relapse of the disease.
Key Points
Question
What are the prognostic factors in neurosarcoidosis, and what is the association of immunosuppressive treatment with relapse of the disease?
Findings
In this cohort study of 234 patients with neurosarcoidosis, encephalic and peripheral nervous system involvement were associated with worsening of the functional score. Immunosuppressive therapies, (ie, intravenous cyclophosphamide, methotrexate sodium, and infliximab) in these patients are associated with lower relapse rates.
Meaning
The presence of encephalic or peripheral nervous system involvement in neurosarcoidosis should affect the decision to use immunosuppressive therapies to help lower relapse rates.
Abstract
Importance
Prognostic factors are lacking in neurosarcoidosis (NS), and the association of immunosuppressive treatments with outcomes are unclear.
Objectives
To identify prognostic factors of and analyze the association of immunosuppressive treatment with relapse of NS.
Design, Setting, and Participants
In this retrospective study, a cohort of 234 patients fulfilled the diagnostic criteria for NS in a tertiary referral center in Paris, France, from January 1, 1990, through December 31, 2015. The median follow-up was 8 years (range, 2 months to 23 years).
Main Outcomes and Measures
All neurologic and extraneurologic data and treatments were analyzed. Functional outcomes measured by the absolute value and the variation from baseline of the Expanded Disability Status Scale (EDSS) score at 60 months after the diagnosis, overall survival, and relapse-free survival (RFS) were assessed. Analyses were stratified by the period of NS diagnosis (1990-1999 vs 2000-2015).
Results
The 234 patients undergoing assessment included 117 women (50.0%) and 117 men (50.0%); median age was 42 years (interquartile range, 32-53 years). The probable 10-year survival rate was 89% (95% CI, 84%-94%). Older age (hazard ratio [HR] per 10 years, 1.64; 95% CI, 1.19-2.27; P = .003), peripheral nervous system involvement (HR, 6.75; 95% CI, 2.31-19.7; P < .001), and higher baseline EDSS score (HR per point, 1.21; 95% CI, 1.06-1.39; P = .005) were associated with mortality. The estimated 10-year RFS rate was 14% (95% CI, 9%-22%) for all relapses and 28% (95% CI, 20%-38%) for neurologic relapses. Encephalic involvement was associated with shorter neurologic RFS (HR, 2.35; 95% CI, 1.44-3.83; P < .001). A lower risk for relapse was associated with cyclophosphamide (HR, 0.26; 95% CI, 0.11-0.59; P = .001), methotrexate sodium (HR, 0.47; 95% CI, 0.25-0.87; P = .02), and infliximab (HR, 0.16; 95% CI, 0.02-1.24; P = .08) treatments. Follow-up was greater than 60 months in 160 patients (68.4%). An elevated baseline EDSS score (odds ratio [OR] per point, 1.92; 95% CI, 1.55-2.37; P < .001), tobacco use (OR, 3.64; 95% CI, 1.36-9.73; P = .01), encephalic symptoms (OR, 3.04; 95% CI, 1.11-8.38; P = .03), and less than 4 extraneurologic sarcoidosis localizations (OR, 3.06; 95% CI, 1.04-8.98; P = .04) were associated with an EDSS value of at least 2.5 at 60 months. Encephalic involvement (16 of 17 patients [94.1%]; P = .008) and peripheral nervous system involvement (5 of 17 patients [29.4%]; P = .03) were associated with worsening of the EDSS score at 60 months.
Conclusions and Relevance
This study identifies putative factors affecting morbidity and mortality in patients with NS. Immunosuppressive therapies (ie, intravenous cyclophosphamide, methotrexate, and infliximab) in these patients may be associated with lower relapse rates.
Introduction
Sarcoidosis is a multisystem granulomatous disease of unknown origin with an overall prevalence of 10 per 100 000 people in the United States. Various genetic and environmental factors have been shown to be involved in its pathogenesis. Although sarcoidosis primarily affects the lungs and the lymphatic nodes, involvement of the nervous system, known as neurosarcoidosis (NS), occurs in about 5% of all cases of sarcoidosis. The central nervous system (CNS) and the cranial nerves are predominantly affected in patients with NS, but any part of the nervous system can be involved. Neurosarcoidosis is a chronic disease that progresses in the course of years. The prognosis is variable; although some patients will recover completely after corticosteroid therapy, others will experience multiple relapses and develop severe functional sequelae. The rarity and heterogeneity of the disease and its protracted course have prevented the achievement of thorough prognostic and therapeutic studies. No clear prognostic factors of NS have been identified yet, and therapeutic strategies remain highly empirical. In this retrospective study of a large cohort of patients with NS, we aimed to identify prognostic factors associated with survival, long-term functional prognosis, and relapses and to assess the association of immunosuppressive drugs with relapse.
Methods
Patients
We retrospectively collected all data from 690 patients with systemic sarcoidosis who were diagnosed and followed up in a single referral center at Groupe Hospitalier La Pitié-Salpêtrière, a university hospital in Paris, France, from January 1, 1965, through February 28, 2015. For the present study, all patients meeting the criteria of Zajicek et al for NS and whose neurologic symptoms had appeared in 1990 or later were selected. The institutional review board of the Assistance Publique–Hôpitaux de Paris approved this study and waived the need for informed consent.
Definite NS was diagnosed when the clinical presentation was suggestive of NS, other possible diagnoses had been ruled out, and positive histologic findings in the nervous system were present. Probable NS was defined by the presence of a clinical syndrome suggestive of NS with laboratory support for CNS inflammation (ie, elevated levels of protein and/or cells in cerebrospinal fluid [CSF], the presence of oligoclonal bands, and/or magnetic resonance imaging [MRI] evidence compatible with NS), and the exclusion of alternative diagnoses. A diagnosis of NS was possible when the clinical presentation was suggestive of NS, alternative diagnoses had been ruled out, and the aforementioned criteria had not been met. With use of results of the physical examination, MRI of the brain and spine, and electroneuromyographic nerve conduction studies, all neurologic symptoms were classified based on involvement of the encephalon, cranial nerves, spine, peripheral nervous system (PNS), and muscle. No test for the presence of a small-fiber neuropathy was performed. Extraneurologic symptoms were recorded according to the organs affected by the sarcoidosis. Abnormal CSF white blood cell values were defined as at least 5/μL and CSF protein levels, as at least 50 mg/dL.
A relapse was defined as the occurrence of any new neurologic or nonneurologic symptom or biological abnormality (eg, urinary sediment or abnormal liver function test results) attributed to sarcoidosis by the patient’s referring physician. Whenever thought to be appropriate, the relapse was confirmed by radiologic (MRI of the brain and/or spine or cardiac MRI) or pathologic evidence (eg, skin, kidney, or lacrimal duct biopsy). Any new radiologic lesion or the expansion of a previously existing lesion was considered to be confirmation of the relapse if it was congruent with the considered symptoms. The worsening of a previously existing symptom was considered to be a relapse if accompanied by other new symptoms or by congruent radiologic changes. Whenever considered necessary, a biopsy was performed in the incriminated site (eg, salivary gland, lacrimal duct, skin, or kidney), and the relapse was confirmed if histopathologic analysis revealed features consistent with sarcoidosis (ie, well-defined noncaseating granuloma). In the case of PNS or muscle relapses, worsening of electroneuromyographic variables was considered to be supplementary evidence of relapse. In dubious cases, other causes were excluded by appropriate tests (eg, MRI of the brain and spine).
We used the Expanded Disability Status Scale (EDSS) scores (possible range, 0-10, with higher scores indicating worsening disability) at the first visit and after 60 months of follow-up to assess long-term neurologic impairment. The EDSS score was primarily driven by ambulation and autonomy in daily activities. An absolute EDSS value of at least 2.5 at 60 months after NS onset was considered to define significant long-term functional impairment. To analyze the progression of the functional impairment, we calculated the differences of EDSS scores between baseline and month 60. Significant clinical worsening was defined as an increase in the EDDS score of at least 1 point. Outcomes were further assessed by vital and relapse-free survival.
The referral physicians had the discretion to switch or to adapt the dosages of immunosuppressant medications as they saw fit with regard to the clinical situation within the following ranges: methotrexate sodium, 0.3 to 0.4 mg/kg/d; mycophenolic acid, 2.0 to 3.0 g/d; and azathioprine sodium, 50 to 150 mg/d. Whenever used, intravenous cyclophosphamide was administered at the dose of 1 g/mo and infliximab at 5 mg/kg at 0, 2, and 6 weeks and every 8 weeks thereafter.
Statistical Analyses
Continuous variables are presented as medians and interquartile range (IQR). They were compared between groups using the Wilcoxon rank sum or the Kruskal-Wallis test, whenever appropriate. Categorical variables are presented with counts and percentages and were compared using the Fisher exact test or the χ2 test.
The date of the NS diagnosis was estimated to be the sarcoidosis diagnosis date (if the neurologic signs occurred concomitant with or before the sarcoidosis diagnosis) or the date of the first neurologic signs (if they occurred after the sarcoidosis diagnosis). The EDSS score at 60 months after the NS diagnosis was described in all patients with a follow-up longer than 60 months (n = 160). Factors associated with an EDSS score of at least 2.5 at 60 months were studied in conditional logistic regression models, stratified on the period of NS diagnosis (1990-1999 vs 2000-2015), and adjusted for the baseline EDSS value. The EDSS results were also categorized into 3 groups according to the changes between baseline and month 60, including stable (no difference), worsening (increase of ≥1), and improvement (decrease of ≥1). The association of the EDSS score variation with covariates was studied at month 60 of the follow-up, using the Kruskal-Wallis test for continuous variables and Fisher exact test for categorical variables.
Overall survival (OS) was defined as the time lapsed from the date of NS diagnosis to the date of death or last follow-up. Relapse-free survival (RFS) was defined as the time lapsed from the date of NS diagnosis to the date of sarcoidosis relapse, death, or last follow-up, whichever occurred first. Neurologic and nonneurologic relapses were included for survival analyses. Overall survival and RFS were estimated using the Kaplan-Meier method, and survival functions were compared using the log-rank test. Univariate analyses were performed in Cox proportional hazards regression models to identify factors associated with OS and RFS. The proportional hazards assumption and log-linearity assumption for quantitative variables were assessed. Factors associated with the risk for neurologic localization of the first relapse were also examined within a competing risks framework, with nonneurologic relapse and death without relapse as competing events, using Cox proportional hazards regression models for cause-specific hazards. For analyses of EDSS scores, RFS, and neurologic relapses, multivariate models were selected by backward stepwise selection on Akaike criterion, using variables that were significant at a 10% level in univariate analysis. The association among RFS and neurologic relapse risk and sequences of NS treatments was examined using the approach of Andersen described in Therneau and Grambsch to Cox proportional hazards regression models, accounting for potential correlations between observations within each patient. Models for univariate and multivariate analyses of time-to-event end points were stratified on the period of NS diagnosis (1990-1999 vs 2000-2015).
All tests were 2-sided, and P < .05 was considered to be significant. Analyses were performed using the R statistical platform (version 3.2.2; https://cran.r-project.org).
Results
Characteristics of Patients With NS
From our cohort of 690 patients with systemic sarcoidosis, 234 (117 women [50.0%] and 117 men [50.0%]; including 125 [53.4%] white) met the criteria for NS (median age, 42 years [IQR, 32-53 years]). The median follow-up was 8 years (range, 2 months to 23 years), and patients had 1 to 12 follow-up visits during the observation period. A follow-up of at least 60 months was attained in 160 patients (68.4%).
The main demographic data are summarized in Table 1; extraneurologic features are given in eTable 1 in the Supplement. Most patients presented with multisystem involvement of the sarcoidosis, with 164 (70.1%) having 2 or more extraneurologic sites, including the heart (84 of 194 [43.3%]), eyes (67 of 234 [28.6%]), lymph nodes (67 of 234 [28.6%]), and skin and/or mucosa (49 of 234 [20.9%]). Of 33 patients (14.1%) with definite NS, histologic evidence was obtained from the brain or dura (13 patients), peripheral nerve (8 patients), or muscle (12 patients). Of 142 patients (60.7%) with probable NS, 135 (95.1%) had biopsy-proven sarcoidosis.
Table 1. Characteristics of 234 Patients With NS.
| Characteristic | Patientsa |
|---|---|
| General | |
| Period of NS diagnosis | |
| 1990-1999 | 55/234 (23.5) |
| 2000-2015 | 179/234 (76.5) |
| Age at NS diagnosis, median (IQR), y | 42 (32-53) |
| Male sex | 117/234 (50.0) |
| Ethnic background | |
| White | 125/234 (53.4) |
| African or Caribbean | 58/234 (24.8) |
| North African | 45/234 (19.2) |
| Other | 6/234 (2.6) |
| Active smoking habit | 54/231 (23.1) |
| Sarcoidosis classificationb | |
| Definite | 33/234 (14.1) |
| Probable | 142/234 (60.7) |
| Possible | 59/234 (25.2) |
| Neurologic | |
| EDSS score, median (IQR) [range]c | |
| Baseline | 2.0 (1.0-4.0) [0-9.5] |
| 60 mo | 1.0 (0.0-3.0) [0-9.5] |
| Cranial nerve involvementd | 86/234 (36.8) |
| VII | 24/234 (10.3) |
| VI | 22/234 (9.4) |
| II | 23/234 (9.8) |
| V | 11/234 (4.7) |
| VIII | 14/234 (6.0) |
| Others | 4/234 (1.7) |
| Encephalic involvement | 130/234 (55.6) |
| Headache | 70/234 (29.9) |
| Cognitive disorders | 43/234 (18.4) |
| Sensory | 42/234 (17.9) |
| Pyramidal tract | 33/234 (14.1) |
| Neuroendocrine dysfunction | 32/234 (13.7) |
| Psychiatric symptoms | 18/234 (7.7) |
| Seizures | 17/234 (7.3) |
| Cerebellar syndrome | 15/234 (6.4) |
| Vestibular syndrome | 15/234 (6.4) |
| Intracranial hypertension | 10/234 (4.3) |
| Stroke | 6/234 (2.6) |
| Myelopathy | 62/234 (26.5) |
| PNS involvement | 24/234 (10.3) |
| Myopathy | 19/234 (8.1) |
| CSF protein level >50 mg/dL | 117/191 (61.3) |
| CSF white blood cells >5/mm3 | 119/191 (62.3) |
| Neuropathic features on ENMG | 35/61 (57.4) |
| Myopathic features on ENMG | 11/61 (18.0) |
| Abnormal MRI finding | |
| Brain | 128/194 (66.0) |
| Spinal cord | 56/81 (69.1) |
Abbreviations: CSF, cerebrospinal fluid; EDSS, Expanded Disability Status Scale; ENMG, electroneuromyography; IQR, interquartile range; MRI, magnetic resonance imaging; NS, neurosarcoidosis; PNS, peripheral nervous system.
Unless otherwise indicated, data are expressed as number/total number (percentage) of patients. Smaller denominators indicate number of patients with data available. Percentages have been rounded and may not total 100.
Classified according to Zajicek et al.
Possible scores range from 0 to 10, with higher scores indicating greater disability.
Includes more than 1 cranial nerve for some patients.
The main neurologic features are detailed in Table 1. Symptoms related to the involvement of the encephalon were noted in 130 patients (55.6%); the spinal cord, 62 (26.5%); cranial nerves, 86 (36.8%); PNS, 24 (10.3%); and muscles, 19 (8.1%). Samples of CSF showed elevated levels of white blood cells or protein in 146 of 191 patients (76.4%). The MRI examinations displayed NS lesions at the brain level in 128 of 194 patients (66.0%) and at the spinal cord level in 56 of 81 patients (69.1%). Electroneuromyographic examinations revealed peripheral nerve dysfunction in 35 of 61 patients (57.4%) and muscle involvement in 11 of 61 patients (18.0%). All but 3 patients received immunomodulatory treatments at some point during their follow-up, including oral corticosteroids alone (170 patients who received oral corticosteroids alone at least once) or in combination with immunosuppressive drugs (178 patients, including 99 who received intravenous cyclophosphamide; 97, methotrexate; 55, mycophenolic acid; 36, hydroxychloroquine; 24, infliximab; and 14, azathioprine). The main adverse effects related to corticosteroids and the immunosuppressive treatments were obesity (32 of 234 [13.7%]), osteoporosis (20 of 234 [8.5%]), infections (17 of 234 [7.3%]), diabetes (13 of 234 [5.6%]), tuberculosis (12 or 234 [5.1%]), and high blood pressure (8 of 234 [3.4%]).
Prognostic Factors
Survival
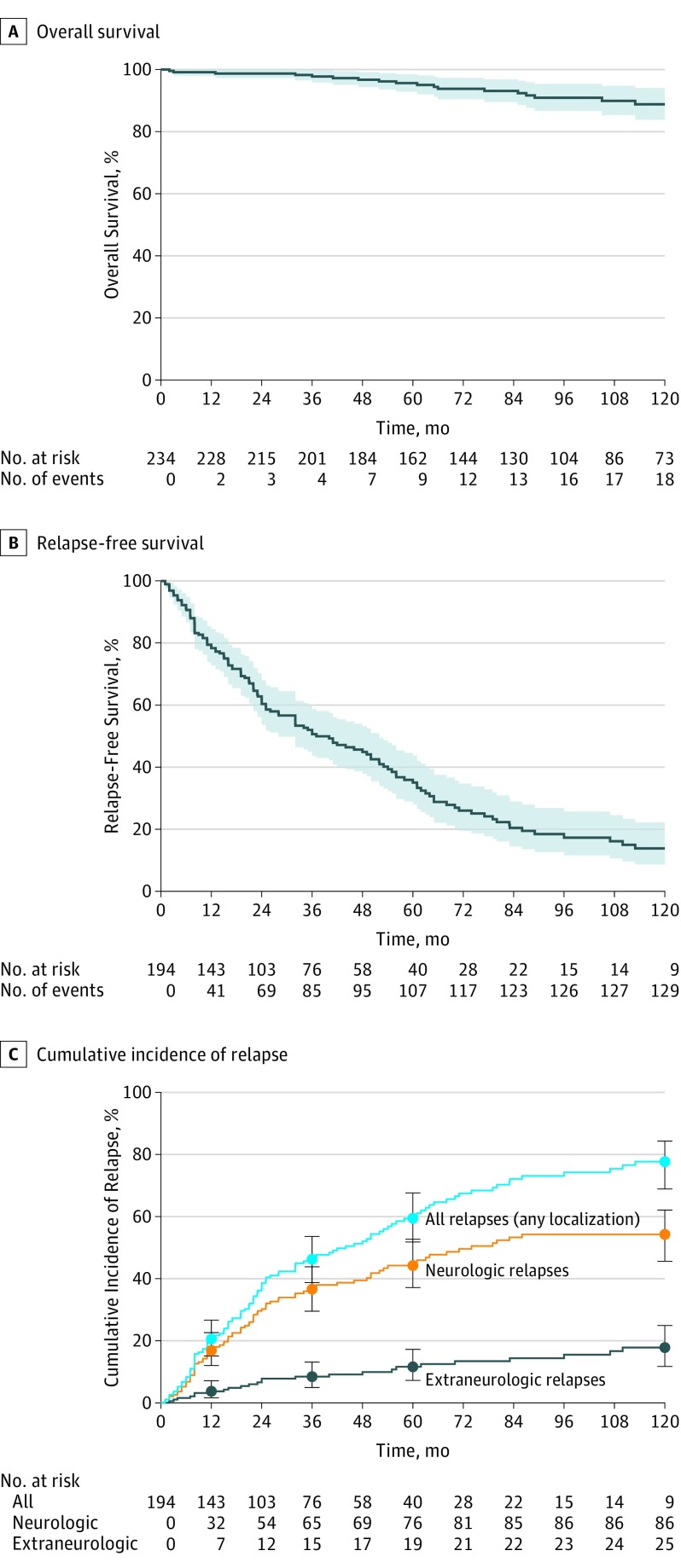
Twenty-one of 234 patients died during follow-up, corresponding to a probable OS at 10 years from the NS diagnosis of 89% (95% CI, 84%-94%) (eFigure and Figure, A). The causes of death were NS (n = 6), cardiovascular (n = 4), CNS disease not related to NS (n = 3), sepsis (n = 3), cancer (n = 3), and unknown (n = 3), with some patients having more than 1 cause. Univariate analysis of the mortality-associated factors is found in Table 2. Multivariate analysis revealed age (hazard ratio [HR] per 10 years, 1.64; 95% CI, 1.19-2.27; P = .003), PNS involvement (HR, 6.75; 95% CI, 2.31-19.7; P < .001), and higher baseline EDSS (HR per point, 1.21; 95% CI, 1.06-1.39; P = .005) to be associated with fatal outcomes. Analysis of survival stratified by the period of NS diagnosis are given in eTables 2 and 3 in the Supplement.
Figure. Kaplan-Meier Curves for Survival and Relapse in the Cohort With Neurosarcoidosis.
A and B, Solid line indicates probable survival; shaded areas, 95% CI. C, Error bars indicate 95% CI.
Table 2. Main Characteristics Associated With Overall and Relapse-Free Survival and Neurologic Relapsesa.
| Characteristic | Overall Survival | Relapse-Free Survival | Neurologic Relapse | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Deaths/Patients | HR (95% CI) | P Value | No. of Relapses/Patients | HR (95% CI) | P Value | No. of Relapses/Patients | HR (95% CI)b | P Value | |
| General | |||||||||
| EDSS score at baseline per point | NA | 1.22 (1.07-1.40) | .003 | NA | 0.99 (0.93-1.05) | .69 | NA | 0.99 (0.93-1.07) | .84 |
| Age at NS diagnosis per 10 y | NA | 1.43 (1.06-1.93) | .02 | NA | 0.91 (0.81-1.04) | .16 | NA | 0.82 (0.70-0.97) | .02 |
| Male sex | 12/117 | 1.19 (0.49-2.89) | .69 | 64/97 | 0.90 (0.64-1.28) | .56 | 43/97 | 1.04 (0.68-1.59) | .86 |
| Ethnic background | |||||||||
| White | 13/125 | 1 [Reference] | NA | 76/102 | 1 [Reference] | NA | 52/102 | 1 [Reference] | NA |
| African or Caribbean | 6/58 | 1.05 (0.40-2.79) | .92 | 32/48 | 0.68 (0.44-1.05) | .08 | 20/48 | 0.72 (0.43 to 1.20) | .21 |
| North African and other | 2/51 | 0.49 (0.11-2.18) | .35 | 28/44 | 1.02 (0.66-1.58) | .93 | 16/44 | 0.82 (0.47 to 1.44) | .49 |
| Smoking | 5/54 | 0.90 (0.33-2.50) | .85 | 39/49 | 1.18 (0.80-1.72) | .40 | 24/49 | 1.06 (0.66-1.70) | .81 |
| Sarcoidosis classificationc | |||||||||
| Definite | 5/33 | 1 [Reference] | NA | 23/31 | 1 [Reference] | NA | 14/31 | 1 [Reference] | NA |
| Probable or possible | 16/201 | 0.55 (0.20-1.51) | .24 | 113/163 | 0.86 (0.54-1.35) | .51 | 74/163 | 0.90 (0.50 to 1.59) | .71 |
| Biopsy-proven sarcoidosis | 16/201 | 0.55 (0.20-1.51) | .24 | 99/141 | 0.75 (0.51-1.10) | .14 | 61/141 | 0.70 (0.44-1.11) | .13 |
| Extraneurologic Involvement | |||||||||
| >3 Nonneurologic sites involved | 3/55 | 0.49 (0.14-1.70) | .26 | 35/46 | 1.06 (0.72-1.57) | .76 | 22/46 | 1.02 (0.63-1.67) | .92 |
| Abnormal chest radiographic finding | 17/162 | 1.75 (0.58-5.23) | .32 | 100/137 | 1.00 (0.68-1.47) | >.99 | 63/137 | 0.91 (0.57-1.46) | .70 |
| Heart | 5/84 | 0.53 (0.18-1.54) | .24 | 52/72 | 1.19 (0.81-1.73) | .37 | 36/72 | 1.13 (0.72-1.77) | .61 |
| General symptoms | 7/82 | 0.82 (0.33-2.03) | .66 | 51/67 | 1.15 (0.81-1.63) | .45 | 39/67 | 1.43 (0.94-2.19) | .098 |
| Eye | 4/67 | 0.55 (0.18-1.64) | .28 | 42/53 | 1.16 (0.80-1.69) | .42 | 25/53 | 0.97 (0.61-1.55) | .91 |
| Lymph nodes | 9/67 | 2.00 (0.83-4.84) | .12 | 47/59 | 1.50 (1.05-2.16) | .03 | 29/59 | 1.28 (0.82-2.01) | .28 |
| Cutaneous and mucosal | 3/49 | 0.57 (0.17-1.97) | .37 | 27/40 | 0.77 (0.50-1.18) | .22 | 16/40 | 0.71 (0.41-1.23) | .22 |
| Liver or spleen | 4/41 | 0.87 (0.29-2.63) | .80 | 28/37 | 1.07 (0.70-1.63) | .76 | 15/37 | 0.89 (0.51-1.55) | .67 |
| Joints | 1/36 | 0.27 (0.035-1.99) | .20 | 23/29 | 0.96 (0.61-1.51) | .86 | 15/29 | 1.04 (0.59-1.82) | .89 |
| Exocrine glands | 4/35 | 1.34 (0.44-4.07) | .60 | 22/28 | 0.89 (0.56-1.42) | .62 | 10/28 | 0.64 (0.33-1.24) | .18 |
| Ear, nose, and throat | 0/16 | NA | .22d | 8/11 | 1.90 (0.91-3.95) | .09 | 6/11 | 1.92 (0.82-4.47) | .13 |
| Kidney | 0/7 | NA | .38d | 6/7 | 1.50 (0.63-3.62) | .36 | 3/7 | 1.60 (0.50-5.15) | .43 |
| Digestive tract | 1/7 | 1.35 (0.18-10.1) | .77 | 5/7 | 0.89 (0.36-2.22) | .81 | 2/7 | 0.63 (0.15-2.55) | .51 |
| Bones | 0/6 | NA | .47d | 3/4 | 0.89 (0.28-2.82) | .85 | 2/4 | 0.90 (0.22-3.68) | .88 |
| Neurologic Involvement | |||||||||
| CNS localization | 17/170 | 1.51 (0.50-4.53) | .46 | 103/140 | 1.59 (1.05-2.39) | .03 | 70/140 | 1.80 (1.06-3.06) | .03 |
| Encephalon | 14/143 | 1.18 (0.47-2.96) | .72 | 90/117 | 1.88 (1.30-2.74) | <.001 | 64/117 | 2.35 (1.44-3.83) | <.001 |
| Myelopathy | 6/62 | 0.96 (0.37-2.49) | .93 | 33/48 | 0.83 (0.56-1.24) | .37 | 21/48 | 0.82 (0.50-1.35) | .43 |
| Cranial nerve | 6/86 | 0.70 (0.27-1.82) | .46 | 57/72 | 1.03 (0.72-1.47) | .87 | 38/72 | 1.20 (0.78-1.85) | .40 |
| PNS | 7/24 | 4.38 (1.69-11.3) | .002 | 19/22 | 1.40 (0.85-2.32) | .19 | 11/22 | 1.20 (0.63-2.30) | .58 |
| Myopathy | 3/19 | 2.22 (0.65-7.60) | .20 | 13/18 | 1.54 (0.86-2.74) | .15 | 9/18 | 1.47 (0.73-2.94) | .28 |
| Abnormal MRI finding of the brain or spinal cord | 13/154 | 1.05 (0.14-8.11) | .96 | 91/129 | 1.20 (0.55-2.63) | .65 | 65/129 | 1.37 (0.50-3.77) | .54 |
| CSF protein level >50 mg/dL | 14/117 | 4.57 (1.03-20.3) | .046 | 76/99 | 1.16 (0.78-1.73) | .47 | 54/99 | 1.36 (0.84-2.21) | .21 |
| CSF white blood cells >5/μL | 11/119 | 1.53 (0.53-4.40) | .43 | 72/96 | 1.32 (0.90-1.96) | .16 | 52/96 | 1.45 (0.90-2.32) | .13 |
| Elevated serum ACE level | 11/85 | 1.96 (0.72-5.31) | .18 | 48/73 | 0.87 (0.58-1.30) | .49 | 28/73 | 0.73 (0.45-1.19) | .21 |
Abbreviations: ACE, angiotensin-converting enzyme; CSF, cerebrospinal fluid; CNS, central nervous system; EDSS, Expanded Disability Status Scale; HR, hazard ratio; MRI, magnetic resonance imaging; NA, not applicable; NS, neurosarcoidosis; PNS, peripheral nervous system.
SI conversion factors: To convert protein to grams per liter, multiply by 0.01; white blood cells to ×109 per liter, multiply by 0.001.
All estimations stratified on period of NS diagnosis (1990-1999 vs 2000-2015).
Uses the Cox proportional hazards regresssion model for cause-specific HRs (neurologic relapse, nonneurologic relapse, relapse with unavailable localization, and death as mutually competing risks).
Classified according to Zajicek et al.
Calculated using the log-rank test, stratified on period (1990-1999 vs 2000-2015); estimation of HR using a Cox proportional hazards regression model was not performed owing to the absence of events in the subgroups with kidney (n = 7) or bone (n = 5) involvement vs 23 of 227 and 23 of 229 in the subgroups without kidney or bone involvement, respectively.
Relapses
During the follow-up, 136 patients had at least 1 event (ie, 88 neurologic relapses, 27 nonneurologic relapses, 8 relapses with unavailable localization, and 13 deaths without relapse). The neurologic and nonneurologic RFS rate at 10 years was 14% (95% CI, 9%-22%) (Figure and eFigure in the Supplement), and the neurologic RFS rate at 10 years was 28% (95% CI, 20%-38%). Cumulative incidences of neurologic and nonneurologic relapses were 17% (95% CI, 12%-23%) and 4% (95% CI, 2%-7%), respectively, at 1 year; 37% (95% CI, 30%-44%) and 8% (95% CI, 5%-13%), respectively, at 3 years; 45% (95% CI, 37%-53%) and 12% (95% CI, 7%-17%), respectively, at 5 years; and 54% (95% CI, 46%-62%) and 18% (95% CI, 12%-25%), respectively, at 10 years (Figure, C). Univariate analysis of the factors associated with poorer RFS (any localization) is shown in Table 2. Multivariate analysis showed encephalic symptoms to be independently associated with a poorer RFS (HR, 1.83; 95% CI, 1.26-2.66; P = .002). Among encephalic symptoms, hydrocephalus was associated with a poorer RFS (HR, 2.47; 95% CI, 1.46-4.17; P < .001). Results were consistent when considering only the risk for neurologic relapse (Table 2 and eTable 4 in the Supplement) because encephalic symptoms were associated with a higher risk for a neurologic relapse (HR, 2.35; 95% CI, 1.44-3.83; P < .001). The effect of immunosuppressive treatments on the relapse risk during a treatment course is detailed in Table 3 for all relapses (any localization) and neurologic relapses. Considering the risk for relapses (any localization), a decreased risk for relapse was strongly associated with administration of intravenous cyclophosphamide (HR, 0.18; 95% CI, 0.09-0.37; P < .001) and infliximab (HR, 0.31; 95% CI, 0.11-0.82; P = .02), compared with the absence of treatment. A lower risk for relapse was also associated with treatment with glucocorticoids (HR, 0.59; 95% CI, 0.39-0.90; P = .01) and methotrexate (HR, 0.48; 95% CI, 0.29-0.79; P = .004), compared with no treatment. Hydroxychloroquine was significantly associated with a decreased relapse rate (HR, 0.29; 95% CI, 0.14-0.63; P = .002) when used alone, in combination with glucocorticoids, or with glucocorticoids plus methotrexate. The administration of mycophenolic acid was not associated with a change of the relapse rate, whereas treatment with azathioprine led to a nonsignificant increase of the relapse rate (HR, 1.40; 95% CI, 0.55-3.53; P = .48). A decreased risk for neurologic relapses was strongly associated with administration of intravenous cyclophosphamide (HR, 0.26; 95% CI, 0.11-0.59; P = .001) and methotrexate (HR, 0.47; 95% CI, 0.25-0.87; P = .02) compared with the absence of treatment. Hydroxychloroquine was significantly associated with a decreased relapse rate (HR, 0.37; 95% CI, 0.15-0.92; P = .03) when used alone, in combination with glucocorticoids, or with glucocorticoids plus methotrexate. Use of infliximab was associated with a nonsignificant decreased risk for relapse (HR, 0.16; 95% CI, 0.02-1.24; P = .08). Only 1 neurologic relapse among the 28 treatment sequences occurred with infliximab.
Table 3. Hazards for Any Relapses (Any Localization) and Neurologic Relapses According to Immunosuppressive or Immunomodulatory Treatments.
| Treatment | No. of Relapses/Therapeutic Sequences | HR (95%CI) | P Value |
|---|---|---|---|
| Any relapsea | |||
| None | 38/87 | 1 [Reference] | NA |
| Glucocorticoid alone | 85/254 | 0.59 (0.39 to 0.90) | .01 |
| Methotrexate sodium | 44/125 | 0.48 (0.29 to 0.79) | .004 |
| Azathioprine sodium | 8/14 | 1.40 (0.55 to 3.53) | .48 |
| Hydroxychloroquineb | 16/59 | 0.29 (0.14 to 0.63) | .002 |
| Mycophenolic acid | 26/64 | 0.67 (0.37 to 1.23) | .20 |
| Intravenous cyclophosphamide | 11/120 | 0.18 (0.09 to 0.37) | <.001 |
| Infliximab | 4/28 | 0.31 (0.11 to 0.82) | .02 |
| Neurologic relapsec | |||
| None | 20/87 | 1 [Reference] | NA |
| Glucocorticoid alone | 58/254 | 0.68 (0.38 to 1.23) | .20 |
| Methotrexate | 26/125 | 0.47 (0.25 to 0.87) | .02 |
| Azathioprine | 6/14 | 1.88 (0.69 to 5.14) | .22 |
| Hydroxychloroquineb | 12/59 | 0.37 (0.15 to 0.92) | .03 |
| Mycophenolic acid | 14/64 | 0.58 (0.25 to 1.34) | .20 |
| Intravenous cyclophosphamide | 10/120 | 0.26 (0.11 to 0.59) | .001 |
| Infliximabd | 1/28 | 0.16 (0.021 to 1.24) | .08 |
Abbreviations: HR, hazard ratio; NA, not applicable.
Analysis was stratified on period of neurosarcoidosis (NS) diagnosis (1990-1999 vs 2000-2015).
Includes alone (n = 15), combined with corticosteroids (n = 30), or combined with corticosteroids plus methotrexate (n = 15).
Calculated as cause-specific HRs for neurologic relapse; analysis was stratified on period of NS diagnosis (1990-1999 vs 2000-2015).
For infliximab, the very low number with neurologic relapse should be emphasized and the HR interpreted with caution.
Functional Outcomes
Of the 160 patients with sufficient follow-up, 56 (35.0%) had an EDSS score of at least 2.5 at 60 months after the diagnosis (Table 4). Univariate analysis of the factors associated with poorer long-term functional outcomes are shown in Table 4. Multivariate analysis showed that an EDSS score of at least 2.5 at month 60 was significantly associated with tobacco use at diagnosis (odds ratio [OR], 3.64; 95% CI, 1.36-9.73; P = .01), encephalic symptoms (OR, 3.04; 95% CI, 1.11-8.38; P = .03), less than 4 extraneurologic sarcoidosis localizations (OR, 3.06; 95% CI, 1.04-8.98; P = .04), and elevated baseline EDSS scores (OR per point, 1.92; 95% CI, 1.55-2.37; P < .001) and inversely associated with a multisystem extension of sarcoidosis at diagnosis (defined as >3 extraneurologic localizations) (OR, 0.33; 95% CI, 0.11-0.96; P = .04). At month 60, 17 of 160 patients (10.6%) had a worsening of their EDSS score compared with baseline (Table 4) that was associated with encephalic (16 of 17 patients [94.1%]; P = .008) and PNS involvement (5 of 17 patients [29.4%]; P = .03). Analysis of functional outcomes stratified by the period of NS diagnosis are given in eTables 5 and 6 in the Supplement.
Table 4. EDSS Score of Patients With NS After a 60-Month Follow-upa.
| Variable | EDSS Score ≥2.5b | Changes in EDSS Score From Baseline to Month 60 | ||||||
|---|---|---|---|---|---|---|---|---|
| No. of Patients | No. (%) With EDSS Score ≥2.5 | OR (95%CI) | P Value | No. (%) of Patients | P Value | |||
| Improved | Stable | Worse | ||||||
| All patients | 160 | 56 (35.0) | NA | NA | 62 (38.8) | 81 (50.6) | 17 (10.6) | NA |
| General Characteristic | ||||||||
| EDSS at baseline (OR per point)c |
160 | NA | 1.82 (1.50-2.21) | <.001 | 4 (2-7) | 1 (0-2) | 1 (0-2) | <.001 |
| Age at NS diagnosis (OR per 10 y)c |
160 | NA | 0.74 (0.53-1.03) | .07 | 42 (35-53) | 41 (30- 53) | 34 (28-43) | .21 |
| Male sex | 84 | 33 (39.3) | 1.29 (0.56-3.00) | .55 | 32 (51.6) | 45 (55.6) | 7 (41.2) | .58 |
| Ethnic background | ||||||||
| White | 94 | 34 (36.2) | 1 [Reference] | NA | 33 (53.2) | 48 (59.3) | 13 (76.5) | NA |
| African or Caribbean | 42 | 15 (35.7) | 0.86 (0.33-2.27) | .76 | 19 (30.6) | 21 (25.9) | 2 (11.8) | .56 |
| North African/other | 24 | 7 (29.1) | 0.52 (0.14-1.98) | .34 | 10 (16.1) | 12 (14.8) | 2 (11.8) | |
| Smoking | 41 | 20 (48.8) | 2.96 (1.19-7.32) | .02 | 13 (21.0) | 21 (25.9) | 7 (41.2) | .24 |
| Sarcoidosis classificationd | .26 | |||||||
| Definite | 25 | 11 (44.0) | 1 [Reference] | NA | 13 (21.0) | 9 (9.9) | 3 (17.6) | NA |
| Probable or possible | 135 | 45 (33.3) | 0.97 (0.32-2.98) | .96 | 49 (79.0) | 72 (88.9) | 14 (82.4) | .62 |
| Biopsy-proven sarcoidosis | 117 | 39 (33.3) | 0.62 (0.25-1.55) | .31 | 47 (75.8) | 59 (72.8) | 11 (64.7) | |
| Extraneurologic Involvement | ||||||||
| >3 Nonneurologic sites involved | 46 | 10 (21.7) | 0.38 (0.14-1.05) | .06 | 16 (25.8) | 25 (30.9) | 5 (29.4) | .82 |
| Abnormal chest radiograph finding | 112 | 39 (34.8) | 0.71 (0.29-1.76) | .46 | 46 (74.2) | 52 (64.2) | 14 (82.4) | .25 |
| Heart | 57 | 20 (35.1) | 0.70 (0.28-1.75) | .44 | 22 (35.5) | 28 (34.6) | 7 (41.2) | .97 |
| General symptoms | 56 | 13 (23.2) | 0.66 (0.27-1.62) | .37 | 22 (35.5) | 25 (30.9) | 9 (52.9) | .22 |
| Eye | 49 | 14 (28.6) | 1.26 (0.50-3.15) | .63 | 13 (21.0) | 28 (34.6) | 8 (47.1) | .07 |
| Lymph node | 49 | 15 (30.6) | 0.44 (0.16-1.20) | .11 | 16 (25.8) | 27 (33.3) | 6 (35.3) | .57 |
| Cutaneous and mucosal | 35 | 10 (28.6) | 0.46 (0.16-1.38) | .17 | 12 (19.4) | 22 (27.2) | 1 (5.9) | .14 |
| Liver or spleen | 32 | 8 (25.0) | 0.58 (0.20-1.69) | .32 | 11 (17.7) | 18 (22.2) | 3 (17.6) | .81 |
| Exocrine | 27 | 8 (29.6) | 1.07 (0.36-3.22) | .90 | 8 (12.9) | 16 (19.8) | 3 (17.6) | .56 |
| Joints | 26 | 6 (23.1) | 0.47 (0.13-1.69) | .25 | 11 (17.7) | 12 (14.8) | 3 (17.6) | .82 |
| Ear, nose, and throat | 10 | 3 (30.0) | 1.21 (0.24-6.14) | .82 | 3 (4.8) | 5 (6.2) | 2 (11.8) | .57 |
| Kidney | 7 | 1 (14.3) | 0.59 (0.06-5.51) | .65 | 4 (6.5) | 2 (2.5) | 1 (5.9) | .43 |
| Digestive tract | 6 | 1 (16.7) | 0.18 (0-9.3) | .40 | 1 (1.6) | 5 (6.2) | 0 | .30 |
| Bone | 4 | 0 | NA | .30e | 2 (3.2) | 2 (2.5) | 0 | >.99 |
| Elevated serum ACE level | 64 | 23 (35.9) | 0.88 (0.35-2.2) | .78 | 28 (45.2) | 31 (37.8) | 5 (29.4) | .47 |
| Neurologic Involvement | ||||||||
| CNS localization | 118 | 43 (36.4) | 2.51 (0.86-7.29) | .09 | 42 (67.8) | 59 (72.8) | 17 (100) | .01 |
| Encephalon | 99 | 38 (38.4) | 2.82 (1.07-7.41) | .04 | 35 (56.5) | 48 (59.3) | 16 (94.1) | .008 |
| Spinal cord | 49 | 15 (30.6) | 0.71 (0.29-1.77) | .47 | 16 (25.8) | 29 (35.8) | 4 (23.5) | .38 |
| Cranial nerve | 65 | 24 (36.9) | 1.19 (0.51-2.75) | .69 | 26 (41.9) | 28 (34.6) | 11 (64.7) | .07 |
| PNS | 16 | 8 (50.0) | 2.17 (0.58-8.1) | .25 | 5 (8.1) | 6 (7.4) | 5 (29.4) | .03 |
| Muscle | 12 | 5 (41.7) | 1.24 (0.27-5.63) | .78 | 6 (9.7) | 5 (6.2) | 1 (5.9) | .82 |
| Abnormal MRI finding of the brain or spinal cord | 102 | 43 (42.2) | 0.71 (0.14-3.48) | .67 | 41 (66.2) | 47 (58.0) | 14 (82.4) | .78 |
| CSF protein level >50 mg/dL | 81 | 44 (54.3) | 2.54 (0.97-6.62) | .06 | 30 (48.4) | 38 (46.9) | 13 (76.5) | .51 |
| CSF white blood cells >5/μL | 79 | 39 (49.4) | 1.42 (0.58-3.5) | .44 | 35 (56.5) | 31 (38.3) | 13 (76.5) | .02 |
Abbreviations: ACE, angiotensin-converting enzyme; CNS, central nervous system; CSF, cerebrospinal fluid; EDSS, Expanded Disability Status Scale; MRI, magnetic resonance imaging; NA, not applicable; NS, neurosarcoidosis; OR, odds ratio; PNS, peripheral nervous system.
Possible scores range from 0 to 10, with higher scores indicating greater disability.
Using conditional logistic regression models, stratified on period of NS diagnosis (1990-99 vs 2000-15) and adjusted on baseline EDSS score.
Changes in EDSS are given as median (interquartile range).
Classified according to Zajicek et al.
Calculated using the Fisher exact test; estimation of the OR using a logistic regression model was not performed owing to the absence of a 60-month EDSS score of at least 2.5 in the subgroup of patients with bone involvement (n = 4) vs 56 of 156 with an EDSS score of at least 2.5 (35.9%) in the subgroup without bone involvement.
Discussion
We aimed to identify long-term prognostic factors and to assess the effects of immunosuppressive treatment on the main outcomes in patients with NS. Considering that most of our patients had a chronic and relapsing disease, outcomes were evaluated using OS and RFS (any localization and neurologic relapses). We also assessed the functional outcomes using EDSS scores at baseline and after 60 months of follow-up. In the present study, which to our knowledge is one of the largest published cohorts of patients with NS, we found that (1) the probable 10-year survival rate was good (89%) and mortality was associated with age, PNS involvement, and a higher baseline EDSS score; (2) the 10-year relapse-rate was high (86.2%), and encephalic involvement was associated with a higher risk for neurologic relapse; (3) some immunosuppressive drugs were associated with decreased neurologic and nonneurologic relapse rates (ie, intravenous cyclophosphamide, methotrexate, and infliximab); and (4) long-term functional impairment was associated with tobacco use, an elevated baseline EDSS score, and encephalic involvement and was inversely associated with diffuse extraneurologic sarcoidosis at diagnosis, whereas worsening of the EDSS score at 60 months was associated with encephalic and PNS involvement.
A fatal outcome was observed in 21 patients (11.1% probability of death at 10 years). Most deaths were not due to sarcoidosis neurologic injury, suggesting that NS might be a marker of an overall aggressive sarcoidosis with potentially lethal extraneurologic involvement. Death was associated with increased age, similar to previous findings in pulmonary sarcoidosis; higher baseline EDSS scores; and PNS involvement. This last finding should be taken with caution because, owing to the conflation of PNS relapses with CNS symptoms, some of the relapses involving PNS may have been missed. However, PNS involvement, a reportedly rare and heterogeneous aspect of NS, could have been considered by physicians as a nonsevere form of sarcoidosis (compared with those having CNS involvement), leading to less aggressive treatment.
The high relapse rate in our study (86.2% had at ≥1 relapse or died, and 72.4% had ≥1 neurologic relapse or died after 10 years of follow-up) is likely to have been favored by the long follow-up and the frequent multiorgan involvement. Encephalic involvements were associated with more frequent relapses, and among them, hydrocephalus was associated with a poorer RFS independently of the occurrence of other encephalic symptoms. Therefore, patients with such presentations should be treated more aggressively. Previous studies have suggested that early immunosuppressive therapy might be beneficial in patients with NS. In the present study, some immunosuppressive drugs were associated with lower risks for neurologic and nonneurologic relapse (ie, intravenous cyclophosphamide, methotrexate, and infliximab), although cyclophosphamide and infliximab were mostly used as second-line therapies in refractory NS. Conversely, mycophenolic acid and azathioprine were not shown to have beneficial effects. A recent publication also suggested that treatment with mycophenolic acid is associated with a shorter RFS compared with methotrexate treatment. With regard to azathioprine, the sample size in our study (14 sequences of treatment) was probably too small and may lack a sufficient power to make a definite conclusion about its effect in patients with NS. Treatment with hydroxychloroquine was found to be associated with fewer relapses, which is likely owing to this drug being used in sarcoidosis with a more benign course (eg, joint, cutaneous, and mucosal symptoms) and is mostly combined with immunosuppressants when severe organ involvement is present. Although these results should be interpreted with caution because (1) treatments were not randomized in the observational cohort in our study and associations between outcome and treatments are subject to confounding factors and (2) sample sizes of some treatments groups were small, therefore limiting the power of analyses, they suggest that treatment with intravenous cyclophosphamide, methotrexate, or infliximab may be beneficial to patients with NS. Despite a frequent use of corticosteroids and immunosuppressants, the rate of adverse effects remained low, highlighting a benefit-risk balance in favor of long-term immunosuppression in patients with NS. Such an approach could be particularly important if patients with NS show factors that we found to be associated with relapse, such as encephalic involvement.
Significant long-term functional impairment in the NS cohort in our study was associated with an elevated baseline EDSS score, a smoking habit, and encephalic involvement and was inversely associated with a diffuse form of sarcoidosis (involving >3 extraneurologic sites). The association of poor functional outcomes with an elevated EDSS score at baseline is similar to recent findings from a small cohort with spinal cord sarcoidosis and suggests that patients with severe impairment at the onset of the disease may benefit from early aggressive therapy. The worsening of the EDSS status was associated with symptoms related to lesions at the encephalic level, which may be linked to the increased risk for relapse and to the immediate functional effects of such involvement. Conversely, PNS involvement was also associated with an increase of the final EDSS score, which suggests a more aggressive disease with multiple involvements of the CNS. Despite the acknowledged severity of myelitis in patients with NS, we did not find myelopathy to be associated with poorer outcomes. This result might reflect progressive improvement of myelitis-induced functional impairment in the long term. However, this hypothesis needs to be confirmed by dedicated cohort studies.
Limitations
We assessed the functional outcomes in our study using the EDSS because of the absence of functional scales dedicated to neurologic outcomes in inflammatory systemic diseases. This choice may have underweighted some aspects of NS, notably pituitary dysfunction, cognitive impairment, and uveitis, and we cannot exclude some prognostic factors associated with these specific features having eluded our analysis. Nonetheless, the EDSS has the advantage of reflecting some important aspects of the disease, notably motor dysfunction and loss of autonomy. Moreover, some part of the disability may have come from other sarcoidosis injuries than neurologic involvement, such as cardiac failure, respiratory insufficiency, or skeletal involvement. Such aspects of the disease are beyond the scope of this study and therefore were not directly assessed, although they might have indirectly affected the EDSS scoring.
We acknowledge some other limitations of the present study. Owing to the rarity of the disease, we were compelled to analyze retrospective data with attendant well-known limitations, although the large size of our cohort still allowed us to analyze statistical associations. The fact that our department is dedicated to internal medicine may have led to a referral bias, explaining some of the characteristics of our cohort (ie, the predominance of multisystemic forms of sarcoidosis and CNS involvement and the frequent involvement of >1 neurologic site). Considering the limits of the EDSS already discussed, the use of combinable scales evaluating the main aspects of NS will be needed in future prospective studies. The heterogeneity of our patients, which reflects the clinical variety of NS during the inclusion period, required the use of complex statistical models. Our statistical models did not allow us to study the association of immunosuppressive treatments with the vital and functional outcome of patients with NS. In addition, as mentioned above, immunosuppressive treatments were not randomized in our study. Only randomized trials would allow causal conclusions regarding the associations of treatments and relapse.
Conclusions
Tobacco use, an elevated baseline EDSS score, and encephalic and PNS involvement were found to be associated with poorer functional outcomes in patients with NS. Encephalic symptoms were associated with more frequent relapses. Being older, having an elevated baseline EDSS score, and having PNS involvement were associated with an increased mortality rate. Our results show that in patients with NS, immunosuppressive therapies with intravenous cyclophosphamide, methotrexate, or infliximab are associated with lower relapse rates, especially when predictive factors of poor outcome or relapses are present. Future prospective studies are needed to confirm our results.
eTable 1. Extraneurologic Characteristics of 234 Patients With NS
eTable 2. Overall and Relapse-Free Survival 1990-1999 (n = 55)
eTable 3. Overall and Relapse-Free Survival 2000-2015 (n = 179)
eTable 4. Factors Associated With the Risk for Neurologic and Nonneurologic Relapse in Patients With Neurosarcoidosis
eTable 5. Functional Outcomes at 60 Months 1990-1999 (n = 55)
eTable 6. Functional Outcomes at 60 Months 2000-2015 (n = 179)
eFigure. Overall and Relapse-Free Survival According to the Period of Neurosarcoidosis Diagnosis
References
- 1.Ungprasert P, Carmona EM, Utz JP, Ryu JH, Crowson CS, Matteson EL. Epidemiology of sarcoidosis 1946-2013: a population-based study. Mayo Clin Proc. 2016;91(2):183-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zajicek JP, Scolding NJ, Foster O, et al. Central nervous system sarcoidosis: diagnosis and management. QJM. 1999;92(2):103-117. [DOI] [PubMed] [Google Scholar]
- 3.Chapelon C, Ziza JM, Piette JC, et al. Neurosarcoidosis: signs, course and treatment in 35 confirmed cases. Medicine (Baltimore). 1990;69(5):261-276. [PubMed] [Google Scholar]
- 4.Gascón-Bayarri J, Mañá J, Martínez-Yélamos S, Murillo O, Reñé R, Rubio F. Neurosarcoidosis: report of 30 cases and a literature survey. Eur J Intern Med. 2011;22(6):e125-e132. [DOI] [PubMed] [Google Scholar]
- 5.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology. 1983;33(11):1444-1452. [DOI] [PubMed] [Google Scholar]
- 6.Therneau T, Grambsch PM. Modelling Survival Data: Extending the Cox Model. 2nd ed New York, NY: Springer; 2000:185-190. [Google Scholar]
- 7.Joseph FG, Scolding NJ. Neurosarcoidosis: a study of 30 new cases. J Neurol Neurosurg Psychiatry. 2009;80(3):297-304. [DOI] [PubMed] [Google Scholar]
- 8.Pawate S, Moses H, Sriram S. Presentations and outcomes of neurosarcoidosis: a study of 54 cases. QJM. 2009;102(7):449-460. [DOI] [PubMed] [Google Scholar]
- 9.Stern BJ, Krumholz A, Johns C, Scott P, Nissim J. Sarcoidosis and its neurological manifestations. Arch Neurol. 1985;42(9):909-917. [DOI] [PubMed] [Google Scholar]
- 10.Swigris JJ, Olson AL, Huie TJ, et al. Sarcoidosis-related mortality in the United States from 1988 to 2007. Am J Respir Crit Care Med. 2011;183(11):1524-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burns TM, Dyck PJB, Aksamit AJ, Dyck PJ. The natural history and long-term outcome of 57 limb sarcoidosis neuropathy cases. J Neurol Sci. 2006;244(1-2):77-87. [DOI] [PubMed] [Google Scholar]
- 12.Scott TF, Yandora K, Valeri A, Chieffe C, Schramke C. Aggressive therapy for neurosarcoidosis: long-term follow-up of 48 treated patients. Arch Neurol. 2007;64(5):691-696. [DOI] [PubMed] [Google Scholar]
- 13.Bitoun S, Bouvry D, Borie R, et al. Treatment of neurosarcoidosis: a comparative study of methotrexate and mycophenolate mofetil. Neurology. 2016;87(24):2517-2521. [DOI] [PubMed] [Google Scholar]
- 14.Durel C-A, Marignier R, Maucort-Boulch D, et al. Clinical features and prognostic factors of spinal cord sarcoidosis: a multicenter observational study of 20 biopsy-proven patients. J Neurol. 2016;263(5):981-990. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Extraneurologic Characteristics of 234 Patients With NS
eTable 2. Overall and Relapse-Free Survival 1990-1999 (n = 55)
eTable 3. Overall and Relapse-Free Survival 2000-2015 (n = 179)
eTable 4. Factors Associated With the Risk for Neurologic and Nonneurologic Relapse in Patients With Neurosarcoidosis
eTable 5. Functional Outcomes at 60 Months 1990-1999 (n = 55)
eTable 6. Functional Outcomes at 60 Months 2000-2015 (n = 179)
eFigure. Overall and Relapse-Free Survival According to the Period of Neurosarcoidosis Diagnosis