This cohort study measures ozone concentration to assess its association with cardiovascular disease risk in 89 healthy adults.
Key Points
Questions
Which cardiovascular disease pathophysiologic mechanisms might explain the epidemiologic association between ozone and cardiovascular mortality, and at what concentrations are these mechanisms associated with ozone?
Findings
In this cohort study of 89 healthy adults, ozone exposure was associated with markers of platelet activation and increased blood pressure. Ozone concentrations were lower than the levels capable of influencing pulmonary function, which is the main basis for current regulatory standards.
Meaning
Standards for safe ozone exposure should take into account its association with cardiovascular disease risk, which appears to occur at lower levels of ozone exposure than respiratory effects.
Abstract
Importance
Exposure to ozone has been associated with cardiovascular mortality, but the underlying biological mechanisms are not yet understood.
Objective
To examine the association between ozone exposure and cardiopulmonary pathophysiologic mechanisms.
Design, Setting, and Participants
A longitudinal study involving 89 healthy adult participants living on a work campus in Changsha City, China, was conducted from December 1, 2014, to January 31, 2015. This unique quasiexperimental setting allowed for better characterization of air pollutant exposure effects because the participants spent most of their time in controlled indoor environments. Concentrations of indoor and outdoor ozone, along with the copollutants particulate matter, nitrogen dioxide, and sulfur dioxide, were monitored throughout the study period and then combined with time-activity information and filtration conditions of each residence and office to estimate 24-hour and 2-week combined indoor and outdoor mean exposure concentrations. Associations between each exposure measure and outcome measure were analyzed using single-pollutant and 2-pollutant linear mixed models controlling for ambient temperature, secondhand smoke exposure, and personal-level time-varying covariates.
Main Outcomes and Measures
Biomarkers indicative of inflammation and oxidative stress, arterial stiffness, blood pressure, thrombotic factors, and spirometry were measured at 4 sessions.
Results
Of the 89 participants, 25 (28%) were women and the mean (SD) age was 31.5 (7.6) years. The 24-hour ozone exposure concentrations ranged from 1.4 to 19.4 parts per billion (ppb), corresponding to outdoor concentrations ranging from 4.3 to 47.9 ppb. Within this range, in models controlling for a second copollutant and other potential confounders, a 10-ppb increase in 24-hour ozone was associated with mean increases of 36.3% (95% CI, 29.9%-43.0%) in the level of platelet activation marker soluble P-selectin, 2.8% (95% CI, 0.6%-5.1%) in diastolic blood pressure, 18.1% (95% CI, 4.5%-33.5%) in pulmonary inflammation markers fractional exhaled nitric oxide, and 31.0% (95% CI, 0.2%-71.1%) in exhaled breath condensate nitrite and nitrate as well as a −9.5% (95% CI, −17.7% to −1.4%) decrease in arterial stiffness marker augmentation index. A 10-ppb increase in 2-week ozone was associated with increases of 61.1% (95% CI, 37.8%-88.2%) in soluble P-selectin level and 126.2% (95% CI, 12.1%-356.2%) in exhaled breath condensate nitrite and nitrate level. Other measured biomarkers, including spirometry, showed no significant associations with either 24-hour ozone or 2-week ozone exposures.
Conclusions and Relevance
Short-term ozone exposure at levels not associated with lung function changes was associated with platelet activation and blood pressure increases, suggesting a possible mechanism by which ozone may affect cardiovascular health.
Introduction
Airborne particulate matter with a diameter of 2.5 µm or less (PM2.5) has often been reported to be associated with the cardiovascular toxic effects of air pollution mixtures and ozone (O3) with respiratory illness.1,2 However, there is increasing evidence that O3, independent of PM2.5, is also associated with cardiovascular mortality.3,4 For example, O3 has been associated with increases in mortality due to embolism and thrombosis,5 ischemic heart disease,3 heart failure,4 and stroke.6 However, not all studies reporting associations of O3 with cardiovascular outcomes found that the associations were independent from particulate matter and other gaseous pollutants, such as nitrogen dioxide (NO2) and sulfur dioxide (SO2).7,8 Other studies have found nonsignificant or seemingly “beneficial” associations between O3 and cardiovascular outcomes.9,10 The uncertainty in epidemiologic findings of O3 cardiovascular effects is further enhanced by the limited understanding of biological mechanisms underlying the epidemiologic associations.11
The relative uniformity of lifestyle patterns and proximity of participants living and working together at a work campus in Changsha City, China, presented a unique opportunity to monitor pollutants in indoor and outdoor air and to measure changes in the levels of biomarkers pertinent to cardiorespiratory disease risk. The cardiovascular biomarkers used in this study have all been associated with air pollution exposure in previous studies,12 although not necessarily with O3 specifically. This study also includes lung function measures that have consistently been associated with O3 concentration in short-term, controlled-exposure experiments.11 In addition, this setting facilitated the manipulation of indoor PM2.5 and O3 concentrations through the use of various filtration technologies in all the participants’ residences and offices. Filtration provided an opportunity to generate different temporal patterns between O3 and PM2.5 exposures, which helped to disentangle the effects of these 2 major pollutants.
Methods
Study Subjects
We recruited 89 healthy white-collar workers living and working at the Broad Company Campus (Broad Town) in suburban Changsha, China. Each participant completed a questionnaire and underwent basic metabolic blood panel screenings for previous medical conditions to ensure a healthy baseline status. All participants had to be older than 18 years, work in one of 2 Broad Town offices, spend at least 4 nights each week in the dormitories, and be free from major chronic diseases as self-reported by the participants. In addition, abnormal blood lipid levels or markers of kidney, liver, or other metabolic dysfunction were grounds for exclusion. The ethics committee of the Shanghai First People’s Hospital and the campus institutional review board of Duke University approved the study protocol. Explicit written consent was obtained from all participants.
The present exploration focusing on pathophysiologic mechanisms of O3 cardiovascular effects used data collected in field experiments in which indoor PM2.5 concentrations were manipulated through varying filtration technologies to remove particulate matter entering the offices and dormitory rooms. These filters included minibag filters, electrostatic precipitators, and high-efficiency particulate air filters, which had a PM2.5 removal efficiency of approximately 50%, 60%, and >99%, respectively. Electrostatic precipitators generated approximately 13.5 parts per billion (ppb) of incidental O3 in the downstream air ducts, corresponding to a 3-ppb increase in steady state indoor concentrations13 (eMethods in the Supplement). On different dates, 1 or more of these filtration technologies were used in the offices and dormitories.
Outcome Measurements
We measured the following biomarkers: exhaled breath condensate (EBC) malondialdehyde (MDA) for pulmonary oxidative stress14; fractional exhaled nitric oxide (FeNO)15 and the sum of EBC nitrite and nitrate (EBCNN) for pulmonary inflammation14; forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), and FEV1:FVC ratio for lung function; C-reactive protein (CRP) for systemic inflammation16; urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) for oxidative stress17; brachial systolic blood pressure (SBP) and diastolic blood pressure (DBP) for vascular tone18; brachial augmentation index (AI) and carotid-femoral pulse wave velocity (PWV) for arterial stiffness19; soluble P-selectin (sCD62P) for platelet activation20; and von Willebrand factor (VWF) for hemostasis.21
Each participant was assessed 4 times over a 9-week period (from December 2, 2014, to January 30, 2015), with about 2 weeks in between assessments. Efforts were made to conduct all sessions for each participant on the same day of the week and at the same time of day, although work schedules necessitated some rescheduling. No sessions were scheduled within a day following a trip off campus.
Each session started with a venous blood and urine collection at 8 am, followed throughout the day by any order of breath and EBC collections as well as blood pressure and arterial stiffness measurements, with spirometry always conducted last. Blood samples were centrifuged, and plasma aliquots were stored at −30°C before analysis for sCD62P, CRP, and VWF using an enzyme-linked immunosorbent assay method (R&D Systems for CRP and sCD62P; RayBiotech for VWF). Urine samples were immediately frozen for later solid-phase extraction and liquid chromatography–mass spectrometry analysis for 8-OHdG.22 Breath sample collection and analyses, spirometry, blood pressure, AI, and PWV measurements were conducted using standard procedures (eMethods in the Supplement).
Exposure Measurements
Outdoor PM2.5, O3, NO2, and SO2 concentrations were measured at a government station 4.5 km from the study site. This station was predominantly upwind of the study site, and there were few major roadways and other local air pollution sources between the station and the study site. A comparison of the concentrations measured simultaneously at both sites showed a slope of 1.03 and R2 of 0.998 for PM2.5 and a slope of 0.97 and R2 of 0.988 for O3 in least squares linear regressions. Indoor PM2.5 mass concentration and O3 concentration were continuously measured in the 2 involved offices during the day and in 2 dormitory rooms per night using field-calibrated (against a primary method at the study location) nephelometers for PM2.5 concentration measurement (SidePak AM510; TSI Inc) and a UV absorption monitor for O3 concentration measurement (Model 205; 2B Tech) (eMethods in the Supplement). These measurements were used to establish indoor/outdoor (I/O) ratios taking into account known indoor sources and filtration conditions (see eTable 2 in the Supplement), which were later used to estimate hourly averages for PM2.5 and O3 concentrations outside of the monitoring times. These I/O ratios were found to be within 25% of measured I/O ratios during monitoring times.
Because NO2 and SO2 were not affected by the filtration conditions, these were not measured indoors. Instead, I/O ratios of 0.8 for NO2 and 0.5 for SO2 were assumed for all indoor spaces on the basis of physical characteristics of the dormitories and offices coupled with previous relevant literature.23,24 Time-activity questionnaire data were integrated with indoor and outdoor O3 and PM2.5 hourly mean concentrations to calculate 24-hour exposure concentrations. The assumed I/O ratios for unknown indoor environments were 0.35 for O3 and 0.8 for PM2.5 according to previous findings in lightly sealed indoor spaces25,26 and expectations for structures in the Changsha region. Because the study was organized with approximately 2-week breaks between biomarker measurements, 2-week exposure concentrations were estimated as representative of “subchronic” exposure effects (eMethods in the Supplement).
Statistical Analysis
Depending on data distribution, values of certain biomarkers were natural logarithm–transformed in statistical analyses (Supplementary Methods in the Supplement). Linear mixed models with participant-specific intercepts were used to analyze concentration-response associations between each biomarker and each exposure measure. The use of participant-specific intercepts in mixed models accounts for the correlation between within-participant repeated measurements and precludes the need to control for participant characteristics that do not change between measurements (eg, age). Two-pollutant models for all combinations of 24-hour or 2-week exposures were performed to test whether the associations observed in the single-pollutant models remained after controlling for a second pollutant. All models controlled for 24-hour mean ambient temperature and time spent in a room with a smoker as an approximation of secondhand smoke exposure. Because we expected an unequal influence of certain covariates on the biomarkers, we used a backward stepwise model selection method to select the following additional covariates for each model: respiratory infection status, menstruation status, day of the week, and hours since the participant last ate (see covariates in eTable 5 in the Supplement). Multiple testing–corrected P values were obtained using the Benjamini-Yekutieli method of reducing the false discovery rate while allowing for an arbitrary dependence structure in the predictor and outcome variables.27 A 2-sided P = .05 indicated statistical significance. In Benjamini-Yekutieli multiple testing correction, the new, higher statistical significance level was P = .05.
Sensitivity analyses included models excluding all smokers and ex-smokers, all observations reporting secondhand smoke exposure, or outlying values from the models. We also assessed 5 separate models by controlling for 1 of the following in the main model: (1) all 4 of the aforementioned stepwise method-selected covariates, (2) curvilinear associations with temperature using a cubic spline, (3) relative humidity, (4) wind direction and speed, and (5) interaction between a building filtration condition and a pollutant. All calculations were made using the “nlme,” “openair,” and “stats” packages in R version 3.2.3 (R Project for Statistical Computing).
Results
Participant Characteristics
Study participant characteristics are listed in Table 1. Of the 89 participants, 25 (28%) were women and the mean (SD) age was 31.5 (7.6) years. Eighty-one of the 89 participants (91%) completed all 4 visits; 3 participants withdrew from the study after the first visit, and 5 participants completed 3 visits. Of the 343 total observations, only 8 (2.3%) were omitted because they corresponded with time-activity questionnaires that were not filled in, and an additional 2 (0.6%) were omitted because medications that might affect biomarker outcomes had been taken by the participants. All participants who attended at least 1 visit were included in the analyses.
Table 1. Characteristics of 89 Study Participants.
| Characteristic | Value |
|---|---|
| Age, mean (SD) [range], y | 31.5 (7.6) [22-52] |
| Female, No. (%) | 25 (28.1) |
| BMI, mean (SD) | 22.3 (2.7) |
| Current smokers, No. (%) | 15 (16.9) |
| Ex-smokers, No. (%) | 6 (6.7) |
| Pack-years for current smokers and ex-smokers, mean (SD)a | 0.87 (2.48) |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
A pack-year is a measure of chronic smoking equal to 1 pack of cigarettes (20 cigarettes) per day smoked for an entire year.
Air Pollution Exposure
Table 2 gives summary statistics for the outdoor and total exposure concentrations averaged over the 24-hour and 2-week period prior to each biomarker sampling time. The 24-hour O3 exposure concentrations corresponded to the daily maximum 8-hour rolling mean (SD) outdoor concentrations of 28.2 (15.6) ppb, which was lower than the daily maximum 8-hour O3 concentrations associated with cardiovascular mortality4 but within the range of concentrations associated with some cardiovascular risk indicators (eg, decreased heart rate variability and fibrinolysis).28 In contrast, the 24-hour PM2.5 exposure concentrations, corresponding to mean (SD) outdoor 24-hour concentrations of 91.20 (53.97) µg/m3, were higher than typical PM2.5 concentrations associated with increased cardiorespiratory mortality risk.29 The 24-hour NO2 and SO2 exposure concentrations, corresponding to mean (SD) outdoor concentrations of 28.62 (8.23) ppb for NO2 and 11.68 (2.95) ppb for SO2, were comparable to levels that have been previously associated with cardiovascular mortality.30,31
Table 2. Air Pollutant Outdoor and Estimated Exposure Measure Concentrations.
| Pollutant | Time Frame | Type of Measurement | Median (Range) | Mean (SD) |
|---|---|---|---|---|
| O3, ppb | 24 h | Outdoor | 19.07 (4.33-47.91) | 21.67 (14.28) |
| Exposure | 4.59 (1.45-19.45) | 6.71 (4.31) | ||
| 2 wk | Outdoor | 21.36 (12.20-34.89) | 22.66 (7.37) | |
| Exposure | 6.95 (4.46-13.28) | 7.84 (2.29) | ||
| PM2.5, µg/m3 | 24 h | Outdoor | 70.33 (16.25-210.70) | 91.20 (53.97) |
| Exposure | 26.64 (3.18-155.01) | 38.69 (30.69) | ||
| 2 wk | Outdoor | 84.43 (56.31-126.88) | 90.25 (23.72) | |
| Exposure | 40.64 (12.98-81.32) | 43.90 (15.08) | ||
| NO2, ppb | 24 h | Outdoor | 28.27 (15.47-47.37) | 28.62 (8.23) |
| Exposure | 22.80 (12.53-39.90) | 23.22 (6.73) | ||
| 2 wk | Outdoor | 30.99 (25.43-39.26) | 31.29 (3.14) | |
| Exposure | 25.20 (20.62-32.13) | 25.48 (2.56) | ||
| SO2, ppb | 24 h | Outdoor | 11.04 (7.41-20.34) | 11.68 (2.95) |
| Exposure | 5.70 (3.71-10.80) | 6.14 (1.63) | ||
| 2 wk | Outdoor | 11.26 (8.22-16.66) | 12.16 (2.99) | |
| Exposure | 6.22 (4.20-9.77) | 6.50 (1.60) |
Abbreviations: NO2, nitrogen dioxide; O3, ozone; PM2.5, particulate matter with a diameter of 2.5 µm or less; ppb, parts per billion; SO2, sulfur dioxide.
Ozone was the only pollutant measured to have negative correlations with other pollutants, with the 2-week O3 exposure more strongly negatively correlated (eTable 4 in the Supplement). In contrast, PM2.5, NO2, and SO2 concentrations were all positively correlated with each other. The 24-hour and 2-week exposure concentrations for all the pollutants were positively correlated with their respective outdoor concentrations.
Biomarker Responses
Biomarker concentrations and values are summarized in Table 3. Using these values in single-pollutant models, we found that a 10-ppb increase in 24-hour O3 exposure concentration (Figure 1) was associated with significant increases in FeNO (24.1%; 95% CI, 11.0%-38.8%), EBCNN (53.8%; 95% CI, 23.6%-91.5%), 8-OHdG (13.5%; 95% CI, 2.6%-25.6%), SBP (3.1%; 95% CI, 1.4%-4.8%), DBP (4.4%; 2.5%-6.3%), and sCD62P (36.2%; 95% CI, 30.6%-42.0%) levels and with significant decreases in EBC MDA level (−26.3%; 95% CI, −37.9% to −12.4%) and AI (−10.2%; 95% CI, −17.6% to −2.8%). For 2-week O3 exposure concentration, a 10-ppb increase was associated with significant increases in FeNO (47.2%; 95% CI, 15.9%-86.9%), EBCNN (158.9%; 95% CI, 61.2%-315.8%), 8-OHdG (37.0%; 95% CI, 11.1%-69.0%), SBP (8.7%; 95% CI, 5.0%-12.3%), DBP (10.1%; 95% CI, 6.2%-14.0%), and sCD62P (100.9%; 95% CI, 84.3%-118.9%) level as well as with significant decreases in EBC MDA level (−52.0%; 95% CI, −67.0% to −30.4%) and AI (−26.5%; 95% CI, −42.1% to −10.8%) and increases in FEV1 (2.6%; 95% CI, 0.02%-5.2%), and FVC (2.2%; 95% CI, 0.04%-4.3%) levels. After multiple testing correction, the following associations became nonsignificant: the association between 24-hour O3 concentration and AI; the association between 2-week O3 concentration and FeNO level; and the associations between both averaging times of O3 concentration and 8-OHdG, FEV1, and FVC levels.
Table 3. Biomarker Summary Statistics Across All Visits.
| Biomarker | Minimum | Median (IQR) | Mean (SD) | Maximum |
|---|---|---|---|---|
| EBC MDA, µM | 1.6 | 20.4 (13.5) | 22.4 (14.0) | 118.6 |
| FeNO, ppb | 1.1 | 6.0 (5.0) | 7.2 (4.5) | 34.8 |
| EBCNN, ng/mL | 301.0 | 1409.7 (1811.8) | 2910.7 (4935.1) | 46657.2 |
| FEV1, L | 1.9 | 3.2 (0.7) | 3.2 (0.5) | 4.6 |
| FVC, L | 2.3 | 3.9 (1.0) | 3.9 (0.7) | 5.9 |
| FEV1:FVC, % | 59.7 | 81.9 (7.1) | 82.0 (6.2) | 98.3 |
| 8-OHdG, ng/mLa | 0.5 | 4.0 (3.7) | 4.4 (2.3) | 14.4 |
| SBP, mm Hg | 90 | 111 (13.0) | 112.3 (10.8) | 159 |
| DBP, mm Hg | 51 | 63 (8.0) | 64.6 (7.0) | 89 |
| AI, % | 0 | 15.3 (9.3) | 16.0 (6.7) | 40.0 |
| PWV, m/s | 4.3 | 6.3 (1.2) | 6.4 (1.1) | 14.3 |
| CRP, ng/mL | 12.5 | 260.5 (450.4) | 870.9 (2035.4) | 18764.2 |
| sCD62P, ng/mL | 10.9 | 22.9 (9.3) | 24.3 (7.5) | 60.4 |
| VWF, µg/mL | 3.5 | 30.1 (20.5) | 32.3 (15.7) | 82.8 |
Abbreviations: 8-OHdG, urinary 8-hydroxy-2′-deoxyguanosine; AI, augmentation index; CRP, C-reactive protein; DBP, diastolic blood pressure; EBC MDA exhaled breath condensate malondialdehyde; EBCNN, EBC nitrite and nitrate; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; ppb, parts per billion; PWV, pulse wave velocity; SBP, systolic blood pressure; sCD62P, soluble P-selectin; VWF, von Willebrand factor.
SI conversion factor: To convert CRP to nanomoles per liter, multiply by 0.0095.
Concentrations of 8-OHdG are adjusted by specific gravity.
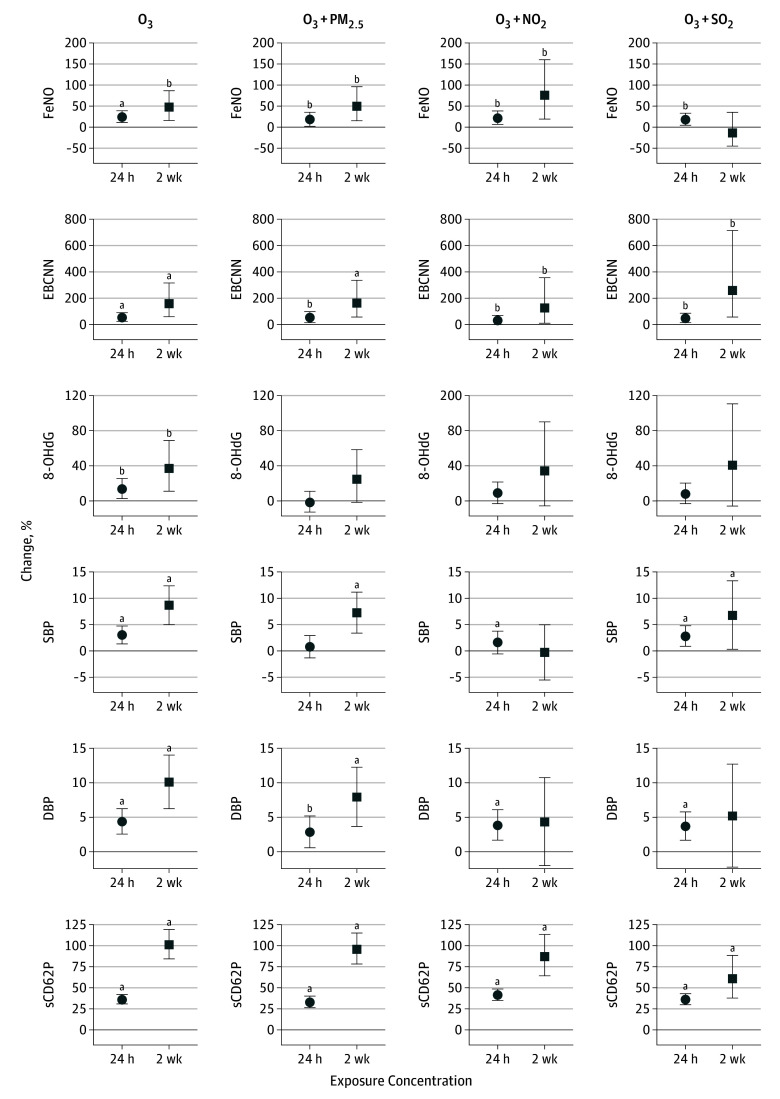
Figure 1. Mean Percentage of Change in Biomarkers and 95% CIs Associated With a 10-Unit Increase in 24-Hour and 2-Week Exposure Concentrations From Single-Pollutant Model Results.
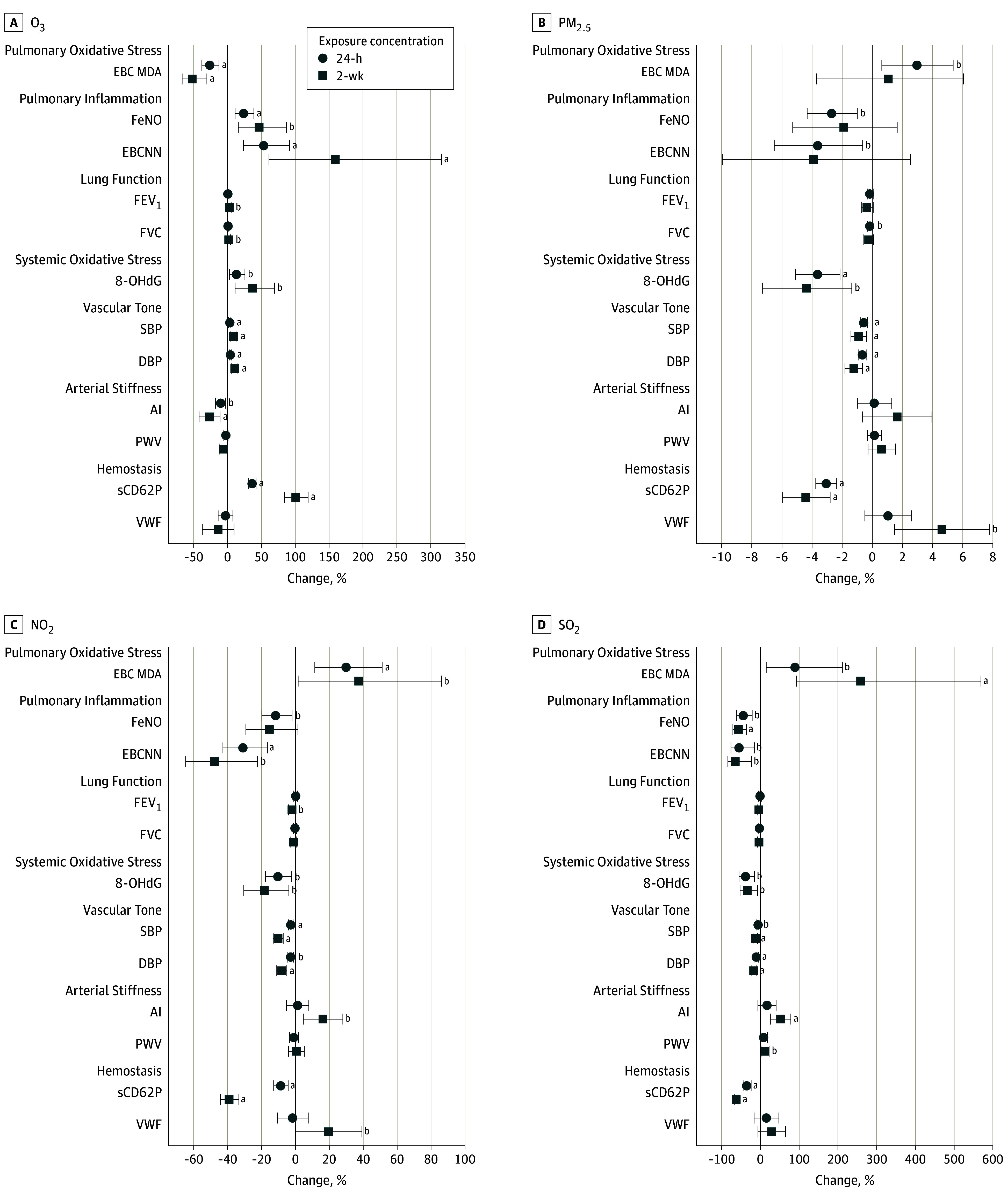
Biomarkers are organized by mechanistic category, first showing pulmonary oxidative stress (exhaled breath condensate malondialdehyde [EBC MDA]), then pulmonary inflammation (fractional exhaled nitric oxide [FeNO] and the sum of EBC nitrite and nitrate [EBCNN]), lung function (forced expiratory volume in the first second [FEV1] and forced vital capacity [FVC]), systemic oxidative stress (urinary 8-hydroxy-2′-deoxyguanosine [8-OHdG]), vascular tone (systolic and diastolic blood pressure [SBP and DBP]), arterial stiffness (augmentation index [AI] and pulse wave velocity [PWV]), and hemostasis (soluble P-selectin [sCD62P] and von Willebrand factor [VWF]). NO2 indicates nitrogen dioxide; O3, ozone; PM2.5, particulate matter with a diameter of 2.5 µm or less; and SO2, sulfur dioxide.
aP < .05 after Benjamini-Yekutieli multiple testing correction.
bP < .05.
In contrast, the single-pollutant models showed that PM2.5, NO2, and SO2 exposure concentrations all appear to be associated with fewer biomarkers than O3 exposure concentration. Each of these pollutants shared various positive associations with EBC MDA level and AI, with only the 2-week NO2 exposure concentration significantly associated with increases in EBC MDA level (29.9%; 95% CI, 11.5%-51.2%) as well as the 2-week SO2 exposure concentration significantly associated with increases in EBC MDA level (258.9%; 95% CI, 92.4%-569.4%) and AI (53.1%; 95% CI, 26.9%-79.3%) after multiple testing correction. Before but not after multiple testing correction, 2-week NO2 exposure concentration was significantly associated with adverse changes in EBC MDA level (37.5%; 95% CI, 1.5%-86.2%) and FEV1 level (−2.0%; 95% CI, −3.9% to −0.04%), 2-week SO2 exposure concentration was associated with a significant worsening of PWV (12.4%; 95% CI, 1.8%-22.9%), 24-hour PM2.5 exposure concentration was associated with a significant decrease in FVC (−0.2%; 95% CI, −0.3% to −0.005%), and 2-week PM2.5 exposure concentration was associated with a significant increase in VWF (4.6%; 95% CI, 1.5%-7.8%).
Figure 2 illustrates the influence of controlling for a second copollutant on the positive associations that O3 exposure had with FeNO, EBCNN, 8-OHdG, SBP, DBP, and sCD62P measurements. These biomarkers are highlighted as the ones showing positive associations with O3 in the single-pollutant models. All other 2-pollutant model results can be found in eFigures 1 through 4 in the Supplement. Only the O3 associations with sCD62P level remained significant after multiple testing correction and after controlling for a copollutant, although controlling for NO2 and SO2 exposure concentration decreased the effect size of the 2-week associations. For the 24-hour O3 and DBP, FeNO, and EBCNN associations as well as the association between 2-week O3 and EBCNN, the associations maintained a similar effect size. However, significance was lost after multiple testing correction. In terms of negative (beneficial) associations with O3 exposure concentration, only the association between 24-hour O3 and AI maintained a consistent effect size in all 2-pollutant models, although this did not remain significant after multiple testing correction.
Figure 2. Single-Pollutant and 2-Pollutant Model Results for Ozone 24-Hour and 2-Week Exposure Concentration Associations.
Associations are evident with fractional exhaled nitric oxide [FeNO], the sum of exhaled breath condensate nitrite and nitrate [EBCNN], urinary 8-hydroxy-2′-deoxyguanosine [8-OHdG], systolic blood pressure [SBP], diastolic blood pressure [DBP], and soluble P-selectin [sCD62P]. NO2 indicates nitrogen dioxide; O3, ozone; PM2.5, particulate matter with a diameter of 2.5 µm or less; and SO2, sulfur dioxide. Error bars indicate 95% CIs.
aP < .05 after Benjamini-Yekutieli multiple testing correction.
bP < .05.
For PM2.5, the association between 2-week PM2.5 exposure concentration and increased VWF values remained significant in all the 2-pollutant models, although only before multiple testing correction. In addition, the 24-hour PM2.5 negative (beneficial) associations with 8-OHdG, SBP, and DBP remained significant in 2-pollutant models, although the DBP association became nonsignificant after multiple testing correction. For NO2, only the negative (beneficial) associations between the 2-week exposure and each of SBP and DBP remained significant in all the 2-pollutant models, and this DBP association was only significant before multiple testing correction. In addition, controlling for O3 exposure concentration showed an increase in sCD62P level associated with 24-hour NO2 that was significant only before correction. Finally, only the 2-week SO2 positive association with AI and negative associations with FeNO and sCD62P levels remained significant, although not after multiple testing correction. Neither the FEV1:FVC ratio nor the CRP level was significantly associated with exposure to O3 or other pollutants in any of our statistical models.
The results of the sensitivity analyses did not change our conclusions from the main analysis. The sensitivity analysis models generally had higher Akaike and Bayes information criteria, which are indicators of model overfitting. Therefore, we report the results from the main model analyses.
Discussion
This study’s principal finding is that O3 exposures were positively associated with sCD62P level but not lung function and pulmonary inflammation after controlling for copollutants and multiple testing correction. An independent O3 association (ie, after copollutant adjustment) was observed with blood pressure, although this association did not remain significant after multiple testing correction. This finding suggests that O3 exposure may enhance cardiovascular disease risk via platelet activation and increased blood pressure at concentrations lower than those required to cause lung function impairment. This result provides mechanistic evidence for the epidemiologic association observed previously between cardiorespiratory mortality and O3 exposure at levels largely below the current US Environmental Protection Agency ambient air quality standards, the values of which are partially based on the lower limits of observed lung function decrements following O3 exposures.32
To our knowledge, this study is the first to show positive associations between low-level O3 exposure and sCD62P level, a biomarker of platelet activation20 linked to deep venous thrombosis33 and associated with increased risk for many cardiovascular diseases.20 The sCD62P level associations with both the 24-hour and 2-week O3 exposure remained robust to multiple testing P value correction and in all models, suggesting a strong association independent of other pollutants. Although the association between O3 exposure and the sCD62P level in blood has not been previously observed, an acute 2-hour exposure to 200-ppb O3 was associated with a significant upregulation in surface P-selectin level on airway vascular endothelial cells.34 As a possible mechanism by which O3 exposure leads to platelet activation and sCD62P upregulation, lung epithelial cells exposed in vitro to lipid ozonation products produced by O3 interactions with airway lipids have been shown to produce platelet-activating factor.35 In terms of O3 exposure associations with other thrombogenic factors, previous evidence of a link between ambient levels of O3 comparable to those seen in this study and a procoagulant state includes associations between factor VII coagulant activity and annual mean O3 concentration36 and between 24-hour mean O3 concentration and increased levels of fibrinolysis inhibitor PAI-1 (or plasminogen activator inhibitor-1).28 At higher concentrations (>70 ppb), an association between ambient O3 exposure and a procoagulant state has also been suggested by increases in fibrinogen level and VWF.37
Another result relevant to the effect of O3 exposure on cardiovascular disease risk is the association between O3 exposure and both SBP and DBP levels, although these associations appeared less robust in the multiple testing–corrected 2-pollutant models compared with the association between O3 and sCD62P. Two previous studies found that O3 exposure was negatively (beneficially) or not associated with blood pressure,38,39 but a recent study has shown a positive association between O3 and both SBP and DBP measurements at slightly higher exposure concentrations.40 A possible mechanism underlying an effect on blood pressure is increased sensitivity to serotonin-induced vasoconstriction and decreased sensitivity to acetylcholine-induced vasodilation, as was shown recently with acute O3 exposures in rats.41 Associations between hypertension and sCD62P level have been shown previously,42 potentially pointing to common mechanisms linking hypertension and platelet activation that could be involved in the associations of O3 exposure with sCD62P level and blood pressure.
In addition, we found associations between O3 exposure and biomarkers reflecting pulmonary inflammation (FeNO and EBCNN) that remained significant in the 2-pollutant models but not after multiple testing correction. Previously, FeNO has been positively associated with exposure to outdoor O3,28,43 and airway NO2 production by nitric oxide synthase has been shown to play an important role in mediating airway inflammation in response to acute O3 exposure in rodents.44 The EBCNN level reflects NO2 metabolism in the airways.14 Although the EBCNN level has not previously been associated with ambient O3 exposure in humans,45 acute O3 exposures in rodents have resulted in increases in airway lavage fluid nitrate.46 These results suggest a mechanistic link between pulmonary inflammatory effects and downstream cardiovascular effects, in which we propose that products of ozonation reactions or oxidative inflammatory mediators propagate inflammatory signals throughout the circulation.
For the other measured pollutants, we observed associations that were significant before but not after multiple testing correction in the 2-pollutant models between VWF level and 2-week PM2.5 exposure concentration and between AI and 2-week SO2 exposure concentration. Previous studies have suggested a correlation between PM2.5 concentration and increased endothelial cell dysfunction as indicated by VWF levels.47 Although SO2 concentration has not previously been associated with AI, it has been previously associated with the related arterial stiffness marker PWV.48 In contrast to previous studies,12 the PM2.5, NO2, and SO2 exposures were, to a large extent, not significantly associated with most of the biomarkers after controlling for copollutants in this study. Previous studies have suggested a plateau in the association between PM2.5 and health effects at high concentrations29; thus, the observed concentration changes may have been at levels too high to effect measurable biomarker changes. Counterintuitively, these pollutants even showed beneficial associations with 8-OHdG level (with 24-hour PM2.5 concentration only), sCD62P level (with 2-week SO2 concentration only), and SBP level (with 24-hour PM2.5 and 2-week NO2 concentrations only) that were robust to controlling for copollutants and multiple testing correction. Given that protective effects are not expected according to the literature and biological plausibility, it is likely that these seemingly beneficial associations are artifacts of these pollutants being positively correlated with some other factors that were negatively correlated with the biomarkers.
It is possible that pollutants correlated with O3 exposure, including O3-derived products, could also be implicated in these novel associations between low-level O3 exposure and cardiovascular disease risk biomarkers. Ozone reacts quickly with various unsaturated organic compounds in the indoor environment to produce highly reactive gaseous and condensed phase products, such as ultrafine particles.49 Although the evidence for cardiopulmonary effects of exposure to O3-initiated reaction products is inconclusive at environmental concentrations,50 there is evidence supporting a link between ultrafine particles and cardiovascular outcomes, including platelet activation and SBP level.47 However, blood pressure has also been associated with personal exposure to O3 independent of ultrafine particle concentrations.40 Ultimately, this study cannot determine whether the observed associations between cardiovascular outcomes and O3 are caused by the effects of O3 exposure itself or its reaction products.
Limitations
This natural experiment used the unique exposure conditions of participants spending most of their time in controlled indoor environments. However, it has several limitations. It is impossible to completely rule out the influence of potential uncontrolled confounders, limiting the ability to make causal determinations because of the reliance on uncontrolled longitudinal associations. In addition, our estimates of 2-week exposure concentrations were based on data from four 24-hour questionnaires, a method that is less precise than measuring exposure over a full 2-week period but more precise than simply using ambient concentrations. Although the 24-hour O3, PM2.5, NO2, and SO2 exposures were well characterized, unmeasured pollutants (eg, products of O3-initiated reactions) or other unmeasured factors may explain more of the variation in the observed biomarker-pollutant relationships. Uncontrolled residual confounding may have influenced the pollutant-biomarker associations observed in the study, including those counterintuitive associations.
Conclusions
This study provides mechanistic support to previously observed associations between low-level O3 exposure and cardiovascular disease outcomes. The findings offer insights into possible underlying biological mechanisms, namely, platelet activation and increases in blood pressure. Given that global tropospheric O3 concentration is rising,51 it is imperative to determine how to minimize its harms on health.
eMethods. Supplementary Methods
eTable 1. Study Subject Characteristics Between Groups With Different Filtration Conditions
eTable 2. Filtration- and Location-Specific PM2.5 I/O Ratios
eTable 3. Key Parameter Values for Estimating the Contribution of the ESP to Indoor O3
eTable 4. Spearman Correlation Coefficients for Outdoor Pollutant Concentrations and Exposure Measures
eTable 5. Covariates Used in the Models for Each Biomarker
eFigure 1. All Single and Two-Pollutant Model Results for O3
eFigure 2. All Single and Two-Pollutant Model Results for PM2.5
eFigure 3. All Single and Two-Pollutant Model Results for NO2
eFigure 4. All Single and Two-Pollutant Model Results for SO2
References
- 1.Jerrett M, Burnett RT, Pope CA III, et al. Long-term ozone exposure and mortality. N Engl J Med. 2009;360(11):1085-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarnat SE, Winquist A, Schauer JJ, Turner JR, Sarnat JA. Fine particulate matter components and emergency department visits for cardiovascular and respiratory diseases in the St. Louis, Missouri-Illinois, metropolitan area. Environ Health Perspect. 2015;123(5):437-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crouse DL, Peters PA, Hystad P, et al. Ambient PM2.5, O3, and NO2 Exposures and Associations with Mortality over 16 Years of Follow-Up in the Canadian Census Health and Environment Cohort (CanCHEC). Environ Health Perspect. 2015;123(11):1180-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turner MC, Jerrett M, Pope CA III, et al. Long-Term Ozone Exposure and Mortality in a Large Prospective Study. Am J Respir Crit Care Med. 2016;193(10):1134-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoek G, Brunekreef B, Fischer P, van Wijnen J. The association between air pollution and heart failure, arrhythmia, embolism, thrombosis, and other cardiovascular causes of death in a time series study. Epidemiology. 2001;12(3):355-357. [DOI] [PubMed] [Google Scholar]
- 6.Hong YC, Lee JT, Kim H, Ha EH, Schwartz J, Christiani DC. Effects of air pollutants on acute stroke mortality. Environ Health Perspect. 2002;110(2):187-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipsett MJ, Ostro BD, Reynolds P, et al. Long-term exposure to air pollution and cardiorespiratory disease in the California teachers study cohort. Am J Respir Crit Care Med. 2011;184(7):828-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stieb DM, Judek S, Burnett RT. Meta-analysis of time-series studies of air pollution and mortality: effects of gases and particles and the influence of cause of death, age, and season. J Air Waste Manag Assoc. 2002;52(4):470-484. [DOI] [PubMed] [Google Scholar]
- 9.Bentayeb M, Wagner V, Stempfelet M, et al. Association between long-term exposure to air pollution and mortality in France: a 25-year follow-up study. Environ Int. 2015;85:5-14. [DOI] [PubMed] [Google Scholar]
- 10.Carey IM, Atkinson RW, Kent AJ, van Staa T, Cook DG, Anderson HR. Mortality associations with long-term exposure to outdoor air pollution in a national English cohort. Am J Respir Crit Care Med. 2013;187(11):1226-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown J, Bowman C. Integrated Science Assessment for Ozone and Related Photochemical Oxidants. Washington, DC: US Environmental Protection Agency; 2013. [Google Scholar]
- 12.Zhang J, Zhu T, Kipen H, et al. ; HEI Health Review Committee . Cardiorespiratory biomarker responses in healthy young adults to drastic air quality changes surrounding the 2008 Beijing Olympics. Res Rep Health Eff Inst. 2013;(174):5-174. [PMC free article] [PubMed] [Google Scholar]
- 13.Xiang J, Weschler CJ, Mo J, Day D, Zhang J, Zhang Y. Ozone, electrostatic precipitators, and particle number concentrations: correlations observed in a real office during working hours. Environ Sci Technol. 2016;50(18):10236-10244. [DOI] [PubMed] [Google Scholar]
- 14.Liang Y, Yeligar SM, Brown LA. Exhaled breath condensate: a promising source for biomarkers of lung disease. ScientificWorldJournal. 2012;2012:217518. doi: 10.1100/2012/217518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones SL, Kittelson J, Cowan JO, et al. The predictive value of exhaled nitric oxide measurements in assessing changes in asthma control. Am J Respir Crit Care Med. 2001;164(5):738-743. [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107(3):363-369. [DOI] [PubMed] [Google Scholar]
- 17.Ren C, Fang S, Wright RO, Suh H, Schwartz J. Urinary 8-hydroxy-2′-deoxyguanosine as a biomarker of oxidative DNA damage induced by ambient pollution in the Normative Aging Study. Occup Environ Med. 2011;68(8):562-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sesso HD, Stampfer MJ, Rosner B, et al. Systolic and diastolic blood pressure, pulse pressure, and mean arterial pressure as predictors of cardiovascular disease risk in men. Hypertension. 2000;36(5):801-807. [DOI] [PubMed] [Google Scholar]
- 19.Laurent S, Cockcroft J, Van Bortel L, et al. ; European Network for Non-invasive Investigation of Large Arteries . Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588-2605. [DOI] [PubMed] [Google Scholar]
- 20.Blann AD, Nadar SK, Lip GY. The adhesion molecule P-selectin and cardiovascular disease. Eur Heart J. 2003;24(24):2166-2179. [DOI] [PubMed] [Google Scholar]
- 21.Sonneveld MA, Cheng JM, Oemrawsingh RM, et al. Von Willebrand factor in relation to coronary plaque characteristics and cardiovascular outcome. Results of the ATHEROREMO-IVUS study. Thromb Haemost. 2015;113(3):577-584. [DOI] [PubMed] [Google Scholar]
- 22.Wang CJ, Yang NH, Chang CC, Liou SH, Lee HL. Rapid and simple one-step membrane extraction for the determination of 8-hydroxy-2′-deoxyguanosine in human plasma by a combination of on-line solid phase extraction and LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(30):3538-3543. [DOI] [PubMed] [Google Scholar]
- 23.Nazaroff WW, Gadgil AJ, Weschler CJ. Critique of the use of deposition velocity in modeling indoor air-quality. In: Nagda NL, ed. Modeling of Indoor Air Quality and Exposure. West Conshohocken, PA: American Society of Testing & Material; 1993:81-104. [Google Scholar]
- 24.Yocom JE. Indoor-outdoor air quality relationships: a critical review. J Air Pollut Control Assoc. 1982;32(5):500-520. [DOI] [PubMed] [Google Scholar]
- 25.Chen C, Zhao B. Review of relationship between indoor and outdoor particles: I/O ratio, infiltration factor and penetration factor. Atmos Environ. 2011;45(2):275-288. doi: 10.1016/j.atmosenv.2010.09.048 [DOI] [Google Scholar]
- 26.Weschler CJ. Ozone in indoor environments: concentration and chemistry. Indoor Air. 2000;10(4):269-288. [DOI] [PubMed] [Google Scholar]
- 27.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29(4):1165-1188. [Google Scholar]
- 28.Chuang KJ, Chan CC, Su TC, Lee CT, Tang CS. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med. 2007;176(4):370-376. [DOI] [PubMed] [Google Scholar]
- 29.Pope CA III, Burnett RT, Turner MC, et al. Lung cancer and cardiovascular disease mortality associated with ambient air pollution and cigarette smoke: shape of the exposure-response relationships. Environ Health Perspect. 2011;119(11):1616-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burnett RT, Stieb D, Brook JR, et al. Associations between short-term changes in nitrogen dioxide and mortality in Canadian cities. Arch Environ Health. 2004;59(5):228-236. [DOI] [PubMed] [Google Scholar]
- 31.Pope CA III, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287(9):1132-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.US Environmental Protection Agency . Policy Assessment for the Review of the Ozone National Ambient Air Quality Standards. Research Triangle Park, NC: US Environmental Protection Agency, Office of Air and Radiation, Office of Air Quality Planning and Standards, Health and Environmental Impacts Division, Ambient Standards Group; 2014. [Google Scholar]
- 33.Vandy FC, Stabler C, Eliassen AM, et al. Soluble P-selectin for the diagnosis of lower extremity deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2013;1(2):117-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blomberg A, Mudway IS, Nordenhäll C, et al. Ozone-induced lung function decrements do not correlate with early airway inflammatory or antioxidant responses. Eur Respir J. 1999;13(6):1418-1428. [DOI] [PubMed] [Google Scholar]
- 35.Kafoury RM, Pryor WA, Squadrito GL, Salgo MG, Zou X, Friedman M. Induction of inflammatory mediators in human airway epithelial cells by lipid ozonation products. Am J Respir Crit Care Med. 1999;160(6):1934-1942. [DOI] [PubMed] [Google Scholar]
- 36.Green R, Broadwin R, Malig B, et al. Long- and short-term exposure to air pollution and inflammatory/hemostatic markers in midlife women. Epidemiology. 2016;27(2):211-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao D, Heiss G, Chinchilli VM, et al. Association of criteria pollutants with plasma hemostatic/inflammatory markers: a population-based study. J Expo Anal Environ Epidemiol. 2005;15(4):319-328. [DOI] [PubMed] [Google Scholar]
- 38.Brook RD, Urch B, Dvonch JT, et al. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension. 2009;54(3):659-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffmann B, Luttmann-Gibson H, Cohen A, et al. Opposing effects of particle pollution, ozone, and ambient temperature on arterial blood pressure. Environ Health Perspect. 2012;120(2):241-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weichenthal S, Hatzopoulou M, Goldberg MS. Exposure to traffic-related air pollution during physical activity and acute changes in blood pressure, autonomic and micro-vascular function in women: a cross-over study. Part Fibre Toxicol. 2014;11:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paffett ML, Zychowski KE, Sheppard L, et al. Ozone inhalation impairs coronary artery dilation via intracellular oxidative stress: evidence for serum-borne factors as drivers of systemic toxicity. Toxicol Sci. 2015;146(2):244-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verhaar MC, Beutler JJ, Gaillard CA, Koomans HA, Fijnheer R, Rabelink TJ. Progressive vascular damage in hypertension is associated with increased levels of circulating P-selectin. J Hypertens. 1998;16(1):45-50. [DOI] [PubMed] [Google Scholar]
- 43.Khan A, Staimer N, Tjoa T, Galassetti P, Blake DR, Delfino RJ. Relations between isoprene and nitric oxide in exhaled breath and the potential influence of outdoor ozone: a pilot study. J Breath Res. 2013;7(3):036007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inoue H, Aizawa H, Nakano H, et al. Nitric oxide synthase inhibitors attenuate ozone-induced airway inflammation in guinea pigs: possible role of interleukin-8. Am J Respir Crit Care Med. 2000;161(1):249-256. [DOI] [PubMed] [Google Scholar]
- 45.Huang W, Wang G, Lu SE, et al. Inflammatory and oxidative stress responses of healthy young adults to changes in air quality during the Beijing Olympics. Am J Respir Crit Care Med. 2012;186(11):1150-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jang AS, Choi IS, Lee JU. Neuronal nitric oxide synthase is associated with airway obstruction in BALB/c mice exposed to ozone. Respiration. 2003;70(1):95-99. [DOI] [PubMed] [Google Scholar]
- 47.Gong J, Zhu T, Kipen H, et al. Comparisons of ultrafine and fine particles in their associations with biomarkers reflecting physiological pathways. Environ Sci Technol. 2014;48(9):5264-5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lenters V, Uiterwaal CS, Beelen R, et al. Long-term exposure to air pollution and vascular damage in young adults. Epidemiology. 2010;21(4):512-520. [DOI] [PubMed] [Google Scholar]
- 49.Weschler CJ. Ozone’s impact on public health: contributions from indoor exposures to ozone and products of ozone-initiated chemistry. Environ Health Perspect. 2006;114(10):1489-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rohr AC. The health significance of gas- and particle-phase terpene oxidation products: a review. Environ Int. 2013;60:145-162. [DOI] [PubMed] [Google Scholar]
- 51.Blunden J, Arndt DS. State of the climate in 2014. Bull Am Meteorol Soc. 2015;96(7):ES1-ES32. doi: 10.1175/2015BAMSStateoftheClimate.1 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplementary Methods
eTable 1. Study Subject Characteristics Between Groups With Different Filtration Conditions
eTable 2. Filtration- and Location-Specific PM2.5 I/O Ratios
eTable 3. Key Parameter Values for Estimating the Contribution of the ESP to Indoor O3
eTable 4. Spearman Correlation Coefficients for Outdoor Pollutant Concentrations and Exposure Measures
eTable 5. Covariates Used in the Models for Each Biomarker
eFigure 1. All Single and Two-Pollutant Model Results for O3
eFigure 2. All Single and Two-Pollutant Model Results for PM2.5
eFigure 3. All Single and Two-Pollutant Model Results for NO2
eFigure 4. All Single and Two-Pollutant Model Results for SO2